Abstract
Background
Most evidence of target‐organ damage in hypertension (HTN) is related to the kidneys and heart. Cerebrovascular and cognitive impairment are less well studied. Therefore, this study analyzed changes in cognitive function in patients with different stages of hypertension compared to nonhypertensive controls.
Methods and Results
In a cross‐sectional study, 221 (71 normotensive and 150 hypertensive) patients were compared. Patients with hypertension were divided into 2 stages according to blood pressure (BP) levels or medication use (HTN‐1: BP, 140–159/90–99 or use of 1 or 2 antihypertensive drugs; HTN‐2: BP, ≥160/100 or use of ≥3 drugs). Three groups were comparatively analyzed: normotension, HTN stage 1, and HTN stage 2. The Mini–Mental State Examination, Montreal Cognitive Assessment, and a validated comprehensive battery of neuropsychological tests that assessed 6 main cognitive domains were used to determine cognitive function. Compared to the normotension and HTN stage‐1, the severe HTN group had worse cognitive performance based on Mini–Mental State Examination (26.8±2.1 vs 27.4±2.1 vs 28.0±2.0; P=0.004) or Montreal Cognitive Assessment (23.4±3.7 vs 24.9±2.8 vs 25.5±3.2; P<0.001). On the neuropsychological tests, patients with hypertension had worse performance in language, processing speed, visuospatial abilities, and memory. Age, hypertension stage, and educational level were the best predictors of cognitive impairment in patients with hypertension in different cognitive domains.
Conclusions
Cognitive impairment was more frequent in patients with hypertension, and this was related to hypertension severity.
Keywords: cognition, cognitive impairment, high blood pressure, hypertension
Subject Categories: Hypertension, High Blood Pressure, Cognitive Impairment
Introduction
Increased blood pressure (BP) is the leading risk factor for premature death, stroke, and heart disease worldwide.1 In 2000, the world was estimated to have nearly 1 billion people with hypertension (HTN) with an increase to 1.56 billion by 2025.2
Prevalence of dementia increases exponentially with increasing age,3 doubling with every 5‐year increase after age 65. In higher‐income countries, prevalence is 5% to 10% in those aged 65+ years and is usually greater among women than men, primarily because women live longer. Within the United States, a higher prevalence has been reported in African‐American and Latino/Hispanic than in white non‐Hispanic populations. Global systematic reviews and meta‐analyses suggest that the prevalence of dementia is lower in sub‐Saharan Africa and higher in Latin America than in the rest of the world.3, 4 According to Chaves et al,5 in Brazil the incidence of mild cognitive impairment (MCI) is 13.2 per 1000 person‐years and for Alzheimer's disease (AD) is 14.8 per 1000 person‐years.
MCI is an intermediate state between normal cognition and dementia. Although specific changes in cognition are observed in normal aging, there is increasing evidence that some forms of cognitive impairment are recognizable as an early manifestation of dementia.6 Several criteria for, and subtypes of, MCI have been proposed.7 In general, these criteria include a measurable deficit in cognition in at least 1 domain, in the absence of dementia or impairment in activities of daily living.
Both HTN and dementia are common in elderly individuals.8 Among people aged ≥65 years, the prevalence of dementia is ≈8%, and the prevalence of HTN is ≈65%.2, 9 The relation of BP with cognitive function and dementia has recently received much attention in epidemiological studies.10 The findings of cross‐sectional studies of BP and cognitive function have varied greatly in their results. Some studies found higher rates of cognitive impairment associated with elevated BP,11 others with low BP,12 and others documented a U‐shaped relationship.13
Epidemiological data from the Framingham study14 has suggested that no association exists between BP and cognitive performance measured concurrently; however, when the data were reanalyzed longitudinally, the average BP over 20 years was inversely related to cognitive performance.15 Two further longitudinal studies have shown a link between midlife or later‐life HTN and subsequent cognitive impairment.11, 16
Reasons for discrepancies between cross‐sectional and longitudinal studies may include the tendency for BP levels to change with onset of dementia,16, 17 inclusion of individuals with established cerebrovascular disease in cross‐sectional studies, and effects of BP‐lowering therapy on cognitive function. Except for 1 small study with 25 older patients with severe HTN, there are no data on cognitive function in either older or younger patients with HTN where the effects of cerebrovascular disease have been excluded18 and the severity of HTN has been addressed in the analyses.
The hypothesis of the present study was that (1) cognitive performance in patients with HTN without manifested cerebrovascular disease would be more impaired than that in normotensive individuals and (2) worse cognitive scores would be observed with increasing BP level.
Methods
Of ≈724 patients (199 normotensive and 525 hypertensive) screened for this cross‐sectional study, we enrolled 221 (71 normotensive and 150 hypertensive) patients. Cognitive function was assessed using the Mini–Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA), and a battery of neuropsychological tests.
Patients from the Hypertension Unit of the Heart Institute of São Paulo University (São Paulo, Brazil) were consecutively recruited from June 2013 to December 2015. The normotension group participants were recruited from patients without cardiovascular disease followed yearly at the Heart Institute as part of a protocol for cardiovascular assessment.19
Patients with the following conditions were excluded: aged <18 years; overt cerebrovascular disease (previous stroke or transient ischemic attack); diabetes mellitus; current smoker; arrhythmias; heart failure with left ventricular dysfunction; known neurodegenerative or psychiatric disease; and illiteracy. The number of school years completed was used to assess educational level. The local ethics committee approved the protocol, and all participants gave written informed consent.
BP Measurement
Brachial systolic (SBP) and diastolic BP (DBP) was assessed with an Omron automatic device (HEM‐705 CP model) in the right upper arm, with the subject seated, after resting for 5 minutes following the recommendations of the VI Brazilian HTN guidelines.20 A mean of 3 measurements with a 1‐minute interval between was calculated and used to determine SBP and DBP in each patient.
