Abstract
BACKGROUND
Sinonasal mucosal melanoma (SNMM) is a rare neoplasm with a poor prognosis.
METHODS
Retrospective analysis was conducted on 78 patients with localized SNMM treated at MSKCC (1998–2013). Demographic, tumor, imaging and treatment factors were recorded and survival and disease-control outcomes were analyzed.
RESULTS
Median overall survival (OS) and disease-specific survival (DSS) were 32 and 50 months. Median local recurrence-free survival (LRFS) and distant recurrence-free survival (DRFS) were 43 and 12 months. Multivariate analysis demonstrated greater OS in nasal cavity tumors and earlier T-stage. Radiotherapy was associated with significantly greater LRFS (5-year, 35% vs. 59%, p=0.01) but no difference in OS. Post-radiotherapy positron-emission tomography (PET) response was associated with greater OS.
CONCLUSIONS
Distant metastasis is the predominant mode of recurrence in SNMM, but local recurrence remains common. Radiotherapy is associated with improved local control, but no survival benefit. The prognostic value of post-radiotherapy PET imaging warrants further investigation.
Keywords: Mucosal Melanoma, Sinonasal, Nasal Cavity, Paranasal Sinus, Radiotherapy, Positron emission tomography
INTRODUCTION
Mucosal melanoma of the nasal cavity or paranasal sinuses represents a rare type of melanoma with a poor prognosis. Less than 1% of all melanomas in the USA arise from the mucosal surfaces of the head and neck, with an incidence of 0.71/million people.1 Due to high rates of local recurrence and distant metastasis, sinonasal mucosal melanoma (SNMM) has a poor prognosis and a reported 5-year survival of 13–45%.2
The majority of patients present with localized disease; treatment traditionally consists of surgery, the extent of which is often limited by anatomic barriers and effect on quality of life. Surgery is often followed by adjuvant radiotherapy (RT) in an effort to improve locoregional control, although evidence for RT benefit remains limited with retrospective series demonstrating improved local control but no benefit in survival.3 There is limited consensus for SNMM staging, with a variety of systems proposed, and only recent release of a SNMM-specific system in the AJCC 7th edition.4 Prognostic factors suggested include age, stage, histological features, gender, and tumor location (sinus vs. nasal), although the clinical significance of these factors has not been consistently demonstrated across series.5 In addition, incorporation of molecular pathology and imaging has become more prevalent despite significant data to support its use or implications.
Due to the rarity of SNMM, treatment decisions are often based on case reports and small retrospective studies, which frequently span decades to maximize the number of patients, and therefore combine disease sites, extent of disease, and treatment modalities. Here, we report a large retrospective analysis of patients treated at Memorial Sloan Kettering Cancer Center with localized, non-metastatic mucosal melanoma of the nasal cavity or paranasal sinuses at initial presentation from 1998 to 2013 with modern diagnostic and treatment techniques.
MATERIALS AND METHODS
Patients
After obtaining institutional IRB approval, patients were identified using a prospectively maintained institutional melanoma database. All patients with pathologically confirmed mucosal melanoma of the sinus or nasal cavity between 1998 and 2013 with at least 1 visit at Memorial Sloan Kettering Cancer Center without evidence of distant metastasis or lymph node involvement and who underwent surgical resection were selected. Patients previously included in MSKCC’s published series (n=3) were excluded6. Charts were reviewed for demographics, tumor characteristics, treatment plan, initial and follow-up imaging, and documentation of recurrence or death. Radiation equivalent-dose calculations were performed using an α/β ratio of 2.5. SNMM was confirmed histopathologically at MSKCC for all patients.
Statistical Analysis
Proportions were compared using Fisher’s exact test. Kaplan-Meier curves were used to estimate survival rates (with asymmetric 95% confidence intervals) and median survival times. Survival curves were generated to estimate overall survival (OS), disease-specific survival (DSS), recurrence-free survival (RFS), local RFS, loco-regional RFS, and distant RFS. Survival analyses were performed using a landmark starting time of 12 weeks post-surgery to account for treatment differences. Survival curves were directly compared between groups using the log-rank test. Univariate and multivariate hazard ratios (HR) were generated using Cox proportional hazard models. Statistical analysis was conducted using Graphpad Prism v6.0c and R (CRAN) with the survival analysis package.
RESULTS
Demographics, Tumor and Staging Characteristics
Between 1998 and 2013, 78 SNMM patients without evidence of regional or distant metastasis were identified. Fifty-one percent were female (Table 1). Median age was 68 years (range, 34 to 91 years). Racial distributions are shown in Table 1. Median follow-up time was 21 months (range, 0 to 178 months). In patients alive at last follow-up, median length of follow-up was 41 months (range, 2–178).
TABLE 1.
