Summary
Background
Structured routines aimed at eating and sleep have been successfully employed in weight loss interventions for children. Although such routines are discussed in lifestyle modification programmes for adults, they are not a primary focus.
Purpose
The purpose of this study is to determine if establishing healthy eating and sleep routines may improve outcomes in a behavioural weight loss (BWL) intervention.
Methods
Twenty‐five overweight/obese participants (age = 52.4 ± 9.8; body mass index = 33.5 ± 4.1) were randomly assigned to either a 4‐week routine‐based intervention (ROU) targeting regular eating and sleep or an education control before beginning an 18‐week BWL intervention.
Results
Routine‐based intervention participants reported adhering to eating routines, with increased ‘on‐schedule’ eating (p = 0.007) and decreased ‘off‐schedule’ eating (p = 0.002) but showed no change in ‘on‐schedule’ sleep (p = 0.74). However, contrary to our hypothesis, ROU participants lost less weight than controls after 6 weeks of BWL (2.3 ± 2.5 vs. 4.6 ± 2.6 kg, p = 0.04) and achieved only modest weight loss over the full 18 weeks (ROU: 3.2 ± 4.6 vs. education control: 5.8 ± 5.7 kg, p = 0.23).
Conclusions
Focusing initially on establishing healthy sleep and eating routines led to poorer, rather than better, subsequent weight loss outcomes. Further studies using a longer initial intervention period or focusing on only sleep or eating behaviour are needed to determine whether establishing routines for eating and sleep behaviours can enhance weight loss in adults.
Keywords: Behavioural weight loss intervention, multiple behaviour change, obesity, weight loss
Introduction
Establishing and adhering to a healthy routine is beneficial for many health‐related behaviours 1, 2, 3. Routines may serve to help make behaviours such as healthy eating, sleep and activity habitual and part of one's lifestyle and may assist in regulating biological rhythms. Regulation of biological rhythms is central to healthy functioning and may be necessary to avoid potential negative outcomes such as obesity 4. Routines may be particularly beneficial in regulation of circadian (i.e. daily) rhythms such as sleep–wake and feeding cycles that are thought to play a role in obesity 5. Thus, routines are a promising strategy for obesity treatment.
Paediatric studies demonstrate the potential utility of routine‐based approaches related to the circadian cycle for weight regulation, including planning family meal times, working on sleep schedules and achieving adequate sleep and limiting daily screen time 6, 7. In these studies, routines have been shown to be beneficial for weight outcomes 8, 9, 10. Given that eating regularly and following a consistent sleep routine are also associated with body weight in adults 11, 12, 13, addressing routines in adults may similarly promote weight loss; however, this has not previously been tested.
Eating regularly (i.e. following a consistent pattern of meal/snack times), and following a consistent sleep schedule (i.e. consistent bedtimes and wake times that promote adequate sleep), may contribute to weight control by helping to regulate circadian rhythms as well as through psychological and physiological mechanisms. For example, research suggests that skipping meals or reduced meal frequency (less than three meals per day) negatively affects appetite control 14. Perceived hunger and desire to eat have been shown to increase, while perceived satiety decreases when individuals wait to consume all calories in one dinner meal rather than eating at regularly spaced meals throughout the day, even when the same total calories are consumed 15. Therefore, by scheduling eating episodes at set intervals throughout the day, obese individuals may be able to better regulate hunger and appetite. Moreover, research with animals suggests that keeping a regular feeding schedule may aid metabolism and contribute to weight control 16, 17. In one particular study, even when keeping caloric intake equal between two groups, those animals who ate on an irregular schedule gained weight compared with those on a more regimented routine 17. In humans, irregular meal frequency has been shown to disturb energy metabolism in lean 18, 19 and obese women 20 and can disrupt glucose regulation 21. Thus, establishing a routine in which obese participants eat at pre‐specified times of day may reduce the negative appetite consequences and suboptimal metabolism that can occur with meal skipping or irregular meal frequency and consequently enhance outcomes in a behavioural weight loss (BWL) programme.
