Abstract
Research on the neural substrates of drug reward, withdrawal and relapse has yet to be translated into significant advances in the treatment of addiction. One potential reason is that this research has not captured a common feature of human addiction: progressive social exclusion and marginalization. We propose that research aimed at understanding the neural mechanisms that link these processes to drug seeking and drug taking would help to make addiction neuroscience research more clinically relevant.
A large gap exists between the promise of neuroscientific approaches to addiction and what they have delivered. Animal models have helped to identify neural substrates of drug addiction, including those mediating drug reward and reinforcement, protracted withdrawal, craving and relapse1–7. Models in which laboratory animals self-administer drugs8 are widely regarded as valid because rodents and monkeys learn to self-administer most drugs abused by humans9. Some medications that are used clinically to treat addiction decrease relapse in laboratory animals6,10, showing that animal models of addiction can have postdictive validity. There is also recent, although more-limited, evidence for the true predictive validity of animal models (BOX 1).
Box 1. Translational studies in addiction.
Some data are available to support postdictive validity of commonly used animal models in addiction. For instance, several medications that have established clinical efficacy in addiction have been found to decrease drug seeking in the reinstatement model, an animal model of drug relapse and craving100. These include naltrexone and acamprosate for alcoholism, methadone and buprenorphine for opiate addiction, and varenicline for nicotine addiction6,10.
There are also some recent indications for the potential predictive validity of animal models. For example, the α2-adrenergic agonist clonidine decreased stress-induced reinstatement of heroin and cocaine seeking in rats101. These observations led to human studies showing that clonidine decreased stress-induced cocaine craving in a human laboratory setting102 and also modestly decreased heroin lapse in a clinical trial103. Similarly, the observation that the glucocorticoid receptor antagonist mifepristone decreased alcohol dependence-induced increases in alcohol intake in rats104 led to a clinical study showing that the antagonist decreased both cue-induced alcohol craving and alcohol intake in alcohol-addicted patients105. There is also evidence that an unexpected behavioural phenomenon identified in a rat model — time-dependent increases in cue-induced drug seeking after withdrawal, termed ‘incubation’ of drug craving106 — generalizes to human addiction107. Recent data also suggest that rats given access to both heroin and cocaine express a preference for intravenous heroin in the home environment and cocaine outside the home environment, and this observation prospectively generalized to human drug users108. Finally, exposure to the memory retrieval-extinction procedure decreased conditioned drug effects and drug seeking in rat models of relapse, as well as drug craving in abstinent heroin users109.
However, these recent ‘translational’ advances, as well as numerous other preclinical studies aimed at identifying novel treatments of addiction, are yet to result in any US Food and Drug Administration (FDA)-approved pharmacotherapies for addiction. The ‘positive’ translational advances mentioned above are also the exception rather than the rule in the addiction field; drug targets identified in animal models110 often do not translate to successful clinical trials111. Most notably, since the 1970s65, neurobiological addiction research and theory have concentrated on the role of dopamine transmission within the mesocorticolimbic dopamine reward system5,112,113. This line of research seemed to point to targets for rational, mechanistically based addiction therapies. However, pharmacological interventions targeting the mesocorticolimbic dopamine system have so far yielded disappointing results in clinical trials114,115. These results and other evidence from human studies have led some to question the prevailing dogma that dopamine has a critical role in addiction across drug classes116,117.
More recently, the focus in preclinical addiction research shifted to the role of drug-induced alterations in glutamate transmission within the mesocorticolimbic dopamine system in drug craving and relapse118,119. However, results from a recent translational, double-blind, placebo-controlled study using N-acetylcysteine — a drug that normalizes cocaine-induced changes in glutamatergic transmission in the nucleus accumbens and decreases relapse and reinstatement of drug seeking in many preclinical studies4 — were negative120.
Similarly, based on rodent studies on the effects of corticotropin-releasing factor receptor 1 (CRFR1) antagonists on dependence-induced alcohol intake, alcohol-withdrawal symptoms and stress-induced reinstatement of alcohol seeking2,121, blockade of this receptor was thought to hold great promise as an anti-stress mechanism for alcoholism treatment2,11,19,41,121. However, when it recently became possible to evaluate this mechanism clinically, CRFR1 blockade was shown to be ineffective against stress-induced craving in human alcoholics, despite painstaking efforts to ensure target engagement122,123. These negative results probably represent a ‘mechanism failure’ rather than a ‘molecule failure’, as several CRFR1 antagonists also failed in development for other stress-related psychiatric disorders in which they had strong preclinical validation124,125 or have in fact been found to exacerbate rather than attenuate fear responses in healthy human subjects126.
Nevertheless, the humbling truth is that the neuroscience of addiction has yet to have a marked impact on clinical treatment11. This can be attributed only in part to practical barriers affecting the treatment of addiction, such as reimbursement issues, inadequate knowledge about available medications or ideologically motivated views that addiction should not be treated with drugs. The most-effective pharma cotherapies currently available for addiction — methadone and buprenorphine maintenance, which are used to treat opioid addiction — were discovered in the 1960s12 and 1970s13, an era preceding neuroscientific attempts to understand the mechanisms of addiction. For cocaine addiction, the only effective treatment to date — contingency management14 — is based on conditioning principles that date back to Pavlov and Skinner. This state of affairs has led some in the addiction field to question the brain-disease model of addiction15, to suggest that addiction research needs to shift away from its current emphasis on neurobiology and to propose that issues of harmful substance use should instead be addressed through policy measures16. We disagree with this view. In this Opinion article, we propose a way forward and focus on one possible reason for the disconnection between addiction neuroscience research and clinical advances: the relatively limited extent to which social factors have been integrated into neurobiological addiction research.