Patient and tumor characteristics
| All patients | No Radiotherapy | Radiotherapy | |
|---|---|---|---|
| No. of patients (%) | 78 | 14 (18%) | 64 (82%) |
| Gender | |||
| Male | 38 (49%) | 6 (43%) | 32 (50%) |
| Female | 40 (51%) | 8 (57%) | 32 (50%) |
| Age, median (range) | 68 (34–91) | 68 (34–89) | 67 (41–91) |
| Ethnicity | |||
| White | 69 (88%) | 10 (77%) | 59 (92%) |
| Black | 4 (5%) | 1 (8%) | 3 (5%) |
| Hispanic | 2 (3%) | 1 (8%) | 1 (2%) |
| Asian | 2 (3%) | 1 (8%) | 1 (2%) |
| Tumor location | |||
| Sinus | 26 (33%) | 8 (57%) | 44 (64%) |
| Nasal cavity | 52 (67%) | 6 (43%) | 17 (36%) |
| Tumor size, median (range, cm) | 2.5 (0.4–6.8) | 2.65 (1.5–6.0) | 2.5 (0.4–6.8) |
| AJCC stage (all patients N0M0)* | |||
| MMHN T3 | 39 (50%) | 8 (57%) | 31 (50%) |
| MMHN T4a | 29 (37%) | 4 (29%) | 25 (40%) |
| MMHN T4b | 8 (10%) | 2 (14%) | 6 (10%) |
| NCPS T1 | 31 (40%) | 7 (50%) | 24 (39%) |
| NCPS T2 | 10 (13%) | 1 (7%) | 9 (15%) |
| NCPS T3 | 21 (27%) | 2 (14%) | 19 (31%) |
| NCPS T4 | 14 (18%) | 4 (29%) | 10 (16%) |
| Margin Status | |||
| Negative | 30 (38%) | 6 (43%) | 24 (38%) |
| Positive | 24 (31%) | 3 (21%) | 21 (33%) |
| Unknown | 24 (31%) | 5 (36%) | 19 (29%) |
P>0.05 for all comparisons between no radiotherapy and radiotherapy subgroups.
Two patients could not be staged.
Abbreviations: AJCC, American Joint Committee on Cancer; MMHN, mucosal melanoma of the head and neck; NCPS, nasal cavity and paranasal sinuses.
Tumors were located in the nasal mucosa in 52 patients (67%) and in the sinus in 26 patients (33%). All patients underwent surgery. Surgery was performed at MSKCC in 44 patients (56%). Margins were negative in 30 patients (38%), positive in 24 (31%) and unknown in 24 (31%). Median tumor size was 2.5 cm (range, 0.4–6.8 cm).
Stage was determined according to the AJCC system for mucosal melanoma of the head and neck (MMHN) and carcinoma of the nasal cavity and paranasal sinuses (Table 1). Two patients could not be staged based on available information. Overall, 39 (50%) patients had MMHN T3 disease with 29 (37%) and 8 (10%) having T4a and T4b disease. Higher T stage was associated with inferior OS in univariate analysis (HR, 0.53; 95% CI, 0.36–0.81), DSS (0.47, 0.30–0.75), and DRFS (0.43, 0.29–0.64) (Fig. 3). A similar but weaker association was observed for the nasal cavity and paranasal sinuses system (Table 2). Multivariate analysis confirmed the association of MMHN stage with OS, DSS and DRFS independent of age and subsite (Table 3). Tumors were also staged by the Prasad microstaging system with 40 patients (67%) found to have invasion into the lamina propria alone and 20 patients (33%) with invasion into the deep tissue (18 patients could not be staged).7 No association was observed between depth of invasion and OS, DSS, LRFS or DRFS.
Figure 3.
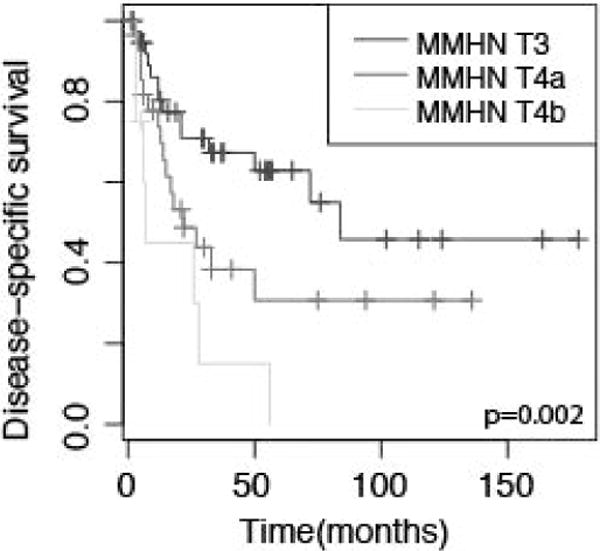
Kaplan-Meier curves depicting disease specific survival for patients stratified by T-stage according to the AJCC mucosal melanoma of the head and neck (MMHN) staging system. p value represents log-rank comparison.
TABLE 2.
Survival Analysis and Prognostic Factors Univariate Analysis
| Overall Survival | Disease-Specific Survival | Local Recurrence-Free Survival | Distant Recurrence-Free Survival | |
|---|---|---|---|---|
| Median (months) | 32 | 50 | 43 | 12 |
| % at 3 years (95% CI) | 48 (37–60) | 51 (40–64) | 57 (44–72) | 38 (26–49) |
| % at 5 years (95% CI) | 31 (18–43) | 40 (27–54) | 36 (19–53) | 28 (16–39) |
| Hazard ratios (univariate analysis) | ||||
| Age >68 years | – | 2.14* | 2.03 | 2.25* |
| Subsite (nasal) | 2.51* | 2.52* | – | – |
| Size >2.5 cm | 0.5 | – | – | 0.35* |
| MMHN T classification | 0.53* | 0.47* | – | 0.43* |
| NCPS T classification | 0.80* | 0.79 | – | 0.80* |
| Margins positive | – | – | – | 0.73 |
| Radiotherapy | – | – | 2.57* | – |
| Chemoradiotherapy | – | – | 0.17* | – |
| Post-radiotherapy PET SUV>4 | 0.42* | 0.43 | 0.34* | 0.50 |
P<0.05 for reported hazard ratios
Hazard ratio >1 indicated improved survival with factor.
Abbreviations: MMHN, mucosal melanoma of the head and neck; NCPS, nasal cavity and paranasal sinuses; PET, positron emission tomography; SUV, standardized uptake value
TABLE 3.
