Abstract
Background
We studied (1) the rates of stroke or systemic embolism and bleeding in patients with atrial fibrillation and peripheral artery disease (PAD) and (2) the efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation with and without PAD.
Methods and Results
The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial randomized 18 201 patients with atrial fibrillation to apixaban or warfarin for stroke/systemic embolism prevention; 884 (4.9%) patients had PAD at baseline. Patients with PAD had higher unadjusted rates of stroke and systemic embolism (hazard ratio [HR] 1.73, 95% CI 1.22–2.45; P=0.002) and major bleeding (HR 1.34, 95% CI 1.00–1.81; P=0.05), but after adjustment, no differences existed in rates of stroke and systemic embolism (HR 1.32, 95% CI 0.93–1.88; P=0.12) and major bleeding (HR 1.03, 95% CI 0.76–1.40; P=0.83) compared with patients without PAD. The risk of stroke or systemic embolism was similar in patients assigned to apixaban and warfarin with PAD (HR 0.63, 95% CI 0.32–1.25) and without PAD (HR 0.80, 95% CI 0.66–0.96; interaction P=0.52). Patients with PAD did not have a statistically significant reduction in major or clinically relevant nonmajor bleeding with apixaban compared with warfarin (HR 1.05, 95% CI 0.69–1.58), whereas those without PAD had a statistically significant reduction (HR 0.65, 95% CI 0.58–0.73; interaction P=0.03).
Conclusions
Patients with PAD in ARISTOTLE had a higher crude risk of stroke or systemic embolism compared with patients without PAD that was not present after adjustment. The benefits of apixaban versus warfarin for stroke and systemic embolism were similar in patients with and without PAD. These findings highlight the need to optimize the treatment of patients with atrial fibrillation and PAD.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00412984.
Keywords: apixaban, atrial fibrillation, bleeding, peripheral artery disease, stroke, systemic embolism
Subject Categories: Arrhythmias, Atrial Fibrillation, Anticoagulants, Peripheral Vascular Disease
Introduction
The rates of both peripheral artery disease (PAD) and atrial fibrillation (AF) are increasing, with an estimated 33.5 million people with AF and 202 million with PAD worldwide.1, 2 Patients with PAD and AF are at increased risk of stroke. Stroke risk scores have been developed for patients with AF and, with the exception of the recently created ABC risk score, are based on clinical variables.3 The CHA2DS2‐VASc risk score for stroke and systemic embolism incorporates vascular disease, which includes myocardial infarction (MI), aortic plaque, and PAD. The inclusion of vascular disease in the CHA2DS2‐VASc risk score emphasizes the importance of PAD as a risk factor in patients with AF.4, 5 Nevertheless, there are limitations to incorporating PAD into the CHA2DS2‐VASc scoring system, including a small number (N=62 [5.7%]) of patients with PAD in the validation cohort and the exclusion of patients with PAD on oral anticoagulation at baseline.4, 5 Recent data from a large PAD cohort on oral anticoagulation at baseline found no statistically significant difference in risk of stroke or systemic embolism in patients with PAD but did find a treatment effect on bleeding risk with type of oral anticoagulant therapy.6 The use of non–vitamin K antagonist oral anticoagulants in the treatment of AF is increasing; however, little is known about the effectiveness and safety of these newer agents in patients with PAD.7, 8, 9
In the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial comparing apixaban and warfarin in patients with AF, apixaban was superior to warfarin in preventing stroke and systemic embolism and reducing major bleeding.10 In this analysis of ARISTOTLE, we aimed to evaluate (1) the association of PAD with clinical outcomes (stroke, systemic embolism, and bleeding) and (2) the effectiveness and safety of apixaban and warfarin in patients with AF with and without PAD.
Methods
ARISTOTLE was a randomized, double‐blind, double‐dummy trial comparing apixaban with dose‐adjusted warfarin in 18 201 patients with AF and at least 1 risk factor for stroke. The study design was described previously.11 Patients were randomly assigned to twice‐daily dosing of apixaban 5 mg or dose‐adjusted warfarin (target International Normalized Ratio 2.0–3.0). Key exclusion criteria were AF due to a reversible cause, moderate or severe mitral stenosis, conditions other than AF requiring anticoagulation, stroke within the previous 7 days, need for aspirin at a dose of >165 mg per day or both aspirin and clopidogrel, and severe renal insufficiency. The trial was designed and approved by a steering committee and approved by appropriate ethics committees at all sites. All patients provided written informed consent.
