Abstract
Background
Common carotid artery and internal carotid artery intima‐media thicknesses (IMT) are associated with coronary heart disease (CHD) and increase with age. Using age, sex, and race/ethnicity IMT percentiles may improve CHD prediction when added to Framingham risk factors and coronary artery calcium score. We study these possibilities in the Multi‐Ethnic Study of Atherosclerosis (MESA), a multi‐ethnic cohort of whites, Chinese, blacks, and Hispanics.
Methods and Results
IMT data were acquired in the age range 45 to 84 years. Common carotid artery and internal carotid artery IMT, sex, and race/ethnic specific normative values were calculated for each MESA participant and combined as an IMT score. Multivariable Cox‐proportional hazards models and logistic regression models were generated with CHD as outcome adding the IMT score to (1) a base model with Framingham risk factors, sex, race/ethnicity and (2) the base model with coronary artery calcium added. Harrell's C‐statistics and area under the curve were estimated. Median follow‐up was 10.2 years (interquartile range: 9.7, 10.7 years) with 429 first‐time CHD events. Mean age was 62.1 years and 52.6% of participants were women. IMT score increased the base area under the curve from 0.7210 to 0.7396 (P=0.0008) and with positive coronary artery calcium score added to the model, from 0.7627 to 0.7714 (P=0.02).
Conclusions
A carotid IMT score based on normative data incrementally adds to Framingham risk factors and a positive calcium score in predicting first‐time CHD in an ethnically diverse cohort.
Keywords: atherosclerosis, cardiovascular outcomes, carotid artery, coronary artery calcification, coronary artery disease, epidemiology, intima‐media thickness, risk assessment, risk factors, ultrasound
Subject Categories: Cardiovascular Disease, Risk Factors, Biomarkers, Ultrasound, Diagnostic Testing
Introduction
Common carotid artery (CCA) wall intima‐media thickness (IMT) is a noninvasive ultrasound measurement associated with cardiovascular events.1 IMT can be measured in the CCA and in the carotid bulb/proximal internal carotid artery (ICA). IMT measurements made at these 2 locations likely represent separate phenotypes since their patterns of associations with risk factors are different.2, 3, 4 For example, ICA IMT, a measurement that includes plaque, has shown stronger associations with coronary heart disease (CHD) events than CCA IMT.5, 6 These observations and the results of a recent meta‐analysis1 showing a lack of substantial improvement in CHD risk prediction after adding common carotid IMT alone to risk factors suggest that the role of ICA IMT needs further evaluation.
A plausible limitation to the use of common and internal carotid IMT as a clinical tool is the lack of age‐specific values.2, 7 Attempts to generate diagnostic cut points that account for age have previously focused on the common carotid IMT8, 9 and not the internal carotid IMT. Population‐based percentile values for anthropomorphic variables such as height and weight are routinely used to monitor growth. These age‐specific normative data are created using approaches that compensate for the often skewed distribution of these variables.10 Once these curves are generated, a given individual's value can be compared to peers of the same age while taking into consideration how the variable changes with age. This approach is also applicable to common and internal carotid IMT measurements and can be used to generate sex‐ and race‐ethnic specific normative values.
Another metric of subclinical cardiovascular disease, the Agatston coronary artery calcium (CAC) score, is strongly associated with CHD events.11 While CAC has a stronger association with cardiovascular events than carotid IMT,12 the question remains whether IMT offers incremental predictive value once CAC is accounted for.
We hypothesized that a combined common and ICA IMT percentile score would offer incremental value to Framingham CHD risk factors and CAC score for predicting incident coronary artery events. We pursued this hypothesis in a multi‐ethnic cohort of non‐Hispanic whites, blacks, Hispanics, and Chinese, the Multi‐Ethnic Study of Atherosclerosis (MESA).
Materials and Methods
Population
MESA enrolled 6814 men and women aged 45 to 84 years without a history of clinical cardiovascular disease at baseline between July 2000 and August 2002 at 6 US sites.13 The MESA cohort includes non‐Hispanic whites, blacks, Hispanic, and Chinese participants. Participants were excluded if they had a weight above 300 lb, were pregnant, or had any medical conditions that would prevent long‐term participation. The Institutional Review Boards of all collaborating institutions approved the study design. All participants gave informed consent.
Risk Factors and Anthropomorphic Variables
Age, sex, race/ethnicity, and medical history were self‐reported. Use of antihypertensive and lipid‐lowering medications was also recorded. Level of education was obtained and classified as the following: (1) less than high school, (2) high school, (3) college or equivalent, and (4) advanced degree. Current smoking was defined as self‐report of 1 or more cigarettes in the last 30 days. Seated resting systolic and diastolic blood pressures were measured as the average of the last 2 of 3 measurements made with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL).
Glucose and lipids were measured after a 12‐hour fast. Serum glucose was measured by rate reflectance spectrophotometry on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc, Rochester, NY). Diabetes mellitus was determined by the use of hypoglycemic medications or according to the 2003 American Diabetes Association fasting criteria (glucose values of 126 mg/dL or more).14 Total cholesterol was measured using a cholesterol oxidase method (Roche Diagnostics), as was high‐density lipoprotein after precipitation of non–high‐density lipoprotein cholesterol with magnesium/dextran.
Carotid Artery Measures
The participants were imaged supine with their head rotated 45° away from the side being imaged, and the images were recorded on superVHS videotape. The CCA was imaged at 45° from the vertical with the beginning of the bulb shown to the left of the image. The ICA was imaged in 3 projections centered on the ICA flow divider: anterior, lateral (at 45°), and posterior. Sonographers were instructed to make slight adjustments to the imaging plane in order to capture the largest wall thickness (plaque), whether it was located on the near or far wall of the carotid bulb or proximal ICA. A matrix array probe (M12L, General Electric, Waukesha, WI) was used with the frequency set at 13 MHz for the CCA and 9 MHz for the ICA, and with 2 focal zones at a frame rate of 32 frames‐per‐second.
