Abstract
Background
In an integrated care model, involving primary care providers (PCPs) and obesity specialists, telehealth may be useful for overcoming barriers to treating childhood obesity.
Objective
To conduct a pilot study comparing BMI changes between two arms: 1) PCP in-person clinic visits plus obesity specialist tele-visits (PCP visits + Specialist tele-visits) and 2) PCP in-person clinic visits only (PCP visits only), with ongoing tele-consultation between PCPs and obesity specialists for both arms.
Methods
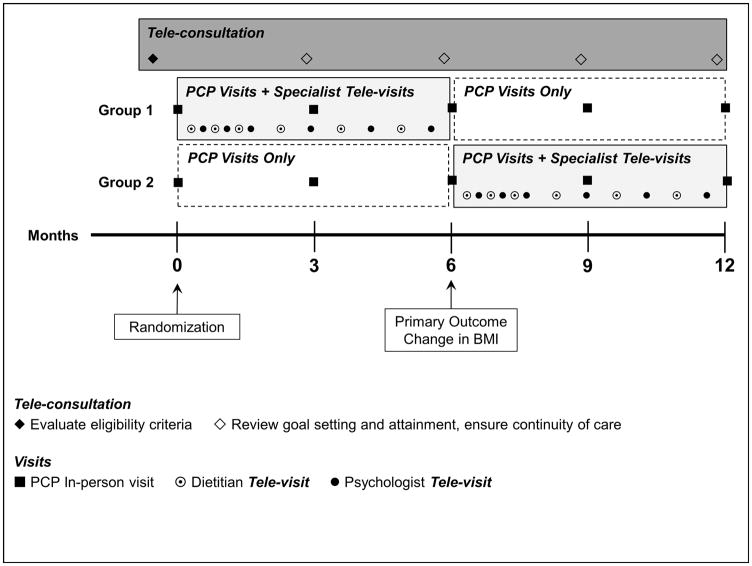
Patients (N=40, 10–17 years, BMI ≥95th percentile) were randomized to Group 1 or 2. Both groups had PCP visits every 3 months for 12 months. Using a cross-over protocol, Group 1 had PCP visits + Specialist tele-visits during the first 6 months and PCP visits only during the second 6 months, and Group 2 followed the opposite sequence. Each of 12 tele-visits was conducted by a dietitian or psychologist with a patient and parent.
Results
Retention rates were 90% at 6 months and 80% at 12 months. BMI (z-score) decreased more for Group 1 (started with PCP visits + Specialist tele-visits) vs. Group 2 (started with PCP visits only) at 3 months (−0.11 vs. −0.05, P=0.049), following frequent tele-visits. At 6 months (primary outcome), BMI was lower than baseline within Group 1 (−0.11, P=0.0006) but not Group 2 (−0.06, P=0.08); however, decrease in BMI at 6 months did not differ between groups. After cross-over, BMI remained lower than baseline for Group 1 and dropped below baseline for Group 2.
Conclusion
An integrated care model utilizing telehealth holds promise for treating children with obesity.
Keywords: Childhood obesity, dietary intervention, integrated care, interdisciplinary care, primary care, telehealth
INTRODUCTION
Multidisciplinary interventions can be efficacious for treating patients with obesity.1 However, inadequate implementation, accessibility, and intensity of such interventions often limit success in clinical settings. Implementation of Expert Committee Recommendations for treating childhood obesity2 often is beyond the capacity of currently established primary care systems.3,4 Weight management clinics, usually located in tertiary care centers, are inaccessible for many families.5–7 In primary care settings, interventions often do not promote significant reductions in BMI, despite changes in self-reported diet, physical activity, or television viewing.8 Disappointing outcomes may relate to insufficient intervention intensity pertaining to frequency and duration of visits, scope of nutrition education, depth of behavioral counseling, and lack of individualized treatment plans.9 Pediatric clinicians cite lack of effective interventions as a barrier to treating patients with obesity.10
Delivery of health information and services via electronic communication technologies, known as telehealth, has been the topic of several recent reports on treatment programs for childhood obesity.11 Most reports describe clinical initiatives developed to provide specialty care remotely for patients attending clinics in rural locations11–13 or school-based settings.11,14 Telehealth also has been used to convene learning networks whereby clinical teams share information and experiences with other teams and also consult with obesity specialists.15 However, very few randomized controlled trials (RCTs)16 have been conducted to evaluate an integrated care model utilizing telehealth to treat children with obesity, with collaboration among team members from different disciplines and across locations.17
In our pilot study, we used telehealth to promote collaboration between primary care providers (PCPs) and obesity specialists in treating children with obesity. Obesity specialists provided tele-consultation to primary care providers (PCPs) and thus were indirectly involved in patient care. Specialists also conducted tele-visits and thereby interacted directly with patients in their homes. We compared changes in BMI between two study arms: 1) PCP in-person clinic visits plus obesity specialist tele-visits (PCP visits + Specialist tele-visits) and 2) PCP in-person visits only (PCP visits only), with ongoing tele-consultation between PCPs and obesity specialists for both arms. To our knowledge, this pilot study is the first RCT using telehealth to provide individualized care for obesity directly to children in their homes, in addition to consultation among PCPs and obesity specialists.