Patients with HTN were divided into 2 levels of HTN severity, according to their BP levels or medication use (HTN‐1: BP, 140–159/90–99 or use of 1 or 2 antihypertensive drugs; HTN‐2: BP, ≥160/100 or use of ≥3 drugs). Three groups were comparatively analyzed: normotension, HTN stage 1, and HTN stage 2.
Cognitive Function Evaluation
Mini–Mental State Examination
The MMSE is a commonly used 30‐point scale for assessing cognitive function in orientation, registration, attention and calculation, recall, language, and praxis. MMSE administration was performed according to existing standards.21 Because participants had heterogeneous educational levels, cutoffs were adjusted to the level of education. The following cutoff scores were used to identify abnormal cognition in this study: ≤21 for the patients with <8 years of education; ≤23 for those with 9–11 years of education; and ≤25 for those with ≥12 years of education.22
Montreal Cognitive Assessment
The MoCA is a rapid screening instrument to identify MCI.23 It assesses attention and concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculations, and orientation. The total possible score is 30 points, and a score of ≥26 is considered normal. A previous validation study in Brazil suggested 25 points as the ideal cutoff for MCI identification.24 To counterbalance the effect of lower education, 1 point was added to the final score of those individuals with <12 years of education.
Neuropsychological Evaluation
The neuropsychological tests were chosen according to the guidelines offered by the National Institute of Neurological Disorders and Stroke‐Canadian Stroke Network standards25 and by the recommendations of the Brazilian Academy of Neurology.26
Procedures and descriptions of the neuropsychological tests used have been published elsewhere.27, 28, 29, 30, 31, 32, 33, 34, 35 The neuropsychological test battery included the Boston Naming Test (BNT),27 Rey Auditory Verbal Learning Test (RAVLT5 = sum of 5 recall trials of 15 words; RAVLT6 = immediate recall after inference; and RAVLT7 = delayed recall after 30 minutes),31 the Rey‐Osterrieth Complex Figure Test copy and delayed recall (REY‐C and REY‐30),32 Semantic Verbal Fluency animal category (VF),28 Phonological Verbal Fluency (FAS),30 Forward and Backward Digit Span Test (FDST and BDST),35 Trail Making Test part A and B (TMT‐A and TMT‐B),29 Clock Drawing Test (CDT),33 and Digit Symbols Substitution Test (DSST).34
We computed scores for global cognition (mean z score of the BNT, RAVLT5, RAVLT6, RAVLT7, REY‐C, REY‐30, VF, FAS, FDST, BDST, TMT‐A, TMT‐B, CDT, and DSST), language (BNT), memory (mean z score of RAVLT5, RAVLT6, RAVLT7, and REY‐30), executive functioning (mean z score of VF, FAS, BDST, and TMT‐B), visuospatial abilities (mean z score of REY‐C and CDT), attention (mean z score of FDST and TMT‐A), and processing speed (DSST).
Statistical Analysis
Data were analyzed with SPSS software (SPSS for Windows 21.0, SPSS, Inc., Chicago, IL). Data distribution was determined using the Kolmogorov–Smirnov test. Continuous variables are presented as mean and SD or as median and range if they are not normally distributed and were analyzed by the independent‐samples t test and Mann–Whitney U test, when suitable. Categorical data are presented as percentages. An ANOVA test was used with Bonferroni post‐hoc comparisons for continuous variables. The Kruskal–Wallis test was used for categorical variables. Pearson's coefficient was used for bivariate correlations. In multivariable regression analyses, all variables with P<0.1 on unadjusted analysis were selected for multivariable linear step‐wise analysis. To account for group differences in education, this variable was included as a covariate in the models. Statistical significance was set at 5%.
Z scores were calculated using normotension as the reference group. Participants were considered to have cognitive impairment if they had scores below −1.5 SD in 1 or more cognitive domains.
Results
In a cross‐sectional study, 221 patients (71 normotensive and 150 hypertensive) were compared. Figure 1 presents the flow chart of the study and the main exclusion reasons. Baseline characteristics are described in Table 1. The 3 groups were similar regarding age, but compared to the normotension and HTN stage 1 groups, the HTN stage 2 group had higher body mass index (BMI) and lower educational level and family income. Diuretics were the most frequently prescribed drugs, followed by calcium‐channel blockers.
Figure 1.
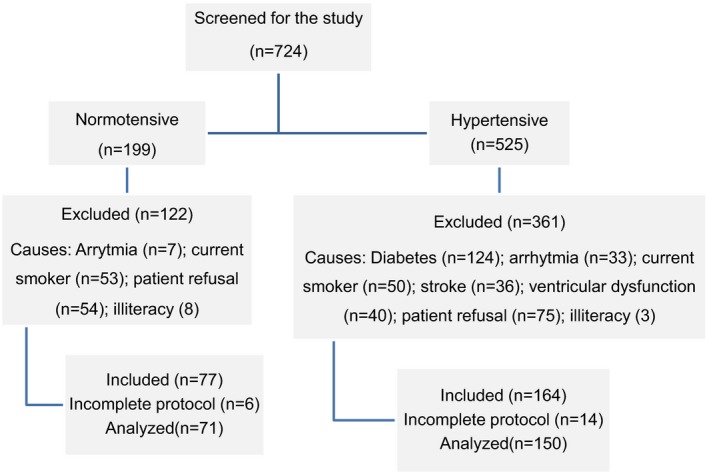
Flow chart of the study population. Patients who did not perform complete neuropsychological protocol have been withdrawn from the final analysis.
Table 1.