Carotid artery measurements were blinded and made at the Ultrasound Reading Center in Boston, MA. Videotaped images were reviewed and image frames that showed clear wall interfaces on an image near to the smallest diameter (end‐diastole) of the artery were digitized into a workstation. Common carotid IMT was measured on near and far walls of the common carotid (1 projection) and the ICA (3 projections) using hand‐drawn continuous tracings of the intima‐lumen and media‐adventitia interfaces that were then processed using a previously described algorithm.15 The average of the mean far wall common carotid IMT and the maximum of the near and far wall internal carotid IMT values seen on either side or projection were used for these analyses.6
We calibrated the IMT measurements for interreader differences by adding previously determined bias terms to a given reader's measurements.16 Blinded replicate scans were performed on 150 participants read by the same readers; intraclass correlation coefficients were 0.92 for CCA IMT and 0.88 for ICA IMT. Interreader reproducibility was assessed on the image sets of 74 participants (intraclass correlation coefficients of 0.81 for CCA IMT and 0.88 for ICA IMT). All paired differences between sets of readings did not show significant divergence from 0.
Derivation of an IMT Score
All measured IMT values were fitted against age, separately for men and women, and for the 4 race/ethnicities, with a program to construct growth references using the LMS method (Pan H, Cole TJ. LMSchartmaker, Version 2.54; http://www.healthforallchildren.co.uk/; 2011). This method is used to generate normative data for anthropomorphic measurements.17 The resultant age‐specific numerical parameters were used to separately calculate the percentile level of the mean far‐wall CCA IMT and of the maximum ICA IMT corresponding to the IMT values at the participant's age. As an example, Figure 1A and 1B, respectively, show the race/ethnic 50% percentile of common and internal carotid IMT for men as a function of age. These 2 percentiles scores (scaled 0–1) were further averaged to yield a global IMT score ((CCA IMT percentile+ICA IMT percentile)/2).
Figure 1.
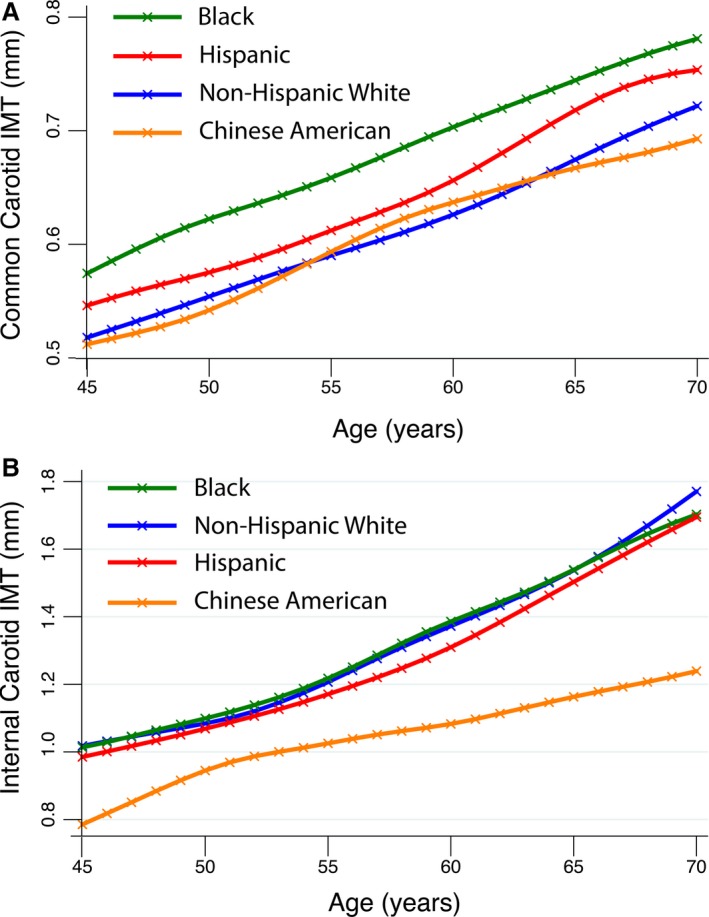
A, These 4 curves represent the fitted median common carotid far wall intima‐media thickness (IMT) values for the 4 race‐ethnicities that are part of the Multi‐Ethnic Study of Atherosclerosis (MESA). There are slight differences. Blacks have consistently higher values followed by Hispanics. Non‐Hispanic whites and Chinese Americans have similar and lower values. B, These 4 curves represent the fitted median internal carotid artery maximum IMT values for the 4 race‐ethnicities that are part of MESA. Non‐Hispanic whites, Hispanics, and blacks have near identical values. Chinese Americans consistently have the lowest values.
CAC Measurement
CAC was measured on cardiac‐gated chest computed tomographic images using either electron‐beam computed tomography scanners (3 centers) or a multidetector computed tomography system (3 centers). All participants were scanned twice, the Agatston calcium scores were averaged, and the results were calibrated against a phantom containing known densities of calcium hydroxyapatite.18 An Agatston CAC score above 0 is considered positive.
Outcomes
Events were identified during follow‐up examinations and by telephone interviews conducted every 9 to 12 months to inquire about interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. Copies were obtained of death certificates and medical records for hospitalizations and outpatient cardiovascular diagnoses. Two physicians from the MESA study events committee independently reviewed all medical records for end point classification and assignment of incidence dates.