Definition of PAD
The presence of PAD was designated by site investigators and reported on the case report form. Those with a history of PAD were included in the PAD cohort. The presence of carotid artery stenosis and aortic aneurysm was captured separately and did not constitute PAD.
Outcomes
The primary efficacy outcome was ischemic or hemorrhagic stroke or systemic embolism. Secondary efficacy outcomes included death from any cause and rate of MI. The primary safety outcome was major bleeding, as defined by the International Society on Thrombosis and Haemostasis (ISTH). Secondary safety outcomes included a composite of major bleeding and clinically relevant nonmajor bleeding. A clinical events committee, whose members were not aware of study group assignments, adjudicated the primary and secondary efficacy and safety outcomes.
Statistical Analysis
The current analysis is a subgroup analysis of the ARISTOTLE population; however, it was not prespecified prior to the completion of the study. Cox proportional hazards regression models were used to assess (1) the association between PAD and efficacy and safety outcomes and (2) the treatment effect of apixaban versus warfarin within subgroups of patients with and without PAD.
All models were stratified by prior vitamin K antagonist use and region. Adjustment variables for each end point were determined by model selection in the full ARISTOTLE cohort. Adjustment variables for stroke or systemic embolism included age; weight; diabetes mellitus; hypertension; moderate valvular disease; prior stroke, transient ischemic attack, or systemic embolism; and type of AF. For the end points of all‐cause and cardiovascular death, adjustment variables included age; sex; systolic blood pressure; diastolic blood pressure; weight; hypertension; moderate valvular disease; left bundle‐branch block; prior MI; prior stroke, transient ischemic attack, or systemic embolism; anemia; smoking; New York Heart Association classification; CHADS2 score; and renal function. Adjustment variables for MI included age, diabetes mellitus, coronary artery disease, prior MI, New York Heart Association classification, and renal function. Adjustment variables for all bleeding end points included age, sex, coronary artery disease, history of MI, prior bleeding, anemia, CHADS2 score, and renal function.
Outcomes are presented as events per 100 patient‐years of follow‐up. Risk relationships are presented as adjusted hazard ratios (HRs) with 95% CIs derived from the adjusted Cox models. Categorical variables were compared for those with and without PAD using chi‐square tests and are presented as count per number not missing (percentage). Continuous variables were compared by Wilcoxon rank sum tests and are presented as medians (25th, 75th percentiles).
Results
Study Population
A total of 18 201 patients were enrolled in the ARISTOTLE trial. PAD status was reported in 17 980 patients. Of those, 884 (4.9%) had PAD at baseline and were included in this analysis. Baseline demographics and patient characteristics according to the presence or absence of PAD are shown in Table 1.
Table 1.
Baseline Demographics and Patient Characteristics by History of PAD
| Variable | PAD (n=884) | No PAD (n=17 096) | P Value |
|---|---|---|---|
| Age, y, median (25th, 75th) | 73 (66.5, 79) | 70 (62, 76) | <0.0001 |
| Female sex | 251/884 (28.4) | 6090/17 096 (35.6) | <0.0001 |
| Region | <0.0001 | ||
| North America | 307/884 (34.7) | 4133/17 096 (24.2) | |
| Latin America | 138/884 (15.6) | 3299/17 096 (19.3) | |
| Europe | 368/884 (41.6) | 6893/17 096 (40.3) | |
| Asia Pacific | 71/884 (8.0) | 2771/17 096 (16.2) | |
| CHADS2 score, mean±SD | 2.66±1.25 | 2.09±1.09 | <0.0001 |
| ≤1 | 164/884 (18.6) | 5929/17 096 (34.7) | |
| 2 | 284/884 (32.1) | 6152/17 096 (36.0) | |