Social epidemiology has established a strong link between poor social integration and behaviours that result in alcohol and drug use17. Although few neuroscientists would negate the importance of these social factors in addiction, aspects of social integration — such as social inclusion or exclusion — have so far typically not been incorporated into neurobiological studies of addiction. We think that the different ways in which social interactions — positive and negative — influence addiction can be incorporated into these studies, and we predict that doing so will be important for understanding addiction. Below, we discuss some aspects of social interactions that we think are important in human addiction, as well as some of the attempts to model the effects of these interactions in experimental animals, mainly focusing on antagonistic interactions and social exclusion. We conclude by proposing that incorporating social factors into animal and human studies of neurobiological mechanisms of addiction will be important for discovering new treatments.
Social exclusion and addiction
Decades ago, data such as those from the Alameda County Human Population Laboratory Study showed that approximately 35% of men at the low end of the social-integration spectrum, but only about 10% at the high end, engaged in behaviours with strong negative effects on health, including heavy use of alcohol and drugs17. Conversely, the level of social integration has been shown to be associated with decreased relapse risk among treatment-seeking drug users18. Social interactions have multiple effects that can be hypothesized to differentially affect addiction. They can provide many of the healthy, non-drug reinforcers that successfully compete with drug rewards and that might also protect against the negative consequences of social stressors. Alternatively, social interactions can be highly stressful when they are of an antagonistic or excluding nature.
Stress provokes relapse to drug seeking in humans11 and laboratory animals19, but stressors typically used in animal models of addiction are discrete, experimenter-imposed events, such as footshocks19. By contrast, in drug users, stressful relapse triggers are typically social; they include, for example, conflicts in the workplace and the family, lack of social support and problems associated with low socioeconomic status20. Even in individuals who are socially well integrated when they start using drugs, continued drug use can often lead to social exclusion, which in turn promotes continued drug use. Specifically, during early stages of drug use, the drug is typically taken in a recreational, impulsive manner. However, as addiction develops, drug use is thought to become increasingly compulsive. As drug use escalates and transitions into compulsive drug use, these individuals typically become increasingly impaired in their ability to function socially. This results in further social marginalization and exclusion — factors that promote further drug use21.
Below, we consider some of the neural mechanisms by which social exclusion might promote addiction. We are not attempting an exhaustive review; rather, we want to show that the study of social factors can be integrated with the predominant neurobiological approaches in current addiction research. The need to integrate social factors applies equally to animal models that attempt to identify neural and molecular substrates of addiction and to human experimental and therapeutic studies.
Neural substrates
Laboratory procedures have been developed that allow the experience of social exclusion to be induced in healthy human volunteers, establishing some of the neural correlates of this state. Experimentally induced social exclusion activates a brain network that overlaps with the pain matrix, which includes the insula22,23. Activity in the insula also correlates with the intensity of subjective craving for most addictive drugs in humans, suggesting that this region may be involved in both social exclusion and drug addiction24. A mechanistic role of insula activation in drug craving is supported by findings that insula lesions caused by stroke25 and insula-targeted transcranial magnetic stimulation26 both reduce cigarette craving and smoking.
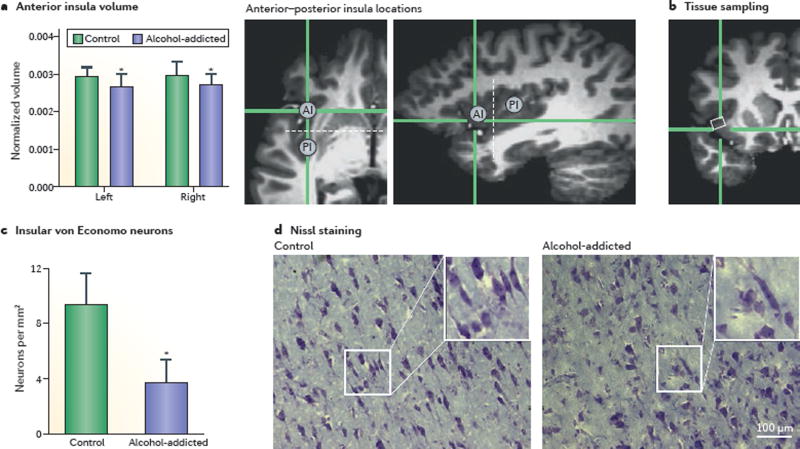
The insula may also have a role in alcoholism. Insula perfusion, as measured by arterial spin-labelling MRI, is progressively decreased in alcohol-addicted individuals27, and alcoholism is also associated with a loss of insula grey matter28 (FIG. 1). This decrease in insula grey matter involves a profound loss of von Economo neurons (FIG. 1), which are large projection neurons found mostly in highly social species and thought to be crucial for prosocial behaviours29. Finally, insula activity, as measured by functional MRI, is associated with important abilities needed for adaptive social behaviours, such as empathy and rational decision making under risk30,31. The importance of these observations for addiction is suggested in the finding that low insula activity in a risk task predicted increased risk of relapse in abstinent methamphetamine users32.
Figure 1. Structural changes in the insula in alcoholics.
Reduced anterior insula (AI) volumes (measured by structural MRI after 3–4 weeks of abstinence) and a pronounced, selective loss of von Economo neurons (measured by histological analysis in post-mortem brains) are observed in alcohol-addicted individuals compared with healthy volunteers (controls). a | Whole-brain normalized volumes (mean ± s.e.m.) of the left and right AI in alcohol-addicted and healthy individuals. The two magnetic resonance (MR) images on the right of the panel show, in two different planes, the location of the measured AI. Dashed white lines indicate a coronal plane through the anterior commissure with y = 0, which represents the estimated border between the AI and posterior insular (PI) divisions. b | An MR image depicting the region (white rectangle) sampled in post-mortem analyses in panel c and panel d. c | Quantitative analysis showing a reduction in the number of von Economo neurons in alcohol-addicted patients compared with healthy controls (mean ± s.e.m.). d | In contrast to the number of von Economo neurons, counts of all (Nissl-stained) neurons in the AI did not differ between groups. *Different from controls; P < 0.05. Adapted from REF. 28, Senatorov, V. V. et al. Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain 2015 138 (1): 69–79, by permission of Oxford University Press.