Demographic, Social, and Clinical Characteristics of Study Participants
| Variable | Normotension (71) | HTN Stage 1 (89) | HTN Stage 2 (61) | P Value |
|---|---|---|---|---|
| Age, mean±SD, y | 52.1±13.8 | 52.3±12.9 | 51.0±10.5 | 0.82 |
| Sex, male, n (%) | 33 (46.5) | 39 (43.8) | 28 (45.9) | 0.94 |
| Race, white, n (%) | 46 (64.8) | 69 (77.5) | 36 (59.0) | 0.038a |
| Married, n (%) | 33 (50.7) | 59 (66.3) | 33 (54.1) | 0.080 |
| Weight, mean±SD, kg | 74.7±16.3 | 77.8±14.6 | 83.2±13.5 | 0.005b |
| Height, mean±SD, m | 1.7±0.1 | 1.7±0.1 | 1.7±0.1 | 0.56 |
| BMI, mean±SD, kg/m2 | 26.9±4.2 | 28.7±4.9 | 30.4±4.6 | <0.001c |
| Education, mean±SD, yr | 12.9±4.0 | 11.3±4.2 | 10.0±4.4 | <0.001c |
| Monthly income, median (range), Rd | 3000.0 (730–20 000) | 2000.0 (600–14 000) | 1900.0 (500–20 000) | <0.001c |
| SBP, mean±SD, mm Hg | 122.1±8.3 | 134.6±13.2 | 150.1±27.9 | <0.001e |
| DBP, mean±SD, mm Hg | 76.7±6.9 | 82.5±9.5 | 92.2±15.2 | <0.001e |
| HTN time, median (range), yr | — | 5.0 (1–33) | 10.0 (1–37) | <0.001 |
| Number of drugs, mean±SD | — | 1.4±0.8 | 3.9±1.2 | <0.001 |
| Controlled HTN, n (%) | — | 50 (56.2) | 22 (36.1) | <0.001 |
| Most‐used drugs | — | |||
| ARB, n (%) | — | 30 (33.7) | 31 (50.8) | 0.037 |
| ACEI, n (%) | — | 31 (34.8) | 28 (45.9) | 0.17 |
| Diuretics, n (%) | — | 41 (46.1) | 55 (90.2) | <0.001 |
| CCB, n (%) | — | 10 (11.2) | 48 (78.7) | <0.001 |
| BB, n (%) | — | 10 (11.2) | 38 (62.3) | <0.001 |
P value refers to comparisons of the means or proportions among the groups by the 1‐way ANOVA, Kruskal–Wallis, and Mann–Whitney tests. ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BB, beta blockers; BMI, body mass index; CCB, calcium‐channel blockers; DBP, diastolic blood pressure; HTN, hypertension; SBP, systolic blood pressure.
HTN‐1 versus HTN‐2.
Normotension versus HTN‐2.
Normotension versus HTN‐1 and HTN‐2.
R (1 USD=3.3 Real).
All groups different.
MoCA and MMSE Scores
The cognitive profile according to the patient's BP level is presented in Table 2. The HTN stage 2 group performed worse than the HTN stage 1 and normotensive individuals did on the MoCA (HTN‐2, mean=23.4±3.7; HTN‐1, mean=24.9±2.8; normotension, mean=25.5±3.2, P=0.004) and on the MMSE (HTN‐2, mean=26.8±2.1; HTN‐1, mean=27.4±2.1; normotension, mean=28.0±2.0, P=0.001).
Table 2.
Cognitive Performance According to Blood Pressure Level
| Variable, Mean±SD | Normotension (71) | HTN Stage 1 (89) | HTN Stage 2 (61) | P Value |
|---|---|---|---|---|
| MMSE | 27.99±1.99 | 27.45±2.04 | 26.66±2.13 | 0.001a |
| MoCA | 25.48±3.21 | 24.93±2.83 | 23.36±3.60 | 0.001b |
| Language | ||||
| BNT | −0.004±0.99 | −0.33±1.24 | −0.44±1.19 | 0.069 |
| Memory | ||||
| RAVLT5 (sum of 5 trials) | 0.001±0.10 | −0.08±1.23 | −0.64±1.18 | 0.003b |
| RAVLT6 | 0.001±1.00 | −0.23±1.40 | −0.71±1.33 | 0.006a |
| RAVLT7 | 0.001±1.00 | −0.14±1.17 | −0.48±1.17 | 0.045a |
| REY‐30 | 0.003±1.00 | −0.09±0.98 | −0.43±0.86 | 0.028a |
| Executive function | ||||
| Verbal fluency animal | −0.001±1.00 | −0.18±0.95 | −0.81±0.69 | <0.001b |
| Backward digit span | −0.006±0.99 | −0.34±0.93 | −0.67±0.74 | <0.001c |
| TMT‐B | −0.01±0.97 | −0.25±0.81 | −0.54±1.17 | 0.009a |
| Phonological verbal fluency | −0.004±1.00 | −0.30±0.94 | −0.72±0.99 | <0.001b |
| Visuospatial abilities | ||||
| REY‐C | −0.004±1.00 | −0.29±1.02 | −0.41±1.16 | 0.071 |
| CDT | −0.003±1.00 | −0.22±1.27 | −0.29±1.15 | 0.32 |
| Attention | ||||
| Forward digit span | −0.002±0.99 | −0.05±0.97 | −0.38±0.65 | <0.035c |
| TMT‐A | 0.00±1.00 | 0.37±1.47 | −1.05±3.90 | 0.033a |
| Processing speed | ||||
| Digit symbol substitution test | 0.002±1.00 | −0.39±0.93 | −0.65±0.85 | <0.001c |
P value refers to comparisons of the means among the groups by the 1‐way ANOVA step‐wise test with Bonferroni post‐hoc analysis. All analyses were adjusted to the education level (years of education). BNT indicates Boston Naming Test; CDT, Clock Drawing Test; HTN, hypertension; MMSE, Mini–Mental State Examination; MoCA, Montreal Cognitive Assessment; RAVLT, Rey Auditory Verbal Learning Test (RAVLT5 = sum of 5 recall trials of 15 words; RAVLT6 = immediate recall after inference; and RAVLT7 = delayed recall after 30 minutes); REY‐C, Rey‐Osterrieth Complex Figure Test copy; REY‐30, Rey‐Osterrieth Complex Figure Test delayed recall; TMT‐A and TMT‐B, Trail Making Test part A and B.