Prognostic Factors Multivariate Analysis
| Overall Survival | Disease-Specific Survival | Local Recurrence-Free Survival | Distant recurrence-Free Survival | |
|---|---|---|---|---|
| Age >68 years | – | – | 5.24* | – |
| Subsite (nasal) | 3.41* | 3.45* | – | – |
| MMHN T classification | 0.43* | 0.43* | – | 0.43* |
| Radiotherapy | – | – | 2.81* | – |
| Post-radiotherapy PET SUV>4 | 0.24* | 0.21* | 0.17* | 0.46 |
P<0.05 for reported hazard ratios
Hazard ratio >1 indicated improved survival with factor.
Abbreviations: MMHN, mucosal melanoma of the head and neck; PET, positron emission tomography; SUV, standardized uptake value
Disease Course and Prognostic Factors
Sixty-one patients (78%) experienced recurrence during follow-up (Fig. 1). Recurrence was first observed at distant sites in 26 patients (33%); 18 (23%) had isolated local recurrence at the surgical site. Ten patients (13%) demonstrated regional lymph node recurrence, of which 4 (5%) were isolated. Distant recurrence was ultimately observed in 48 patients (66%).
Figure 1.
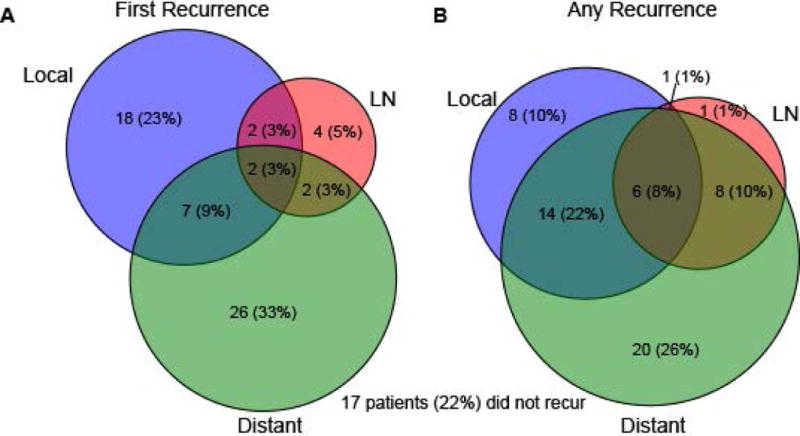
Venn diagrams depicting number and percent of patients with local, lymph node (LN) and distant recurrence at first evidence of recurrence (A) or overall during followup. 17 patients (22%) did not recur during followup.
Kaplan-Meier survival curves were generated for OS, DSS, RFS, local RFS (LRFS), loco-regional RFS, and distant RFS (DRFS) (Fig. 2, data not shown). Median OS was 32 months; median DSS was 50 months (Table 2). Median LRFS and DRFS were 43 and 12 months, respectively. Five-year OS was 31% (95% CI, 18–43) with 40% (95% CI, 27–54) DSS.
Figure 2.
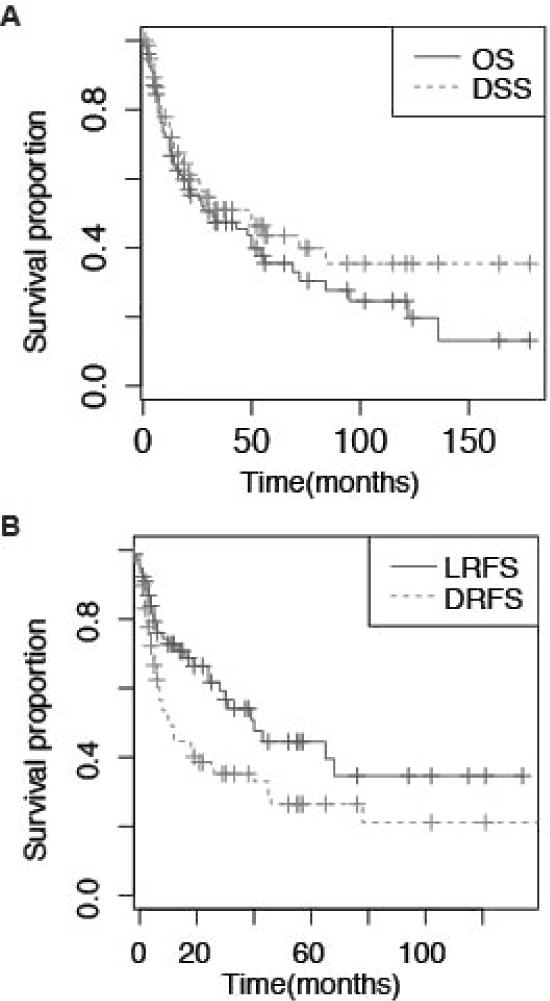
Kaplan-Meier survival curves depicting (A) overall survival (OS) and disease-specific survival (DSS); (B) local recurrence-free survival (LRFS) and distant recurrence-free survival (DRFS) in all patients (n=78). Time is represented in months from landmark of 12 weeks after surgery.
Univariate analysis of potential prognostic factors is shown in Table 2. Tumor in the nasal cavity rather than the paranasal sinuses was associated with greater OS and DSS (HR, 2.51 and 2.52, respectively; 95% CI, 1.4–4.5 and 1.3–4.9). Five-year OS and DSS for nasal cavity tumors were 40% (95% CI, 27–58) and 48% (35–68) vs 9% (2–53) and 16% (35–75) for the sinuses. Age greater than the median age of 68 was associated with greater DSS (HR, 2.14; 95% CI, 1.1–4.1) and DRFS (HR, 2.25; 1.3–4.0). No significant associations were observed for gender, margin status, or complete resection. Increased size was associated with decreased DRFS (HR, 0.35; 95% CI, 0.17–0.72) but not LRFS or OS.
