Abstract
Background
Contrast‐induced acute kidney injury (CI‐AKI) was traditionally defined as an increase in serum creatinine (sCr) after contrast media exposure. Recently, serum cystatin C (sCyC) has been proposed as an alternative to detect acute changes in renal function. The clinical implications of combining sCyC and sCr to diagnose CI‐AKI remain unknown.
Methods and Results
One thousand seventy‐one consecutive patients undergoing coronary angiography/intervention were prospectively enrolled. SCyC and sCr were assessed at baseline and 24 to 48 hours after contrast media exposure. CI‐AKI determined by sCr (CI‐AKI sCr) was defined as an sCr increase greater than 0.3 mg/dL or 50% from baseline. Major adverse events at 12 months were assessed. CI‐AKI sCr developed in 25 patients (2.3%). Twelve‐month follow‐up was available for 1063 patients; major adverse events occurred in 61 patients (5.7%). By receiver operating characteristic curve analysis, an sCyC increase of greater than 15% was the optimal cutoff for CI‐AKI sCr detection, which occurred in 187 patients (17.4%). To evaluate the use of both sCyC and sCr as CI‐AKI diagnostic criteria, we stratified patients into 3 groups: no CI‐AKI, CI‐AKI detected by a single marker, and CI‐AKI detected by both markers. Multivariable logistic regression revealed that the predictability of major adverse events increased in a stepwise fashion in the 3 groups (no‐CI‐AKI group as the reference, CI‐AKI detected by a single marker: odds ratio=2.25, 95% CI: 1.24–4.10, P<0.01; CI‐AKI detected by both markers: odds ratio=10.00, 95% CI: 3.13–31.91, P<0.001).
Conclusions
Combining sCyC and sCr to diagnose CI‐AKI would be beneficial for risk stratification and prognosis in patients after contrast media exposure.
Keywords: contrast‐induced acute kidney injury, diagnosis, prognosis, risk stratification
Subject Categories: Nephrology and Kidney
Introduction
Contrast‐induced acute kidney injury (CI‐AKI), a powerful predictor of unfavorable clinical outcomes,1 was traditionally defined as an increase in serum creatinine (sCr) after contrast media (CM) exposure.2 Although sCr is an insensitive indicator during acute changes in renal function,3 measurement of sCr is the only approach to detect AKI recommended by guidelines because of the strong association between sCr and disease severity.4, 5 Recently, serum cystatin C (sCyC) was proposed as an alternative to sCr for diagnosing AKI.6, 7 CyC is a 122‐amino acid, nonglycosylated protein that is generated at a constant rate by nucleated cells and is renally cleared. Therefore, sCyC level is determined by the glomerular filtration rate.8 Metabolic and kinetic evidence suggests that the half‐life of CyC is shorter than Cr, which results in a more rapid increase of sCyC concentration during acute‐phase kidney injury.9, 10 For this reason, sCyC might be a more sensitive AKI marker. However, use of sCyC level for CI‐AKI diagnosis remains under debate.11 The main purposes of this study were to (1) assess the optimal sCyC cutoff point to detect CI‐AKI; (2) test whether combining sCyC and sCr for AKI diagnosis provides extra benefit by integrating the advantage of each marker; and (3) determine the clinical implications of using both markers.
Materials and Methods
Design and Participants
Between July 2013 and December 2014, consecutive patients over 18 years of age who were scheduled to undergo coronary angiography/intervention in the cardiac center of Renji Hospital, a tertiary‐care academic center in China, were prospectively recruited. Exclusion criteria were pregnancy, end‐stage renal disease on maintenance dialysis, recent exposure to CM (within 2 days before/after procedure), cardiac shock, and concomitant use of nephrotoxic agents. Nonionic, low‐osmolality CM and intravenous hydration were used in all patients. The duration and volume of hydration were determined at the discretion of the physicians. The study protocol was approved by the Institutional Research Ethics Board. Informed consent was obtained from all participants.
Data Collection, Biomarker Measurement, and Follow‐Up
Prespecified demographic and clinical data were recorded for each participant. Blood samples for biomarker measurement were collected at baseline and postprocedure day 2 (at the time of routine morning blood collection). Peripheral blood was collected into heparin lithium‐anticoagulant tubes and plasma was separated by centrifugation at 2600 g for 10 minutes. SCr and sCyC were measured in the central biochemistry laboratory of Renji Hospital. SCyC was quantified by latex particle–enhanced immuneturbidimetry using a fully automatic chemistry analyzer (Hitachi 7180) with Norudia Cystatin C kit (Sekisui Medical Technology Co., Ltd.). All the participants were scheduled to follow up until 12 months postprocedure by outpatient clinic visit or telephone interview. Reported events were carefully evaluated and recorded.