Normotension versus HTN‐2.
HTN‐2 versus normotension and HTN‐1.
Control versus group 1 and group 2.
Neuropsychological Evaluation Performance
The normotensive group performed better than the HTN groups in the majority of cognitive tests, with significance differences mainly between the control and the most severe HTN group (Table 2). However, after the educational‐level adjustment, only the following variables retained statistical differences: MoCA (P=0.027); RAVLT5 (P=0.040); Animal Fluency Test (P<0.001); Backward Digit Span (P=0.024); phonological verbal fluency (P=0.021); and DSST (P=0.047).
Both MoCA and neuropsychological evaluation (NPE) were better screening tools than MMSE for cognitive impairment (Figure 2). The frequencies of cognitive impairment by different tools were, respectively, normotension (7%, 25%, and 24%); HTN stage 1 (9%, 33%, and 35%), and HTN stage 2 (14%, 50%, and 45%) for MMSE, MoCA, and NPE. Table 3 shows cognitive performance according to the cognitive domains investigated comparing the 3 groups. The HTN stage 2 group performed worse than the other 2 groups, with differences mainly observed in global cognitive function, memory, executive functioning, and processing speed. These differences remained significant even after adjustment for educational level: global function (P=0.002); memory (P=0.036); executive function (P<0.001); and processing speed (P=0.047).
Figure 2.
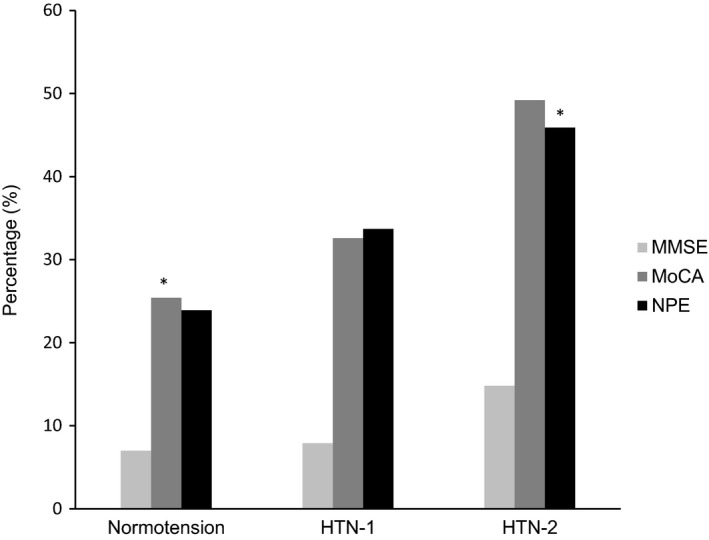
Percentage of participants with cognitive impairment. HTN indicates hypertension; MMSE, Mini–Mental State Examination; MoCA, Montreal Cognitive Assessment; NPE, neuropsychological evaluation. *Normotension versus HTN‐2 (P<0.05).
Table 3.
NPE Compound Scores in Specific Cognitive Domains According to Blood Pressure Levels
| Cognitive Domain, Mean±SD | Normotension (71) | HTN Stage 1 (89) | HTN Stage 2 (61) | P Value |
|---|---|---|---|---|
| Global cognitive function | −0.002±0.61 | −0.23±0.70 | −0.59±0.60 | <0.001a |
| Language | −0.004±0.99 | −0.33±1.24 | −0.44±1.19 | 0.069 |
| Memory | 0.007±0.77 | −1.13±1.03 | −0.56±0.92 | 0.002a |
| Executive function | −0.005±0.76 | −0.27±0.66 | −0.69±0.62 | <0.001a |
| Visuospatial abilities | −0.002±0.88 | −0.25±0.92 | −0.35±0.93 | 0.073 |
| Attention | −0.001±0.74 | −0.21±0.95 | −0.47±0.64 | 0.004b |
| Processing speed | 0.002±1.00 | −0.39±0.93 | −0.65±0.85 | <0.001c |
P value refers to comparisons of the means among the groups by the 1‐way ANOVA step‐wise test with Bonferroni post‐hoc analysis. All analyses were adjusted to education level (year of education). HTN indicates hypertension; NPE, neuropsychological evaluation.
HTN‐2 versus normotension and HTN‐1.
Normotension versus HTN‐2.
Normotension versus HTN‐1 and HTN‐2.
Alterations on NPE in patients with HTN were mainly because of the impairment in language (16%), followed by processing speed (14%), visuospatial abilities (13%), and memory (10%) domains.
Correlations Between Clinical and Cognitive Variables
Unadjusted analysis (Table 4) showed that family income and educational level positively correlated with cognitive function, mainly on MoCA and NPE tests. Conversely, age, HTN level, and SBP had negative correlations with cognitive tests in different domains. In the multivariable analysis (Table 5), age, educational level, and HTN stage independently predicted the cognitive impairment either on the MoCA or the NPE tests. The odds ratio of cognitive impairment on different tests in patients with HTN was, respectively, MMSE (1.58; 95% CI, 0.55–4.49); MoCA (1.91; 95% CI, 1.02–3.57), and NPE (2.00; 95% CI, 1.01–3.78).
Table 4.