| ≥3 | 436/884 (49.3) | 5015/17 096 (29.3) | |
| Presenting characteristics | |||
| Systolic BP, mm Hg, median (25th, 75th) | 130 (120, 140) | 130 (120, 140) | 0.2785 |
| Weight, kg, median (25th, 75th) | 83.0 (71.5, 95.8) | 82.0 (70.0, 95.5) | 0.1417 |
| Moderate or severe renal impairment | 228/884 (25.8) | 2745/17 021 (16.1) | <0.0001 |
| Baseline comorbidities | |||
| History of stroke, TIA, or systemic embolism | 295/884 (33.4) | 3189/17 096 (18.7) | <0.0001 |
| Carotid disease (bruit, stent, stenosis, endarterectomy) | 169/884 (19.1) | 441/17 096 (2.6) | <0.0001 |
| Nonparoxysmal AF type | 745/884 (84.3) | 14 470/17 093 (84.7) | 0.7609 |
| Prior warfarin/VKA | 553/884 (62.6) | 9721/17 096 (56.9) | 0.0008 |
| Coronary artery disease | 523/884 (59.2) | 5469/17 092 (32.0) | <0.0001 |
| Myocardial infarction | 257/884 (29.1) | 2297/17 094 (13.4) | <0.0001 |
| CHF within 3 months or LVEF ≤40% | 363/884 (41.1) | 6000/17 096 (35.1) | 0.0003 |
| Diabetes mellitus | 325/884 (36.8) | 4172/17 096 (24.4) | <0.0001 |
| Hypertension | 813/884 (92.0) | 14 991/17 096 (87.7) | 0.0001 |
| Current smoking | 89/884 (10.1) | 1378/17 079 (8.1) | 0.0343 |
| Moderate or worse valvular heart disease | 239/884 (27.0) | 2953/17 094 (17.3) | <0.0001 |
| History of bleeding | 213/884 (24.1) | 2794/17 093 (16.3) | <0.0001 |
| History of fall within previous year | 77/828 (9.3) | 669/15 451 (4.3) | <0.0001 |
| Medications | |||
| ACEI or ARB | 668/877 (76.2) | 12 053/16 817 (71.7) | 0.0039 |
| Beta blocker | 603/877 (68.8) | 10 767/16 817 (64.0) | 0.0044 |
| Aspirin | 345/884 (39.0) | 5231/17 096 (30.6) | <0.0001 |
| Clopidogrel | 33/884 (3.7) | 300/17 096 (1.8) | <0.0001 |
ACEI indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BP, blood pressure; CHF, congestive heart failure; LVEF, left ventricular ejection fraction; PAD, peripheral artery disease; TIA, transient ischemic attack; VKA, vitamin K antagonist.
Participants with PAD were more likely to be older, male, and current smokers. They were more likely to have a history of diabetes mellitus, MI, coronary artery disease, carotid artery disease, or moderate or worse valvular heart disease and to have been on warfarin previously and had a higher risk of bleeding and fall within the previous year. Patients with PAD were more likely to be on aspirin, clopidogrel, or any aspirin or thienopyridine at baseline, at 1 year of follow‐up, and at the time of study drug discontinuation (Table 2).
Table 2.
Antiplatelet Use at Baseline and During Follow‐up
| PAD (All) | No PAD (All) | PAD (Apixaban) | PAD (Warfarin) | No PAD (Apixaban) | No PAD (Warfarin) | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| Patients, n | 884 | 17 096 | 442 | 442 | 8564 | 8532 |
| ASA | 345 (39.0) | 5231 (30.6) | 160 (36.2) | 185 (41.9) | 2668 (31.2) | 2563 (30.0) |
| Clopidogrel | 33 (3.7) | 300 (1.8) | 21 (4.8) | 12 (2.7) | 146 (1.7) | 154 (1.8) |
| Any ASA or thienopyridine | 376 (42.5) | 5477 (32.0) | 179 (40.5) | 197 (44.6) | 2786 (32.5) | 2691 (31.5) |
| At 1 year | ||||||
| Patients, n | 793 | 15 976 | 398 | 395 | 8037 | 7939 |
| ASA | 222 (28.0) | 3311 (20.7) | 95 (23.9) | 127 (32.2) | 1713 (21.3) | 1598 (20.1) |
| Clopidogrel | 34 (4.3) | 238 (1.5) | 18 (4.5) | 16 (4.1) | 124 (1.5) | 114 (1.4) |
| Any ASA or thienopyridine | 247 (31.1) | 3484 (21.8) | 110 (27.6) | 137 (34.7) | 1800 (22.4) | 1684 (21.2) |
| At time of study drug discontinuationa | ||||||
| Patients, n | 884 | 17 096 | 442 | 442 | 8564 | 8532 |
| ASA | 263 (29.8) | 3559 (20.8) | 115 (26.0) | 148 (33.5) | 1828 (21.3) | 1731 (20.3) |
| Clopidogrel | 31 (3.5) | 244 (1.4) | 18 (4.1) | 13 (2.9) | 113 (1.3) | 131 (1.5) |
| Any ASA or thienopyridine | 289 (32.7) | 3742 (21.9) | 130 (29.4) | 159 (36.0) | 1914 (22.3) | 1828 (21.4) |
ASA indicates aspirin; PAD, peripheral artery disease.
Or at the time of last contact.
