Abstract
Background
Heterosexual sex has become the dominant transmission route in China. Recently studies reported high heterogeneity in heterosexual transmission risk in resource-limited countries. The aim of this study was to summarize the risk of HIV transmission among Chinese serodiscordant couples.
Methods
A systematic review and meta-analysis of observational studies of heterosexual HIV transmission among serodiscordant couples in China was conducted. Two reviewers conducted a literature search using the China National Knowledge Infrastructure (CNKI), Chinese Medical Current Contents (CMCC), and Medline databases. Pooled transmission estimates per 100 person-years (PY) were calculated using a random-effects model. Meta-regression analysis and subgroup analysis stratified by study design, transmission direction and period of antiretroviral therapy (ART) availability were conducted to assess the factors associated with transmission.
Results
Eleven eligible studies were identified reporting on 11 984 couples and 405 HIV transmission events. HIV transmission risk from HIV-positive individuals to heterosexual partners was 1.68 (95% CI 0.74–2.62) per 100 PY. Study design did not reach statistical significance in meta-regression analysis. The pooled female-to-male transmission estimate was 1.11 (95% CI 0.09–2.14) per 100 PY and male-to-female transmission estimate was 1.43 (95% CI 0.19–2.68) per 100 PY. The pooled estimate for those before the availability of the Chinese National Free Antiretroviral Therapy Program (2.13 (95% CI 0.00–4.63) per 100 PY) was higher than that for those after the implementation of this program (1.44 (95% CI 0.62–2.26) per 100 PY).
Conclusions
Transmission estimates in China were lower than other developing countries, but higher than developed countries. Research that better defines HIV secondary transmission rates and the associated behavioral, treatment adherence, and health-related risk factors among heterosexual serodiscordant couples in China is needed.
Keywords: human immunodeficiency virus, heterosexual transmission
Since the beginning of the HIV/AIDS epidemic in China, the Chinese Ministry of Health has taken extensive measures to control the spread of the virus. Illegal blood and plasma stations have been shut down and infection through blood transfusions has been essentially eliminated. Methadone maintenance treatment was scaled-up in 2004 to prevent HIV transmission among injecting drug users (IDUs). Moreover, in 2003 the Chinese government announced the “Four Free One Care” program, which secures free access to voluntary counseling and testing (VCT), free antiretroviral therapy (ART), and free prevention of mother to child transmission (PMTCT).1–5 In recent years, heterosexual sex has surpassed injection drug use as the primary mode of HIV transmission in China.6,7
In 2007, the State Council AIDS Working Committee Office and the United Nations released a report stating that 37.9% of all new infections of HIV/AIDS in China have been transmitted through heterosexual sex. Today, the Chinese HIV epidemic is generally contained to several high-risk groups, including former plasma donors (FPD), men who have sex with men (MSM), and IDUs. Although each group’s defining HIV risk factor is not heterosexual sex, heterosexual sex serve as a bridge to spread HIV from these high risk populations to the general population. Previous studies have found high heterogeneity in heterosexual per-act transmission risk across serodiscordant couples in low-income countries.8,9 Quantifying the infectivity in resource limited settings is crucial, especially in countries like China, where the large population and limited access to healthcare in some areas could potentially compound the impact of a generalized HIV/AIDS epidemic. To better understand the epidemiology of HIV in China and improve the understanding of epidemiology of HIV/AIDS worldwide, we systematically reviewed the risk of heterosexual HIV transmission among serodiscordant couples in China.
METHODS
Search strategy and selection criteria
The China National Knowledge Infrastructure (CNKI), Chinese Medical Current Contents (CMCC), and Medline databases were systematically searched for observational studies of HIV transmission in heterosexual serodiscordant couples in China; CNKI includes conference abstracts and master’s and doctoral level dissertations, which were also eligible for inclusion in the meta-analysis. Combinations of the keywords “China” (only used in non-Chinese based database (Medline)) and “HIV” or “AIDS” or “human immunodeficiency virus” or “acquired immunodeficiency syndrome” and “heterosexual transmission” or “secondary transmission” or “couples” or “spouse” or “family transmission” or “discordant” or “transmission” were used to search. Original articles, dissertations, and conference abstracts reporting on observational studies of HIV transmission in serodiscordant couples were included.