Unadjusted Analysis Between Clinical Variables and Cognitive Tests in the Hypertensive Group
| Variable | MMSE | MoCA | Language | Memory | Executive Function | Visuospatial | Attention | Processing Speed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | |
| Age | −0.07 | 0.38 | −0.19 | 0.12 | −0.10 | 0.22 | −0.34 | <0.001 | −0.20 | 0.01 | −0.18 | 0.03 | −0.32 | <0.001 | −0.45 | <0.001 |
| HTN time | 0.02 | 0.78 | −0.06 | 0.46 | 0.09 | 0.30 | −0.14 | 0.10 | −0.02 | 0.81 | −0.16 | 0.04 | −0.14 | 0.09 | −0.09 | 0.28 |
| Family income | 0.14 | 0.08 | 0.24 | 0.004 | 0.27 | 0.001 | 0.11 | 0.17 | 0.31 | <0.001 | 0.14 | 0.09 | 0.22 | 0.006 | 0.11 | 0.17 |
| Education | 0.44 | <0.001 | 0.38 | <0.001 | 0.37 | <0.001 | 0.29 | <0.001 | 0.56 | <0.001 | 0.33 | <0.001 | 0.48 | <0.001 | 0.44 | <0.001 |
| Controlled BP | −0.14 | 0.19 | −0.17 | 0.04 | −0.07 | 0.43 | −0.12 | 0.13 | −0.05 | 0.54 | −0.06 | 0.47 | −0.06 | 0.49 | −0.10 | 0.22 |
| HTN stage | −0.19 | 0.02 | −0.24 | 0.003 | −0.04 | 0.59 | −0.21 | 0.01 | −0.31 | <0.001 | −0.05 | 0.53 | −0.15 | 0.06 | −0.14 | 0.09 |
| SBP | −0.11 | 0.18 | −0.18 | 0.03 | −0.08 | 0.34 | −0.10 | 0.24 | −0.21 | 0.76 | −0.04 | 0.59 | −0.08 | 0.31 | −0.04 | 0.64 |
| DBP | −0.03 | 0.97 | −0.08 | 0.36 | 0.01 | 0.93 | −0.01 | 0.87 | 0.01 | 0.92 | 0.05 | 0.52 | 0.04 | 0.65 | 0.12 | 0.16 |
BP indicates blood pressure; DBP, diastolic blood pressure; HTN, hypertension; SBP, systolic blood pressure; MMSE, Mini–Mental State Examination; MoCA, Montreal Cognitive Assessment.
Table 5.
Multivariable Linear Regression Analysis With Cognitive Parameters as Dependent Variables
| Variable | Parameter | ||
|---|---|---|---|
| B | 95% CI for B | P Value | |
| Memory | |||
| Age | −0.026 | (−0.039, −0.014) | <0.001 |
| Education level | 0.046 | (0.011, 0.081) | 0.011 |
| HTN stage | −0.403 | (−0.705, −0.101) | 0.013 |
| Executive function | |||
| Education level | 0.082 | (0.061, 0.103) | <0.001 |
| HTN stage | −0.315 | (−0.495, −0.134) | 0.001 |
| Visuospatial abilities | |||
| Education level | 0.087 | (0.066, 0.109) | <0.001 |
| Attention | |||
| Age | −0.016 | (−0.026, −0.006) | 0.001 |
| Education level | 0.084 | (0.057, 0.112) | <0.001 |
| Processing speed | |||
| Age | −0.028 | (−0.030, −0.018) | <0.001 |
| Education level | 0.077 | (0.048, 0.105) | <0.001 |
B indicates unstandardized model coefficients to indicate how much the dependent variable varies with an independent variable when all other independent variables are held constant. Consider the effect of age in this example. The unstandardized coefficient, B1, for age is equal to −0.026. This means that for each 1‐year increase in age, there is a decrease in memory of 0.026; HTN indicates hypertension.
A subanalysis of cognitive performance in those patients with high level of education (≥9 years schooling years) is presented in Table 6. Even in this subgroup of patients, where the education level might have a possible protective effect, cognitive performance worsened in line with the severity of BP level.
Table 6.
Cognitive Performance in Patients With High Level of Education (≥9 Years) According to the Blood Pressure Levels
| Cognitive Domain, Mean (SD) | Normotension (61) | HTN Stage 1 (64) | HTN Stage 2 (39) | P Value |
|---|---|---|---|---|
| MMSE | 28.30±1.77 | 27.88±1.82 | 27.36±1.97 | 0.048a |
| MoCA | 26.13±2.53 | 25.42±2.41 | 24.64±2.93 | 0.020a |
| Global cognitive function | 0.14±0.49 | −0.05±0.59 | −0.31±0.44 | <0.001b |
| Language | −0.09±0.89 | −0.08±0.95 | −0.28±1.29 | 0.21 |
| Memory | 0.14±0.70 | −0.04±1.04 | −0.31±0.76 | 0.047a |
| Executive function | −0.12±0.72 | −0.10±0.62 | −0.42±0.56 | <0.001c |
| Visuospatial abilities | 0.18±0.60 | −0.07±0.70 | −0.13±0.67 | 0.039a |
| Attention | 0.14±0.61 | −0.08±0.62 | −0.26±0.54 | 0.003c |
| Processing speed | 0.16±0.90 | −0.15±0.87 | −0.37±0.88 | 0.010a |
P value refers to comparisons of the means among the groups by the 1‐way ANOVA step‐wise test with Bonferroni post‐hoc analysis. All analyses were adjusted to education level (year of education). Global cognitive function on neuropsychological evaluation. HTN indicates hypertension; MMSE, Mini–Mental State Examination; MoCA, Montreal Cognitive Assessment.
Normotension versus HTN‐2.
Normotension versus HTN‐1 and HTN‐2.
HTN‐2 versus normotension and HTN‐1.
Discussion
Our data show that patients with HTN had poorer cognitive performance than controls in all cognitive tests, which seems to be related to severity of HTN. Age, HTN stage, and educational level were the variables that most robustly predicted cognitive impairment in our study.