Outcomes in Patients With and Without PAD
Rates of efficacy end points and corresponding HRs for patients with PAD compared with patients without PAD are shown in Table 3. The overall unadjusted rate of stroke or systemic embolism was higher in patients with versus without PAD (HR 1.73, 95% CI 1.22–2.45; P=0.002), but after adjustment for differences in patient characteristics, no difference was present in rates of stroke or systemic embolism (HR 1.32, 95% CI 0.93–1.88; P=0.12). Those with PAD had an increased risk of all‐cause death (HR 1.36, 95% CI 1.11–1.67; P=0.003) and cardiovascular death (HR 1.44, 95% CI 1.08–1.90; P=0.01) compared with those without PAD.
Table 3.
Association Between PAD and Outcomes
| Outcomes | PAD Events (Rate) | No PAD Events (Rate) | Unadjusted HR (95% CI) | Unadjusted P Value | Adjusted HR (95% CI) | Adjusted P Value |
|---|---|---|---|---|---|---|
| Efficacy end points | ||||||
| Stroke or systemic embolism | 34 (2.17) | 440 (1.40) | 1.73 (1.22–2.45) | 0.0022 | 1.32 (0.93–1.88) | 0.1227 |
| All‐cause death | 108 (6.71) | 1151 (3.58) | 1.97 (1.61–2.40) | <0.0001 | 1.36 (1.11–1.67) | 0.0033 |
| Cardiovascular death | 58 (3.60) | 586 (1.82) | 2.13 (1.62–2.79) | <0.0001 | 1.44 (1.08–1.90) | 0.0117 |
| MI | 19 (1.20) | 171 (0.54) | 2.02 (1.26–3.26) | 0.0037 | 1.25 (0.77–2.03) | 0.3722 |
| Safety end points | ||||||
| ISTH major bleeding | 47 (3.42) | 731 (2.56) | 1.34 (1.00–1.81) | 0.0504 | 1.03 (0.76–1.40) | 0.8256 |
| ISTH major or clinically relevant nonmajor bleeding | 92 (6.85) | 1380 (4.93) | 1.38 (1.12–1.71) | 0.0027 | 1.12 (0.90–1.39) | 0.3114 |
| Clinically relevant nonmajor bleeding | 45 (3.30) | 710 (2.50) | 1.29 (0.96–1.75) | 0.0957 | 1.10 (0.81–1.50) | 0.5416 |
| GUSTO moderate or severe bleed | 36 (2.61) | 486 (1.69) | 1.57 (1.12–2.21) | 0.0089 | 1.22 (0.86–1.72) | 0.2709 |
| TIMI major or minor bleed | 41 (2.98) | 560 (1.95) | 1.56 (1.13–2.14) | 0.0063 | 1.22 (0.88–1.70) | 0.2226 |
| ISTH major bleeding type | ||||||
| Required transfusion | 27 (1.96) | 294 (1.02) | 1.80 (1.21–2.68) | 0.0036 | 1.32 (0.88–1.98) | 0.1840 |
| Required a medical or surgical intervention to stop bleeding | 12 (0.86) | 218 (0.76) | 1.15 (0.64–2.06) | 0.6383 | 0.97 (0.54–1.76) | 0.9242 |
| Required changes in antithrombotic therapy | 33 (2.39) | 360 (1.25) | 1.86 (1.30–2.66) | 0.0007 | 1.41 (0.98–2.04) | 0.0665 |
| Required hospitalization | 29 (2.10) | 338 (1.17) | 1.76 (1.21–2.58) | 0.0035 | 1.28 (0.86–1.89) | 0.2178 |
| Fatal bleeding | 2 (0.14) | 17 (0.06) | 2.87 (0.65–12.62) | 0.1624 | 1.94 (0.42–8.94) | 0.3960 |
| ISTH major bleeding site | ||||||
| Intracranial | 9 (0.65) | 165 (0.57) | 1.35 (0.69–2.64) | 0.3869 | 1.19 (0.60–2.35) | 0.6250 |
GUSTO indicates Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries; HR, hazard ratio; ISTH, International Society on Thrombosis and Haemostasis; MI, myocardial infarction; PAD, peripheral artery disease; TIMI, Thrombolysis in Myocardial Infarction.