A CHD definition similar to that used in the Framingham Study was used in these analyses19: incident angina, myocardial infarction and resuscitated cardiac arrest, and death following either a coronary artery event or a coronary intervention.
Statistical Analyses
The mean and SD values of continuous variables and the distribution of dichotomous variables as percentages in each group were calculated. We excluded 314 participants (from the original cohort of 6814) from the analyses because of missing ultrasound measurements or risk factor data.
A baseline multivariable Cox proportional hazards regression model was created with race/ethnicity and sex added to the components of the Framingham risk score for CHD: age, diabetes mellitus, smoking, systolic blood pressure, high‐density lipoprotein‐cholesterol, and total cholesterol. We tested the predictive value of the respective participant's CCA and ICA IMT percentiles by separately adding these variables to the baseline model. We also evaluated the predictive value of the combined IMT score by adding this variable to (1) the baseline model and (2) the baseline model with CAC score (0 or >0) added as a predictor variable. Validity of the proportional hazards models was determined using Schoenfeld residuals. Calibration was estimated using the Gronnesby and Borgan score.20 The Harrell's C‐statistics were compared to estimate increase in predictive value. In a sensitivity analysis, we also added CAC score (0 or >0), lipid lowering, blood pressure lowering therapy, and education to the baseline model and tested the predictive value of the model when the IMT score was included.
We applied the same analytic strategy this time with multivariable logistic regression models using CHD as outcome and included as predictor variables race/ethnicity and sex added to the components of the Framingham risk score for CHD and the CAC score (0 or >0). Goodness of fit was verified by the Hosmer and Lemeshow test (Figures S1 through S3). Receiver operating characteristic curves were generated and the areas under the curves (AUCs) estimated and compared. In a sensitivity analysis we also studied the effect of adding IMT score to a more complete model with CAC score (0 or >0), lipid‐lowering therapy, blood pressure lowering therapy, and education added to the base model.
Statistical analyses were performed using Stata 11.2 (StataCorp, College Station, TX). Level of statistical significance was set at P≤0.05. Net Reclassification Improvement (NRI) was calculated with the help of a Stata add‐on from the Uppsala Clinical Research Center: (http://www.ucr.uu.se/en/). Cut points for 10‐year events were set at 6% and 20% according to the Framingham Heart Study as described by Pencina et al.21
Results
Median follow‐up was 10.2 years (interquartile range: 9.7, 10.7 years). Of 6814 MESA participants, 6739 had a carotid artery examination at baseline. Of these, far‐wall mean CCA IMT values were obtained in 6721 (99.7%) individuals and the maximum ICA IMT in 6628 (98.4%), with both these measurements obtained in 6614 participants (98.2%). Of these, 114 participants did not have complete risk factor profiles, resulting in a final analytic sample of 6500 (Table S1). The mean age of the cohort was 62.1 years, and 52.6% were women; the race/ethnicity distribution is shown in Table 1. There were 429 incident CHD events, classified as follows: angina, 181; myocardial infarction, 160; resuscitated coronary event, 22; and coronary deaths, 66.
Table 1.
Means and Distributions of Selected Variables in MESA for the Analytic Sample (n=6500)
| Variable | Valuea |
|---|---|
| Age, y | 62.1 (10.2) |
| Sex (woman) | 3421 (52.6%) |
| Race/ethnicity | |
| White | 2529 (38.9) |
| Chinese | 787 (12.1%) |
| Black | 1762 (27.1%) |
| Hispanic | 1422 (21.9%) |
| Education | |
| No high school | 1153 (17.8%) |
| High school | 3035 (46.7%) |
| College or equivalent | 1133 (17.4%) |
| Advanced degree | 1179 (18.1%) |
| Diabetes mellitus (yes) | 617 (9.5%) |
| Smoker (yes) | 849 (13.1%) |
| Systolic blood pressure, mm Hg | 126.5 (21.5) |
| Total cholesterol, mg/dL | 194.2 (35.6) |
| HDL‐cholesterol, mg/dL | 60.0 (14.8) |
| Hypertension medications (yes) | 2375 (36.5%) |
| Lipid‐lowering therapy (yes) | 1044 (16.1%) |
| Common carotid IMT, mmb | 0.675 (0.204) |
| Internal carotid IMT, mmb | 1.610 (0.996) |
| CAC score (>0) | 3259 (50.1%) |
| CHD events | 429 (6.6%) |
| Median follow‐up with interquartile values, years | 10.2 (9.7, 10.7) |
CAC indicates coronary artery calcium; CHD, coronary heart disease; HDL, high‐density lipoprotein; IMT, intima‐media thickness; MESA, Multi‐Ethnic Study of Atherosclerosis.
Values in parentheses are percentages for ordinal variables and standard deviations for continuous variables with the exception of follow‐up intervals that represent the interquartile ranges.
Three‐decimal precision is given so that the IMT values can also be read as microns by multiplying by 1000.
IMT Score Added to Framingham Risk Factors
Table 2 summarizes the hazard ratios obtained when respectively adding common carotid IMT percentile, ICA IMT percentile, and the combined IMT score to the baseline Cox‐proportional hazards model with the Framingham risk factors, sex, and race/ethnicity as predictors. The combined IMT score had a stronger hazard ratio than either of the 2 variables by themselves. The respective addition of each IMT percentile variable significantly increased the C‐statistic (Table 3). The biggest effect was for the combined IMT score, which significantly increased (P<0.001) the C‐statistic of the base model from 0.7276 to 0.7457, for a net increase of 0.0180 (95% CI: 0.0082, 0.0279). As seen in Tables S2 through S4, in all models the IMT percentile was a strong independent predictor of CHD events, as were the Framingham risk factors and sex. In addition, Chinese participants showed a significantly lower hazard ratio as compared to whites, while differences between whites, blacks, and Hispanics were of borderline statistical significance.