A subset of patients had diagnostic genomic testing for mutations of the KIT, BRAF, NRAS, and GNAQ genes.8 The proportion of patients with mutations were 2/29 (7%), 1/27 (4%), 6/24 (25%), and 0/13 (0%), respectively. Overall, 9/30 (30%) tumors tested harbored a mutation for one gene; no tumors contained mutations in multiple tested genes. No significant association was observed between mutational status and tumor subsite, disease recurrence, or survival.
Radiation Therapy
Of the 78 patients with localized SNMM, 64 (82%) were selected for adjuvant or definitive radiation post-surgery. Adjuvant RT was planned in 53 (68%) patients while 11 (32%) received definitive RT for incompletely resected gross residual disease. Radiation was performed at MSKCC in 37 patients (58%). Radiation targeted nasal cavity and/or paranasal sinuses in most patients, with 5 patients (8%) also receiving neck radiation. Intensity-modulated radiation therapy (IMRT) was used in 45 (70%) patients, with the remaining receiving three-dimensional conformal RT. Median dose was 30 Gy (range, 20–70.8) with a median dose per fraction of 6 Gy (1.2–8). The median number of fractions was 5 (range, 3–59) with 43 (67%) patients receiving hypofractionated RT. Calculated median equivalent dose in 2 Gy fractions was 57 Gy (range, 33–74).
No significant differences in gender, age, ethnicity, tumor location, stage, tumor size, or margin status were observed between patients receiving and not receiving RT (Table 1). LRFS was greater in patients receiving RT vs not (Fig. 4, P=0.01). The 5-year LRFS in patients who did not receive RT was 35% (95% CI, 16–76%) compared with 59% (45–77%) in patients who did. In RT patients, 66% never recurred locally compared to 29% in patients who did not receive RT. However, no difference was observed in OS or DSS. Multivariate analysis including age and stage confirmed the association of increased LRFS in RT patients (Table 3; HR, 2.70; 95% CI, 1.3–6.0). Among RT patients, there was no significant association with hypofractionation, equivalent total dose, or RT technique and local recurrence.
Figure 4.
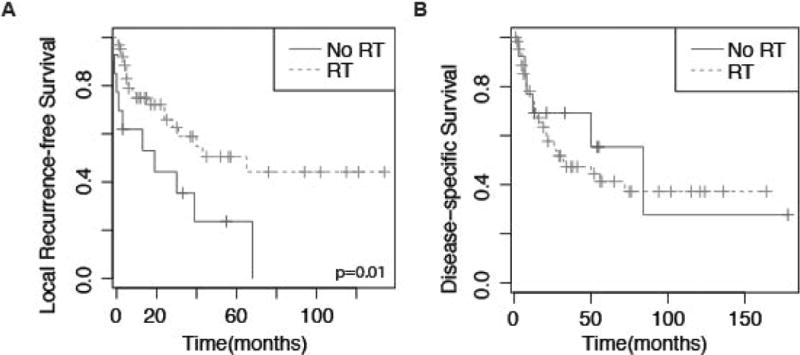
Kaplan Meier curves depicting (A) local recurrence-free survival for patients stratified by whether patient received radiation treatment (RT); (B) disease-specific survival for patients stratified by RT. p value represents log-rank comparison.
Only 6 patients received chemotherapy combined with RT (temozolomide). The addition of chemotherapy was associated with a median LRFS of 5 months compared with 65 months for RT alone. This small subset was not associated with a significant difference in margin status, complete resection, or stage, although tumor size was significantly greater (data not shown). No difference was observed in OS or DSS in patients receiving chemotherapy.
PET Imaging
18FDG-PET scans were performed in a subset of the patients 2–4 weeks before surgery, during radiotherapy planning (4–6 weeks after surgery), and/or 10–12 weeks after the completion of radiation therapy (Table 4). Of 38 patients with preoperative PET studies, 25 (64%) demonstrated significant FDG uptake at the primary tumor (standardized uptake value [SUV]> 4.0). After surgery, 9/20 (45%) exhibited FDG uptake, and 15/41 (37%) demonstrated PET abnormality at the primary tumor after RT. No significant association was observed between preoperative or postoperative PET findings with disease recurrence or survival (n=39 and 20, respectively). However, in patients who had PET performed post-RT (n=41), there was a significant association between longer OS, DSS, and LRFS (Table 2 and Fig. 5) in patients without FDG uptake (SUV<4.0). Median DSS for PET-negative disease was 56 months vs 14 months for PET-positivity after radiation. Multivariate analysis showed significant associations independent of age, stage, and tumor subsite (Table 3).
TABLE 4.
18FDG-PET Imaging in Sinonasal Mucosal Melanoma
| n | SUV>4 (%) | Median SUV | |
|---|---|---|---|
| Initial | 39 | 25 (64%) | 8.05 |
| Postoperative | 20 | 9 (45%) | 4.6 |
| Post-radiotherapy | 41 | 15 (37%) | 5.55 |
Abbreviation: SUV, standardized uptake value.
Figure 5.