Two authors independently reviewed the abstracts and titles of searched articles to determine eligibility. Eligible studies reported heterosexual transmission or had sufficient information to derive an estimate, reported follow-up time, and were conducted between January 1985 and January 2010. The search was updated to December 2010 to include the most recently published research. Case reports, reviews, and modeling papers were excluded and all included papers were restricted to English or Chinese because both reviewers are fluent in these languages. When information in the abstract was not sufficient to determine eligibility, the full text of the article was reviewed. Serodiscordant couples were defined as one HIV positive partner and one HIV negative partner. Included studies must have epidemic evidence that seroconversion was not due to injection drug use, commercial blood plasma donation, blood transfusion, or homosexual sex with a man. A study could only be included if the exposure time to the HIV positive partner could be extracted. A third reviewer (WANG Ning) was consulted when a disagreement between the first two reviewers arose. The reference lists of all articles meeting the exclusion criteria were carefully examined to identify other articles that may meet the inclusion criteria. If multiple publications reported estimates based on the same study population, the largest or most recent sample was used (largest sample size used over the most recent).
Data extraction
The full text of all abstracts meeting the inclusion criteria were retrieved and information was extracted regarding the study design, location and date, as well as the risk of the index group (HIV-positive partners) and transmission data. In prospective studies, serodiscordant couples were followed until seroconversion occurred with exposure time considered the difference between seroconversion date and index partner’s diagnosis date. For retrospective studies, the exposure time was calculated as the time between HIV diagnosis date in the first partner and the HIV diagnosis date in the second partner. Since the seroconversion time of prospective studies is more precise than retrospective studies, it was hypothesized that the effect size is varied based on study design. When available, male-to-female and female-to-male transmission estimates, condom use data, and ART data were also extracted. When additional information was needed, we tried to contact the authors of included studies. We relied solely on the published data when the authors could not be reached.
Statistical analysis
The pooled transmission estimates per 100 person-years (PY) and 95% confidence intervals (95% CI) were calculated using a random-effects model based on the DerSimonian-Laird method.10,11 A value of 0.000001 was substituted for zero transmission.9 Some studies reported the transmission rate as a percentage, but the transmission rate was calculated in terms of PY using the extracted follow-up time. If the calculated lower limit of the 95% CI for transmission rate was less than zero, a zero value was used in its place. I2 and the Cochran’s Q statistic were used to assess the heterogeneity of the included studies overall and within each subgroup. A random effects model was used for the calculation of the pooled estimate to reduce bias caused by heterogeneity. Sensitivity analysis was conducted to assess the individual influence of each study on the pooled transmission estimate. Subgroup analyses were performed to explore differences in transmission risk according to study design, sex, and ART. Finally, univariate meta-regression analysis was conducted to determine other sources of heterogeneity.12 All analyses were conducted using STATA version 10 (Stata Corporation, College Station, USA).
RESULTS
Literature search
Figure 1 shows the results of the literature search. The search terms yielded 1023 publications, including articles, conference abstracts and theses. In all, 949 publications were excluded because they were not relevant. The abstracts and full texts of 74 publications were thoroughly reviewed and 46 were excluded because of lack of epidemic evidence of infection from heterosexual transmission. Of the 28 remaining articles, another 14 were excluded because exposure time could not be calculated.
Figure 1.
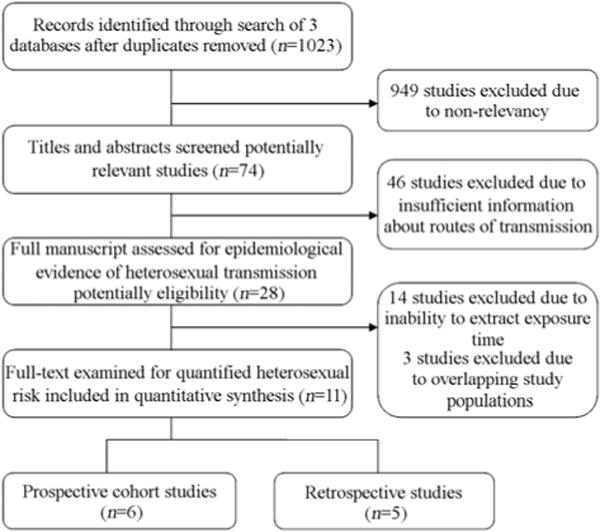
Identification and selection of eligible studies.