Table 2.
Results of Cox Proportional Hazards Model Showing the Association of the Mean Common Carotid, Maximum Internal Carotid Artery, and Combined IMT Scores With CHD
| Variable | Hazard Ratio | Lower 95% CI | Upper 95% CI | P Values |
|---|---|---|---|---|
| Common carotid artery IMT percentile (scaled 0–1) | ||||
| Not adjusted | 3.19 | 2.25 | 4.53 | <0.001 |
| Adjusted for age, sex, and race/ethnicity | 3.28 | 2.31 | 4.66 | <0.001 |
| Fully adjusted a | 2.43 | 1.70 | 3.47 | <0.001 |
| Internal carotid IMT score (scaled 0–1) | ||||
| Not adjusted | 3.38 | 2.43 | 4.69 | <0.001 |
| Adjusted for age, sex, and race/ethnicity | 3.36 | 2.42 | 4.66 | <0.001 |
| Fully adjusted | 2.58 | 1.83 | 3.62 | <0.001 |
| Combined IMT score (scaled 0–1) | ||||
| Not adjusted | 6.09 | 4.02 | 9.24 | <0.001 |
| Adjusted for age, sex, and race/ethnicity | 6.29 | 4.13 | 9.58 | <0.001 |
| Fully adjusted | 4.24 | 2.74 | 6.57 | <0.001 |
CHD indicates coronary heart disease; HDL, high‐density lipoprotein; IMT, intima‐media thickness.
Adjusted for age, sex, race/ethnicity, smoking status, presence of diabetes mellitus, systolic blood pressure, total cholesterol, and HDL‐cholesterol.
Table 3.
Change in C‐Statistic With the Addition of Carotid Artery IMT Percentile
| C‐Statistic/Difference | Lower 95% CI | Upper 95% CI | P Values | |
|---|---|---|---|---|
| Base modela | 0.7276 | 0.7058 | 0.7494 | <0.001 |
| Model with common carotid percentile added | ||||
| C‐Statistic value | 0.7396 | 0.7182 | 0.7611 | <0.001 |
| Difference in C‐statistic | 0.0120 | 0.0047 | 0.0194 | 0.001 |
| Model with internal carotid percentile added | ||||
| C‐statistic value | 0.7387 | 0.7175 | 0.7600 | <0.001 |
| Difference in C‐statistic | 0.0111 | 0.0024 | 0.0198 | 0.003 |
| Model with combined IMT score added | ||||
| C‐statistic value | 0.7457 | 0.7245 | 0.7669 | <0.001 |
| Difference in C‐statistic | 0.0180 | 0.0082 | 0.0279 | <0.001 |
HDL indicates high‐density lipoprotein; IMT, intima‐media thickness.
Adjusted for age, sex, race/ethnicity, smoking status, presence of diabetes mellitus, systolic blood pressure, total cholesterol, and HDL‐cholesterol.
The baseline logistic regression model with Framingham risk factors, sex, and race/ethnicity had an AUC of 0.7210 (95% CI: 0.6983, 0.7437). Adding CCA IMT percentile significantly increased (P=0.002) the AUC area to 0.7340, while adding the ICA IMT percentile significantly increased (P=0.026) the AUC to 0.7317. Finally, the AUC significantly increased (P=0.0008) to 0.7396 (95% CI: 0.7174, 0.7617) when the combined IMT score was added to the baseline model (Figure 2). The Kaplan–Meier failure plots are shown for the cut points in risk score of 0.25, 0.5, and 0.75 (Figure 3).
Figure 2.
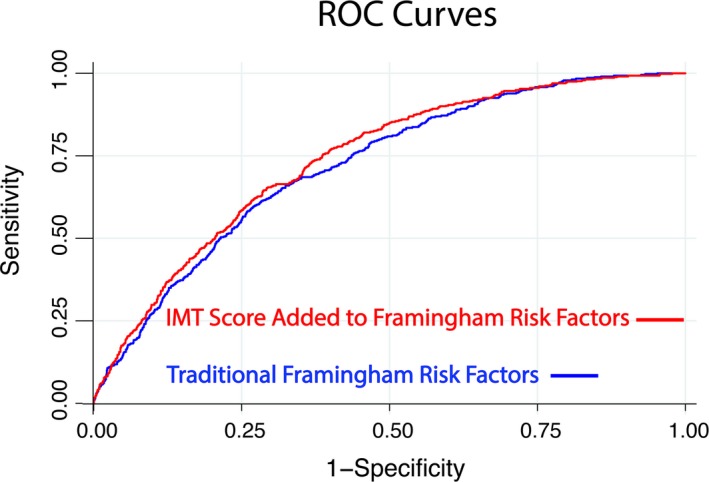
These 2 receiver operating characteristic (ROC) curves show the effect of adding an intima‐media thickness (IMT) score to a base model with Framingham risk factors. The area under the curve of the base model is 0.7210 (95% CI, 0.6983, 0.7437) and increases (P=0.0008) to 0.7396 (95% CI, 0.7174, 0.7617) when IMT score is added.
Figure 3.