Definitions
Estimated glomerular filtration rate was calculated by applying the Levey modification of the Modification of Diet in Renal Disease Study equation. The CI‐AKI risk score (Mehran risk score) was calculated according to the algorithm reported by Mehran et al.12 CI‐AKI as determined by sCr (CI‐AKIsCr) was defined as an increase greater than 0.3 mg/dL or 50% in sCr from baseline within 48 hours after CM exposure, which differs from the Kidney Disease: Improving Global Outcomes (KDIGO) definition by not incorporating oliguria as evidence of CI‐AKI.4 CI‐AKI as determined by sCyC was identified as CI‐AKIsCyC. Major adverse events (MAEs) were defined as the composite of specified events including all‐cause death, myocardial infarction, stroke, ischemia‐driven revascularization, and nephropathy requiring dialysis.
Statistical Analyses
The continuous variables were presented as the mean±SD or median (with 25th and 75th percentiles) and categorical variables as percentages. For continuous variables, comparisons between groups were made using the t test and analysis of variance for normally distributed data and the Wilcoxon test and Kruskal–Wallis test for non‐normally distributed data. Categorical data were compared using the χ2 test and Fisher's exact test, as appropriate. The diagnostic accuracy of the sCyC increment above baseline for predicting CI‐AKI as determined by sCr was evaluated by receiver operating characteristic curve analysis. The optimal cutoff value was chosen using Youden's index. Whether the increment of sCyC change was an independent predictor of MAEs was determined by multivariable logistic regression analysis using Firth's penalized‐likelihood estimation as the number of MAEs was relative small.13 Finally, a new definition of CI‐AKI (CI‐AKINew) was proposed using both sCr and sCyC (Table 1). By this definition, participants were further stratified into 3 groups as follows: no CI‐AKI, CI‐AKI detected by a single marker, and CI‐AKI detected by both markers. Baseline characters, Mehran risk score, and MAEs incidence were compared across the 3 groups. CI‐AKINew was included as a covariate in the multivariable logistic regression model using Firth's penalized‐likelihood estimation to assess whether the new definition of CI‐AKI was an independent predictor of MAEs. Interaction effects between CI‐AKINew and other covariates were assessed. If statistically significant, the interaction term was included into the regression model. P<0.05 was considered significant throughout the analyses. All analyses were performed using SPSS 19.0 and R 3.3.2 software.
Table 1.
New Definition of CI‐AKI Using Both sCr and sCyC
| Group | Definition | Risk Stratification |
|---|---|---|
| Group 1 | No CI‐AKI: sCr increase <0.3 mg/dL and 50% from baseline; and sCyC increase <15% from baseline. | No risk |
| Group 2 | CI‐AKI detected by a single marker: fulfill only 1 of criteria as below: (1) sCr increase ≥0.3 mg/dL or 50% from baseline; (2) sCyC increase ≥15% from baseline. | Potential risk |
| Group 3 | CI‐AKI detected by both markers: sCr increase ≥0.3 mg/dL or 50% from baseline; and sCyC increase ≥15% from baseline. | High risk |
CI‐AKI indicates contrast‐induced acute kidney injury; sCr, serum creatinine; sCyC, serum cystatin C.
Results
Participant Characteristics
A total of 1071 participants were included in the study. Baseline characteristics of participants are listed in Table 2. Median sCr concentration in these participants significantly increased from baseline after CM exposure (Table 2). CI‐AKIsCr occurred in 25 participants (2.3%). Twelve‐month follow‐up information was available for 1063 participants (99.3%). MAEs occurred in 61 participants (5.7%, Table 3).
Table 2.