Thus, findings to date point to the insula as a site of overlap for brain responses involved in the processing of social inclusion and exclusion, and drug craving. Future research should establish whether these two processes are mechanistically linked through insula-related circuits. Insula activity has been observed in many tasks that probe multiple, seemingly unrelated cognitive and affective functions. However, there have been interesting recent attempts to integrate these observations into a unified conceptual framework of insula function. These attempts have focused on the handling of risk31 and the attribution of salience33 — functions that are of particular relevance in addiction.
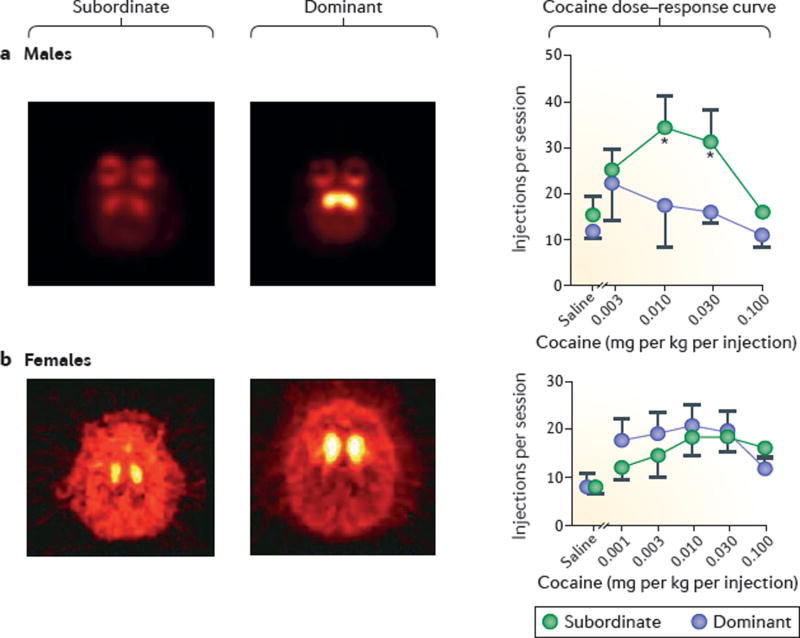
It is unlikely that the insula alone can account for the effects of social exclusion on addiction. Striatal dopamine function seems to be critical as well, at least in males. In subordinate male monkeys, low availability of striatal dopamine D2/3 receptors (D2/3Rs; commonly used imaging agents do not distinguish between these closely related subtypes) is associated with increased cocaine self-administration34 (FIG. 2). In a possible human correlate, low striatal D2/3R availability is associated with low social status and low perceived social support in healthy volunteers35, and low availability of D2/3Rs is a consistent observation in drug users, across drug classes36. We speculate that the development of drug addiction involves a progressive decrease in D2/3R availability caused by both the drug use and the resulting social exclusion.
Figure 2. Sex differences in the effects of social hierarchy on cocaine self-administration in non-human primates.
a | Effects of social hierarchy on the striatal availability of dopamine D2/3 receptors (D2/3Rs) and on cocaine self-administration in socially housed male cynomolgus monkeys. Positron emission tomography (PET) images (transverse plane) were obtained with the D2/3R–imaging agent [18F]fluoroclebopride, and cocaine self-administration was maintained under a schedule of reinforcement in which every 30 lever presses led to the delivery of cocaine infusion (fixed-ratio 30). Subordinate males have lower D2/3R availability and show higher rates of cocaine self-administration than do dominant males, as observed in the dose–response curves. Brighter and lighter colours in the scan images on the left represent increased binding of [18F]fluoroclebopride, suggesting higher D2/3R availability. b | In a separate experiment, it was shown that social hierarchy resulted in social-rank-related differences in D2/3R availability (as assessed using PET) but did not affect maintenance of cocaine self-administration in female cynomolgus monkeys, with similar levels of self-administration being observed in subordinate and dominant monkeys (see dose– response curves; the number of infusions per session in the females was limited to 30). Data are depicted as mean ± s.e.m. Panel a is adapted from REF. 34, Nature Publishing Group. Panel b is adapted with permission from REF. 95, Elsevier.
The findings regarding the role of the insula and striatum in social exclusion might be related, as the two structures are anatomically and functionally connected. In non-human primates, anterior insular regions project to the ventromedial part of the ventral striatum37. In humans, recent evidence shows that connectivity between these structures is functionally involved in risk preferences when pursuing rewards. Specifically, activity in the ventral striatum and anterior insula inversely predicts risky choices, and individuals with stronger anatomical connectivity between the right anterior insula and ventral striatum (measured using MRI-based tractography) demonstrated less-risky gambling choices38.
The anterior insula also has extensive reciprocal connections with the amygdala complex39,40, and these connections might link social stress with drug seeking as well. Amygdala activation is crucial for initiating stress-induced alcohol-seeking behaviour in rats41, and reductions in functional connectivity between the insula and the amygdala have been reported in alcohol-addicted individuals42.