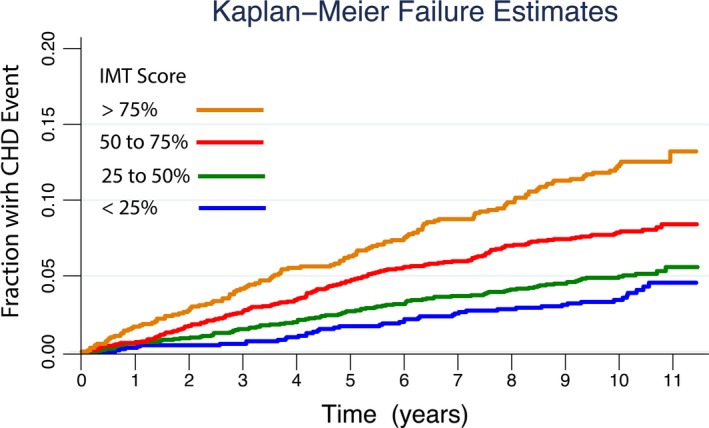
Unadjusted Kaplan–Meier failure curves showing the increased cumulative incidence of coronary heart disease (CHD) by intima media thickness (IMT) score percentiles as a function of time since baseline IMT measurement. All participants were free of cardiovascular disease at baseline. The actual IMT percentile score cut points are shown in the legend and are scaled 0% to 100% instead of 0 to 1 for ease of interpretation.
The net reclassification improvement for the combined IMT score (Table S5) was 4.9% (P=0.024) for an upward reclassification of events of 2.8% (12/429) and downward reclassification of nonevents of 2.1% (130/6071). Restricting the analysis to participants who were in the intermediate risk category (6–20%) gave an NRI of 11.5% (Table S5).
IMT Score Added to Framingham Risk Factors and CAC Score
After entering CAC score in the model including sex, race/ethnicity, and traditional Framingham risk factors, the predictive value of the combined carotid IMT score remained highly significant (P<0.001), but was attenuated, with the hazard ratio decreasing from 4.24 to 3.15 (Table 4). In addition, the IMT score significantly increased the C‐statistic by 0.009 (P=0.005) after adjustment for the same variables shown in Table 4. For the multivariable logistic regression models, the AUC increased significantly (P=0.018) from 0.7627 (95% CI; 0.7419, 0.7836) to 0.7714 (95% CI; 0.7506, 0.7923) and the NRI was 5.0% (Table S6). Restricting the analysis to participants who were in the intermediate risk category (6–20%) gave an NRI of 11.5% (Table S6).
Table 4.
Multivariable Cox Proportional Hazards Ratios for Coronary Heart Disease According to Age, Sex, Race/Ethnicity, Traditional Framingham Risk Factors, and CAC Score (0 or >0) and IMT Percentile Score
| Variable | Hazard Ratio | Lower 95% CI | Upper 95% CI | P Values |
|---|---|---|---|---|
| Age, y | 1.03 | 1.02 | 1.04 | <0.001 |
| Sex (woman) | 1.65 | 1.33 | 2.05 | <0.001 |
| Race/ethnicity | ||||
| White (referent) | ||||
| Chinese | 0.55 | 0.38 | 0.79 | 0.001 |
| Black | 0.94 | 0.74 | 1.20 | 0.625 |
| Hispanic | 0.89 | 0.69 | 1.14 | 0.353 |
| Smoker (yes) | 1.43 | 1.09 | 1.88 | 0.011 |
| Diabetes mellitus (yes) | 1.58 | 1.26 | 1.98 | <0.001 |
| Systolic blood pressurea | 1.18 | 1.07 | 1.29 | 0.001 |
| Total cholesterola | 1.11 | 1.01 | 1.22 | 0.029 |
| HDL‐cholesterola | 0.86 | 0.76 | 0.98 | 0.02 |
| Positive CAC score | 3.95 | 2.97 | 5.27 | <0.001 |
| Carotid IMT score (scaled 0–1) | 3.15 | 2.05 | 4.85 | <0.001 |
CAC indicates coronary artery calcium; HDL, high‐density lipoprotein; IMT, intima‐media thickness.
Normalized to standard deviation values of the respective distributions: 21.5 mm Hg for systolic blood pressure, 35.6 mg/dL for total cholesterol, and 14.8 mg/dL for HDL‐cholesterol.
Supplemental Analyses
We also investigated the effect of adding the IMT score to a baseline model that included age, sex, race/ethnicity, systolic blood pressure, total cholesterol, high‐density lipoprotein‐cholesterol, smoking, diabetes mellitus, lipid‐lowering therapy, blood pressure lowering therapy, education, and calcium score (0 or >0). The predictive value of the combined carotid IMT score was slightly attenuated, the hazard ratio decreasing from 3.15 to 2.98 (Table S7). The increases in both the C‐statistic (P=0.014) and in the AUC (P=0.018) remained statistically significant (Table S8). The NRI (Table S9) was 4.6% and also significant (P<0.005).
Discussion
We have shown that mean far wall CCA and maximum internal carotid IMT percentiles presented as normative values are independent predictors of CHD events and that their combination as an IMT score adds significantly to CHD event prediction after adjusting for Framingham risk factors and CAC score (0 or >0).
We opted to generate IMT normative data by calculating individual IMT percentiles that factored in the effect of age since IMT has been shown to increase with age.8, 9 We did so by adopting an approach used by the World Health Organization to generate normative data for anthropomorphic measurements such as height as a function of age.10 We found that both common and internal carotid IMT increased with age and that there were race/ethnic differences (Figure 1A and 1B). We also observed that ICA IMT subjectively showed less difference between race/ethnicities, with only Chinese Americans showing lower values than the other groups (Figure 1B). After deriving the equations describing the distribution of IMT values as a function of age for both sexes and the 4 ethnicities in MESA, we calculated a given individual's common carotid and ICA IMT percentile. We then combined them into an IMT score and tested the ability of these 3 measures to predict CHD events in models where the Framingham risk factors were entered. We found that the combined IMT score was a consistent predictor of CHD events and gave a greater increment in the C‐statistic than either the CCA or ICA IMT percentile values alone (Table 2). We also found that the hazards ratios for IMT percentile values were the same for the unadjusted and the sex and race‐ethnic adjusted models predicting CHD events. This suggests that the derived IMT percentiles contain the key variance components linked to age, sex, and race‐ethnicity.