Finally, another three studies were excluded because they reported on the same populations as other included studies. We identified 11 eligible studies reporting on 11 984 couples and 405 HIV transmission events in five provinces. The authors of three potentially eligible studies were contacted; one replied and no additional information was provided.
Study characteristics
Table presents the characteristics of all included studies. Reviewed studies were conducted between 1992 and 2009. Six of the included publications were prospective cohort studies of serodiscordant couples and five were retrospective studies of previously serodiscordant couples.13–23 Among prospective studies, the longest follow-up time was 2.5 years and 11.2 years for retrospective studies. The included studies were conducted in Henan, Yunnan, Hebei and Xinjiang provinces, Beijing municipality, and two studies defined the location as “high-prevalence area,” but did not identify the precise locations. Study populations ranged from 22 couples in a study conducted in Xinjiang to 8664 couples in a study conducted in Henan. Transmission estimates extracted from reviewed studies ranged from 0 per 100 PY in both Henan and Beijing to 32.5 per 100 PY in Xinjiang.
Table.
Characteristics of eligible studies
| Studies | Location | Study design | Risk group of index case | Frequency of HIV tests (per year) | Index case | Total enrolled | Total follow-up (person years) | Study date | Chinese National ART Program | Female/male transmission | Male/female transmission |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen SL18 | Hebei | Retrospective | Blood recipient | N/A | 19 | 110 | 741 | 2008 | After | 17/89 | 2/21 |
| Cui ZL17 | Henan | Retrospective | FPD | N/A | 22 | 350 | 4200 | 2007 | After | 11/161 | 11/189 |
| Duan S14 | Yunnan | Prospective | IDU | 1 | 2 | 49 | 97 | 2002–2005 | After | N/A | 2/49 |
| Gui XE19 | High-prevalence area | Retrospective | FPD Blood recipient | N/A | 10 | 103 | 515 | 2002 | Before | N/A | N/A |
| Li JY20 | Henan | Prospective | FPD | 0.5, 1.0, 2.5 | 0 | 52 | 66 | 2002–2005 | After | N/A | N/A |
| Mao YR13 | Xinjiang | Prospective | General population | 0.5 | 8 | 22 | 24.6 | 1997–2000 | Before | 0/1 | 8/21 |
| Liu XX16 | Henan | Prospective | FPD | 0.5 | 165 | 8664 | 24 998 | 2005–2008 | After | N/A | N/A |
| Wang L23 | Henan | Retrospective | FPD | N/A | 84 | 1927 | 4918 | 2006–2008 | After | 49/1092 | 35/835 |
| Yang RR22 | High-prevalence area | Retrospective | FPD Blood recipient | N/A | 94 | 420 | 4704 | 2005–2009 | After | 67/288 | 27/132 |
| Zhang K21 | Beijing | Prospective | Sexual transmission and Blood recipient | 0.5 | 0 | 37 | 18.5 | 2000 | Before | N/A | N/A |
| Zheng XW15 | Yunnan | Prospective | IDU | 1 | 1 | 48 | 48 | 1992–1993 | Before | N/A | N/A |
FPD: former plasma donor; IDU: injecting drug user.
Each individual study was also subject to bias. The accuracy of the diagnosis date varied across studies. The infected dates of FPD or blood transmission are more accurate than those who were infected through other routes of transmission. In studies of FPD, the year 1995 was used instead of the diagnosis date since the infection most likely occurred around 1995 due to unsafe plasma donation practices.2 There were insufficient data to allow estimation of summary rates of transmission through sexual intercourse without condoms or to stratify according to frequency of sex, viral load, and CD4 count.
Figure 2 summarizes the individual and pooled transmission rates of the 11 included studies. The heterogeneity across transmission estimates was significant (P <0.001, I2=91.7%). The overall HIV transmission risk from HIV positive partners to heterosexual partners, irrespective of other sexually transmitted infections, was 0.19 per 100 PY (95% CI 0.10–0.29).
Figure 2.