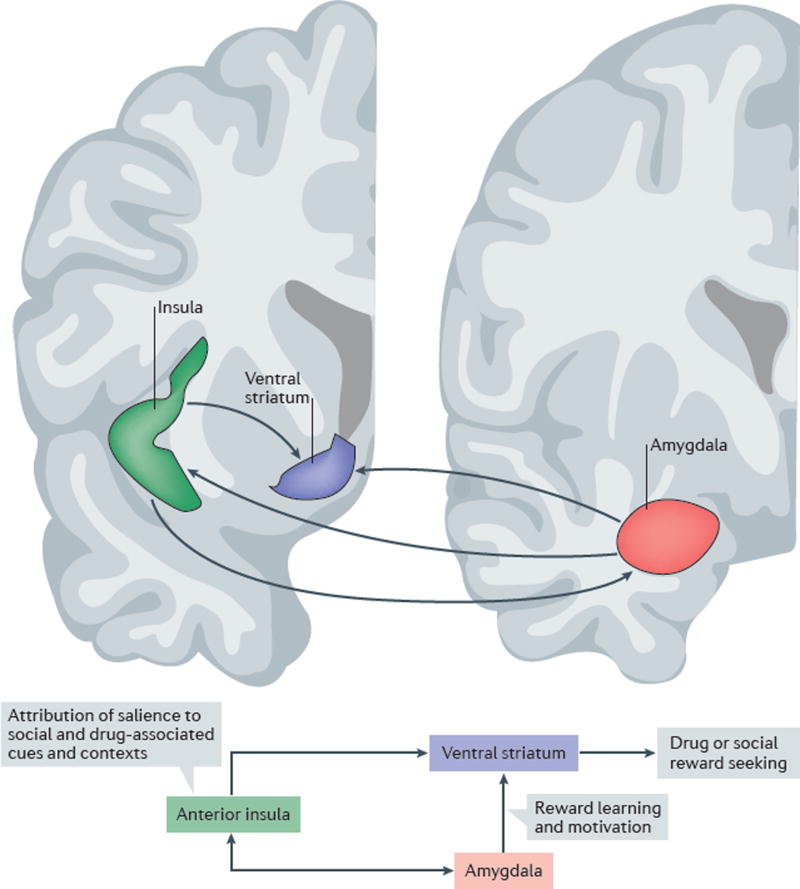
Thus, the social ‘spiral of distress’ is a complex process, and one that is probably influenced by the activity of multiple circuits. On the basis of the observations reviewed above, we propose that a circuit including the anterior insula, ventral striatum and parts of the amygdala complex may contribute to this process (FIG. 3).
Figure 3. Schematic representation of hypothesized core circuitry linking social exclusion to drug seeking.
Drug seeking is a complex behaviour, and multiple circuits are likely to have a role in the ability of social and drug-associated cues and contexts to gain access to the affective and motivational processes involved in addiction. The insula and its afferent and efferent projections play a key part in integrating the motivational relevance of salient interoceptive and visceromotorstimuli and executing appropriate (adaptive) behaviours based on this integration33. A network including the insula seemsto be involved in responsesto social exclusion22,24. Established connections among the anterior insula, the amygdala complex and the ventral striatum suggest that these structures form an important circuit in linking social exclusion to drug seeking. The anterior insula (green), a key element of the so-called social pain matrix, is closely interconnected with the amygdala (red) and the ventral striatum (purple)33,37,39,40. We propose that these connections form a networkthat is important for linking social stimuli to the affective and motivational aspects of drug seeking and drug taking. In the early stages of addiction, activity of the anterior insula that is induced by social exclusion may directly promote drug craving. Overtime, drug-induced changes in insula function are hypothesized to result in an impaired ability to appropriately attribute salience to social versus drug-related cues and contexts24,28,32,127. Figure is adapted from an image provided courtesy of S. N. Haber, University of Rochester, New York, USA.
Endogenous opioids
Endogenous opioids, through actions on µ-opioid receptors (MORs), play an important part in the reward associated with affiliative behaviours and counteract the aversive consequences of social stress; these roles of endogenous opioids are thought to involve the insula, amygdala and ventral striatum22,43,44. Mice lacking MORs show profound deficits in attachment45. In non-human primates, systemic administration of MOR antagonists increases rates of grooming and other affiliative behaviours in what is thought to be an attempt to compensate for blockade of MOR function46, whereas low doses of MOR agonists dampen or eliminate distress responses to social separation of dog puppies47. In addition, functional genetic variation at the locus that encodes the MOR moderates mother–infant attachment in monkeys48 and in humans49.
In humans, MORs are highly expressed in the insula50, and MORs in this structure have a role in social processes. In healthy volunteers, functional genetic MOR variation moderates levels of subjective distress and insula activity in response to social-exclusion stress51. A recent positron emission tomography study further showed that social acceptance is associated with MOR activation in the anterior insula and the amygdala52. A key role for endogenous opioids in the link between addiction and social pain has recently been proposed53. In that context, it is interesting that decreased endogenous opioid release was found in individuals with pathological gambling disorder, a behavioural addiction in which brain changes are not confounded by the pharmacological effects of illicit drug use54.
Combining the neuroanatomical and neurochemical observations above, we propose a set of testable hypotheses. If the aversive experience of social rejection and exclusion can be buffered by MOR activation, possibly within the insula–amygdala– ventral striatum circuit (FIG. 3), then people with impaired endogenous opioid function, exposure to social stress, or both will experience relief of distress when taking drugs that directly or indirectly activate MORs. Examples of drugs that directly activate MORs are opiates such as heroin and oxycodone. Alcohol, which has long been postulated as a self-medication agent for people with difficulties in experiencing social attachment55, also increases endogenous opioid levels in humans56. Thus, social integration, by restoring normal function of endogenous opioid systems, can decrease the need to activate these systems through alcohol or opioid use and subsequently can decrease drug use and relapse. Improving the social integration of drug users through opportunities for housing, jobs and meaningful relationships is therefore not merely a nonspecific intervention but rather a neurobiologically specific and critically important way to decrease drug use.