Rates of bleeding end points and corresponding HRs for patients with PAD compared with those without PAD are also shown in Table 3. Crude rates of ISTH major bleeding were higher in patients with versus without PAD (HR 1.34, 95% CI 1.00–1.81; P=0.05), but no difference in event rates was present after adjustment (HR 1.03, 95% CI 0.76–1.40; P=0.83) between patients with and without PAD. Clinically overt bleeding was more common in patients with PAD compared with those without PAD (HR 1.61, 95% CI 1.13–2.30; P=0.008), and patients with PAD were more likely to have mucosal bleeding (HR 1.58, 95% CI 1.07–2.33; P=0.02) and gastrointestinal bleeding (HR 1.72, 95% CI 1.11–2.67; P=0.02) compared with those without PAD. There was no statistically significant difference in intracranial bleeding (HR 1.19, 95% CI 0.60–2.35; P=0.63) in patients with PAD compared with those without PAD.
Apixaban Versus Warfarin in Patients With and Without PAD
The effect of apixaban versus warfarin for the prevention of stroke or systemic embolism was similar in patients with PAD (HR 0.63, 95% CI 0.32–1.25) and without PAD (HR 0.80, 95% CI 0.66–0.96; interaction P=0.52) (Figures 1 and 2). The effect of apixaban versus warfarin on all‐cause death was also similar in patients with PAD (HR 1.03, 95% CI 0.71–1.51) and without PAD (HR 0.88, 95% CI 0.78–0.99; interaction P=0.42).
Figure 1.
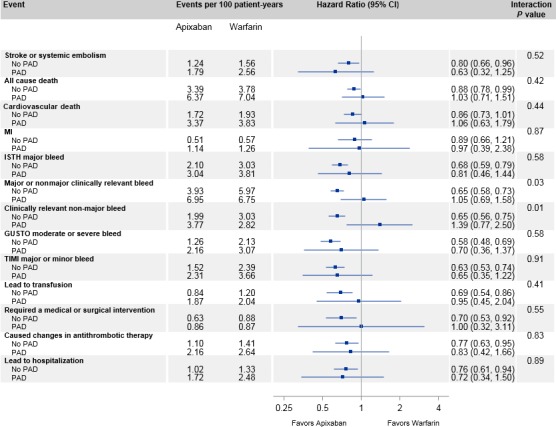
Treatment effects according to PAD. GUSTO indicates Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries; ISTH, International Society on Thrombosis and Haemostasis; MI, myocardial infarction; PAD indicates peripheral artery disease; TIMI, thrombolysis in myocardial infarction.
Figure 2.
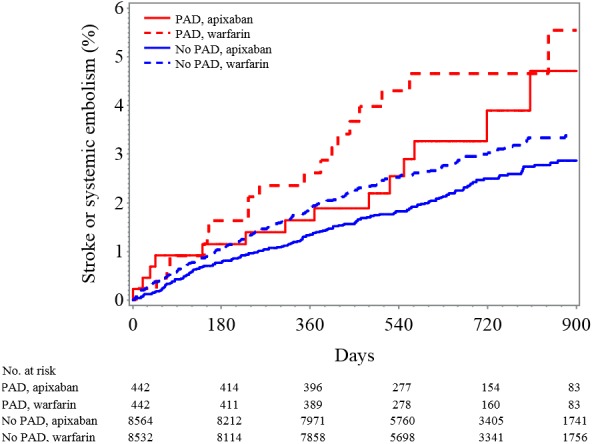
Kaplan–Meier plot for cumulative rate of stroke or systemic embolism in patients classified by PAD and treatment assignment. PAD indicates peripheral artery disease.
Patients with PAD appeared to have no difference in ISTH major bleeding with apixaban compared with warfarin (HR 0.81, 95% CI 0.46–1.44) versus those without PAD (HR 0.68, 95% CI 0.59–0.79; interaction P=0.58) (Figure 1). Patients with PAD had no statistically significant reduction in major or clinically relevant nonmajor bleeding (HR 1.05, 95% CI 0.69–1.58) with apixaban compared with warfarin, whereas those without PAD had a statistically significant reduction in major or clinically relevant nonmajor bleeding (HR 0.65, 95% CI 0.58–0.73; interaction P=0.03) with apixaban compared with warfarin. Similar results were seen for the outcome of clinically relevant nonmajor bleeding alone (apixaban versus warfarin: HR 1.39, 95% CI 0.77–2.50; patients with versus without PAD: HR 0.65, 95% CI 0.56–0.75; interaction P=0.01) (Figure 3, Table S1).
Figure 3.