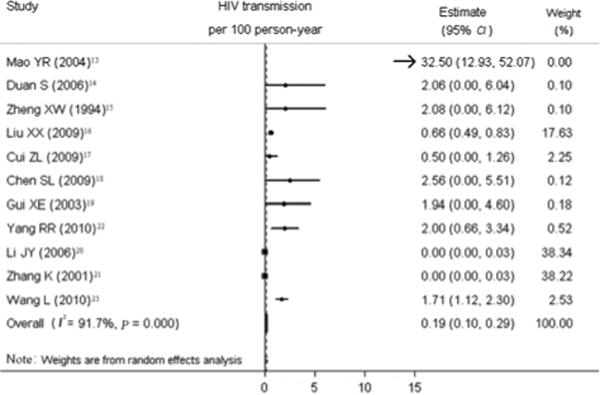
Forest plot of individual and pooled HIV transmission estimates (per 100 person-years).
Sensitivity analysis
To evaluate the influence of each study on the overall pooled estimate, sensitivity analysis was performed calculating the overall risk removing one study at a time (Figure 3). Two studies conducted in Henan and one study conducted in Beijing had the greatest influence on the pooled HIV secondary transmission rate. The other eight studies remained within the 95% CI of overall pooled estimate. When these three studies were removed from the analysis, the pooled estimate of transmission risk was 1.68 (95% CI 0.74–2.62) per 100 PY (Figure 4). Heterogeneity of effects was assessed using I2 (68.5%) and Q-test (P=0.005).
Figure 3.
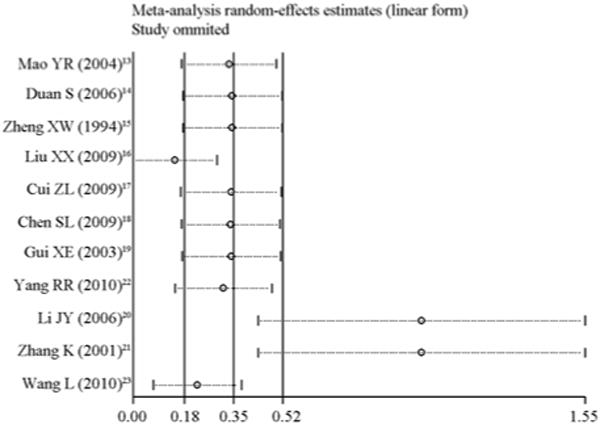
Sensitivity analysis.
Figure 4.
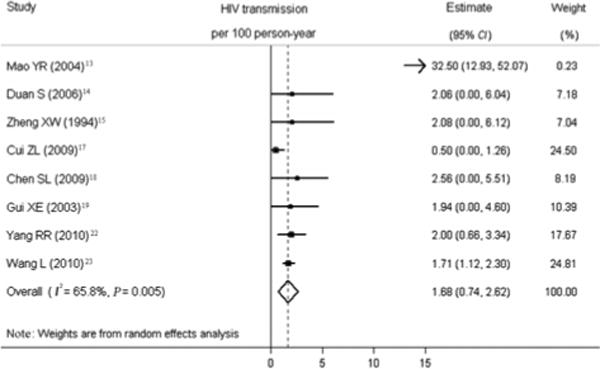
Forest plot of individual and pooled HIV transmission estimates for included studies.
Subgroup analyses
Figure 5 illustrates the results of subgroup analyses. Of the eight transmission estimates included, five estimates were from retrospective studies and three estimates were from prospective studies. The pooled transmission estimate for prospective studies (4.37 (95% CI 0–10.19) per 100 PY) was higher than it was for retrospective studies (1.46 (95% CI 0.66–2.26) per 100 PY), but in meta-regression analysis, the difference in study design did not reach statistical significance.
Figure 5.
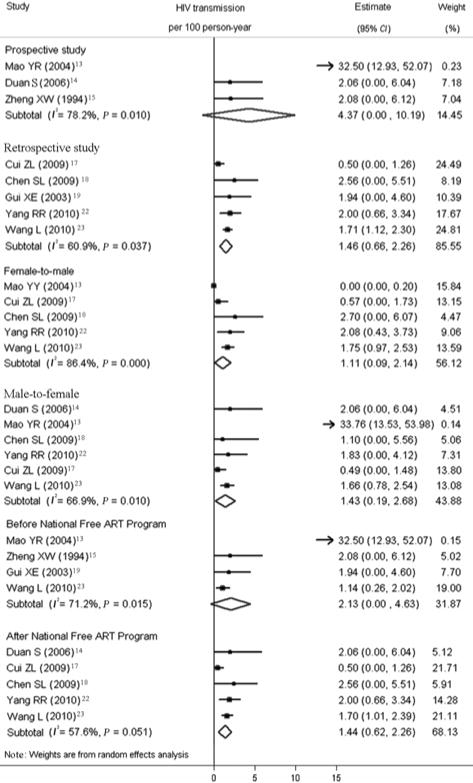
Forest plot of transmission estimates stratified by study design, sex, and ART use.