Clinical Characteristics (n=1071)
| Variables | |
|---|---|
| Age, y | 64.8±10.2 |
| Male, n (%) | 713 (66.6%) |
| Current smoking, n (%) | 345 (32.2%) |
| Diabetes mellitus, n (%) | 364 (34.0%) |
| Hypertension, n (%) | 698 (65.2%) |
| Prior MI or stroke, n (%) | 228 (21.3%) |
| Acute MI, n (%) | 127 (11.9%) |
| Blood pressure, mm Hg | |
| Systolic | 134±19 |
| Diastolic | 77±11 |
| NYHA Grade III–IV, n (%) | 57 (5.3%) |
| Drugs | |
| ACEI/ARB | 524 (48.9%) |
| Statins | 922 (86.1%) |
| PCI, n (%) | 636 (59.4%) |
| Volume of CM, mL | 100 (60–150) |
| Baseline eGFR, mL/min per 1.73 m2 | 96.6 (80.5–114.4) |
| ≥90, n (%) | 644 (60.1%) |
| 60 to 89, n (%) | 358 (33.4%) |
| 30 to 59, n (%) | 64 (6.0%) |
| <30, n (%) | 5 (0.5%) |
| Mehran risk score | 4.0 (2.0–6.0) |
| ≤5, n (%) | 747 (69.7%) |
| 6 to 10, n (%) | 264 (24.7%) |
| ≥11, n (%) | 60 (5.6%) |
| sCr, mg/dL | |
| Baseline | 0.79 (0.67–0.94) |
| 24 to 48 hours post CM exposure | 0.82 (0.68–0.98)a |
| sCyC, mg/dL | |
| Baseline | 0.96 (0.82–1.17) |
| 24 to 48 hours post CM exposure | 0.97 (0.83–1.16) |
Continuous values are expressed as mean±SD or median (with 25th and 75th percentiles); categorical values are expressed as total number and proportion of the global population (in parentheses). ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CM, contrast media; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; sCr, serum creatinine; sCyC, serum cystatin C.
P<0.001, compared with sCr at baseline.
Table 3.
Occurrence of MAEs at 12‐Months Follow‐Up (n=1063)
| Event | |
|---|---|
| All‐cause death, n (%) | 1 (0.09%) |
| MI, n (%) | 2 (0.2%) |
| Stroke, n (%) | 6 (0.6%) |
| Ischemia‐driven revascularization, n (%) | 54 (5.1%) |
| Nephropathy requiring chronic dialysis, n (%) | 0 (0%) |
| MAEs, n (%) | 61 (5.7%) |
Categorical values are expressed as total number and proportion of the global population (in parentheses). MAEs indicates major adverse events; MI, myocardial infarction.
Performance of sCyC in the Diagnosis of CI‐AKI
Median sCyC concentration did not significantly change from baseline after CM exposure (Table 2). The distributions in sCyC level changes after CM exposure are presented in Table 4. By receiver operating characteristic curve analysis, the change in sCyC from baseline significantly predicted the development of CI‐AKIsCr (area under curve=0.856, 95% CI=0.779–0.936, P<0.001, [Figure 1]). To optimize both sensitivity and specificity, an sCyC increase greater than 15% after CM exposure, which had an 80% sensitivity and 83% specificity, was chosen as the optimal cutoff value (Table 4). Using this definition, participants who developed CI‐AKIsCyC had higher Mehran risk scores and experienced more MAEs compared with participants without CI‐AKIsCyC (Figure 2). Multivariable logistic regression analysis revealed that an sCyC increase greater than 15% was an independent predictor of 12‐month MAEs (adjusted odd ratios=3.13, 95% CI: 1.82–5.38, P<0.001).
Table 4.
Distribution in sCyC Level Changes After CM Exposure and Relationship With CI‐AKIsCr
| Changes in sCyC | Proportion, n (%) | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Youden Index |
|---|---|---|---|---|---|---|
| ≥5% | 388 (36.2%) | 84 | 65 | 5.4 | 99.4 | 0.49 |
| ≥10% | 259 (24.2%) | 80 | 77 | 7.7 | 99.4 | 0.57 |
| ≥15% | 187 (17.4%) | 80 | 83 | 9.6 | 99.4 | 0.63 |
| ≥25% | 114 (10.6%) | 68 | 90 | 14.9 | 99.2 | 0.58 |
Categorical values are expressed as total number and proportion of the global population (in parentheses). CI‐AKIsCr indicates contrast‐induced acute kidney injury as determined by serum creatinine; CM, contrast media; NPV, negative predictive value; PPV, positive predictive value; sCyC, serum cystatin C.
Figure 1.