Social cognition
Impairments of cognitive function are both risk factors for and consequences of drug use57,58. Cognitive impairments affect functions that may not be viewed as primarily social per se but nevertheless have an impact on the ability to function socially. For example, drug users tend to make intertemporal choices favouring small, immediate drug rewards over more-advantageous but temporally more-distant outcomes59. Indeed, such steep delay discounting is an established psychological trait of addicted individuals60 and impedes rehabilitation, the ability to comply with social norms and the achievement of healthy personal goals. Furthermore, empathy and recognition of emotions in others are impaired in drug users61,62, and these deficits pose strong challenges to rehabilitation.
We hypothesize that impaired decision making and social exclusion form the elements of a vicious cycle of their own. Homelessness, a social problem strongly associated with drug addiction63, represents an extreme of social exclusion and low access to resources. Impaired value-based decision making (such as making disadvantageous intertemporal choices) contributes to and maintains this condition; consistently choosing to buy drugs today over paying rent next week contributes to the risk of homelessness. But a lack of resources that is characteristic of homelessness per se promotes disadvantageous decision making of the kind commonly observed in drug users64. We know of no research that directly tests whether social exclusion contributes to impaired decision making in addiction, but we think that this will be important to examine in future studies.
Animal models of addiction
Animal models have been in use for many years to study neural substrates of addiction, in the hope of identifying mechanisms that could be targeted for treatment. The operant drug self-administration model has long been a cornerstone of preclinical research on addiction. In this model, over 90% of single-housed rodents or monkeys will voluntarily self-administer opiates or psycho-stimulants8,65. This percentage, which was originally cited as evidence that these drugs are highly addictive, was realized over time to reflect a shortcoming of the model, because it is about four- to fivefold higher than the percentage of human drug users who transition from initial drug use to compulsive use66. Therefore, when reassessing the way we have approached animal models, this model should be one of the first examined.
Indeed, among humans exposed to addictive drugs such as heroin, cocaine and alcohol, transition to addiction is the exception rather than the rule, occurring in about 20% of the people exposed to these drugs66. Genetic vulnerability67 and a lack of access to non-drug alternatives68 are risk factors for the transition to compulsive use. Furthermore, in animal models, addiction cannot be equated with operant drug self-administration as such, because all commonly used diagnostic criteria require that there be continued use despite adverse consequences. These insights have been incorporated into animal models7,69–71, producing results that have led to re-evaluations of earlier results from traditional drug self-administration7,70.
For example, when rats or monkeys are given unlimited access, 24 hours a day, to psychostimulant drugs but no other alternative, competing rewards, most die of overdose within several weeks72,73. By contrast, more than 80% of rats given extended daily access to cocaine or methamphetamine stop or significantly decrease self-administration when given a mutually exclusive choice between the drug and a non-drug reward, such as palatable food74,75, or when drug self-administration also results in intermittent footshock punishment76,77. This consideration of contingencies — that is, the availability of non-drug choices or the adverse consequences of choosing drug — has been a good start to refining animal models of addiction. What should come next, we think, is to consider social factors78–80.
Modelling social factors
In this Opinion article, we focus on social exclusion, but readers should be aware that animal studies of addiction have examined other social phenomena and other stressors, such as maternal separation, early social isolation, food restriction, restraint, intermittent footshock and social defeat79–82. However, with some exceptions19,80,83, these lines of research have not been integrated with research aimed at identifying the underlying neural mechanisms of addiction. A probable reason for this is that the effects of many stressors on drug self-administration are relatively weak, procedure-dependent and often not reproducible across laboratories81,82. For example, a seemingly promising finding was that oral morphine intake and preference were markedly higher in single-housed rats than in rats housed with conspecifics in a large, enriched environment (known as the ‘Rat Park’)84. However, the interpretation of these data turned out to be problematic. Oral morphine, which has a bitter taste, is only a weak reinforcer to rats and is rarely preferred over water85. Subsequent attempts to demonstrate the Rat Park or ‘social housing’ effect with intravenous opiates, which are stronger reinforcers, were unsuccessful86.
More-robust effects of isolated versus social housing have been seen on home-cage intake of alcohol87. The alcohol data also suggest something more subtle: socially housed rats develop social ranks and, among these rats, alcohol intake varies according to social rank69. This work was part of the background for more-recent studies on the inhibitory effect of environmental enrichment on cocaine self-administration and relapse in rats81,88. In social animals, low rank (which can be viewed as a correlate of social exclusion78,89) has been implicated to be a determinant of drug self-administration. Subordinate male rats and monkeys drink more alcohol than their dominant conspecifics, and subordinate rats drink more etonitazene (a potent opiate agonist) than their dominant conspecifics87,90–92. However, in rats, these social-rank differences in drug intake persisted for only the first few months of alcohol or opiate availability; after many months of drug access, all rats, independent of social status, consumed high amounts of the drugs87,91.
In animal models of cocaine addiction, the picture is more complicated and there are major species differences. In rats, social rank has no effect on cocaine self-administration and reinstatement of cocaine seeking93, whereas, in cynomolgus monkeys, subordinate males self-administer more cocaine than do their dominant conspecifics34. The differences in cocaine self-administration in the monkeys that are related to social rank seem to involve striatal D2/3Rs; social housing increased striatal D2/3R availability in dominant but not subordinate male monkeys, and this increase seemed to initially protect the dominant male monkeys from self-administering high amounts of cocaine (FIG. 2a). These data are consistent with findings in humans that a high availability of striatal D2/3Rs confers resistance to addiction94.