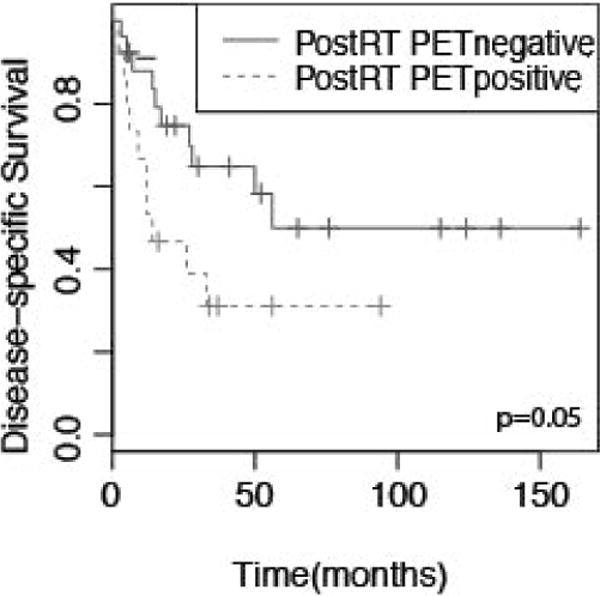
Kaplan-Meier curves depicting disease specific survival for patients stratified by 18FDG-PET uptake (positive indicates SUV>4). p value represents log-rank comparison.
DISCUSSION
SNMM is an uncommon cancer with poor outcomes. Due to its rarity, the literature largely consists of retrospective series that are often small, span numerous decades, and include diverse patient populations.9–12 Most of these reports include patients with oral cavity or pharyngeal mucosal melanoma, which has a distinct disease course.13 Although a majority of SNMM patients present with localized disease initially,14 lymph node and distant metastatic disease are often included in series despite management differences and markedly different clinical behavior. We describe one of the largest single-institutional series of 78 patients with localized SNMM at presentation treated homogenously over a recent 15-year period.5
All patients underwent attempted surgical resection. Of note, we observed no association between pathological margin status post-surgery and either local control or OS. This is particularly important given the anatomical complexity of the sinonasal region and the difficulty of obtaining complete resection, without compromising function, cosmesis, and quality of life. These results contrast with a recent report demonstrating margin status as a prognostic factor for OS, but agree with several other reports showing no significant association.10,15 Difficulty determining margin status in SNMM, given the three-dimensional cavity and the complex resection, may contribute to the variability in outcomes based on margin status.
Localized SNMM was associated with a poor prognosis, with 5-year OS of 31% and DSS of 40%, consistent with previous studies.15–18 Poor survival in SNMM is generally attributed to the high rate of distant metastasis, which developed in 66% of patients in our series. Recurrence in the regional lymph nodes was a rare event, consistent with prior studies.9,19
Patel et al.6 previously reported a series of 59 patients with mucosal melanoma of the head and neck treated at MSKCC between 1978 and 1998, 35 with SNMM. This allows for direct comparison of treatment outcomes at the same large major referral center. Incorporation of more modern surgical, imaging, and treatment might have been expected to improve SNMM outcomes. However, there were no major differences in the patterns of recurrence and 5-year OS and DSS: 35% and 47% vs 31% and 40%, respectively, in our series.
The subsite of mucosal melanoma has been suggested to be prognostic.20 Sinus tumors have been associated with worse local control, thought to be secondary to their tendency to present in a more advanced state as patients remain asymptomatic compared with tumors of the nasal cavity.11,21 We observed a significantly longer OS and DSS for nasal cavity tumors versus sinus tumors independent of tumor stage in multivariate analysis, suggesting an underlying difference with more aggressive course. It may be suggested that resectability of the tumor may differ by location; however, location of the tumor was not associated with improved local control, but only with overall and DSS.
Until recently, SNMM was staged using the Ballantyne system for melanoma.22 However, a large proportion of SNMM patients, including all in this study, present with localized disease initially, making this system of little prognostic value. The AJCC staging system for nasal cavity and paranasal sinus tumors excluded mucosal melanomas.12,15,23 In the seventh edition of the AJCC staging system (2009), MMHN classification was established.4 In our study, prognostic value in OS was observed for both AJCC staging systems, but the MMHN system appeared to be more significant and offered a better estimation of OS and DSS. These results agree with several retrospective studies2,16,18,24 and support the use of the AJCC MMHN staging system.
Analysis of pathological features has also been suggested to improve prognostication with some association of histological features and more aggressive disease course. Prasad et al. proposed a staging system for SNMM based on the degree of invasion into the tissue after analysis demonstrated no significant association on multivariate analysis with other pathological features.7,25 We observed no association between clinical course with the Prasad microstage on univariate or multivariate analysis, suggesting that histologic characteristics may be less important for prognosis than overall T stage.
Proportions of mutations in KIT, BRAF, and NRAS genes were similar to those previously described in 56 patients, with NRAS most prevalent.8,26 There was no significant difference in the frequency of mutations in sinus and nasal cavity tumors. While mutational status did not confer prognostic value in our study, perhaps the value of mutational analysis likely resides in future attempts at therapy targeted at these pathways.27
The role of RT has remained controversial for SNMM. Eighty-two percent (64/78) of patients with localized SNMM at MSKCC received adjuvant or definitive RT after surgery, consistent with common practice based on retrospective series.3,28 Although limited numbers of patients did not receive adjuvant RT, our results agree with previous reports demonstrating an association of local control with adjuvant radiotherapy, but no clear benefit in OS or DSS.6,15,17,24,29–33 RT was associated with a lower rate of local recurrence, but, presumably as a consequence, more patients presented with distant metastases at first recurrence. Selection bias for more aggressive disease may be present among patients chosen for RT, although no associations were observed with factors analyzed. There was no clear treatment strategy followed for which patients received RT and thus a selection bias may still be present. If a bias was present this would presumably make the observed local control difference more pronounced but may have resulted in obscuring an OS difference. Conclusive data is therefore lacking for whether treatment with postoperative RT is indicated in patients with localized SNMM. While no benefit in survival has been shown in any retrospective series, a substantial improvement of local control and potential mitigation of the morbidity of local recurrence was demonstrated in our study and several other retrospective series.17,30
Hypofractionation of RT has been suggested for head and neck mucosal melanoma due to the ability of melanoma cells to repair sublethal damage34,35 Improved local control has been observed in some small studies33,36 while others failed to show benefit.37 Although a significant proportion of patients received hypofractionated RT, we observed no association with local control or OS, consistent with the prospective randomized RTOG trial of cutaneous melanoma that demonstrated no benefit on response rates.38 Although we observed no significant association between dose and local control in our analysis, dose escalation has been suggested to improve local control. In addition, the use of charged particle therapy for nasal and paranasal tumors has been suggested and a recent large meta-analysis demonstrated improved overall survival and local control at longest followup39. Yanagi et al. reported on 72 patients with MMHN treated on 3 prospective studies delivering 52.8 – 63 GyE in 16 fractions with 5 year local control of 84.1% and overall survival of 27%.40 In another study, Demizu et al. compared carbon ion and proton therapy (65–70.2 GyE in 26 fractions) in a retrospective analysis of 62 patients with MMHN and demonstrated 59% and 71% 2 year local control, respectively.41 The local control rates observed in these studies suggest that there may be some benefit in higher dose particle radiotherapy.