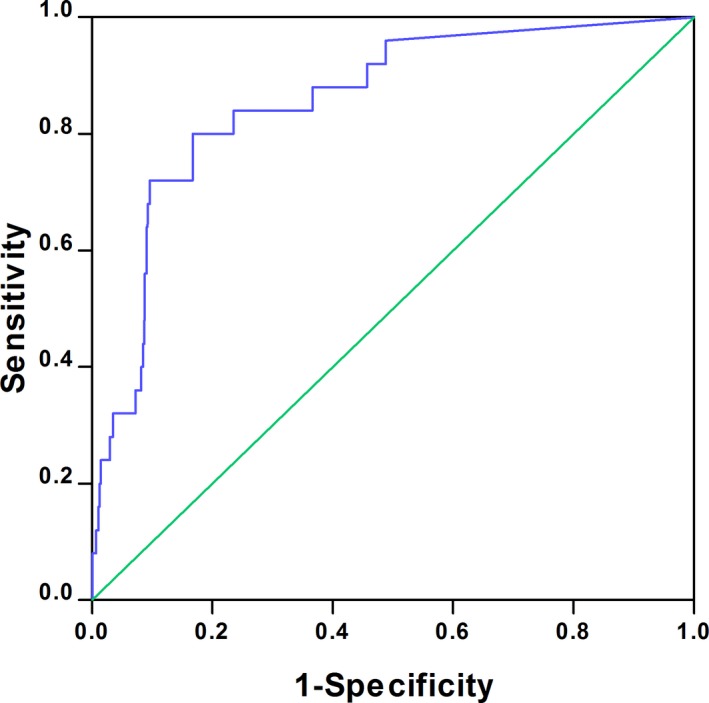
Receiver operating characteristic curve and AUC showing the diagnostic performance of sCyC for CI‐AKI detection. AUC=0.856 (P<0.001). AUC indicates area under the curve; CI‐AKI, contrast‐induced acute kidney injury; sCyC, serum cystatin C.
Figure 2.
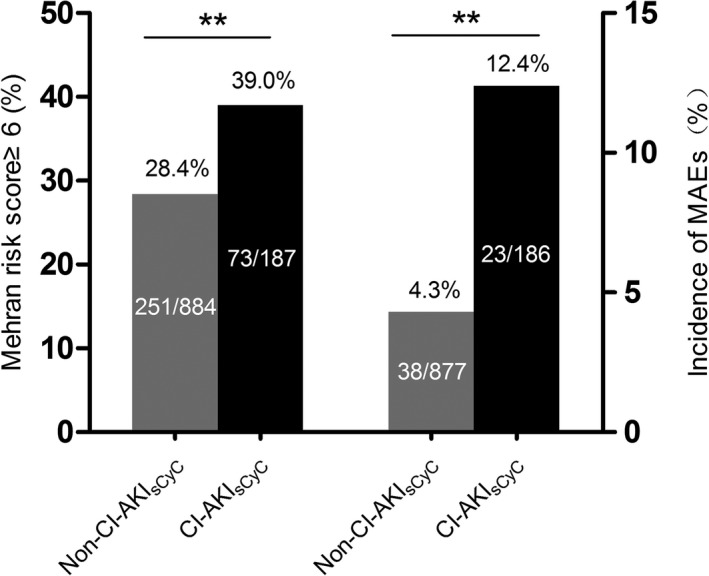
Portion of patients with Mehran risk score greater than 6 and incidence of MAEs in the CI‐AKI sCyC group and the non‐CI‐AKI sCyC group. CI‐AKI sCyC indicates contrast‐induced acute kidney injury defined by serum cystatin C; MAEs, major adverse events. **P<0.01.
Clinical Implications of the New Definition of CI‐AKI
Based on the new definition of CI‐AKI listed in Table 1, participants were further stratified into 3 groups. The baseline characteristics revealed the risk of developing CI‐AKI increased across the 3 groups (Table 5). Participants in the no‐CI‐AKI group had the lowest Mehran risk scores, and participants in the group with CI‐AKI detected by both markers had the highest Mehran risk scores (Figure 3). A similar pattern was observed in the occurrence of MAEs. The occurrence of MAEs was lowest in the no‐CI‐AKI group and the highest in participants with CI‐AKI detected by both markers (Figure 3). By multivariable logistic regression analysis, no significant interaction effects existed between new definition of CI‐AKI and other covariates. A significant correlation was found between MAEs and the new definition of CI‐AKI. Taking the no‐CI‐AKI group as the reference, CI‐AKI detected by a single marker (adjusted odds ratio=2.25, 95% CI: 1.24–4.10, P<0.01) and CI‐AKI detected by both markers (adjusted odds ratio=10.00, 95% CI: 3.13–31.91, P<0.001) were independent predictors of MAEs at 12 months (Table 6).
Table 5.