METHODS
Collaboration
Teams from Wareham Pediatric Associates (WPA; 2 physicians, 1 nurse practitioner, 3 nurses, 2 practice administrators) and Boston Children’s Hospital (BCH; clinical researchers, psychologist, dietitians, physicians) collaborated on this pilot study. Wareham, Massachusetts is about 50 miles south of Boston. At the time of the study, this practice met National Committee for Quality Assurance (NCQA) criteria for a Level 2 patient-centered medical home. Ten providers served ~5,600 patients (~2,100 within the 10- to 17-year-old age range), and more than 30% of office visits were for Medicaid patients. The practice identified childhood obesity as an unmet need in the community and volunteered for participation in the study. Three providers then were selected based on an interest in improving their knowledge and care of children with obesity. Together, the teams from WPA and BCH specified enrollment criteria, operationalized outcomes assessments, conceptualized treatment strategies, standardized study protocols, and carried out the study.
Study Design
The study design is presented in Figure 1. We randomly assigned patients to Group 1 or Group 2 for 12 months, with assignment stratified by age (10–13 y, 14–17 y) and sex. Both groups had in-person clinic visits every 3 months for PCP treatment, reflecting the standard follow-up frequency established at WPA. During the week prior to each visit, PCPs participated in tele-consultation with obesity specialists to discuss treatment strategies. According to a cross-over protocol, Group 1 also had tele-visits with obesity specialists (PCP visits + Specialist tele-visits) during the first 6 months, and Group 2 received this treatment during the second 6 months. The primary outcome was change in BMI at 6 months. Participants received $25 and $50 gift cards at 6 and 12 months, respectively, as compensation for their time and effort. The study was approved by the BCH Institutional Review Board. Parents or guardians (henceforth referred to as parents) provided written informed consent, and patients provided written assent. The study was conducted between February 2013 and January 2015.
Figure 1.
Study Design
Patients
All study patients were recruited from the patient pool at WPA. We implemented a multistep protocol to explain the study to patients/parents and evaluate eligibility: 1) telephone screening by BCH staff, 2) informational visit and measurement of weight and height by WPA providers and staff, and 3) tele-consultation involving the teams at BCH and WPA to review all eligibility criteria and determine whether participation in the study was an appropriate course of treatment, or if the patient should be referred for subspecialty medical or mental health care. Medical care for all study patients was directed by PCPs who had the opportunity for weekly tele-consultation with a pediatric endocrinologist to discuss potential evaluation and treatment of comorbidities for the duration of the study.
Inclusion criteria included age between 10 and 17 years and BMI ≥95th percentile for sex and age.18 Exclusion criteria included known obesity comorbidities requiring medical intervention (e.g., type 2 diabetes), inability to actively participate in treatment (e.g., physical or cognitive limitations), major medical illness, use of medication or supplement known to affect body weight, unstable home environment (homeless, temporary living situation, lack of working phone or electricity) which was deemed likely to impede participation in the study, diagnosed eating disorder, untreated significant depression or anxiety, or self-reported suicidal ideation in the past month. Personnel conducting recruitment, enrollment, and random assignment were masked to sequence.
Treatments
Dietary Intervention and Messaging
A low-glycemic load diet has been found to be efficacious in previous studies,19–21 represents the cornerstone of dietary intervention in the obesity clinic at BCH, and thus was the selected approach for this study. Emphasis was on consuming reasonable portions of non-starchy vegetables, legumes, fruits, minimally processed grains, unsweetened beverages, lean sources of protein, and sources of healthful fat. We translated nutrition science to intervention messages and developed tools for explaining and assisting patients in operationalizing these messages. The main messages for the PCP and obesity specialist treatments were: 1) Eat balanced meals and 2) Eat paired snacks (i.e., low-glycemic carbohydrate paired with protein and/or fat, such as apple with peanut butter). For the obesity specialist treatment, an additional message was: 3) Limit high glycemic foods to 0–1 servings per day. Protocols and educational materials were designed to foster consistency in language between treatments and thus enhance coordination among all members of the care team with regard to sharing assessments, care plans, and progress toward achieving target dietary behaviors. Although diet was the primary focus of treatment, obesity specialists and PCPs also encouraged increased physical activity (e.g., family recreational activities, after-school programs) and decreased sedentary time (i.e., time spent watching television or using other entertainment media).
PCP Treatment during In-person Clinic Visits
For nutrition education and goal setting, PCPs utilized a booklet that was developed for this pilot study to align with the obesity specialist treatment (described below). The booklet contained the aforementioned messages with regard to healthful eating, a plate model to provide instruction on balanced meals, a “mix and match” paradigm to convey paired snacks, guidance on portion sizes, pictures of foods categorized according to the colors of a traffic light to aid families in making healthful choices, and a checklist to facilitate goal setting. Visits were about 30 minutes in duration. During this time, PCPs discussed current dietary behaviors and provided assistance in developing plans to achieve goals. PCPs shared information regarding their patients with obesity specialists during tele-consultation (described below).
Obesity Specialist Treatment during Tele-visits
The patient and a parent had 12 tele-visits over 6 months, alternating between a dietitian and psychology fellow (henceforth referred to as psychologist, for brevity), as shown in Figure 1. A six-week intensive phase of weekly visits was followed by twice monthly follow-up visits. Dietitian visits were 1 hour during the intensive phase and 30 minutes thereafter. All psychologist visits were 1 hour. The dietitian was responsible for ongoing dietary assessment, diagnosis of nutrition problems, and nutrition education and dietary counseling. Nutrition education was guided by sequential learning objectives mapped to desired dietary behaviors. The psychologist was responsible for assessing environmental, behavioral, cognitive, emotional, and familial variables influencing level of adherence to the dietary prescription, consistent with a cognitive behavioral framework.