However, an opposite pattern emerges in female monkeys: dominant females will learn to self-administer cocaine at lower doses than do subordinates, suggesting that the dominant females are more sensitive to the reinforcing effects of cocaine. This occurs despite higher striatal D2/3R availability in dominant female monkeys than in subordinates, similar to what had been observed in male monkeys95 (FIG. 2b). These findings suggest that social rank has different effects on cocaine self-administration in males and females, and that striatal D2/3R expression (and, by implication, function) has a minimal role in female self-administration of cocaine78. Given the increasing awareness that sex differences need to be considered for most human behaviours96, these findings are of considerable interest.
Conclusions and implications
Confrontational and punitive attitudes to drug addiction remain widespread. We hope that, at a minimum, neurobiological insights into the mechanisms through which social exclusion promotes addiction can foster an understanding that such attitudes are counter productive. This, in turn, should help to reduce the stigma of addiction and promote better-informed policies. In addition, we hope that incorporating the considerations that we have discussed can stimulate research, ultimately resulting in improved treatments.
In the search for biological mechanisms that could be targeted by novel treatments, addiction needs to be defined as a pattern of self-administration that is dysfunctional and that occurs in spite of adverse consequences and/or of the availability of non-drug reinforcers. Social functioning needs to be assessed as both a determinant of this behaviour and as a variable affected by it. Critical species differences in social behaviour, as well as individual differences within species — including sex differences — need to be considered. Potential treatments need to be assessed for their ability to influence the ‘addictive’ behaviour in the vulnerable laboratory animals that display it. Combining all of this into any single study is a lot to ask, but incorporating more than one of these components into modern neuroscience studies of addiction in animal models is an achievable goal7,97.
To maximize chances of success, preclinical research needs to work in concert with translational studies on social exclusion in drug users. Several of the methodologies that have been successfully used in healthy volunteers can be used in addiction research, and some of these lend themselves to being combined with functional brain imaging, whereby the neural underpinnings of processes linking social factors to addictive behaviours can be mapped98. Pharmacological manipulations targeting opioid systems could initially help to clarify causal links between social exclusion, distress reactions and drug use. Eventually, this line of research could help to evaluate candidate treatments99 and, most importantly, bring forward novel treatments.
For clinicians, it is hard to spend a day with patients who struggle with addictive disorders without being struck by the impaired social interactions and lack of access to resources that frequently become the dominant themes of their lives. The central role of these factors in clinical addiction needs to be reflected in both basic and clinical addiction neuroscience. Despite ambitious attempts to elucidate some of these processes in animal models80,97, our understanding of their neural and molecular mechanisms remains vastly insufficient.
Acknowledgments
The writing of this article was supported in part by a grant from the Swedish Research Council (M.H.), the Intramural Research Program of the US National Institutes of Health and the US National Institute on Drug Abuse (D.H.E. and Y.S.) and grants DA010584 and DA017763 (M.A.N.). The authors are grateful to S. N. Haber at the University of Rochester, New York, USA, and her co-workers for producing and providing Figure 3. The authors apologize to their many friends and colleagues for not citing many reviews and empirical papers relevant to the topic of their paper, owing to format restrictions.
Glossary
- Compulsive drug use
Continued use of a drug despite (known) adverse consequences
- Contingency management
A treatment based on systematic reinforcement of a desired, clinically beneficial behaviour
- Craving
The subjective experience of a strong desire to consume a particular substance, to experience its effects or to avoid the symptoms of its withdrawal
- Drug addiction
A clinical condition in which an individual knowingly continues to pursue and consume a chemical substance in a manner that is harmful to that individual or to others
- Pain matrix
A term proposed for a set of brain structures, including the anterior insula and the dorsal anterior cingulate cortex, that are consistently shown by functional MRI to be activated during physical pain
- Postdictive validity
The ability to retrospectively demonstrate an established human phenomenon in an animal model
- Predictive validity
In the context of medications development, the extent to which a drug effect in laboratory animals prospectively predicts therapeutic effects of the same drug in humans
- Protracted withdrawal
The affective symptoms of drug withdrawal — including low mood, elevated anxiety and increased sensitivity to stress — that persist beyond the time frame of acute physical withdrawal (which typically does not last beyond 3–7 days).
- Reinstatement
In the context of addiction research, the resumption of drug seeking after extinction of the drug-reinforced responding, induced by exposure to priming doses of drug, drug cues or stressors
- Relapse
Resumption of drug taking after achieving abstinence
- Social defeat
A type of social stress used in laboratory-animal studies that is typically induced by placing a rodent in a cage with an unfamiliar rodent that is expected to attack and defeat the intruder, owing to increased strength, aggression or established dominance
- Social integration
A central concept of sociology, developed by the French sociologist Émile Durkheim, that refers to the web of relationships and interactions — family, kinship groups, traditions or economic activity — through which individuals are connected to each other to form a societysocial exclusion is defined as a failure of this process.
Footnotes
Competing interests statement
The authors declare no competing interests.
Contributor Information
Markus Heilig, Center for Social and Affective Neuroscience, Department of Clinical and Experimental Medicine (IKE), Linköping University, SE-581 83 Linköping, Sweden.
David H. Epstein, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, Maryland 21044, USA
Michael A. Nader, Department of Physiology and Pharmacology, Wake Forest School of Medicine, Winston-Salem, North Carolina 27157, USA
Yavin Shaham, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, Maryland 21044, USA.