Comparisons of Clinical Characteristics in Patients Stratified by Composite of sCyC and sCyC (n=1071)
| Variables | No CI‐AKI (n=877) | CI‐AKI Detected by a Single Marker (n=176) | CI‐AKI Detected by Both Markers (n=18) | P Value |
|---|---|---|---|---|
| Age, y | 64.9±9.9 | 64.0±11.5 | 69.6±12.4 | 0.074 |
| Male, n (%) | 580 (66.1%) | 120 (68.2%) | 13 (72.2%) | 0.764 |
| Current smoking, n (%) | 282 (32.2%) | 60 (34.1%) | 3 (16.7%) | 0.349 |
| Diabetes mellitus, n (%) | 293 (33.4%) | 63 (35.8%) | 8 (44.4%) | 0.594 |
| Hypertension, n (%) | 569 (64.9%) | 118 (67.0%) | 11 (61.1%) | 0.804 |
| Prior MI or stroke, n (%) | 179 (20.4%) | 41 (23.3%) | 8 (44.4%) | 0.037 |
| Acute MI, n (%) | 97 (11.1%) | 26 (14.8%) | 4 (22.2%) | 0.125 |
| Blood pressure, mm Hg | ||||
| Systolic | 134±19 | 135±19 | 142±20 | 0.136 |
| Diastolic | 77±11 | 77±10 | 78±11 | 0.895 |
| NYHA Grade III–IV, n (%) | 42 (4.8%) | 11 (6.3%) | 4 (22.2%) | 0.014 |
| Drugs | ||||
| ACEI/ARB | 423 (48.2%) | 88 (50.0%) | 13 (72.2%) | 0.125 |
| Statins | 751 (85.6%) | 154 (87.7%) | 17 (94.4%) | 0.594 |
| PCI, n (%) | 513 (58.5%) | 110 (62.5%) | 13 (72.2%) | 0.329 |
| Volume of CM, mL | 100 (60–150) | 120 (60–160) | 150 (100–200) | 0.025 |
| Baseline eGFR, mL/min per 1.73 m2 | 96.1 (80.5–112.9) | 101.6 (83.9–122.9) | 55.2 (43.4–92.1) | <0.001 |
| ≥90, n (%) | 526 (60.0%) | 113 (64.2%) | 5 (27.8%) | 0.011 |
| 60 to 89, n (%) | 308 (35.1%) | 49 (27.8%) | 1 (5.5%) | 0.005 |
| 30 to 59, n (%) | 42 (4.8%) | 13 (7.4%) | 9 (50.0%) | <0.001 |
| <30, n (%) | 1 (0.1%) | 1 (0.6%) | 3 (16.7%) | <0.001 |
| Mehran risk score | 4.0 (1.0–6.0) | 4.0 (2.0–7.0) | 10.5 (5.8–14.0) | <0.001 |
| ≤5, n (%) | 632 (72.1%) | 111 (63.1%) | 4 (22.2%) | <0.001 |
| 6 to 10, n (%) | 207 (23.6%) | 52 (29.5%) | 5 (27.8%) | 0.237 |
| ≥11, n (%) | 38 (4.3%) | 13 (7.4%) | 9 (50.0%) | <0.001 |
| sCr, mg/dL | ||||
| Baseline | 0.79 (0.67–0.94) | 0.76 (0.65–0.90) | 1.33 (0.90–1.62) | <0.001 |
| Post CM | 0.81 (0.67–0.95) | 0.86 (0.71–0.97) | 1.81 (1.42–2.35) | <0.001 |
| sCyC, mg/dL | ||||
| Baseline | 0.98 (0.86–1.19) | 0.80 (0.70–1.02) | 1.62 (0.90–2.37) | <0.001 |
| Post CM | 0.95 (0.81–1.12) | 1.04 (0.89–1.30) | 2.52 (1.34–3.04) | <0.001 |
Continuous values are expressed as mean±SD or median (with 25th and 75th percentiles); categorical values were expressed as total number and proportion of the global population (in parentheses). ACEI indicates angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CI‐AKI, contrast‐induced acute kidney injury; CM, contrast media; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; sCr, serum creatinine; sCyC, serum cystatin C.
Figure 3.