The dietitian and psychologist operated as an interdisciplinary team (distinguished from a multidisciplinary team by more extensive and formal communication and non-hierarchical structure).22 Care was reviewed during weekly meetings of the two providers using a case formulation model23 to conceptualize and flexibly apply empirically-based treatment strategies to the unique presentation of each study patient. Based on intake assessments, the dietitian and psychologist worked together to identify discrepancies between reported patterns of eating and desired dietary behaviors and then develop individualized treatment plans. Treatment targets were variables conceptualized to serve as antecedent or maintaining factors contributing to the primary problem related to dietary intake (e.g., home food environment, patterns of eating, parent behavior management skills). Plans were modified in an iterative process using information gathered during subsequent sessions. Obesity specialists shared information with PCPs during tele-consultation (described below).
Tele-consultation
Teams from WPA and BCH met weekly for tele-consultation to discuss any patients involved with the study. These meetings provided opportunity to share information and experiences. The WPA team offered insights based on in-person clinic visits. The dietitian and psychologist provided written reports of goals and progress every 3 months for each patient who was attending specialist tele-visits. Particular attention was directed towards continuity of care at start and completion of the 6-month obesity specialist treatment involving tele-visits. The teams discussed any concerns about medical or mental health comorbidities and assessed need for supplemental services.
Technology
VidyoDesktop® (Vidyo, Inc., Hackensack, NJ) was used for tele-consultation and tele-visits. During enrollment, parents completed a technology questionnaire regarding access to a computer with adequate Internet connectivity, speakers, and webcam for utilizing VidyoDesktop®. We provided webcams or secure iPads® with 3G Internet service for the duration of the study, if a family did not have adequate technology.
Outcome Measures
Anthropometry and Blood Pressure
The BCH team trained WPA nurses, masked to group assignment, on research protocols for taking anthropometric and blood pressure measurements during clinic visits. Weight and height were measured using a calibrated scale (Model BWB-800, Tanita, Arlington Heights, IL) and stadiometer (Model PE-AIM-101, Perspective Enterprises, Portage, MI), respectively.24 BMI (kg/m2), BMI percentile, and BMI z-score were calculated from sex- and age-specific charts.18 Waist circumference and triceps skinfold were measured using a Gulick measuring tape and Lange caliper (Creative Health Products, Ann Arbor, MI), respectively.24 Blood pressure was measured by auscultation.25
Diet and Physical Activity
A BCH diet technician, masked to group assignment, conducted two recall interviews by telephone at baseline and again at 3, 6, 9, and 12 months to assess dietary intake and physical activity during the 24 hours preceding each telephone call, as previously described.26 Dietary data were collected using Nutrition Data System for Research (NDSR) software versions 2012, 2013, and 2014, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. Final calculations were completed using NDSR version 2014.
Patient/Parent Experience
We invited patients and parents to independently complete an “overall study experience” questionnaire at 6 and 12 months, responding to questions regarding helpfulness of the program and satisfaction using 10-cm visual analog scales with appropriate verbal anchors. We also asked them to complete a “telehealth experience” questionnaire at the end of the obesity specialist treatment involving tele-visits (i.e., at 6 months for Group 1 and 12 months for Group 2).
Statistical Analyses
Baseline characteristics were compared between Groups 1 and 2 using the Fisher exact test for categorical variables and t-test for continuous variables. Changes in BMI and other outcomes were compared between groups using a general linear model, adjusted for sex and age, with an autoregressive covariance structure for the repeated-measures factor (time). Recognizing the clinical utilization of BMI percentile for weight assessment in patients aged ≤15 years, and BMI as a percentile or in units of kg/m2 for those aged 16 to 17 years;27 we specified change in BMI percentile as the primary outcome measure. To overcome potential bias from the skewed distribution of BMI percentiles in this population (median 98th percentile), we performed our analyses on BMI z-scores, which preserve the order of the percentiles but are distributed more appropriately for valid parametric statistical analysis. All available data from each randomized patient, regardless whether he/she adhered to the intervention or completed the study, were included in analyses. Net treatment effects within and between groups were constructed and tested by forming contrasts from parameters of the fitted model.
We compared the 2 groups in parallel to assess change in BMI over the first 6 months (primary outcome), hypothesizing that any decrease in BMI at 6 months would be greater among patients in Group 1 (PCP visits + Specialist tele-visits) compared to Group 2 (PCP visits only). This hypothesis was addressed by the contrast (6-month mean – baseline mean in Group 1) – (6-month mean – baseline mean in Group 2). Contrasts of additional interest were within-group change (6-month mean or 12-month mean – baseline) and between-group comparison of the net change over the whole trial ((12-month mean – baseline mean in Group 1) – (12-month mean – baseline mean in Group 2)). Although the study nominally followed a crossover design, it lacked a washout period between intervention modes and manifestly did not satisfy the critical assumption that the two intervention modes functioned independently of order of administration. We therefore did not estimate or test the conventional crossover contrast, ((6-month mean – baseline mean) – (12-month mean – 6-month mean) in Group 1) minus ((12-month mean – 6-month mean) – (6-month mean – baseline) in Group 2), which would not be meaningful or interpretable in this setting.
Experience data from the visual analog scales were analyzed using t-tests. Two-sided P<0.05 was taken as a statistically significant result.
Data are presented as mean and SD or SE. SAS software (version 9.2, SAS Institute, Cary, NC) was used for all computations.