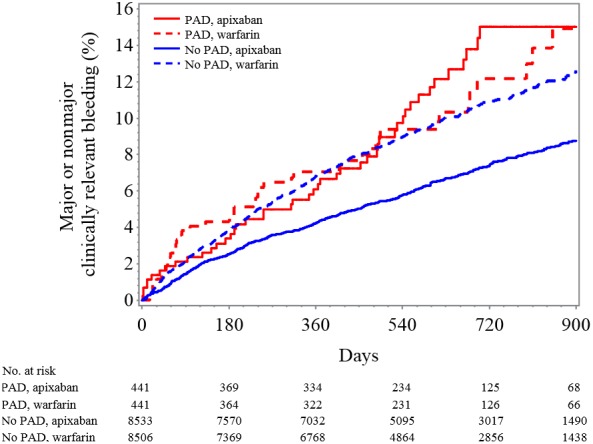
Kaplan–Meier plot for major or clinically relevant nonmajor bleeding classified by PAD status and treatment assignment. PAD indicates peripheral artery disease.
Discussion
As new therapies are introduced and utilized in the treatment of AF, it is imperative to understand the impact of comorbidities such as PAD on clinical outcomes. This analysis of patients with PAD in ARISTOTLE had 4 major findings. First, crude rates of stroke and bleeding were higher among those with PAD, but after adjustment for differences in patient characteristics, there was no difference in rate of stroke or systemic embolism and bleeding in patients with versus without PAD. Second, patients with PAD had an increased risk of all‐cause death and cardiovascular death compared with those without PAD. Third, the effect of apixaban versus warfarin for the prevention of stroke or systemic embolism was consistent in patients with and without PAD. Fourth, the reduction in bleeding with apixaban in the whole trial cohort was not observed in this relatively small subgroup with PAD.
Our first major finding, that the presence of PAD was not statistically significantly associated with stroke or systemic embolism, was not entirely surprising. A simple explanation for this is the low number of stroke or systemic embolic events in patients with PAD (N=32) thus limiting the power to detect a difference. The current results are similar to a post hoc analysis of patients with PAD in ROCKET AF (Rivaroxaban Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation). In ROCKET AF, patients with PAD compared with those without PAD did not have a statistically significant increase in stroke or systemic embolism (HR 1.04, 95% CI 0.72, 1.50; P=0.84).6 Our results are congruent with this prior analysis and suggest that in patients with AF and at least 1 additional risk factor for stroke who are on oral anticoagulant therapy, the presence of PAD is not independently associated with a substantial increase in risk of stroke. For patients not on anticoagulation, whether the presence of PAD (including as a sole factor) is associated with risk of stroke cannot be addressed with our study.
Our second finding, that PAD was associated with a higher risk of all‐cause death and cardiovascular death in patients with PAD compared with those without PAD, is consistent with prior studies and has been seen across many different cardiovascular trials and registries.12 PAD alone has been shown to be a powerful independent predictor of coronary and cerebrovascular disease. In addition, worsening PAD severity has been associated with increased mortality in patients with multiple disease states.13, 14 We were unable to validate this finding in the present analysis because of limited information regarding severity of PAD in the ARISTOTLE data set.
Our third major finding is that the benefit of apixaban versus warfarin for the prevention of stroke or systemic embolism was similar in patients with and without PAD. These results are consistent with the main trial results from ARISTOTLE, which found that apixaban was superior to warfarin in preventing stroke or systemic embolism in the entire study population.10 This helps provide confidence that apixaban can be used effectively to prevent stroke or systemic embolism in patients with AF with and without PAD.
In terms of bleeding risk, patients with PAD did not have a significantly increased risk of major bleeding compared with patients without PAD. In those who had overt bleeding, patients with PAD were more likely than those without PAD to have mucosal or gastrointestinal bleeding, but the overall event rates were low in both groups. This can be explained, at least in part, by the greater use of antiplatelet agents among patients with PAD (Table 2). Patients with PAD, compared with those without PAD, were more likely to be on aspirin, clopidogrel, or any aspirin or thienopyridine at baseline, at 1‐year follow‐up, and at the time of study drug discontinuation. In addition to antiplatelet therapy, the impact of PAD itself (related to something inherent about the disease, the need for invasive procedures, or other factors) may also contribute to an increased bleeding risk.