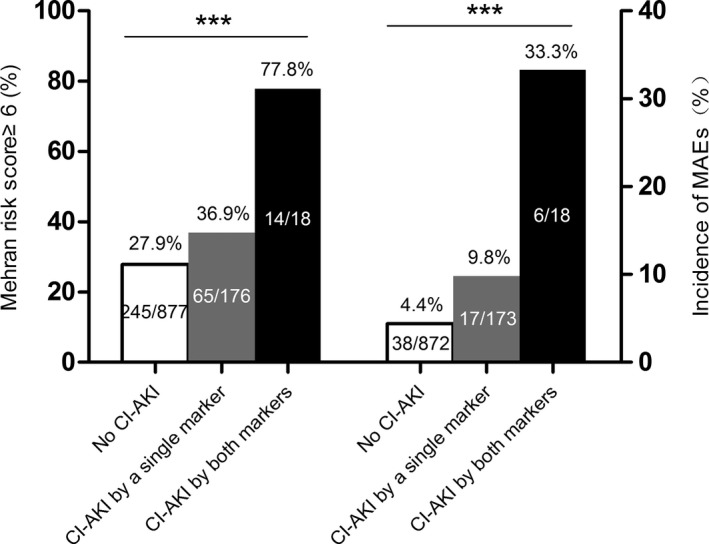
Portion of patients with Mehran risk score greater than 6 and incidence of MAEs in patients stratified by the composite of sCyC and sCr. CI‐AKI indicates contrast‐induced acute kidney injury; MAEs, major adverse events; sCr, serum creatinine; sCyC, serum cystatin C. ***P<0.001.
Table 6.
Predictors of MAEs at 12 Months Follow‐Up by Multivariable Logistic Regression Analysis Using Firth's Penalized‐Likelihood Estimation
| Variables | OR (95% CI) | P Value |
|---|---|---|
| CI‐AKI detected by a single marker | 2.25 (1.24–4.10) | <0.010 |
| CI‐AKI detected by both markers | 10.00 (3.13–31.91) | <0.001 |
| Age ≥75 years | 0.54 (0.19–1.49) | 0.234 |
| Diabetes mellitus | 0.94 (0.43–2.07) | 0.887 |
| Prior or new‐onset MI | 2.26 (1.37–3.73) | 0.001 |
| NYHA Grade III–IV | 0.77 (0.16–3.11) | 0.709 |
| Baseline eGFR | 1.00 (0.99–1.01) | 0.909 |
| Mehran risk score | 1.03 (0.88–1.20) | 0.730 |
CI‐AKI indicates contrast‐induced acute kidney injury; eGFR, estimated glomerular filtration rate; MAEs, major adverse events; MI, myocardial infarction; NYHA, New York Heart Association; OR, odds ratio.
Discussion
CI‐AKI is an increasingly common complication in patients with CM exposure, potentially devastating, and associated with adverse clinical outcomes.14 Although the prevalence of and prevention measures for CI‐AKI have been investigated in multiple clinical trials, conclusions on these topics remain inconsistent.15, 16 A possible explanation for the heterogeneity in results might be the disparity in the definition of CI‐AKI adopted in each study.17 Therefore, new diagnostic criteria of CI‐AKI that could predict adverse clinical outcomes and be commonly adopted are desperately needed. To the best of our knowledge, this is the first study evaluating the use of both sCyC and sCr in diagnosing CI‐AKI in an unselected population receiving coronary angiography/percutaneous intervention. The clinical implications of our findings are important in several respects.
We evaluated the diagnostic performance of sCyC for CI‐AKI. The reliability of sCyC as a biomarker in detecting acute changes in kidney function has been proven in several previous studies.18 In CI‐AKI patients, the rise in sCyC concentrations post‐CM exposure was also observed.9 However, the optimal threshold of sCyC for CI‐AKI diagnosis remains in debate. In a small cohort enrolling 121 patients, a 25% increase of sCyC from baseline within 48 hours post‐CM exposure was used to define CI‐AKI.19 Another cohort of 410 patients with chronic kidney disease indicated that a 10% increase of sCyC is a reliable definition of CI‐AKI.20 In the present study, we found that a 15% increase in sCyC was highly accurate in diagnosing CI‐AKI, with a Youden index up to 0.63. Our data might have several advantages. Firstly, we enrolled consecutive, unselected patients including both chronic kidney disease patients and patients with preserved renal function, which increase the generalizability of study population. Secondly, we adopted KDIGO criteria (an increase greater than 0.3 mg/dL or 50% in sCr from baseline within 48 hours after CM exposure) for CI‐AKI based on sCr. Although contrast‐induced nephropathy (CIN) criteria (an increase greater than 0.5 mg/dL or 25% in sCr) was widely used in multiple trials,16 recent evidence suggests that KDIGO criteria are superior to contrast‐induced nephropathy criteria. A large cohort enrolling 119 554 patients revealed that KDIGO criteria are more robust determinants in predicting mortality compared with contrast‐induced nephropathy criteria.21 Therefore, current guidelines suggests that KDIGO criteria are superior to contrast‐induced nephropathy criteria.4 Adopting KDIGO criteria allowed us to better identify patients at risk. Finally, our data confirmed that patients with a 15% increase in sCyC had higher Mehran risk score, which was a valid predictor for CI‐AKI occurrence. Moreover, a 15% increase in sCyC strongly associated with 12‐month MAEs. Taken together, these data suggest that a 15% increase in sCyC could be served as not only an optimal threshold for CI‐AKI detection, but also a significant predictive factor for clinical outcomes.