Six studies reported transmission risk by sex and these studies were used to calculate pooled estimates of female-to-male and male-to-female transmission. Pooled male-to-female transmission was 1.43 (95% CI 0.19–2.68) per 100 PY and female-to-male transmission was 1.11 (95% CI 0.09–2.14) per 100 PY.
China’s National Free Anretrotiviral Therapy Program began in 2003 and has been rapidly expanded to cover the entire country.24,25 Three studies were conducted before 2003 and five studies were conducted after 2003. Figure 5 shows the individual and pooled estimates of HIV transmission from studies conducted before and after the National Free Anretrotiviral Therapy Program. The pooled estimate for those before the implementation of the National Free Anretrotiviral Therapy Program was 2.13 (95% CI 0.00–4.63) per 100 PY. The pooled estimate for those after the implementation of the National Free ART Program was 1.44 (95% CI 0.62–2.26) per 100 PY.
Meta-regression
Univariate meta-regression analysis was performed to explore potential cofactors, including study design, direction of transmission (male-to-female vs. female-to-male), sample size (≥100 vs. <100), and ART (before the National Free ART Program vs. after the National Free ART Program) that might influence heterogeneity between studies. None of these factors were found to be statistically significant.
DISCUSSION
The overall pooled estimate of heterosexual HIV transmission was 1.68 (95% CI 0.74–2.62) per 100 PY. This estimate is lower than the corresponding rates found in several studies conducted in Africa (5.17–11.80 per 100 PY),26–30 but similar to India (1.22 per 100 PY).31 The differences in these transmission estimates may be associated with cofactors such as condom use, ulcerative sexually transmitted infections (STIs), frequency of sexual contact, and ART use.
A previous study that found transmission rates as high as 11.8 per 100 PY also had high rates of unprotected sex (with 89% never using condoms).30 However, in the studies reviewed in this meta-analysis, condom use varied; in three of the reviewed studies, participants reported never using condoms, while two other studies reported 80% condom use. Ulcerative STIs are of further significance in understanding HIV transmission because they increase the infectivity and susceptibility to transmission. In a recent review of heterosexual transmission, pooled transmission estimates were five times higher when the negative partner had one or more ulcerative STIs.9 The majority of the index cases in the reviewed studies were originally infected through unsanitary plasma donation, blood transfusion, and IDU, but few were infected through sexual transmission. Their partners, who had not engaged in other risky behavior besides heterosexual sex as defined by the exclusion criteria of the study, may also exhibit few risky behaviors outside of sexual contact with their regular partners. STI prevalence in the populations of the reviewed studies may have been low, which may be reflected in the low HIV transmission rate. STIs are an important risk factor for HIV infectivity and susceptibility,32–34 Without measure of prevalent STIs, evaluating the accuracy of reported condom use is difficult and future studies of heterosexual transmission need more thorough measures of condom use and STIs.
The prospective study pooled transmission estimate (4.37 per 100 PY) was higher than the retrospective study estimate (1.46 per 100 PY). Any small difference between transmission estimates may be due to differing risk behaviors of these study populations. The three prospective studies were conducted in areas with large injection drug use epidemics and the five retrospective studies were conducted in areas impacted by unsanitary plasma donations and blood transfusions. However, the difference in transmission estimates between study design type did not reach statistical significance in meta-regression analysis and this is consistent with previous findings.9
Based on the six studies with sufficient information to extract data on direction of transmission, male-to-female and female-to-male transmission estimates were calculated. The male-to-female pooled estimate was 1.43 (95% CI 0.19–2.68) per 100 PY and was higher than the female-to-male pooled estimate of 1.11 (95% CI 0.09–2.14). Both pooled transmission rates are lower than those found in Uganda (male-to-female transmission 12.0 per 100 PY and female-to-male transmission 11.6 per 100 PY)30 and Tanzania (male-to-female 10 per 100 PY, female-to-male 5 per 100 PY).27 In this study, direction of transmission was not significant in univariate meta-regression analysis (P=0.11) and this finding is generally inconsistent with previous research that has been conducted in Europe and the United States that has found significantly higher HIV transmission risk in male-to-female transmission in comparison to female-to-male transmission.35,36 However, transmission estimates from developing countries have generally found a greater female-to-male transmission risk in comparison to that in developed countries37 and some studies of serodiscordant couples in developing countries have not found significant differences in direction of transmission.30,33 Some have hypothesized that higher female-to-male infectivity in resource poor countries is related to high female STI prevalence in these settings,9 but insufficient data in the reviewed studies limit our ability to make the same conclusions.