We specified a priori a sample size of 40 patients, considering practicality and adequacy for a pilot study. Post hoc power calculations indicated that we had 80% power to detect a mean difference of 0.12 SD for change in BMI z-score between the two groups at 6 months.
RESULTS
Enrollment, Randomization, and Retention
We randomly assigned 21 patients to Group 1 and 19 to Group 2 (Supporting Information Figure S1). The two groups did not differ at baseline (Supporting Information Table S1). Among the 40 enrolled patients, mean age (± SD) was 14.3 ± 1.9 years, 31 were female, and 35 were non-Hispanic white. Annual household income varied widely. Overall retention rates were 90% at 6 months and 80% at 12 months. There were no adverse events related to study participation.
Process Measures
Among the 38 patients (21 in Group 1, 17 in Group 2) who started the obesity specialist treatment, 22 had necessary hardware and Internet access for videoconferencing, 3 borrowed only a study webcam, and 13 borrowed a study iPad®. For the 33 patients retained in the study for the duration of the obesity specialist treatment (i.e., 19 through 6 months for Group 1, 14 through 12 months for Group 2), mean (SD) attendance was 5.0 (1.6) for the 6 dietitian tele-visits and 4.9 (1.6) for the 6 psychologist tele-visits.
Outcomes
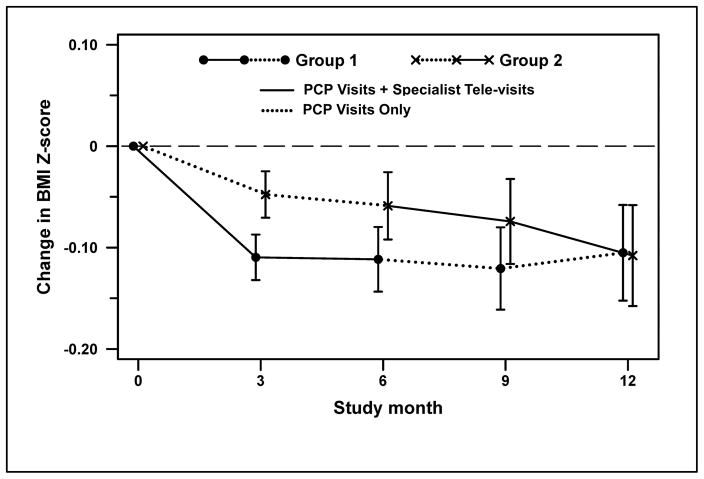
Changes in BMI z-score are displayed in Figure 2, and all anthropometric data are presented in Table 1. At 3 months, we noted a greater decrease in BMI (z-score) for Group 1 (started with PCP visits + Specialist tele-visits) vs. Group 2 (started with PCP visits only) (−0.11 vs. −0.05, P=0.049), following the period of most frequent tele-visits for Group 1. Within-group analyses indicated a significant decrease in BMI at 6 months for Group 1 (−0.11, P=0.0006) but not Group 2 (−0.06, P=0.08). However, decrease in BMI at 6 months did not differ between groups. After cross-over at 6 months, BMI remained significantly different from baseline for Group 1 at 9 months (−0.12, P=0.004) and 12 months (−0.11, P=0.03), despite discontinuation of Specialist tele-visits. BMI decreased with Specialist tele-visits for Group 2 and was significantly different from baseline at 12 months (−0.11, P=0.03). BMI did not differ between groups at the end of the 12-month study. Decreases in waist circumference and triceps skinfold were not different between groups. Blood pressure did not change throughout the study (data not shown).
Figure 2.
Change in BMI Z-score
Table 1.
Anthropometric Measures
| Variable | Group | Baselinea (mean ± SE) | Change from Baselineb (mean ± SE) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Group 1: PCP Visits + Specialist Tele-Visits Group 2: PCP Visits Only |
Group 1: PCP Visits Only Group 2: PCP Visits + Specialist Tele-Visits |
|||||||||
|
|
|
|
||||||||
| 3 months | P c,d | 6 months | P c,d | 9 months | P c,d | 12 months | P c,d | |||
|
|
|
|
|
|
||||||
| BMI z-score | 1 | 2.11 ± 0.07 | −0.11 ± 0.02 | <0.0001 | −0.11 ± 0.03 | 0.0006 | −0.12 ± 0.04 | 0.004 | −0.11 ± 0.05 | 0.03 |
|
|
|
|
|
|
||||||
| 2 | 2.10 ± 0.07 | −0.05 ± 0.02 | 0.04 | −0.06 ± 0.03 | 0.08 | −0.07 ± 0.04 | 0.08 | −0.11 ± 0.05 | 0.03 | |
|
|
|
|
|
|
||||||
| 1 – 2 | −0.06 ± 0.03 | 0.049 | −0.05 ± 0.04 | 0.23 | −0.05 ± 0.05 | 0.38 | 0.003 ± 0.06 | 0.96 | ||
|
|
|
|
|
|
||||||
| BMI Percentile | 1 | 97.9 ± 0.4 | −0.8 ± 0.2 | 0.0001 | −0.8 ± 0.3 | 0.003 | −0.7 ± 0.3 | 0.03 | −0.6 ± 0.4 | 0.10 |