To optimize risk stratification, we combined sCyC and sCr to create new diagnostic criteria for CI‐AKI. Among the entire cohort, 176 of CI‐AKI cases were detected by a single marker (sCyC only [n=169] versus sCr only [n=7]). Subgroup analysis revealed that patients in this subcohort had higher risk profiles and substantially worse long‐term outcomes compared with patients without CI‐AKI by either sCyC‐ or sCr‐based criteria. Another 18 CI‐AKI cases were detected by both sCyC‐ and sCr‐based criteria. A 2‐fold increase in risk of long‐term MAEs was observed in this group of patients. Multivariable logistic regression analysis showed that the predictability of adverse outcomes increased stepwise across the 3 groups. Consistent with the results of a previous study, our data indicated that sCyC is a more sensitive marker than sCr in identifying CI‐AKI cases.20 The disparity between sCyC and sCr in CI‐AKI detection might be because of the properties of CyC, including shorter half‐life, absence of renal tubular secretion, and less affected by volume status.22 Patients with both sCyC‐ and sCr‐based CI‐AKI had poorest outcomes, implying that inclusion of both sCyC and sCr in defining CI‐AKI would increase association between CI‐AKI and outcomes. Just as combining both sCyC and sCr in an equation is the most accurate method for estimating GFR, combining sCyC and sCr to define CI‐AKI might be superior to using a single marker only.23 In conclusion, patients with an abnormal value of a single marker are at potential risk for adverse outcomes, and preventive measures should be employed for these patients to avoid further kidney injury. Moreover, abnormal values of both markers can potentially identify the highest‐risk subset of CI‐AKI patients, in whom careful monitoring for adverse events is required. Overall, our new proposed diagnostic criteria of CI‐AKI would be beneficial for patient stratification and prognosis.
Limitations
This study had several limitations. Firstly, this was a single center study. The sample size precluded us from generating a validation cohort. The result of our data should be confirmed by a further larger multicenter study. Secondly, the hydration protocol and other prevention measures, such as statin use, were not standardized in our study, which may have influenced the development of CI‐AKI.24, 25 Thirdly, albuminuria and other urine markers of CI‐AKI, such as neutrophil gelatinase‐associated lipocalin, were not routinely measured in our center. Data regarding the association between the new definition of CI‐AKI and renal injury markers were unavailable in the present study. Fourthly, 7 patients experienced an acute rise in sCr without sCyC rise. The potential explanations are unknown and the case volume prevented us from performing further analysis. Future study is needed to address the issue whether the difference exist between the patients with an acute rise in sCyC only and patients with an acute rise in sCr only. Finally, no new onset of nephropathy requiring chronic dialysis was observed in the present cohort. The predictability of the new CI‐AKI definition for adverse renal outcomes should be further assessed.
Sources of Funding
This work was supported by a grant from the National Natural Science Foundation of China (number 81600518) and grants from the Shanghai Municipal Commission of Health and Family Planning (numbers 20144Y0112, 2014ZYJB0501, and 20164Y0111).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e004747. DOI: 10.1161/JAHA.116.004747.)
Contributor Information
Ling‐hong Shen, Email: rjshenlinghong@126.com.
Ben He, Email: rjheben@126.com.