Interestingly, our study found a treatment interaction with apixaban compared with warfarin in patients with PAD. Patients with PAD had no statistically significant reduction in major or clinically relevant nonmajor bleeding with apixaban compared with warfarin, whereas those without PAD had a statistically significant reduction in major or clinically relevant nonmajor bleeding. Prior analysis of the ARISTOTLE trial found a similar interaction in patients with diabetes mellitus, which is of interest because PAD and diabetes mellitus are often comorbid conditions.15 Our current results are consistent with a similarly sized post hoc analysis of patients with AF with PAD enrolled in ROCKET AF, in which patients with PAD had a higher risk of major bleeding or clinically relevant nonmajor bleeding with rivaroxaban compared with warfarin.6 Although the use of antiplatelet agents at baseline and during the study may have contributed to this effect, patients with PAD on warfarin were more likely to be on concomitant antiplatelet therapy compared with patients with PAD on apixaban. These findings may not fully represent and explain the impact of antiplatelet agents on bleeding with warfarin and apixaban because patients may have been started on aspirin and/or a thienopyridine after year 1 and before study drug discontinuation. Furthermore, a prior analysis of the ARISTOTLE trial regarding concomitant aspirin use found that apixaban actually caused significantly less bleeding among both aspirin and nonaspirin users compared with warfarin.16 This makes it less likely that concomitant aspirin use alone is driving this interaction. Finally, one should always consider the play of chance when analyzing results from small subgroups like patients with AF and PAD in the ARISTOTLE trial.
Limitations
This analysis has several limitations. First, given its post hoc nature, our results should be interpreted as hypothesis generating rather than definitive. The multivariable model attempts to account for known confounders between the PAD and no PAD cohorts but is unable to account for unmeasured confounders. In addition, this study included a relatively small sample of patients with PAD compared with those without PAD because only 4.9% of patients included in this study had PAD. The power to detect similarities and differences in patient characteristics and baseline demographics is limited. Moreover, the results of our findings may not be generalizable to other non–clinical trial populations. In addition, patients with PAD in our study were identified as determined by site investigators. The severity of PAD in our subgroup is not known. Finally, although cerebrovascular disease was distinguished from PAD on the case report form, the disease burden and symptom status (eg, asymptomatic PAD, intermittent claudication, or critical limb ischemia) of PAD patients were not measured or recorded by site investigators. Because these factors influence morbidity and mortality with PAD, they could lead to imprecise findings in this small group of patients with PAD in ARISTOTLE.
Conclusions
Patients with AF and PAD had higher rates of death and trends toward higher rates of stroke or systemic embolism and bleeding than patients without PAD. The benefits of apixaban compared with warfarin for stroke and death were similar in patients with and without PAD. In the relatively small subgroup of patients with PAD in ARISTOTLE, no statistically significant reduction in major or clinically relevant nonmajor bleeding was observed in the larger population of patients without PAD. These findings highlight the need to optimize the treatment of patients with AF and PAD.
Sources of Funding
The ARISTOTLE trial was funded by Bristol‐Myers Squibb and Pfizer Inc.
Disclosures
Drs Hu, Stevens, and Thomas have nothing to disclose. Dr Lopes has research grants from Bristol Myers Squibb, Glaxo SmithKline. Dr Lopes has consulting fees from Bayer Corporation US, Boehringer Ingelheim, Bristol Myers Squibb, Glaxo SmithKline, Merck, Pfizer, and Portola. Dr Wallentin has research funding from Bristol Myers Squibb/Pfizer, AstraZeneca, Merck & Co., Boehringer Ingelheim, and GlaxoSmithKline. Dr Wallentin receives honorarium from GlaxoSmithKline. He is a consultant/on the advisory board for Bristol Myers Squibb/Pfizer, Abbott, AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim. He receives other financial benefit from Bristol Myers Squibb/Pfizer, AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim. Dr Alexander receives research funding from Bristol Myers Squibb, Boehringer Ingelheim, CSL Behring, National Institutes of Health, Regado Biosciences, Sanofi, Tenex Therapeutics, and Vivus Pharmaceuticals. He receives consulting fees/honorarium from Bristol Myers Squibb, and Duke Private Diagnostic Clinic. Dr Hanna is an employee of Bristol Meyers Squibb. Dr Lewis is in the speaker bureau at Pfizer. Dr Lewis receives honorarium from Pfizer. Dr Lewis is a consultant/on the advisory board for Pfizer, Merck & Co., and AstraZeneca. Dr Verheugt receives honorarium at Bristol Myers Squibb, and Pfizer. Dr Verheugt is a consultant/on the advisory board for Bristol Myers Squibb and Pfizer. Dr Granger receives research funding from Boehringer Ingelheim, Bristol Myers Squibb, Glaxo SmithKline, Medtronic Foundation, Merck & Co., Pfizer, Sanofi‐Aventis, Takeda, The Medicines Company, Astra Zeneca, Daiichi Sankyo, Janssen Pharmaceuticals, Bayer, and Armetheon. Dr Granger receives consulting fees from Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Pfizer, Sanofi‐Aventis, Daiichi Sankyo, Ross Medical Corporation, Janssen Pharmaceuticals, Salix Pharmaceuticals, Bayer, and Gilead. Dr Jones receives research funding from Agency for Healthcare Research and Quality, American Heart Association, AstraZeneca, Bristol‐Myers Squibb, Daiichi‐Sankyo, and Patient‐Centered Outcomes Research Institute.