The relation of HTN to cognitive function is frequently studied by comparing the cognitive performance of people with normal BP (or normotensives) with that of hypertensive patients at 1 time point. Commonly assessed cognitive functions include attention, learning and memory, executive functions (ie, self‐regulatory behaviors like planning and organization, mental flexibility, and response inhibition), visuospatial skills, psychomotor abilities, perceptual skills, and language abilities.36 Results of such studies indicate that increases in BP are associated with incremental reductions in cognitive function. Interestingly, several studies have instead found that low levels of BP are associated with poorer cognitive function. Indeed, some investigations found that both high and low BP were associated with lower levels of cognitive performance.37
The most convincing evidence of a relationship between HTN and cognitive deterioration is derived from a prospective study conducted in the 1960s, when antihypertensive treatment was still infrequent.38 The researchers suggested that “the basis for the cognitive decline associated with aging should be considered secondary to some pathologic processes and not merely as a ‘normal’ aging process.” Results from other studies assessing longitudinally measured BP point in the same direction. In the Honolulu‐Asia Aging Study, high midlife SBP was a predictor of reduced cognitive function in later life, when stroke cases were included.11 Similarly, in the Framingham study, untreated BP levels and chronicity of HTN were inversely related to the composite cognitive score.39
Education, HTN, and Cognitive Impairment
Our data also show that both HTN stage and educational level predicted cognitive impairment, with better performance occurring in those patients with a higher educational level.
Although the relationship between education and cognitive status is well known, evidence regarding whether education moderates the trajectory of cognitive change in later life is conflicting.40
The hypothesis of cognitive reserve asserts that older individuals with greater experiential resources exhibit better cognitive functioning and are able to tolerate higher levels of brain pathology before displaying clinical symptoms.41 One of the most well‐established proxy measures of reserve capacity in the elderly is educational attainment, which is thought to reflect the more‐effective use of brain networks or cognitive paradigms.42 In line with the hypothesis of cognitive reserve, many studies in both North America and Europe have suggested that educational attainment is associated with better cognitive performance and reduced risk for cognitive impairment and dementia in later life.43, 44 On the other hand, some studies found that education is not related to cognitive decline. In a large longitudinal cohort, education was related to cognitive performance, but unrelated to cognitive decline, supporting the hypothesis of passive cognitive reserve with aging.40
Whether HTN could moderate this hypothesis of cognitive reserve is unclear. However, in our analysis, we found that even those patients with higher educational levels (high school and college) had poorer cognitive performance when they were hypertensive. In general, patients with more‐severe hypertension had poorer cognitive performance than the patients in control and stage 1 group irrespective of their educational level.
Cognitive Functions at Different Domains
Alterations on NPE were mainly because of impairment in the language domain followed by impairment in processing speed, visuospatial abilities, and memory. Both NPE and MoCA were better as screening tools for identifying cognitive impairment than was MMSE.
In the early 1990s, the criteria for diagnosis of vascular dementia (VaD) were largely based on those used for AD, which emphasized memory impairment, irreversibility of the deficits, and impaired activities of daily living.45 This definition was felt to be restrictive, because it did not take into consideration cognitive deficits more commonly associated with cerebrovascular lesions, such as executive dysfunction and psychomotor slowing. Therefore, the term vascular cognitive impairment (VCI) was introduced to better reflect the full range of cognitive alterations resulting from vascular factors.46 The concept of VCI has gained wide acceptance and is currently defined as “a syndrome with evidence of clinical stroke or subclinical vascular brain injury and cognitive impairment affecting at least one cognitive domain,”47 VaD being the most severe form of VCI.
In patients with less‐severe cognitive impairment, a more‐distinctive cognitive profile might be discernable. Garrett et al48 reported that the neuropsychological performance of a small group of patients with cognitive impairment (but no dementia) was characterized by disproportionate executive dysfunction and deficits in verbal retrieval. Data from Looi and Sachdev49 also confirm an overall profile of less‐severe memory impairment and greater executive impairment in VaD.
Several investigators have reported a profile of memory impairment that includes better preservation of recognition memory performance relative to free recall in patients with VCI.50, 51 In studies of patients with CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy), who are younger and therefore less likely to have concomitant pathological AD changes, the speed of processing has consistently been identified as impaired, with somewhat less‐pronounced, but significant, deficits in areas of executive performance and attention. It has been suggested that this pattern of impairment represents the core of the cognitive syndrome associated with small‐vessel subcortical ischemic disease.52, 53, 54
Furthermore, in our study, although the use of 25 as the cut‐off point following the value proposed by Memória et al24 in the Brazilian population, the frequency of MCI screened by the MoCA was somewhat high. Whether the HTN status and its severity might have influenced this scenario is unclear.
Many studies have been performed for MoCA validation in different populations, and different cut‐off scores have been proposed.55, 56 Although the cut‐off point used for years of education in the original study on MoCA was 12 years,23 the same we used in the present study, Ng et al56 found that a 1‐point correction was needed in patients with <10 years of education. For these researchers, this change in cut‐off score from that in the original study was affected by the educational system in their country, where the average population spends 10 years in school to obtain a basic education. These differences in cut‐off scores proposed in many studies highlight the importance of conducting population‐specific validations of MoCA to maintain its effectiveness as a screening tool.
Limitations
There is always the possibility of residual confounding variables with observational studies. First, the primary limitation of the cross‐sectional study design is that because the exposure and outcome are simultaneously assessed, there is generally no evidence of a temporal relationship between exposure and outcome.57 That is, although the investigator may determine that there is an association between an exposure and an outcome, there is generally no evidence that the exposure caused the outcome. Of course, if the exposure is a characteristic such as sex or race and the outcome developed over time, the temporal nature of the exposure‐outcome association is more plausible; however, for studies in which the exposure is not an inherent trait, but one that developed over time, causality is often unclear. Second, our study sample is relatively small and our study was carried out in a select group of patients with HTN referred to a university hospital, limiting the generalizability of our findings to other populations. The absence of magnetic resonance imaging is another limitation of this study because we were not able to completely rule out subclinical cerebrovascular disease.