Subgroup analysis of studies conducted before and after the initiation of the National Free Anretrotiviral Therapy Program indicates that the Chinese National Free Anretrotiviral Therapy Program may have lowered the risk of heterosexual HIV transmission. There was a dramatic decrease from 2.13 (95% CI 0–4.63) per 100 PY before 2003 to 1.44 (95% CI 0.62–2.26) per 100 PY after 2003. Estimating the transmission risk among ART users was limited by insufficient information in the reviewed studies. Three studies were conducted before 2003 and the participants in these studies were most likely not on ART because prior to this time very few patient had access to ART.25,38,39 The pooled transmission estimate for those studies conducted prior to 2003 was lower than the corresponding transmission estimate of 5.64 (95% CI 3.28–9.70) per 100 PY reported in a recent review.40 Further research considering ART status and adherence, as well as viral load, is needed to better characterize the HIV epidemic and its transmission among serodiscordant couples.
This study was subject to several limitations. There were insufficient data to stratify transmission rates according to STIs, condom use, sexual behaviors, viral load, CD4 count, ART use, adherence to ART, and other factors that may significantly impact the transmission risk associated with heterosexual HIV transmission among serodiscordant couples. In addition, some relevant studies were excluded because they did not have sufficient information to extract exposure time and transmission estimates. The included studies also did not provide sufficient data on frequency of sex. Previous research indicates that frequency of sex varies between countries.41 This review was also subject to bias due to some of the assumptions used. In retrospective studies, follow-up time was calculated from diagnosis date, but date of infection and diagnosis date likely differ. In this study, we sought to explore the transmission risk associated with heterosexual contact only. However, the reviewed studies were subject to social desirability bias and participants may have been subject to unreported risk factors other than heterosexual with their regular partners that could influence their risk of HIV transmission. The included studies may also not be geographically representative of all serodiscordant couples in China since this analysis depended on available, published studies. This study was also subject to publication bias, as studies with no cases of HIV seroconversion may be less likely to be published.
This meta-analysis summarizes heterosexual transmission among serodiscordant couples in China. The results of this study indicate relatively low transmission among serodiscordant couples in China. This analysis also found high heterogenity across studies. Future research should investigate behavioral, virological, and biological risk factors to better understand and characterize heterosexual transmission of HIV in China.
Acknowledgments
This study was supported by the grants from the Fogarty International Center, National Institutes of Health Office of the Director, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental & Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases Health, through the International Clinical Research Fellows Program at Vanderbilt (No. R24 TW007988) and the Mega-projects of Chinese National Science Research for the 11th Five-Year Plan (No. 2008ZX10001-003).
Contributor Information
Chun-peng ZANG, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, 155 Changbai Road, Changping District, Beijing 102206, China.
Zhong-wei JIA, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, 155 Changbai Road, Changping District, Beijing 102206, ChinaNational Institute of Drug Dependence, 38 Xueyuan Road, Haidian District, Beijing 100083, China.
Katherine Brown, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, 155 Changbai Road, Changping District, Beijing 102206, China.
Kathleen Heather Reilly, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, 155 Changbai Road, Changping District, Beijing 102206, ChinaTulane University Health Sciences Center, School of Public Health and Tropical Medicine, New Orleans, LA, USA.
Jun-jie WANG, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, 155 Changbai Road, Changping District, Beijing 102206, China.
Ning WANG, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, 155 Changbai Road, Changping District, Beijing 102206, China.