|
|
|
|
|
|
||||||
| 2 | 97.9 ± 0.4 | −0.3 ± 0.2 | 0.21 | −0.3 ± 0.3 | 0.34 | −0.3 ± 0.3 | 0.40 | −0.7 ± 0.4 | 0.11 | |
|
|
|
|
|
|
||||||
| 1 – 2 | −0.6 ± 0.3 | 0.04 | −0.5 ± 0.4 | 0.16 | −0.4 ± 0.5 | 0.35 | 0.03 ± 0.5 | 0.95 | ||
|
|
|
|
|
|
||||||
| BMI (kg/m2) | 1 | 32.5 ± 0.9 | −1.1 ± 0.2 | <0.0001 | −1.3 ± 0.4 | 0.001 | −1.5 ± 0.5 | 0.002 | −1.3 ± 0.6 | 0.02 |
|
|
|
|
|
|
||||||
| 2 | 32.3 ± 0.9 | −0.6 ± 0.2 | 0.02 | −0.9 ± 0.4 | 0.02 | −1.2 ± 0.5 | 0.01 | −1.5 ± 0.6 | 0.01 | |
|
|
|
|
|
|
||||||
| 1 – 2 | −0.6 ± 0.3 | 0.10 | −0.4 ± 0.5 | 0.43 | −0.3 ± 0.6 | 0.62 | 0.1 ± 0.7 | 0.85 | ||
|
|
|
|
|
|
||||||
| Weight (kg) | 1 | 89.1 ± 3.0 | −2.5 ± 0.6 | 0.0002 | −2.7 ± 1.0 | 0.005 | −3.1 ± 1.3 | 0.02 | −2.5 ± 1.5 | 0.11 |
|
|
|
|
|
|
||||||
| 2 | 86.4 ± 2.9 | −1.2 ± 0.7 | 0.06 | −1.8 ± 1.0 | 0.08 | −2.7 ± 1.3 | 0.04 | −3.3 ± 1.6 | 0.04 | |
|
|
|
|
|
|
||||||
| 1 – 2 | −1.2 ± 0.8 | 0.15 | −1.0 ± 1.2 | 0.42 | −0.5 ± 1.5 | 0.76 | 0.76 ± 1.7 | 0.66 | ||
|
|
|
|
|
|
||||||
| Height (kg) | 1 | 165.1 ± 1.4 | 0.7 ± 0.3 | 0.02 | 0.8 ± 0.4 | 0.06 | 1.2 ± 0.6 | 0.04 | 1.3 ± 0.7 | 0.05 |
|
|
|
|
|
|
||||||
| 2 | 163.2 ± 1.4 | 0.4 ± 0.3 | 0.15 | 0.8 ± 0.4 | 0.06 | 0.9 ± 0.6 | 0.13 | 1.0 ± 0.7 | 0.14 | |
|
|
|
|
|
|
||||||
| 1 – 2 | 0.3 ± 0.4 | 0.48 | −0.0 ± 0.5 | 0.96 | 0.3 ± 0.6 | 0.66 | 0.3 ± 0.7 | 0.69 | ||
|
|
|
|
|
|
||||||
| Waist Circumference (cm) | 1 | 103.7 ± 2.6 | −2.0 ± 1.1 | 0.08 | −2.9 ± 1.5 | 0.06 | −2.6 ± 1.9 | 0.18 | −2.7 ± 2.2 | 0.22 |
|
|
|
|
|
|
||||||
| 2 | 104.0 ± 2.6 | −1.4 ± 1.1 | 0.21 | −3.2 ± 1.6 | 0.05 | −4.8 ± 2.0 | 0.02 | −5.2 ± 2.3 | 0.03 | |
|
|
|
|
|
|
||||||
| 1 – 2 | −0.6 ± 1.6 | 0.72 | 0.3 ± 2.1 | 0.91 | 2.2 ± 2.6 | 0.42 | 2.5 ± 3.0 | 0.40 | ||
|
|
|
|
|
|
||||||
| Triceps Skinfold (mm) | 1 | 39.9 ± 1.7 | −3.3 ± 1.1 | 0.004 | −2.4 ± 1.5 | 0.11 | −2.7 ± 1.8 | 0.13 | −4.8 ± 1.9 | 0.01 |
|
|
|
|
|
|
||||||
| 2 | 37.7 ± 1.7 | −0.3 ± 1.1 | 0.77 | −0.7 ± 1.6 | 0.66 | −2.2 ± 1.9 | 0.23 | −1.3 ± 2.1 | 0.52 | |
|
|
|
|
|
|
||||||
| 1 – 2 | −2.9 ± 1.6 | 0.07 | −1.7 ± 2.1 | 0.42 | −0.4 ± 2.5 | 0.86 | −3.5 ± 2.7 | 0.21 | ||
Least squares means calculated from the general linear model, with adjustment for sex and age.
Changes from baseline calculated from the general linear model, with adjustment for sex and age.
P values for changes from baseline within each study group are based on tests of the hypothesis that the mean change was zero.
P values for the between-group differences in changes from baseline are based on tests of the hypothesis that the mean change was the same in the two groups.
Dietary glycemic load was significantly different from baseline during the obesity specialist treatment (i.e., at 3 months for Group 1 and 12 months for Group 2) (Supporting Information Table S2). Physical activity did not change throughout the study.
Patient/Parent Experience
Data derived from the “overall study experience” questionnaire revealed that patients in Group 1 (PCP visits + Specialist tele-visits) may have found the program to be more helpful, compared to previous weight loss strategies, than those in the Group 2 (PCP visits only) at 6 months (P=0.06) (Supporting Information Table S3). Patients in Group 1 also were more likely to recommend the study to others at 6 months (immediately following PCP visits + Specialist tele-visits) compared to 12 months (following cross-over to PCP visits only) (P=0.03).