Conclusions
Our data demonstrate that cognitive impairment is more frequent in patients with HTN and is related to the severity of HTN. HTN stage and educational level were the best predictors for cognitive impairment in patients with HTN, and language, processing speed, visuospatial, and memory were the most affected domains.
Perspectives
The present findings support undertaking further studies to elucidate the mechanisms through which HTN is associated with cognitive impairment and intervention studies to determine whether better control of HTN could prevent cognitive decline and dementia. Although the relationship of HTN and cognitive impairment has been reported previously and gained great interest among researchers worldwide in recent years with conflicting results, to the best of our knowledge, studies addressing the severity of HTN and cognitive impairment are lacking. Our data indicate that not only the hypertensive condition, but also its severity is an important factor associated with cognitive impairment in young patients without previous manifested cerebrovascular disease and other factors affecting cognitive function.
Disclosures
This article is part of the doctoral thesis of the postgraduate Program in Cardiology, Faculty of Medicine, University of São Paulo. The authors declare that they have no other competing interests.
Acknowledgments
We appreciate Drs André Borba, Raul Feitosa, Silvia Merlim, Eduardo Sturzeneker Trés, and Ana Paula Gonçalves for their valuable contributions.
(J Am Heart Assoc. 2017;6:e004579. DOI: 10.1161/JAHA.116.004579.)
References
- 1. Joffres M, Falaschetti E, Gillespie C, Robitaille C, Loustalot F, Poulter N, McAlister FA, Johansen H, Baclic O, Campbell N. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross‐sectional study. BMJ Open. 2013;3:e003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 3. Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, dagnosis, and treatment. Clin Geriatr Med. 2014;30:421–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75.e2. [DOI] [PubMed] [Google Scholar]
- 5. Chaves ML, Camozzato AL, Godinho C, Piazenski I, Kaye J. Incidence of mild cognitive impairment and Alzheimer disease in Southern Brazil. J Geriatr Psychiatry Neurol. 2009;22:181–187. [DOI] [PubMed] [Google Scholar]
- 6. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 7. Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand. 2002;106:403–414. [DOI] [PubMed] [Google Scholar]
- 8. Tsivgoulis G, Alexandrov AV, Wadley VG, Unverzagt FW, Go RC, Moy CS, Kissela B, Howard G. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology. 2009;73:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fratiglioni L, De Ronchi D, Agüero‐Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15:365–375. [DOI] [PubMed] [Google Scholar]
- 10. Kuo HK, Sorond F, Iloputaife I, Gagnon M, Milberg W, Lipsitz LA. Effect of blood pressure on cognitive functions in elderly persons. J Gerontol A Biol Sci Med Sci. 2004;59:1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late‐life cognitive function. The Honolulu‐Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 12. Guo Z, Fratiglioni L, Winblad B, Viitanen M. Blood pressure and performance on the Mini‐Mental State Examination in the very old. Cross‐sectional and longitudinal data from the Kungsholmen Project. Am J Epidemiol. 1997;145:1106–1113. [DOI] [PubMed] [Google Scholar]
- 13. Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. [DOI] [PubMed] [Google Scholar]
- 14. Farmer ME, White LR, Abbott RD, Kittner SJ, Kaplan E, Wolz MM, Brody JA, Wolf PA. Blood pressure and cognitive performance. The Framingham Study. Am J Epidemiol. 1987;126:1103–1114. [DOI] [PubMed] [Google Scholar]
- 15. Farmer ME, Kittner SJ, Abbott RD, Wolz MM, Wolf PA, White LR. Longitudinally measured blood pressure, antihypertensive medication use, and cognitive performance: the Framingham Study. J Clin Epidemiol. 1990;43:475–480. [DOI] [PubMed] [Google Scholar]
- 16. Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Odén A, Svanborg A. 15‐year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 17. Harrington F, Saxby BK, McKeith IG, Wesnes K, Ford GA. Cognitive performance in hypertensive and normotensive older subjects. Hypertension. 2000;36:1079–1082. [DOI] [PubMed] [Google Scholar]
- 18. Kalra L, Jackson SH, Swift CG. Effect of antihypertensive treatment on psychomotor performance in the elderly. J Hum Hypertens. 1993;7:285–290. [PubMed] [Google Scholar]
- 19. Antelmi I, Chuang EY, Grupi CJ, Latorre Mdo R, Mansur AJ. Heart rate recovery after treadmill electrocardiographic exercise stress test and 24‐hour heart rate variability in healthy individuals. Arq Bras Cardiol. 2008;90:380–385. [DOI] [PubMed] [Google Scholar]
- 20. Sociedade Brasileira de Cardiologia, Sociedade Brasileira de Hipertensão, Sociedade Brasileira de Nefrologia . VI Brazilian guidelines on hypertension. Arq Bras Cardiol. 2010;95:1–51. [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 22. Brucki SM, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH. Suggestions for utilization of the mini‐mental state examination in Brazil. Arq Neuropsiquiatr. 2003;61:777–781. [DOI] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummimgs JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 24. Memória CM, Yassuda MS, Nakano EY, Forlenza OV. Brief screening for mild cognitive impairment: validation of the Brazilian version of the Montreal Cognitive Assessment. Int J Geriatr Psychiatry. 2013;28:34–40. [DOI] [PubMed] [Google Scholar]