References
- 1.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 4.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 5.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 6.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–457. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology. 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu. Rev. Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- 9.Brady JV. Animal models for assessing drugs of abuse. Neurosci. Biobehav. Rev. 1991;15:35–43. doi: 10.1016/s0149-7634(05)80089-2. [DOI] [PubMed] [Google Scholar]
- 10.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dole VP, Nyswander MA. Medical treatment for diacetylmorphine (heroin) addiction. A clinical trial with methadone hydrochloride. JAMA. 1965;193:646–650. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- 13.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch. Gen. Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 14.Higgins ST, et al. A behavioral approach to achieving initial cocaine abstinence. Am. J. Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- 15.Satel S, Lilienfeld SO. Addiction and the brain-disease fallacy. Front. Psychiatry. 2013;4:141. doi: 10.3389/fpsyt.2013.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalant H. What neurobiology cannot tell us about addiction. Addiction. 2010;105:780–789. doi: 10.1111/j.1360-0443.2009.02739.x. [DOI] [PubMed] [Google Scholar]
- 17.Berkman LF, Kawachi I. Social Epidemiology. Oxford Univ. Press; 2000. [Google Scholar]
- 18.Havassy BE, Hall SM, Wasserman DA. Social support and relapse: commonalities among alcoholics, opiate users, and cigarette smokers. Addict. Behav. 1991;16:235–246. doi: 10.1016/0306-4603(91)90016-b. [DOI] [PubMed] [Google Scholar]
- 19.Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marlatt GA, Baer JS, Donovan DM, Kivlahan DR. Addictive behaviors: etiology and treatment. Annu. Rev. Psychol. 1988;39:223–252. doi: 10.1146/annurev.ps.39.020188.001255. [DOI] [PubMed] [Google Scholar]
- 21.Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. Am. Psychol. 1986;41:765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- 23.Wager TD, et al. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garavan H. Insula and drug cravings. Brain Struct. Funct. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- 25.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinur-Klein L, et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry. 2014;76:742–749. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan EV, et al. A selective insular perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biol. Psychiatry. 2013;74:547–555. doi: 10.1016/j.biopsych.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senatorov VV, et al. Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain. 2015;138:69–79. doi: 10.1093/brain/awu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butti C, Santos M, Uppal N, Hof PR. Von Economo neurons: clinical and evolutionary perspectives. Cortex. 2013;49:312–326. doi: 10.1016/j.cortex.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann. NY Acad. Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Gowin JL, et al. Attenuated insular processing during risk predicts relapse in early abstinent methamphetamine-dependent individuals. Neuropsychopharmacology. 2014;39:1379–1387. doi: 10.1038/npp.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 34.Morgan D, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 35.Martinez D, et al. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol. Psychiatry. 2010;67:275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch. Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 37.Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J. Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leong JK, Pestilli F, Wu CC, Samanez-Larkin GR, Knutson B. White-matter tract connecting anterior insula to nucleus accumbens correlates with reduced preference for positively skewed gambles. Neuron. 2016;89:63–69. doi: 10.1016/j.neuron.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho YT, Ernst M, Fudge JL. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J. Neurosci. 2013;33:14017–14030. doi: 10.1523/JNEUROSCI.0170-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieuwenhuys R. The insular cortex: a review. Prog. Brain Res. 2012;195:123–163. doi: 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- 41.Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orban C, et al. Resting state synchrony in anxiety-related circuits of abstinent alcohol-dependent patients. Am. J. Drug Alcohol Abuse. 2013;39:433–440. doi: 10.3109/00952990.2013.846348. [DOI] [PubMed] [Google Scholar]
- 43.Machin AJ, Dunbar RIM. The brain opioid theory of social attachment: a review of the evidence. Behaviour. 2011;148:985–1025. [Google Scholar]
- 44.Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids social behavior. Neurosci. Biobehav. Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- 45.Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the µ-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 46.Fabre-Nys C, Meller RE, Keverne EB. Opiate antagonists stimulate affiliative behaviour in monkeys. Pharmacol. Biochem. Behav. 1982;16:653–659. doi: 10.1016/0091-3057(82)90432-4. [DOI] [PubMed] [Google Scholar]
- 47.Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: opiates alleviate separation distress. Biol. Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- 48.Barr CS, et al. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc. Natl Acad. Sci. USA. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Copeland WE, et al. Child µ-opioid receptor gene variant influences parent-child relations. Neuropsychopharmacology. 2011;36:1165–1170. doi: 10.1038/npp.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumgartner U, et al. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30:692–699. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 51.Way BM, Taylor SE, Eisenberger NI. Variation in the µ-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc. Natl Acad. Sci. USA. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu DT, et al. Response of the µ-opioid system to social rejection and acceptance. Mol. Psychiatry. 2013;18:1211–1217. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: implications for addiction. Curr. Opin. Neurobiol. 2013;23:473–479. doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mick I, et al. Blunted endogenous opioid release following an oral amphetamine challenge in pathological gamblers. Neuropsychopharmacology. 2016;41:1742–1750. doi: 10.1038/npp.2015.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv. Rev. Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell JM, et al. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci. Transl Med. 2012;4:116ra6. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- 57.Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat. Neurosci. 2012;22:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- 60.MacKillop J, et al. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uekermann J, Daum I. Social cognition in alcoholism: a link to prefrontal cortex dysfunction? Addiction. 2008;103:726–735. doi: 10.1111/j.1360-0443.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 62.Preller KH, et al. Impaired emotional empathy and related social network deficits in cocaine users. Addict. Biol. 2014;19:452–466. doi: 10.1111/adb.12070. [DOI] [PubMed] [Google Scholar]
- 63.North CS, Eyrich KM, Pollio DE, Spitznagel EL. Are rates of psychiatric disorders in the homeless population changing? Am. J. Publ. Health. 2004;94:103–108. doi: 10.2105/ajph.94.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mullainathan S, Shafir E. Scarcity: Why Having Too Little Means So Much. 2013 Times Books. [Google Scholar]
- 65.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 66.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 1994;2:244–268. [Google Scholar]