We received responses to the “telehealth experience” questionnaire from 29 of 33 patients retained in the obesity specialist treatment. Among these patients, 24 responded that they could hear and 27 responded that they could see the psychologist/dietitian, with the quality of communication ranked as “average,” “good,” or “excellent.” The number of visits seemed appropriate to 15 of the respondents. If they had to travel to Boston to see a weight loss specialist instead of attending tele-visits, 18 said that they would have lost more time from school/work. Given the choice between a tele-visit vs. in-person visit with an obesity specialist in the future, 14 would choose a tele-visit and 7 had no preference. Responses from 26 parents were similar to those of the patients. When parents were asked what they would have done if the tele-visits were not available, only 3 would have driven to Boston to see a weight loss specialist, 8 would have made an appointment with a local weight loss specialist, and 15 would not have seen any weight loss specialist.
DISCUSSION
We utilized telehealth in an integrated care model for children with obesity. Tele-consultation was designed to promote communication between obesity specialists and PCPs in an established pediatric primary care practice. Tele-visits with patients in their homes were conceptualized to reduced barriers to obtaining care from an interdisciplinary team of obesity specialists (dietitian, psychologist). Treatments resulted in favorable decreases in BMI, consistent with our study hypothesis. Overall, patients and parents gave high ratings for helpfulness of the treatments in general and goal setting in particular, satisfaction with lifestyle (eating and physical activity) changes, and level of enthusiasm for recommending the study to others. Satisfaction with weight loss was moderate, possibly due to high pre-treatment weight loss expectations.28
Most of the patients enrolled in the study would not have consulted with obesity specialists if the tele-visits were not available. Retention and attendance rates compare favorably with what is typical in weight loss clinics.7 These rates likely can be attributed to strong patient-PCP relationships, collaboration between PCPs and obesity specialists, support from office staff at WPA, and also selective enrollment criteria and monetary incentives that were part of the research protocol. Using the intervention booklet developed for this pilot study, PCPs laid the foundation for the obesity specialist treatment for Group 1 by introducing the low-glycemic load diet and underscoring importance and credibility of the intervention. In addition, PCP treatment, supported by tele-consultation with obesity specialists, promoted an initial decrease in BMI for Group 2, without deviating from the standard follow-up frequency established at WPA. Change in BMI was the same for both groups at 12 months, indicating that it did not matter whether the specialist tele-visits were started soon after enrollment or following 6-months of PCP visits only, likely due in part to continuous involvement of PCPs and consistency of messaging used by PCPs and obesity specialists. Office staff at WPA had long-term relationships with many patients in the study, provided encouragement at visits, and collaborated with research staff at BCH in contacting patients with friendly appointment reminders.
Our findings are consistent with emerging data on patient experience and change in BMI with telehealth. In previous studies, patients in both rural29 and urban14 settings reported generally positive experience with tele-visits, comparable to or exceeding in-person visits. Davis et al.16 conducted a study of 58 patients in one of the few randomized trials using telehealth to treat children with obesity. They compared a group intervention delivered by behavioral health specialists during tele-visits with an in-person intervention delivered by primary care physicians. Among 5- to 11-year-old patients, they observed similar decreases in BMI z-score of 0.12 and 0.15 SD with the respective interventions over 8 months. Of note, the research team sent a list of obesity-related topics to each family-physician dyad, requesting that they discuss the topics during an upcoming visit. Taken together, this study by Davis et al.16 and our pilot study suggest that a PCP visit focused specifically on treating children with obesity, with appropriate supports provided by specialists, may address some of the barriers to successful primary care interventions. Other studies indicate that even modest decreases in BMI z-score, of the magnitude observed in our pilot study and by Davis et al.,16 lead to improvements in psychosocial outcomes30 and cardiometabolic risk factors.31,32
Several study design issues warrant comment. Strengths include a randomized design, high retention rates, close communication between obesity specialists and PCPs, and interdisciplinary case formulation. Primary limitations inherent to a pilot study include small sample size, relatively short duration of treatment, and involvement of only one primary care practice. Selective enrollment criteria and monetary compensation for participation complicates generalizability of results to mainstream clinical practice. Moreover, while this study indicates that behavioral counseling via telehealth can facilitate adherence to dietary recommendations, we did not offer or evaluate psychotherapy via telehealth for disordered eating or serious psychopathology. Underreporting of dietary intake is common in dietary intervention studies of free-living patients, although adjusting other dietary variables for energy intake may partially correct for underreporting.33,34 Patients were predominately non-Hispanic white but diverse with regard to socioeconomic status, reflecting the demographics of Wareham, Massachusetts.
In conclusion, an integrated care model utilizing telehealth holds promise for treating children with obesity, overcoming the barrier of co-location for collaborative care.35 Although we assessed patient/parent experience in terms of study participation, comprehensive evaluation of experience (with particular focus on care integration) was beyond the scope of this pilot study. Future initiatives may include surveying patients/families specifically to evaluate care integration, determining optimal frequency and combination of PCP in-person visits and specialist tele-visits, disentangling the independent effects of various treatment components, and adapting protocols for more widespread dissemination. Furthermore, well designed protocols will be essential to appraise tradeoffs between improved access to treatment with telehealth and potential loss of non-specific therapeutic factors associated with in-person interventions, evaluate financial feasibility and reimbursement mechanisms, and assess psychosocial outcomes and comorbidities over the long term in multi-site studies.