IMT score was a strong independent predictor of events when the Framingham risk factors were taken into account and still a strong and statistically significant predictor of CHD events in a model with calcium score (0 or >0) added. However, the IMT score hazard ratio decreased from 4.24 to 3.15 (Tables 2 and 4) when coronary calcium was added to the model, suggesting that coronary calcium score is a confounder of the association between IMT and CHD. This is consistent with previous analyses since, in MESA, independent associations between IMT and the CAC score have been noted in both cross‐sectional and longitudinal analyses.22
We evaluated the effect of adding the IMT score to 2 prediction models, 1 without and 1 with CAC score, by examining the change in the Harrell's C‐statistic for the multivariable Cox proportional hazards models and the area under the receiver operating curve for multivariable logistic regression models. In all instances, there were statistically significant increases in these metrics. These findings should be contrasted to the ambiguity seen when other novel biomarkers have been evaluated for their incremental value over the Framingham risk factors for predicting CHD events.23, 24, 25 We also note that there has been a question as to which metric should be used to confirm that there is in fact an increment in the predictive power of a new risk factor.26 While it has been argued that the AUC or the C‐statistic may not be sensitive enough to detect a true improvement in risk prediction, there does not appear to be a strong belief that an increase in C‐statistic or AUC yields a false positive result.27, 28 Although the NRI21 has been criticized,26, 29 our NRI results showed both an increase in the up‐reclassification of events and down‐reclassification of nonevents (P<0.05), and were consistent with the AUC and C‐statistics results.
Given the lack of significant difference in the NRI for a model without and with positive CAC included, we looked at the NRI for 2 models: (1) positive CAC alone, and (2) positive CAC with IMT score added to the baseline model. The NRI with the inclusion of positive CAC in the model (Table S10) was 11.1% (P=0.0001) and was 16.1% (P=0.0001) with both positive CAC and IMT added at the same time (Table S11). These results suggest a significant incremental contribution of the IMT score when added to a model with a positive CAC score and Framingham risk factors on the order of half the effect of a positive CAC score. We briefly examined how IMT score could affect the NRI for individuals within the intermediate risk category. We found that the NRI's were 11.5% for these individuals when respectively adding IMT score to risk factors alone and to risk factors with CAC. Whether this can be considered as having any value has yet to be determined. Such an evaluation will likely require more than simply calculating the NRI since it has been found to be dependent on the number of categories and the specific cut points selected.30
It is possible to consider the potential impact of using an IMT score for patient risk stratification. The recent guidelines on the estimation of atherosclerosis cardiovascular disease risk indicated that carotid IMT might not offer added value to the pooled risk equations derived from National Heart, Lung, and Blood Institute–funded observation cohorts.31 That assessment was mostly based on the results of a meta‐analysis1 based on a group of studies with varied common carotid imaging protocols and measurement processes.32 One important missing element to this meta‐analysis was the absence of any ICA IMT measurements.1 We believe that the combination of common and ICA IMT increased the predictive power of the IMT score in addition to taking into consideration its association with age. We have further demonstrated that these measurements are reliably obtainable in almost all individuals having undergone a carotid ultrasound examination.
Our study strengths are the applicability of our findings to a multi‐ethnic cohort, the use of a noninvasive and risk‐free technique to perform our measurements, and the application of a general approach used to generate normative data. We also show that carotid IMT data can be acquired in a reliable fashion (correlation coefficients ≈0.90) at 6 very distinct and geographically dispersed clinical sites, even though the sonographers performing the examinations had various levels of expertise, since there was no requirement for any credentialing or formal certification.
A weakness of our study is the possibility that our IMT imaging process may not be applicable to the general population; however, the imaging protocol used at the 6 MESA clinic sites was derived from that used in a single center, the Framingham Offspring study.6 Another limitation is possible residual confounding, as ours was an observational study.
In conclusion, we have shown that an IMT score can be derived from noninvasive measurements of the common and ICA wall and that this measurement improved the Framingham risk score for predicting CHD events, even after addition of coronary calcium to the model. Because no single cohort has perfect external validity, our findings would require confirmation in other cohorts.
Sources of Funding
This research was supported by contracts N01‐HC‐95159 through N01‐HC‐95167 from the National Heart, Lung, and Blood Institute as well as R01 HL069003 and R01 HL081352 and by grants UL1‐RR‐024156 and UL1‐RR‐025005 from National Center for Research Resources.
Disclosures
Dr O'Leary owns stock in Medpace, Inc, Cincinnati, OH. The remaining authors have no disclosures to report.