References
- 1.Wu Z, Sullivan S, Wang Y, Rotheram-Borus M, Detels R. Evolution of China’s response to HIV/AIDS. Lancet. 2007;369:679–690. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, Rou K, Cui H. The HIV/AIDS epidemic in China: history, current strategies and future challenges. AIDS Educ Prev. 2004;16(Suppl A):7–17. doi: 10.1521/aeap.16.3.5.7.35521. [DOI] [PubMed] [Google Scholar]
- 3.Zhang F, Haberer J, Wang Y, Zhao Y, Ma Y, Zhao D, et al. The Chinese free antiretroviral treatment program: challenges and responses. AIDS. 2007;21:143–148. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 4.Mathers B, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee S, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 5.Yan C, Liau A, Wu ZY. An overview of the history of epidemic of and response to HIV/AIDS in China: achievements and challenges. Chin Med J. 2009;122:2251–2257. [PubMed] [Google Scholar]
- 6.State Council AIDS Working Committee Office and UN Theme Group on HIV/AIDS in China. A joint assessment of HIV/AIDS prevention, treatment and care in China (2007) Beijing: 2007. [Google Scholar]
- 7.Wang L, Wang N, Li D, Jia M, Gao X, Qu S, et al. The 2007 estimates for people at risk for and living with HIV in China: progress and challenges. J Acquir Immune Defic Syndr. 2009;50:414–418. doi: 10.1097/QAI.0b013e3181958530. [DOI] [PubMed] [Google Scholar]
- 8.Downs A, De Vincenzi I. Probability of heterosexual transmission of HIV: relationship to the number of unprotected sexual contacts. J Acquir Immune Defic Syndr. 1996;11:388–395. doi: 10.1097/00042560-199604010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Boily M, Baggaley R, Wang L, Masse B, White R, Hayes R, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks J, Altman D, Bradburn M. Systematic reviews in health care: meta-analysis. 2nd. London: BMJ Publishing Group; 2001. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis; pp. 285–312. [Google Scholar]
- 11.Hartung J, Knapp G. An alternative test procedure for meta-analysis. Meta-analysis: new developments and applications in medical and social sciences. Ashland: Hogrefe & Huber Publishers. 2003:53–69. [Google Scholar]
- 12.Van Houwelingen H, Arends L, Stijnen T. Tutorial in biostatistics advanced methods in meta-analysis: multivariate approach and meta-regression. Tutorials in biostatistics: statistical modelling of complex medical data. Chichester: Wiley. 2004:289–324. [Google Scholar]
- 13.Mao YR, Zheng XW, Re ZY, Pan CD, Guli RZ, Song JQ, et al. An epidemiological study on sexual transmission of human immunodeficiency virus among pre–marital group in Yining city, Xinjiang. Chin J Epid (Chin) 2004;25:322–324. [PubMed] [Google Scholar]
- 14.Duan S, Xiang L, Ye R, Kang Y, Zhao Y, Zhang H, et al. Analysis of HIV infection of spouses after promote the use of condoms in HIV-infected family in Dehong Prefecture. Chin J AIDS STD (Chin) 2006;12:457. [Google Scholar]
- 15.Zheng XW, Zhang J, Qu S, Cheng H, Lin J, Duan S, et al. Cohort study of HIV infection among drug users in Ruili and other counties in Yunnan Province, China. Chin J Epid (Chin) 1994;15:3–5. [PubMed] [Google Scholar]
- 16.Liu XX, Zhu Q, Sun DY, Ma YM, Wang Q, Peng YE, et al. Evaluation of performance on management model for HIV discordant couples in GF3 AIDS program counties of Henan Province. Chin J Dis Control Prev (Chin) 2009;13:491–492. [Google Scholar]
- 17.Cui ZL. Cohort study on household transmission in three high prevalence villages in Henan Province. Beijing: The National Center for AIDS/STD Prevention and Control Chinese Center for Disease Control and Prevention; 2009. [Google Scholar]
- 18.Chen SL, Zhao HR, Zhang YQ, Zhao C, Li B, Miao X, et al. Retrospective cohort study on transmission of human immunodeficiency virus type 1 between spouses. Chin J AIDS STD (Chin) 2009;15:21–23. [Google Scholar]
- 19.Gui XE, Luo J, Zhuang K. Survey on the family transmission of human immunodeficiency virus. Chin J Epid (Chin) 2003;24:396. [Google Scholar]