Very few patients in our series received adjuvant chemotherapy and radiation, consistent with current practice during this time.5 Lian et al. reported a prospective randomized trial of systemic adjuvant high-dose interferon-α2b or temozolomide plus cisplatin compared with observation in 189 patients with localized mucosal melanoma.42 The study included patients with mucosal melanoma of any site, with 43–48% with MMHN and an unknown percentage of SNMM. None of the patients received adjuvant radiation. A benefit in OS and RFS was observed in patients receiving adjuvant chemotherapy compared with observation or high-dose interferon-α2b (median OS, 48.7%, 21.2%, and 40.4%, respectively). Given the small number of patients selected for chemotherapy in our series, conclusions regarding effect are difficult to make.
The role of PET imaging in SNMM has not been clearly established.43 PET scans are used in the staging and follow-up of cutaneous melanoma, with increased sensitivity and specificity over standard imaging.44 Haerle et al reported 10 patients with SNMM who received PET-CT imaging at staging and follow-ups.45 When compared with concurrent CT and MRI imaging, all primary tumors were seen and all regional and distant metastasis were identified except for one cerebral metastasis. Significant FDG uptake was seen initially in 6/10 patients (SUV>4), similar to the proportion in our study. However, due to the limited number of patients, prognostic value of PET avidity could not be assessed.
Inubushi et al reported on 13 patients with primary or recurrent MMHN treated with carbon ion RT in Japan and followed with PET imaging before and after RT.46 A significant association was demonstrated between PET avidity before radiation and OS and distant metastasis, but not with post-RT response. This is in contrast to our results demonstrating a prognostic value to only post-radiation PET avidity both on local control and OS. Our study represents a larger cohort of more uniform patients (n=41) being treated in the adjuvant setting after surgery with traditional photon-based radiotherapy. The prognostic value of PET imaging is consistent with the literature for other mucosal head and neck cancers.47,48 Decreased metabolic activity has been associated with a completed pathologic response in some cases of cutaneous melanoma.49 The striking difference observed in survival in patients with a complete PET response after RT suggests that it may be an important prognostic indicator of potential for durable response in SNMM. Further investigation into PET imaging in SNMM is warranted in a more systematic and prospective approach.
Given the retrospective nature of this study, there are clear limitations including referral bias, variations in treatment modalities, and incomplete data. Selection bias may play a significant role, as treatment strategies are likely reflective of disease characteristics. However, prospective studies in such a rare disease entity are unlikely to be feasible and thus this large cohort can inform clinicians to the benefits and limitations of adjuvant radiation therapy in these patients.
CONCLUSIONS
Our study represents one of the largest single-institutional retrospective series of localized SNMM at a large referral center incorporating molecular pathology and imaging. SNMM is associated with a poor prognosis despite aggressive treatment, with high rates of distant metastasis. Prognostic factors associated with improved OS and DSS included nasal tumors and lower stage. Postoperative RT was associated with improved local control but no benefit in OS or DSS. FDG-PET avidity after RT was associated with decreased LRFS, OS, and DSS. With no significant improvement in disease course despite developments in surgical and radiation technology, prospective clinical trials of systemic therapy seem warranted.
Acknowledgments
Funding sources and conflicts of interest: None.