Supporting information
Table S1. Proportional Hazards Regression Models for Peripheral Artery Disease by Treatment Interactions on ISTH Bleeding End Points
(J Am Heart Assoc. 2017;6:e004699. DOI: 10.1161/JAHA.116.004699.)
An abstract of this work was presented at the American Heart Association Scientific Sessions, November 7 to 11, 2015 in Orlando, FL, and at the American College of Physicians Scientific Sessions, May 5 to 7, 2016, in Washington, DC.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 3. Hijazi Z, Lindbäck J, Alexander JH, Hanna M, Held C, Hylek EM, Lopes RD, Oldgren J, Siegbahn A, Stewart RA, White HD, Granger CB, Wallentin L. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker‐based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 5. Olesen JB, Lip GY, Lane DA, Køber L, Hansen ML, Karasoy D, Hansen CM, Gislason GH, Torp‐Pedersen C. Vascular disease and stroke risk in atrial fibrillation: a nationwide cohort study. Am J Med. 2012;125:826. [DOI] [PubMed] [Google Scholar]
- 6. Jones WS, Hellkamp AS, Halperin J, Piccini JP, Breithardt G, Singer DE, Fox KA, Hankey GJ, Mahaffey KW, Califf RM, Patel MR. Efficacy and safety of rivaroxaban compared with warfarin in patients with peripheral artery disease and non‐valvular atrial fibrillation: insights from ROCKET AF. Eur Heart J. 2014;35:242–249. [DOI] [PubMed] [Google Scholar]
- 7. Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, Petersen P. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292. [DOI] [PubMed] [Google Scholar]
- 8. Goto S, Bhatt DL, Röther J, Alberts M, Hill MD, Ikeda Y, Uchiyama S, D'Agostino R, Ohman EM, Liau CS, Hirsch AT, Mas JL, Wilson PW, Corbalán R, Aichner F, Steg PG. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156:855–863. [DOI] [PubMed] [Google Scholar]
- 9. Winkel TA, Hoeks SE, Schouten O, Zeymer U, Limbourg T, Baumgartner I, Bhatt DL, Steg PG, Goto S, Röther J, Cacoub PP, Verhagen HJ, Bax JJ, Poldermans D. Prognosis of atrial fibrillation in patients with symptomatic peripheral arterial disease: data from the reduction of atherothrombosis for continued health (REACH) registry. Eur J Vasc Endovasc Surg. 2010;40:9–16. [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 11. Lopes RD, Alexander JH, Al‐Khatib SM, Ansell J, Diaz R, Easton JD, Gersh BJ, Granger CB, Hanna M, Horowitz J, Hylek EM, McMurray JJ, Verheugt FW, Wallentin L. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331–339. [DOI] [PubMed] [Google Scholar]
- 12. Rasmussen LH, Larsen TB, Due KM, Tjønneland A, Overvad K, Lip GY. Impact of vascular disease in predicting stroke and death in patients with atrial fibrillation: the Danish Diet, Cancer and Health Cohort study. J Thromb Haemost. 2011;9:1301–1307. [DOI] [PubMed] [Google Scholar]
- 13. Golomb BA. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. [DOI] [PubMed] [Google Scholar]
- 14. Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. [DOI] [PubMed] [Google Scholar]
- 15. Ezekowitz JA, Lewis BS, Lopes RD, Wojdyla DM, McMurray JJ, Hanna M, Atar D, Bahit CM, Keltai M, Lopez‐Sendon JL, Pais P, Ruzyllo W, Wallentin L, Granger CB, Alexander JH. Clinical outcomes of patients with diabetes and atrial fibrillation treated with apixaban: results from the ARISTOTLE trial. Eur Heart J Cardiovasc Pharmacother. 2015;1:86–94. [DOI] [PubMed] [Google Scholar]
- 16. Alexander JH, Lopes RD, Thomas L, Alings M, Atar D, Aylward P, Goto S, Hanna M, Huber K, Husted S, Lewis BS, McMurray JJ, Pais P, Pouleur H, Steg PG, Verheugt FW, Wojdyla DM, Granger CB, Wallentin L. Apixaban vs. warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2014;35:224–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Proportional Hazards Regression Models for Peripheral Artery Disease by Treatment Interactions on ISTH Bleeding End Points