- 20.Li JY, Li L, Li H, Bao Z, Li H, Wang Z, et al. Cohort study on human immunodeficiency virus discordant couples in the countryside of central China. Chin J Epid (Chin) 2006;27:192–195. [PubMed] [Google Scholar]
- 21.Zhang K, Wu H, Xu L. Behavior interventions and health education for HIV-infected people and their spouses. Chin J AIDS STD (Chin) 2001;7:286–288. [Google Scholar]
- 22.Yang R, Gui X, Benoit J, Xiong Y. The comparison of human immunodeficiency virus type 1 transmission between couples through blood or sex in central China. Jpn J Infect Dis. 2010;63:283–285. [PubMed] [Google Scholar]
- 23.Wang L, Ge Z, Luo J, Shan D, Gao X, Ding GW, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr. 2010;55:232–238. doi: 10.1097/QAI.0b013e3181e9b6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Zhang F, Zhao Y, Zang C, Zhao D, Dou Z, et al. Cohort profile: the Chinese national free antiretroviral treatment cohort. Int J Epidemiol. 2010;39:939–937. doi: 10.1093/ije/dyp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, et al. Five-year outcomes of the china national free antiretroviral treatment program. Ann Intern Med. 2009;151:241–251. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter LM, Kamali A, Ruberantwari A, Malamba SS, Whitworth JAG. Rates of HIV-1 transmission within marriage in rural Uganda in relation to the HIV sero-status of the partners. AIDS. 1999;13:1083–1089. doi: 10.1097/00002030-199906180-00012. [DOI] [PubMed] [Google Scholar]
- 27.Hugonnet S, Mosha F, Todd J, Mugeye K, Klokke A, Ndeki L, et al. Incidence of HIV infection in stable sexual partnerships: a retrospective cohort study of 1802 couples in Mwanza Region, Tanzania. J Acquir Immune Defic Syndr. 2002;30:73–80. doi: 10.1097/00042560-200205010-00010. [DOI] [PubMed] [Google Scholar]
- 28.Piot P, Laga M, Ryder R, Perriens J, Temmerman M, Heyward W, et al. The global epidemiology of HIV infection: continuity, heterogeneity, and change. J Acquir Immune Defic Syndr. 1990;3:403–412. [PubMed] [Google Scholar]
- 29.Johnson AM, Laga M. Heterosexual transmission of HIV. AIDS. 1988;2:49–56. doi: 10.1097/00002030-198800001-00008. [DOI] [PubMed] [Google Scholar]
- 30.Quinn T, Wawer M, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 31.Powers K, Poole C, Pettifor A, Cohen M. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baeten J, Richardson B, Lavreys L, Rakwar J, Mandaliya K, Bwayo J, et al. Female-to-male infectivity of HIV-1 among circumcised and uncircumcised Kenyan men. J Infect Dis. 2005;191:546–553. doi: 10.1086/427656. [DOI] [PubMed] [Google Scholar]
- 33.Gray R, Wawer M, Brookmeyer R, Sewankambo N, Serwadda D, Wabwire-Mangen F, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 34.Mastro T, Satten G, Nopkesorn T, Sangkharomya S, Longini I. Probability of female-to-male transmission of HIV-1 in Thailand. Lancet. 1994;343:204–207. doi: 10.1016/s0140-6736(94)90990-3. [DOI] [PubMed] [Google Scholar]
- 35.European Study Group on Heterosexual Transmission of HIV. Comparison of female to male and male to female transmission of HIV in 563 stable couples. BMJ. 1992;304:809–813. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Royce R, Sena A, Cates W, Cohen M. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 37.O’Farrell N. Enhanced efficiency of female-to-male HIV transmission in core groups in developing countries. Sex Transm Dis. 2001;28:84–91. doi: 10.1097/00007435-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F, Pan J, Lan Y, Yi W, Yan Z. Current progress of China’s free ART program. Cell Res. 2005;15:877–882. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 39.Sabin L, DeSilva M, Hamer D, Keyi X, Yue Y, Wen F, et al. Barriers to adherence to antiretroviral medications among patients living with HIV in southern China: a qualitative study. AIDS Care. 2008;20:1242–1250. doi: 10.1080/09540120801918651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 41.Kitchener C, Thompson C. AIDS & contraception. XI International Conference on AIDS; Vancouver: 1996. Trends in sexual behaviour & safer sex perceptions in Europe, USA, Africa & Asia relating to sexual health. [Google Scholar]