References
- 1.McLaughlin CC, Wu X-C, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the US. Cancer. 2005;103(5):1000–1007. doi: 10.1002/cncr.20866. [DOI] [PubMed] [Google Scholar]
- 2.Koivunen P, Bäck L, Pukkila M, et al. Accuracy of the current TNM classification in predicting survival in patients with sinonasal mucosal melanoma. Laryngoscope. 2012;122(8):1734–1738. doi: 10.1002/lary.23343. [DOI] [PubMed] [Google Scholar]
- 3.Krengli M, Jereczek-Fossa BA, Kaanders JHAM, Masini L, Beldì D, Orecchia R. What is the role of radiotherapy in the treatment of mucosal melanoma of the head and neck? Crit Rev Oncol Hematol. 2008;65(2):121–128. doi: 10.1016/j.critrevonc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB, Cancer AJCO . AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 5.Lazarev S, Gupta V, Hu K, Harrison LB, Bakst R. Mucosal Melanoma of the Head and Neck: A Systematic Review of the Literature. Int J Radiat Oncol Biol Phys. 2014;90(5):1108–1118. doi: 10.1016/j.ijrobp.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 6.Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24(3):247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 7.Prasad ML, Patel SG, Huvos AG, Shah JP, Busam KJ. Primary mucosal melanoma of the head and neck: a proposal for microstaging localized, Stage I (lymph node-negative) tumors. Cancer. 2004;100(8):1657–1664. doi: 10.1002/cncr.20201. [DOI] [PubMed] [Google Scholar]
- 8.Zebary A, Jangard M, Omholt K, Ragnarsson-Olding B, Hansson J. KIT, NRAS and BRAF mutations in sinonasal mucosal melanoma: a study of 56 cases. Br J Cancer. 2013;109(3):559–564. doi: 10.1038/bjc.2013.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manolidis S, Donald PJ. Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer. 1997;80(8):1373–1386. doi: 10.1002/(sici)1097-0142(19971015)80:8<1373::aid-cncr3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Bachar G, Loh KS, O’Sullivan B, et al. Mucosal melanomas of the head and neck: experience of the Princess Margaret Hospital. Head Neck. 2008;30(10):1325–1331. doi: 10.1002/hed.20878. [DOI] [PubMed] [Google Scholar]
- 11.Dauer EH, Lewis JE, Rohlinger AL, Weaver AL, Olsen KD. Sinonasal melanoma: a clinicopathologic review of 61 cases. Otolaryngol Head Neck Surg. 2008;138(3):347–352. doi: 10.1016/j.otohns.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Loree TR, Mullins AP, Spellman J, North JH, Hicks WL. Head and neck mucosal melanoma: a 32-year review. Ear Nose Throat J. 1999;78(5):372–375. [PubMed] [Google Scholar]
- 13.Penel N, Mallet Y, Mirabel X, Van JT, Lefebvre J-L. Primary mucosal melanoma of head and neck: prognostic value of clear margins. Laryngoscope. 2006;116(6):993–995. doi: 10.1097/01.mlg.0000217236.06585.a9. [DOI] [PubMed] [Google Scholar]
- 14.Rinaldo A, Shaha AR, Patel SG, Ferlito A. Primary mucosal melanoma of the nasal cavity and paranasal sinuses. Acta Otolaryngol. 2001;121(8):979–982. [PubMed] [Google Scholar]
- 15.Moreno MA, Roberts DB, Kupferman ME, et al. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson Cancer Center. Cancer. 2010;116(9):2215–2223. doi: 10.1002/cncr.24976. [DOI] [PubMed] [Google Scholar]
- 16.Gal TJ, Silver N, Huang B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope. 2011;121(9):2026–2033. doi: 10.1002/lary.21925. [DOI] [PubMed] [Google Scholar]
- 17.Krengli M, Masini L, Kaanders JHAM, et al. Radiotherapy in the treatment of mucosal melanoma of the upper aerodigestive tract: analysis of 74 cases. A Rare Cancer Network study. Int J Radiat Oncol Biol Phys. 2006;65(3):751–759. doi: 10.1016/j.ijrobp.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Shuman AG, Light E, Olsen SH, et al. Mucosal melanoma of the head and neck: predictors of prognosis. Arch Otolaryngol Head Neck Surg. 2011;137(4):331–337. doi: 10.1001/archoto.2011.46. [DOI] [PubMed] [Google Scholar]
- 19.Lund VJ, Howard DJ, Harding L, Wei WI. Management options and survival in malignant melanoma of the sinonasal mucosa. Laryngoscope. 1999;109(2 Pt 1):208–211. doi: 10.1097/00005537-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Freedman HM, DeSanto LW, Devine KD, Weiland LH. Malignant melanoma of the nasal cavity and paranasal sinuses. Arch Otolaryngol. 1973;97(4):322–325. doi: 10.1001/archotol.1973.00780010332008. [DOI] [PubMed] [Google Scholar]
- 21.Kingdom TT, Kaplan MJ. Mucosal melanoma of the nasal cavity and paranasal sinuses. Head Neck. 1995;17(3):184–189. doi: 10.1002/hed.2880170303. [DOI] [PubMed] [Google Scholar]
- 22.Ballantyne AJ. Malignant melanoma of the skin of the head and neck. An analysis of 405 cases. Am J Surg. 1970;120(4):425–431. doi: 10.1016/s0002-9610(70)80001-0. [DOI] [PubMed] [Google Scholar]
- 23.Greene FL, Cancer AJCO, Society AC . AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002. [Google Scholar]
- 24.Vandenhende C, Leroy X, Chevalier D, Mortuaire G. Sinonasal mucosal melanoma: retrospective survival study of 25 patients. J Laryngol Otol. 2012;126(2):147–151. doi: 10.1017/S0022215111002519. [DOI] [PubMed] [Google Scholar]
- 25.Prasad ML, Patel S, Hoshaw-Woodard S, et al. Prognostic factors for malignant melanoma of the squamous mucosa of the head and neck. Am J Surg Pathol. 2002;26(7):883–892. doi: 10.1097/00000478-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Turri-Zanoni M, Medicina D, Lombardi D, et al. Sinonasal mucosal melanoma: Molecular profile and therapeutic implications from a series of 32 cases. Head Neck. 2013;35(8):1066–1077. doi: 10.1002/hed.23079. [DOI] [PubMed] [Google Scholar]