Supporting information
Table S1. Comparison of Risk Factors for Individuals Having Complete Intima‐Media Thickness (IMT) Measurements and Risk Factors (n = 6500) as Compared to Those Without With Missing Variables (n = 314)*
Table S2. Results of Cox Proportional Hazards Model With Time to Coronary Heart Disease Event as Outcome and Common Carotid Artery Intima‐Media Thickness (IMT) Percentile Added to a Base Set of Variables: Sex, Race/Ethnicity, and Traditional Framingham Risk Factors
Table S3. Results of Cox Proportional Hazards Model With Time to Coronary Heart Disease Event as Outcome and Internal Carotid Artery Intima‐Media Thickness (IMT) Percentile Added to a Base Set of Variables: Sex, Race/Ethnicity, and Traditional Framingham Risk Factors
Table S4. Results of Cox Proportional Hazards Model With Time to Coronary Heart Disease Event as Outcome and the Carotid IMT Score Added to a Base Set of Variables: Sex, Race/Ethnicity, and Traditional Framingham Risk Factors
Table S5. Calculated Net Reclassification Improvement (NRI)*† After Adding the Carotid IMT Score to Framingham Risk Factors‡
Table S6. Calculated Net Reclassification Improvement (NRI)*† After Adding the Carotid IMT Score to a Model That Includes Coronary Artery Calcium (CAC) Score (0 or Greater Than 0) and Risk Factors‡
Table S7. Results of Cox Proportional Hazards Model With Time to Coronary Heart Disease Event as Outcome and Coronary Artery Calcium Score (= 0 or > 0) and Carotid Intima‐Media Thickness (IMT) Percentile Score Both Added to en Expanded Set of Variables: Sex, race/Ethnicity, Traditional Framingham Risk Factors, Lipid‐Lowering Medications, Blood Pressure Lowering Medications, and Education
Table S8. Model C‐Statistic and Change in C‐Statistic With the Addition of Carotid IMT Percentile Score to a Model With Variables Listed in Table 7S, Inclusive of Coronary Artery Calcium Score
Table S9. Net Reclassification Improvement (NRI)*† Noted by Adding the Carotid Artery Intima‐Media Thickness (IMT) Percentile Score to a Model That Includes Coronary Artery Calcium (CAC) Score (0 or Greater Than 0) and All Risk Factors‡
Table S10. Net Reclassification Improvement (NRI)*† Upon Adding Positive Calcium Score to Risk Factors‡
Table S11. Net Reclassification Improvement (NRI)*† Upon Adding Positive Calcium Score and Intima‐Media Thickness Percentile Score to Risk Factors‡
Figure S1. Verification of calibration was made of a multivariable logistic regression models with coronary heart disease event as outcome and the following predictor variables: sex, race/ethnicity, age, smoking, diabetes, systolic blood pressure, total‐cholesterol and HDL‐cholesterol. The model passed the Hosmer and Lemeshow test at the p = 0.10 level. Observed and predicted events are displayed graphically.
Figure S2. Verification of calibration was made of a multivariable logistic regression models with coronary heart disease event as outcome and the following predictor variables: sex, race/ethnicity, age, smoking, diabetes, systolic blood pressure, total‐cholesterol and HDL‐cholesterol. The model also passed the Hosmer and Lemeshow test at the p = 0.10 level. Observed and predicted events are displayed graphically.
Figure S3. Verification of calibration was made of a multivariable logistic regression models with coronary heart disease event as outcome and the following predictor variables: sex, race/ethnicity, age, smoking, diabetes, systolic blood pressure, total‐cholesterol and HDL‐cholesterol. The model passed the Hosmer and Lemeshow test at the p = 0.25 level. Observed and predicted events are displayed graphically.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
(J Am Heart Assoc. 2017;6:e004612. DOI: 10.1161/JAHA.116.004612.)
References
- 1. Den Ruijter HM, Peters SAE, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O'Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CDA, Witteman JC, Moons KG, Bots ML. Common carotid intima‐media thickness measurements in cardiovascular risk prediction: a meta‐analysis. JAMA. 2012;308:796–803. [DOI] [PubMed] [Google Scholar]
- 2. O'Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, Tracy R, Gardin JM, Price TR, Furberg CD; for the Cardiovascular Health Study Collaborative research Group . Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Stroke. 1996;27:224–231. [DOI] [PubMed] [Google Scholar]
- 3. OLeary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 4. Polak JF, Person SD, Wei GS, Godreau A, Jacobs DR Jr, Harrington A, Sidney S, O'Leary DH. Segment‐specific associations of carotid intima‐media thickness with cardiovascular risk factors: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Stroke. 2010;41:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima‐media thickness and presence or absence of plaque improves prediction of coronary heart disease. The ARIC (Atherosclerosis Risk in Communities) Study. J Am Coll Cardiol. 2010;55:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB Sr. Carotid‐wall intima‐media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polak JF, O'Leary DH, Kronmal RA, Wolfson SK, Bond MG, Tracy RP, Gardin JM, Kittner SJ, Price TR, Savage PJ. Sonographic evaluation of carotid artery atherosclerosis in the elderly: relationship of disease severity to stroke and transient ischemic attack. Radiology. 1993;188:363–370. [DOI] [PubMed] [Google Scholar]
- 8. Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S; Reference Values for Arterial Measurements C . Reference intervals for common carotid intima‐media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013;34:2368–2380. [DOI] [PubMed] [Google Scholar]
- 9. Howard G, Sharrett AR, Heiss G, Evans GW, Chambless LE, Riley WA, Burke GL. Carotid artery intimal‐medial thickness distribution in general populations as evaluated by B‐mode ultrasound. ARIC Investigators. Stroke. 1993;24:1297–1304. [DOI] [PubMed] [Google Scholar]
- 10. Borghi E, de Onis M, Garza C, Van den Broeck J, Frongillo EA, Grummer‐Strawn L, Van Buuren S, Pan H, Molinari L, Martorell R, Onyango AW, Martines JC; Group WHOMGRS . Construction of the World Health Organization child growth standards: selection of methods for attained growth curves. Stat Med. 2006;25:247–265. [DOI] [PubMed] [Google Scholar]