Supplementary Material
What is already known about this subject
Multidisciplinary interventions can be efficacious for treating patients with obesity.
However, numerous barriers preclude delivery of such interventions in primary care settings.
In an integrated care model, with collaboration among team members from different disciplines and across locations, telehealth may be useful for overcoming barriers to successful treatment.
What this study adds
Within an integrated care model, we used telehealth to 1) facilitate consultation between obesity specialists (dietitians, psychologist, endocrinologist) and primary care providers who treated patients with obesity in their clinical practice (tele-consultation) and 2) provide visits with obesity specialists (registered dietitian, psychologist) directly to patients in their homes (tele-visits).
Both applications of telehealth were feasible and hold promise for implementation within an integrated care model. Tele-visits with obesity specialists, in addition to in-person visits with PCPs who participated in tele-consultation, resulted in decreases in BMI. This pilot study provides a foundation for more definitive studies of an integrated care model, conducted with larger sample sizes and longer intervention periods.
Acknowledgments
Funding Source: New Balance Foundation. DSL was supported by a career award from the National Institute of Diabetes and Digestive and Kidney Diseases (DK082730).
We thank the patients/families who participated in the pilot study; Shawn Farrell and Patrick McCarthy for their telehealth expertise; Michelle Brodeur, Lisa Govoni, and Marianne Iacobucci for conducting assessment visits; Linda Seger-Shippee for conducting the 24-hour dietary and physical activity recall interviews; Lesley Levitt and Meghan Leary for administrative support, scheduling tele-visits, and data entry; Megan Wadman for coordination of clinic schedules, assessments, hardware distribution, and communication with patients; and Robin Zahner for practice support.
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Potential Conflicts of Interest: DSL reported receiving royalties from books on nutrition and obesity. The other authors have no potential conflicts of interest relevant to this article to disclose.
Clinical Trial Registration: ClinicalTrials.gov (NCT01794546) https://clinicaltrials.gov/ct2/results?term=NCT01794546&Search=Search
Author’s contributions
Dr. Fleischman assisted with study design, was the study obesity medical consultant, carried out statistical analyses, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr. Hourigan conceptualized and developed the protocol for the interdisciplinary intervention, was the study psychologist, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr. Lyon assisted in conceptualizing and delivered the PCP treatment, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Ms. Landry designed the nutrition education program, was the study dietitian, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr. Reynolds developed a registry of patients with obesity, designed the patient recruitment strategy, assisted in conceptualizing and delivered the PCP treatment, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Ms. Steltz drafted a clinic manual, designed the data collection instruments, managed the data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Ms. Robinson was the project coordinator, critically reviewed the manuscript, and approved the final manuscript as submitted.
Ms. Keating assisted in conceptualizing and delivered the PCP treatment, critically reviewed the manuscript, and approved the final manuscript as submitted.
Dr. Feldman formulated statistical analyses, advised on data presentation, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr. Antonelli conceptualized the study, provided expertise on integrated care, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr. Ludwig assisted with study design, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr. Ebbeling conceptualized and designed the study, provided study supervision, carried out statistical analyses, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted.
References
- 1.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125:e396–418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 2.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 3.Tanda R, Salsberry P. The impact of the 2007 expert committee recommendations on childhood obesity preventive care in primary care settings in the United States. J Pediatr Health Care. 2014;28:241–50. doi: 10.1016/j.pedhc.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein JD, Sesselberg TS, Johnson MS, et al. Adoption of body mass index guidelines for screening and counseling in pediatric practice. Pediatrics. 2010;125:265–72. doi: 10.1542/peds.2008-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambler KA, Hagedorn DW, Ball GD. Referrals for pediatric weight management: the importance of proximity. BMC Health Serv Res. 2010;10:302. doi: 10.1186/1472-6963-10-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampl S, Paves H, Laubscher K, Eneli I. Patient engagement and attrition in pediatric obesity clinics and programs: results and recommendations. Pediatrics. 2011;128(Suppl 2):S59–64. doi: 10.1542/peds.2011-0480E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obes Rev. 2011;12:e273–81. doi: 10.1111/j.1467-789X.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seburg EM, Olson-Bullis BA, Bredeson DM, Hayes MG, Sherwood NE. A review of primary care-based childhood obesity prevention and treatment interventions. Curr Obes Rep. 2015;4:157–73. doi: 10.1007/s13679-015-0160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell TB, Amaro CM, Steele RG. Pediatric Weight Management Interventions in Primary Care Settings: A Meta-Analysis. Health Psychol. doi: 10.1037/hea0000381. ePub April 19, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes ET, Ebbeling CB, Meyers AF, et al. Pediatric obesity management: variation by specialty and awareness of guidelines. Clin Pediatr (Phila) 2007;46:491–504. doi: 10.1177/0009922806298704. [DOI] [PubMed] [Google Scholar]