- 27.Rapisuwon S, Parks K, Al-Refaie W, Atkins MB. Novel somatic KIT exon 8 mutation with dramatic response to imatinib in a patient with mucosal melanoma: a case report. Melanoma Res. 2014;24(5):509–511. doi: 10.1097/CMR.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 28.Wu AJ, Gomez J, Zhung JE, et al. Radiotherapy after surgical resection for head and neck mucosal melanoma. Am J Clin Oncol. 2010;33(3):281–285. doi: 10.1097/COC.0b013e3181a879f5. [DOI] [PubMed] [Google Scholar]
- 29.Temam S, Mamelle G, Marandas P, et al. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer. 2005;103(2):313–319. doi: 10.1002/cncr.20775. [DOI] [PubMed] [Google Scholar]
- 30.Benlyazid A, Thariat J, Temam S, et al. Postoperative radiotherapy in head and neck mucosal melanoma: a GETTEC study. Arch Otolaryngol Head Neck Surg. 2010;136(12):1219–1225. doi: 10.1001/archoto.2010.217. [DOI] [PubMed] [Google Scholar]
- 31.Owens JM, Roberts DB, Myers JN. The role of postoperative adjuvant radiation therapy in the treatment of mucosal melanomas of the head and neck region. Arch Otolaryngol Head Neck Surg. 2003;129(8):864–868. doi: 10.1001/archotol.129.8.864. [DOI] [PubMed] [Google Scholar]
- 32.Nandapalan V, Roland NJ, Helliwell TR, Williams EM, Hamilton JW, Jones AS. Mucosal melanoma of the head and neck. Clin Otolaryngol Allied Sci. 1998;23(2):107–116. doi: 10.1046/j.1365-2273.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- 33.Wada H, Nemoto K, Ogawa Y, et al. A multi-institutional retrospective analysis of external radiotherapy for mucosal melanoma of the head and neck in Northern Japan. Int J Radiat Oncol Biol Phys. 2004;59(2):495–500. doi: 10.1016/j.ijrobp.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Dewey DL. The radiosensitivity of melanoma cells in culture. Br J Radiol. 1971;44(526):816–817. doi: 10.1259/0007-1285-44-526-816. [DOI] [PubMed] [Google Scholar]
- 35.Bentzen SM, Overgaard J, Thames HD, et al. Clinical radiobiology of malignant melanoma. Radiother Oncol. 1989;16(3):169–182. doi: 10.1016/0167-8140(89)90017-0. [DOI] [PubMed] [Google Scholar]
- 36.Harwood AR, Dancuart F, Fitzpatrick PJ, Brown T. Radiotherapy in nonlentiginous melanoma of the head and neck. Cancer. 1981;48(12):2599–2605. doi: 10.1002/1097-0142(19811215)48:12<2599::aid-cncr2820481211>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Thariat J, Poissonnet G, Marcy P-Y, et al. Effect of surgical modality and hypofractionated split-course radiotherapy on local control and survival from sinonasal mucosal melanoma. Clin Oncol (R Coll Radiol) 2011;23(9):579–586. doi: 10.1016/j.clon.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Sause WT, Cooper JS, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. 1991;20(3):429–432. doi: 10.1016/0360-3016(91)90053-7. [DOI] [PubMed] [Google Scholar]
- 39.Patel SH, Wang Z, Wong WW, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15(9):1027–1038. doi: 10.1016/S1470-2045(14)70268-2. [DOI] [PubMed] [Google Scholar]
- 40.Yanagi T, Mizoe J-E, Hasegawa A, et al. Mucosal malignant melanoma of the head and neck treated by carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74(1):15–20. doi: 10.1016/j.ijrobp.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 41.Demizu Y, Fujii O, Terashima K, et al. Particle therapy for mucosal melanoma of the head and neck. A single-institution retrospective comparison of proton and carbon ion therapy. Strahlenther Onkol. 2014;190(2):186–191. doi: 10.1007/s00066-013-0489-9. [DOI] [PubMed] [Google Scholar]
- 42.Lian B, Si L, Cui C, et al. Phase II randomized trial comparing high-dose IFN-α2b with temozolomide plus cisplatin as systemic adjuvant therapy for resected mucosal melanoma. Clin Cancer Res. 2013;19(16):4488–4498. doi: 10.1158/1078-0432.CCR-13-0739. [DOI] [PubMed] [Google Scholar]
- 43.Murphy G, Hussey D, Metser U. Non-cutaneous melanoma: is there a role for (18)F-FDG PET-CT? Br J Radiol. 2014;87(1040):20140324. doi: 10.1259/bjr.20140324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reinhardt MJ, Joe AY, Jaeger U, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24(7):1178–1187. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 45.Haerle SK, Soyka MB, Fischer DR, et al. The value of 18F-FDG-PET/CT imaging for sinonasal malignant melanoma. Eur Arch Otorhinolaryngol. 2012;269(1):127–133. doi: 10.1007/s00405-011-1664-1. [DOI] [PubMed] [Google Scholar]
- 46.Inubushi M, Saga T, Koizumi M, et al. Predictive value of 3“-deoxy-3-”[18F]fluorothymidine positron emission tomography/computed tomography for outcome of carbon ion radiotherapy in patients with head and neck mucosal malignant melanoma. Ann Nucl Med. 2013;27(1):1–10. doi: 10.1007/s12149-012-0652-x. [DOI] [PubMed] [Google Scholar]
- 47.Paidpally V, Chirindel A, Lam S, Agrawal N, Quon H, Subramaniam RM. FDG-PET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med. 2012;4(6):633–647. doi: 10.2217/iim.12.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheikhbahaei S, Marcus C, Subramaniam RM. 18F FDG PET/CT and Head and Neck Cancer: Patient Management and Outcomes. PET Clin. 2015;10(2):125–145. doi: 10.1016/j.cpet.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Foote M, Burmeister B, Dwyer P, et al. An innovative approach for locally advanced stage III cutaneous melanoma: radiotherapy, followed by nodal dissection. Melanoma Res. 2012;22(3):257–262. doi: 10.1097/CMR.0b013e3283531335. [DOI] [PubMed] [Google Scholar]