References
- 1. Giacoppo D, Madhavan MV, Baber U, Warren J, Bansilal S, Witzenbichler B, Dangas GD, Kirtane AJ, Xu K, Kornowski R, Brener SJ, Genereux P, Stone GW, Mehran R. Impact of contrast‐induced acute kidney injury after percutaneous coronary intervention on short‐ and long‐term outcomes: pooled analysis from the HORIZONS‐AMI and ACUITY Trials. Circ Cardiovasc Interv. 2015;8:e002475. [DOI] [PubMed] [Google Scholar]
- 2. McCullough PA. Contrast‐induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–1428. [DOI] [PubMed] [Google Scholar]
- 3. Sun SQ, Zhang T, Ding D, Zhang WF, Wang XL, Sun Z, Hu LH, Qin SY, Shen LH, He B. Circulating MicroRNA‐188, ‐30a, and ‐30e as early biomarkers for contrast‐induced acute kidney injury. J Am Heart Assoc. 2016;5:e004138 DOI: 10.1161/JAHA.116.004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, Van Biesen W. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast‐induced nephropathy. Nephrol Dial Transplant. 2012;27:4263–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, Almen T, Aspelin P, Bellin MF, Clement O, Heinz‐Peer G. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541. [DOI] [PubMed] [Google Scholar]
- 6. Wald R, Liangos O, Perianayagam MC, Kolyada A, Herget‐Rosenthal S, Mazer CD, Jaber BL. Plasma cystatin C and acute kidney injury after cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 2010;25:3283–3289. [DOI] [PubMed] [Google Scholar]
- 8. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta‐analysis. Am J Kidney Dis. 2002;40:221–226. [DOI] [PubMed] [Google Scholar]
- 9. Rickli H, Benou K, Ammann P, Fehr T, Brunner‐La Rocca HP, Petridis H, Riesen W, Wuthrich RP. Time course of serial cystatin C levels in comparison with serum creatinine after application of radiocontrast media. Clin Nephrol. 2004;61:98–102. [DOI] [PubMed] [Google Scholar]
- 10. Sjostrom P, Tidman M, Jones I. The shorter T1/2 of cystatin C explains the earlier change of its serum level compared to serum creatinine. Clin Nephrol. 2004;62:241–242. [DOI] [PubMed] [Google Scholar]
- 11. Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008–1016. [DOI] [PubMed] [Google Scholar]
- 12. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 13. Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 14. Narula A, Mehran R, Weisz G, Dangas GD, Yu J, Genereux P, Nikolsky E, Brener SJ, Witzenbichler B, Guagliumi G, Clark AE, Fahy M, Xu K, Brodie BR, Stone GW. Contrast‐induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS‐AMI substudy. Eur Heart J. 2014;35:1533–1540. [DOI] [PubMed] [Google Scholar]
- 15. Song W, Zhang T, Pu J, Shen L, He B. Incidence and risk of developing contrast‐induced acute kidney injury following intravascular contrast administration in elderly patients. Clin Interv Aging. 2014;9:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chalikias G, Drosos I, Tziakas DN. Contrast‐induced acute kidney injury: an update. Cardiovasc Drugs Ther. 2016;30:215–228. [DOI] [PubMed] [Google Scholar]
- 17. Slocum NK, Grossman PM, Moscucci M, Smith DE, Aronow HD, Dixon SR, Share D, Gurm HS. The changing definition of contrast‐induced nephropathy and its clinical implications: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am Heart J. 2012;163:829–834. [DOI] [PubMed] [Google Scholar]
- 18. Bellomo R. Decade in review—acute kidney injury: acute kidney injury—a decade of progress. Nat Rev Nephrol. 2015;11:636–637. [DOI] [PubMed] [Google Scholar]
- 19. Ebru AE, Kilic A, Korkmaz FS, Seker R, Sasmaz H, Demirtas S, Biyikli Z. Is cystatin‐C superior to creatinine in the early diagnosis of contrast‐induced nephropathy? A potential new biomarker for an old complication. J Postgrad Med. 2014;60:135–140. [DOI] [PubMed] [Google Scholar]
- 20. Briguori C, Visconti G, Rivera NV, Focaccio A, Golia B, Giannone R, Castaldo D, De Micco F, Ricciardelli B, Colombo A. Cystatin C and contrast‐induced acute kidney injury. Circulation. 2010;121:2117–2122. [DOI] [PubMed] [Google Scholar]
- 21. Parsh J, Seth M, Briguori C, Grossman P, Solomon R, Gurm HS. The optimal definition of contrast‐induced acute kidney injury for prediction of inpatient mortality in patients undergoing percutaneous coronary interventions. Am Heart J. 2016;175:160–167. [DOI] [PubMed] [Google Scholar]
- 22. Soto K, Coelho S, Rodrigues B, Martins H, Frade F, Lopes S, Cunha L, Papoila AL, Devarajan P. Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol. 2010;5:1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, Bell L. Derivation and validation of cystatin C‐based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–230. [DOI] [PubMed] [Google Scholar]
- 24. Zhang T, Shen LH, Hu LH, He B. Statins for the prevention of contrast‐induced nephropathy: a systematic review and meta‐analysis. Am J Nephrol. 2011;33:344–351. [DOI] [PubMed] [Google Scholar]
- 25. Gupta R, Moza A, Cooper CJ. Intravenous hydration and contrast‐induced acute kidney injury: too much of a good thing? J Am Heart Assoc. 2016;5:e003777 DOI: 10.1161/JAHA.116.003777. [DOI] [PMC free article] [PubMed] [Google Scholar]