- 11.Cohen GM, Irby MB, Boles K, Jordan C, Skelton JA. Telemedicine and pediatric obesity treatment: review of the literature and lessons learned. Clin Obes. 2012;2:103–11. doi: 10.1111/j.1758-8111.2012.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irby MB, Boles KA, Jordan C, Skelton JA. TeleFIT: adapting a multidisciplinary, tertiary-care pediatric obesity clinic to rural populations. Telemed J E Health. 2012;18:247–9. doi: 10.1089/tmj.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face-to-face care for pediatric obesity. Telemed J E Health. 2013;19:806–8. doi: 10.1089/tmj.2012.0292. [DOI] [PubMed] [Google Scholar]
- 14.Slusser W, Whitley M, Izadpanah N, Kim SL, Ponturo D. Multidisciplinary pediatric obesity clinic via telemedicine within the Los Angeles metropolitan area: lessons learned. Clin Pediatr (Phila) 2016;55:251–9. doi: 10.1177/0009922815594359. [DOI] [PubMed] [Google Scholar]
- 15.Shaikh U, Nettiksimmons J, Joseph JG, Tancredi D, Romano PS. Collaborative practice improvement for childhood obesity in rural clinics: the Healthy Eating Active Living Telehealth Community of Practice (HEALTH COP) Am J Med Qual. 2014;29:467–75. doi: 10.1177/1062860613506252. [DOI] [PubMed] [Google Scholar]
- 16.Davis AM, Sampilo M, Gallagher KS, Landrum Y, Malone B. Treating rural pediatric obesity through telemedicine: outcomes from a small randomized controlled trial. J Pediatr Psychol. 2013;38:932–43. doi: 10.1093/jpepsy/jst005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebbeling CB, Antonelli RC. Primary care interventions for pediatric obesity: need for an integrated approach. Pediatrics. 2015;135:757–8. doi: 10.1542/peds.2015-0495. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 19.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 20.Papadaki A, Linardakis M, Larsen TM, et al. The effect of protein and glycemic index on children’s body composition: the DiOGenes randomized study. Pediatrics. 2010;126:e1143–52. doi: 10.1542/peds.2009-3633. [DOI] [PubMed] [Google Scholar]
- 21.Spieth LE, Harnish JD, Lenders CM, et al. A low-glycemic index diet in the treatment of pediatric obesity. Arch Pediatr Adolesc Med. 2000;154:947–51. doi: 10.1001/archpedi.154.9.947. [DOI] [PubMed] [Google Scholar]
- 22.Zeiss AM, Steffen A. Interdisciplinary health care teams: the basic unit of geriatric care. In: Carstensen LL, Edelstein BA, Dornbrand L, editors. The Handbook of Clinical Gerontology. Thousand Oaks, CA: Sage Publishing; 1996. pp. 423–50. [Google Scholar]
- 23.Persons JB. The Case Formulation Approach to Cognitive-Behavior Therapy. New York: The Guilford Press; 2008. [Google Scholar]
- 24.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual. 2007. [Google Scholar]
- 25.Luma GB, Spiotta RT. Hypertension in children and adolescents. Am Fam Physician. 2006;73:1558–68. [PubMed] [Google Scholar]
- 26.Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117:673–80. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]
- 27.National Committee for Quality Assurance. [Accessed November 3, 2015];Weight Assessment and Counseling for Nutrition and Physical Activity for Children/Adolescents (WCC) 2010 2015, at http://www.ncqa.org/Portals/0/Weight%20Assessment%20and%20Counseling.pdf.
- 28.Crawford R, Glover L. The impact of pre-treatment weight-loss expectations on weight loss, weight regain, and attrition in people who are overweight and obese: a systematic review of the literature. Br J Health Psychol. 2012;17:609–30. doi: 10.1111/j.2044-8287.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- 29.Mulgrew KW, Shaikh U, Nettiksimmons J. Comparison of parent satisfaction with care for childhood obesity delivered face-to-face and by telemedicine. Telemed J E Health. 2011;17:383–7. doi: 10.1089/tmj.2010.0153. [DOI] [PubMed] [Google Scholar]
- 30.Incledon E, Gerner B, Hay M, Brennan L, Wake M. Psychosocial predictors of 4-year BMI change in overweight and obese children in primary care. Obesity (Silver Spring) 2013;21:E262–70. doi: 10.1002/oby.20050. [DOI] [PubMed] [Google Scholar]
- 31.Kirk S, Zeller M, Claytor R, Santangelo M, Khoury PR, Daniels SR. The relationship of health outcomes to improvement in BMI in children and adolescents. Obes Res. 2005;13:876–82. doi: 10.1038/oby.2005.101. [DOI] [PubMed] [Google Scholar]
- 32.Kolsgaard ML, Joner G, Brunborg C, Anderssen SA, Tonstad S, Andersen LF. Reduction in BMI z-score and improvement in cardiometabolic risk factors in obese children and adolescents. The Oslo Adiposity Intervention Study - a hospital/public health nurse combined treatment. BMC Pediatr. 2011;11:47. doi: 10.1186/1471-2431-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Castro JM. Varying levels of food energy self-reporting are associated with between-group, but not within-subject, differences in food intake. J Nutr. 2006;136:1382–8. doi: 10.1093/jn/136.5.1382. [DOI] [PubMed] [Google Scholar]
- 34.Lau C, Toft U, Tetens I, et al. Association between dietary glycemic index, glycemic load, and body mass index in the Inter99 study: is underreporting a problem? Am J Clin Nutr. 2006;84:641–5. doi: 10.1093/ajcn/84.3.641. [DOI] [PubMed] [Google Scholar]
- 35.Miller BF, Petterson S, Brown Levey SM, Payne-Murphy JC, Moore M, Bazemore A. Primary care, behavioral health, provider colocation, and rurality. J Am Board Fam Med. 2014;27:367–74. doi: 10.3122/jabfm.2014.03.130260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.