- 11. Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima‐media thickness in the prediction of cardiovascular disease incidence: the Multi‐Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 14. Genuth S, Alberti KGMM, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P; Expert Committee on the D and Classification of Diabetes M . Follow‐up report on the diagnosis of diabetes mellitus. [see comment]. Diabetes Care. 2003;26:3160–3167. [DOI] [PubMed] [Google Scholar]
- 15. Polak JF, Pencina MJ, Herrington D, O'Leary DH. Associations of edge‐detected and manual‐traced common carotid intima‐media thickness measurements with Framingham risk factors: the Multi‐Ethnic Study of Atherosclerosis. Stroke. 2011;42:1912–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polak JF, Funk LC, O'Leary DH. Inter‐reader differences in common carotid artery intima‐media thickness: implications for cardiovascular risk assessment and vascular age determination. J Ultrasound Med. 2011;30:915–920. [DOI] [PubMed] [Google Scholar]
- 17. Cole T, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. [DOI] [PubMed] [Google Scholar]
- 18. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of Multi‐Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 19. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 20. Gronnesby J, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–328. [DOI] [PubMed] [Google Scholar]
- 21. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. [see comment]. Stat Med. 2008;27:157–172; discussion 207‐12. [DOI] [PubMed] [Google Scholar]
- 22. Polak JF, Tracy R, Harrington A, Zavodni AEH, O'Leary DH. Carotid artery plaque and progression of coronary artery calcium: the Multi‐Ethnic Study of Atherosclerosis. J Am Soc Echocardiogr. 2013;26:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Melander O, Newton‐Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tzoulaki I, Liberopoulos G, Ioannidis JP. Assessment of claims of improved prediction beyond the Framingham risk score. JAMA. 2009;302:2345–2352. [DOI] [PubMed] [Google Scholar]
- 25. Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton‐Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. [DOI] [PubMed] [Google Scholar]
- 26. Pepe MS, Kerr KF, Longton G, Wang Z. Testing for improvement in prediction model performance. Stat Med. 2013;32:1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. [DOI] [PubMed] [Google Scholar]
- 28. Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48:1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PWF; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Polak JF, O'Leary DH. Carotid intima‐media thickness as surrogate for and predictor of CVD. Glob Heart. 2016;11:295–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of Risk Factors for Individuals Having Complete Intima‐Media Thickness (IMT) Measurements and Risk Factors (n = 6500) as Compared to Those Without With Missing Variables (n = 314)*
Table S2. Results of Cox Proportional Hazards Model With Time to Coronary Heart Disease Event as Outcome and Common Carotid Artery Intima‐Media Thickness (IMT) Percentile Added to a Base Set of Variables: Sex, Race/Ethnicity, and Traditional Framingham Risk Factors
Table S3. Results of Cox Proportional Hazards Model With Time to Coronary Heart Disease Event as Outcome and Internal Carotid Artery Intima‐Media Thickness (IMT) Percentile Added to a Base Set of Variables: Sex, Race/Ethnicity, and Traditional Framingham Risk Factors
Table S4. Results of Cox Proportional Hazards Model With Time to Coronary Heart Disease Event as Outcome and the Carotid IMT Score Added to a Base Set of Variables: Sex, Race/Ethnicity, and Traditional Framingham Risk Factors
Table S5. Calculated Net Reclassification Improvement (NRI)*† After Adding the Carotid IMT Score to Framingham Risk Factors‡
Table S6. Calculated Net Reclassification Improvement (NRI)*† After Adding the Carotid IMT Score to a Model That Includes Coronary Artery Calcium (CAC) Score (0 or Greater Than 0) and Risk Factors‡
Table S7. Results of Cox Proportional Hazards Model With Time to Coronary Heart Disease Event as Outcome and Coronary Artery Calcium Score (= 0 or > 0) and Carotid Intima‐Media Thickness (IMT) Percentile Score Both Added to en Expanded Set of Variables: Sex, race/Ethnicity, Traditional Framingham Risk Factors, Lipid‐Lowering Medications, Blood Pressure Lowering Medications, and Education
Table S8. Model C‐Statistic and Change in C‐Statistic With the Addition of Carotid IMT Percentile Score to a Model With Variables Listed in Table 7S, Inclusive of Coronary Artery Calcium Score
Table S9. Net Reclassification Improvement (NRI)*† Noted by Adding the Carotid Artery Intima‐Media Thickness (IMT) Percentile Score to a Model That Includes Coronary Artery Calcium (CAC) Score (0 or Greater Than 0) and All Risk Factors‡
Table S10. Net Reclassification Improvement (NRI)*† Upon Adding Positive Calcium Score to Risk Factors‡
Table S11. Net Reclassification Improvement (NRI)*† Upon Adding Positive Calcium Score and Intima‐Media Thickness Percentile Score to Risk Factors‡
Figure S1. Verification of calibration was made of a multivariable logistic regression models with coronary heart disease event as outcome and the following predictor variables: sex, race/ethnicity, age, smoking, diabetes, systolic blood pressure, total‐cholesterol and HDL‐cholesterol. The model passed the Hosmer and Lemeshow test at the p = 0.10 level. Observed and predicted events are displayed graphically.
Figure S2. Verification of calibration was made of a multivariable logistic regression models with coronary heart disease event as outcome and the following predictor variables: sex, race/ethnicity, age, smoking, diabetes, systolic blood pressure, total‐cholesterol and HDL‐cholesterol. The model also passed the Hosmer and Lemeshow test at the p = 0.10 level. Observed and predicted events are displayed graphically.
Figure S3. Verification of calibration was made of a multivariable logistic regression models with coronary heart disease event as outcome and the following predictor variables: sex, race/ethnicity, age, smoking, diabetes, systolic blood pressure, total‐cholesterol and HDL‐cholesterol. The model passed the Hosmer and Lemeshow test at the p = 0.25 level. Observed and predicted events are displayed graphically.