- 25. Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke‐Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 26. Frota NAF, Nitrini R. Criteria for the diagnosis of Alzheimer's disease recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Group. 2011;5:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miotto EC, Sato J, Lucia MCS, Camargo CHP, Scaff M. Development of an adapted version of the Boston Naming Test for Portuguese speakers. Rev Bras Psiquiatr. 2010;32:279–282. [DOI] [PubMed] [Google Scholar]
- 28. Rosen WG. Verbal fluency in aging and dementia. J Clin Neuropsychol. 1980;2:135–146. [Google Scholar]
- 29. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed New York, NY: Oxford University Press; 2006:655–677. [Google Scholar]
- 30. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 31. Malloy‐Diniz LF, Lasmar VAP, Gazinelli Lde SR, Fuentes D, Salgado JV. The Rey Auditory‐Verbal Learning Test: applicability for the Brazilian elderly population. Rev Bras Psiquiatr. 2007;29:324–329. [DOI] [PubMed] [Google Scholar]
- 32. Shin M‐S, Park S‐Y, Park S‐R, Seol S‐H, Kwon JS. Clinical and empirical applications of the Rey‐Osterrieth Complex Figure Test. Nat Protoc. 2006;1:892–899. [DOI] [PubMed] [Google Scholar]
- 33. Aprahamian I, Martinelli JE, Neri AL, Yassuda MS. The accuracy of the Clock Drawing Test compared to that of standard screening tests for Alzheimer's disease: results from a study of Brazilian elderly with heterogeneous educational backgrounds. Int Psychogeriatr. 2010;22:64–71. [DOI] [PubMed] [Google Scholar]
- 34. Axelrod BN. Administration duration for the Wechsler Adult Intelligence Scale‐III and Wechsler Memory Scale‐III. Arch Clin Neuropsychol. 2001;16:293–301. [PubMed] [Google Scholar]
- 35. Leung JLM, Lee GTH, Lam YH, Chan RCC, Wu JYM. The use of the Digit Span Test in screening for cognitive impairment in acute medical inpatients. Int Psychogeriatr. 2011;23:1569–1574. [DOI] [PubMed] [Google Scholar]
- 36. Waldstein SR. The relation of hypertension to cognitive function. Curr Dir Psychol Sci. 2003;12:9–12. [Google Scholar]
- 37. Liu H, Gao S, Hall KS, Unverzagt FW, Lane KA, Callahan CM, Hendrie HC. Optimal blood pressure for cognitive function: findings from an elderly African‐American cohort study. J Am Geriatr Soc. 2013;61:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilkie F, Eisdorfer C. Intelligence and blood pressure in the aged. Science. 1971;172:959–962. [DOI] [PubMed] [Google Scholar]
- 39. Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138:353–364. [DOI] [PubMed] [Google Scholar]
- 40. Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SW, Manly JJ. Education does not slow cognitive decline with aging: 12‐year evidence from the Victoria Longitudinal Study. J Int Neuropsychol Soc. 2011;17:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scarmeas N, Stern Y. Cognitive reserve: implications for diagnosis and prevention of Alzheimer's disease. Curr Neurol Neurosci Rep. 2004;4:374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evans DA, Hebert LE, Beckett LA, Scherr PA, Albert MS, Chown MJ, Pilgrim DM, Taylor JO. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54:1399–1405. [DOI] [PubMed] [Google Scholar]
- 44. Prencipe M, Casini AR, Ferretti C, Lattanzio MT, Fiorelli M, Culasso F. Prevalence of dementia in an elderly rural population: effects of age, sex, and education. J Neurol Neurosurg Psychiatry. 1996;60:628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, Moody DM, O'Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E, Bermejo F, Wolf PA, Gorelick PB, Bick KL, Pajeau AK, Bell MA, DeCarli C, Culebras A, Korczyn AD, Bogousslavsky J, Hartmann A, Scheinberg P. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology. 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 46. Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garrett KD, Browndyke JN, Whelihan W, Paul RH, DiCarlo M, Moser DJ, Cohen RA, Ott BR. The neuropsychological profile of vascular cognitive impairment–no dementia: comparisons to patients at risk for cerebrovascular disease and vascular dementia. Arch Clin Neuropsychol. 2004;19:745–757. [DOI] [PubMed] [Google Scholar]
- 49. Looi JC, Sachdev PS. Differentiation of vascular dementia from AD on neuropsychological tests. Neurology. 1999;53:670–678. [DOI] [PubMed] [Google Scholar]
- 50. Tierney MC, Black SE, Szalai JP, Snow WG, Fisher RH, Nadon G, Chui HC. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Arch Neurol. 2001;58:1654–1659. [DOI] [PubMed] [Google Scholar]
- 51. Traykov L, Baudic S, Thibaudet MC, Rigaud AS, Smagghe A, Boller F. Neuropsychological deficit in early subcortical vascular dementia: comparison to Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14:26–32. [DOI] [PubMed] [Google Scholar]
- 52. Peters N, Opherk C, Danek A, Ballard C, Herzog J, Dichgans M. The pattern of cognitive performance in CADASIL: a monogenic condition leading to subcortical ischemic vascular dementia. Am J Psychiatry. 2005;162:2078–2085. [DOI] [PubMed] [Google Scholar]
- 53. Buffon F, Porcher R, Hernandez K, Kurtz A, Pointeau S, Vahedi K, Bousser MG, Chabria H. Cognitive profile in CADASIL. J Neurol Neurosurg Psychiatry. 2006;77:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O'Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry. 2004;75:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24:197–201. [DOI] [PubMed] [Google Scholar]
- 56. Ng A, Chew I, Narasimhalu K, Kandiah N. Effectiveness of Montreal Cognitive Assessment for the diagnosis of mild cognitive impairment and mild Alzheimer's disease in Singapore. Singapore Med J. 2013;54:616–619. [DOI] [PubMed] [Google Scholar]
- 57. Carlson MDA, Morrison RS. Study design, precision, and validity in observational studies. J Palliat Med. 2009;12:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