- 67.Kendler KS, et al. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nat. Neurosci. 2012;15:181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopez-Quintero C, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav. Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci. Biobehav. Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology. 1991;105:169–174. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- 72.Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA. 1985;254:81–83. [PubMed] [Google Scholar]
- 73.Johanson CE, Balster RL, Bonese K. Self-administration of psychomotor stimulant drugs: the effects of unlimited access. Pharmacol. Biochem. Behav. 1976;4:45–51. doi: 10.1016/0091-3057(76)90174-x. [DOI] [PubMed] [Google Scholar]
- 74.Cantin L, et al. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS ONE. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caprioli D, et al. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol. Psychiatry. 2015;78:463–473. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 77.Krasnova IN, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology. 2014;39:2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nader MA, Banks ML. Environmental modulation of drug taking: nonhuman primate models of cocaine abuse and PET neuroimaging. Neuropharmacology. 2014;76:510–517. doi: 10.1016/j.neuropharm.2013.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol. Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol. Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology. 2012;224:33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci. Biobehav. Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 83.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alexander BK, Coambs RB, Hadaway PF. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology. 1978;58:175–179. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- 85.Meisch RA, Carroll ME. In: Methods of Assessing the Reinforcing Properties of Abused Drugs. Bozarth MA, editor. Springer; 1987. pp. 143–161. [Google Scholar]
- 86.Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol. Biochem. Behav. 1989;33:903–907. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- 87.Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol. Biochem. Behav. 1991;38:389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- 88.Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog. Neurobiol. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Blanchard DC, Blanchard RJ. Behavioral correlates of chronic dominance-subordination relationships of male rats in a seminatural situation. Neurosci. Biobehav Rev. 1990;14:455–462. doi: 10.1016/s0149-7634(05)80068-5. [DOI] [PubMed] [Google Scholar]
- 90.Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol. Biochem. Behav. 1987;28:437–442. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- 91.Heyne A. The development of opiate addiction in the rat. Pharmacol. Biochem. Behav. 1996;53:11–25. doi: 10.1016/0091-3057(95)00193-x. [DOI] [PubMed] [Google Scholar]
- 92.Helms CM, McClintick MN, Grant KA. Social rank, chronic ethanol self-administration, and diurnal pituitary-adrenal activity in cynomolgus monkeys. Psychopharmacology. 2012;224:133–143. doi: 10.1007/s00213-012-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jupp B, et al. Social dominance in rats: effects on cocaine self-administration, novelty reactivity and dopamine receptor binding and content in the striatum. Psychopharmacology. 2015;233:579–589. doi: 10.1007/s00213-015-4122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 95.Nader MA, et al. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol. Psychiatry. 2012;72:414–421. doi: 10.1016/j.biopsych.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cahill L. Sex influences on brain and emotional memory: the burden of proof has shifted. Prog. Brain Res. 2010;186:29–40. doi: 10.1016/B978-0-444-53630-3.00003-8. [DOI] [PubMed] [Google Scholar]
- 97.Nader MA, Czoty PW, Gould RW, Riddick NV. Review, Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Phil. Trans. R. Soc. B. 2008;363:3223–3232. doi: 10.1098/rstb.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 99.Czoty PW, Nader MA. Effects of oral and intravenous administration of buspirone on food-cocaine choice in socially housed male cynomolgus monkeys. Neuropsychopharmacology. 2015;40:1072–1083. doi: 10.1038/npp.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 101.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Brain Res. Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 102.Jobes ML, et al. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology. 2011;218:83–88. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kowalczyk WJ, et al. Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: a randomized controlled trial with ecological momentary assessment. Am. J. Psychiatry. 2015;172:760–767. doi: 10.1176/appi.ajp.2014.14081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vendruscolo LF, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vendruscolo LF, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J. Clin. Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bedi G, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol. Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caprioli D, et al. Ambience and drug choice: cocaine-and heroin-taking as a function of environmental context in humans and rats. Biol. Psychiatry. 2009;65:893–899. doi: 10.1016/j.biopsych.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 109.Xue YX, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cunningham KA, Bradberry CW, Chang AS, Reith ME. The role of serotonin in the actions of psychostimulants: molecular and pharmacological analyses. Behav. Brain Res. 1996;73:93–102. doi: 10.1016/0166-4328(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 111.Schmitz JM, et al. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63:207–214. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 112.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 113.Bradberry CW. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology. 2007;191:705–717. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- 114.Anton RF, et al. A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J. Clin. Psychopharmacol. 2008;28:5–12. doi: 10.1097/jcp.0b013e3181602fd4. [DOI] [PubMed] [Google Scholar]
- 115.Tiihonen J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am. J. Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- 116.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 2015;16:305–312. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- 117.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat. Rev. Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci. Biobehav. Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.LaRowe SD, et al. A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am. J. Addict. 2013;22:443–452. doi: 10.1111/j.1521-0391.2013.12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J. Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwako LE, et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwandt ML, et al. The CRF1 antagonist verucerfont in anxious alcohol dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.61. http://dx.doi.org/10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed]
- 124.Coric V, et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress. Anxiety. 2010;27:417–425. doi: 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- 125.Binneman B, et al. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am. J. Psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- 126.Grillon C, et al. The CRH1 antagonist GSK561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacology. 2015;40:1064–1071. doi: 10.1038/npp.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maurage P, et al. Disrupted regulation of social exclusion in alcohol-dependence: an fMRI study. Neuropsychopharmacology. 2012;37:2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]