Similarly, consistent sleep schedules that promote adequate sleep may also enhance weight loss outcomes. Short sleep is associated with increased obesity risk 22, 23, 24, 25, 26. This association may be mediated by the effect of sleep duration on the regulation of hunger‐signalling and satiety‐signalling hormones ghrelin and leptin 27, 28 and/or through glucose regulation 29. Recent reports in children and older adults suggest that variability in sleep schedule is also associated with obesity, independent of total sleep duration 30, 31, 32. Moreover, short sleep is related to reductions in cognitive function and declines in mood 33, 34, which may both contribute to difficulties in carrying out necessary weight management behaviours. Although several studies have suggested this connection between short sleep and obesity, studies investigating the effects of intervening on sleep behaviours to enhance weight loss in adults are needed. To date, one pilot study in overweight/obese adults has examined how an intervention focused on sleep and weight behaviours compares with a standard BWL intervention in a primary care setting 35. Findings suggest a potential benefit to addressing sleep behaviours as participants in the joint sleep and weight intervention lost weight at a faster rate than those in the control group 35.
Given the potential for a consistent healthy sleep and eating schedule to aid in weight control efforts, we examined whether establishing a regular sleep and eating schedule prior to beginning a standard BWL intervention would improve weight loss outcomes. In this pilot randomized controlled trial, participants were randomly assigned to either a novel 4‐week routine‐based intervention (ROU) to work towards (i) eating at regular intervals throughout the day and (ii) following a consistent sleep schedule to achieve 8 h of sleep each night or to a 4‐week education control (EDU) group. The ROU and EDU interventions were limited to 4 weeks duration to allow participants to quickly begin the 18‐week BWL programme. We hypothesized that participants assigned to ROU would achieve better weight losses in the subsequent weight loss programme than those in EDU.
Methods
Participants
A total of 25 individuals participated in this pilot study (Figure 1). To be eligible for the study, participants had to be 21–65 years, have a body mass index (BMI) within 25–45 kg/m2, be weight stable (within 5% of their current reported body weight over the past 6 months), not currently on any weight loss medications or enrolled in other weight loss programmes and report no history of eating disorders, schizophrenia, bipolar disorder or substance abuse. Given the focus on sleep in the routine intervention, those who self‐reported sleeping more than 7 h per night based on typical bedtimes and wake times across the week or those who were shift workers were also excluded. All assessments and treatment sessions were conducted at the Weight Control and Diabetes Research Center in Providence, RI. All participants provided informed consent in accordance with The Miriam Hospital Internal Review Board. This study was registered at clinicaltrials.gov (NCT01428687).
Figure 1.
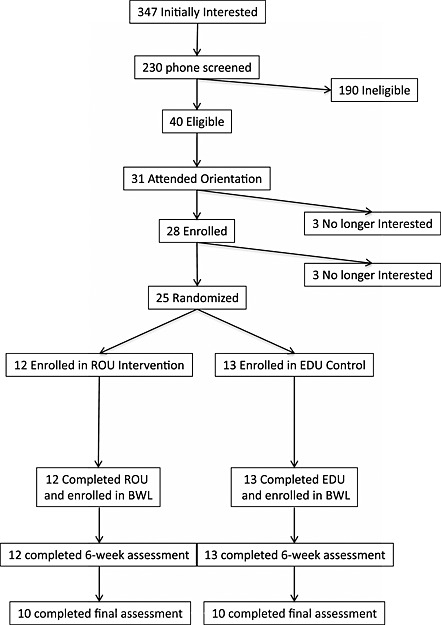
Consort diagram depicting the flow of participants in this randomized controlled study. Common reasons for ineligibility included self‐reported sleep >7 h, inability to attend group sessions, and medication usage.
Study design
Subjects completed baseline assessments (described later) and were then randomly assigned within gender to the routine intervention (ROU; N = 12) or an education only control (EDU; N = 13). Both groups attended weekly 1‐h group sessions for 4 weeks that provided the ROU or EDU content (described later). They were asked not to lose weight during this time. Following the 4‐week ROU intervention or EDU, assessments were repeated. All subjects then went on to participate in an 18‐week BWL intervention with six weekly meetings, followed by six biweekly meetings. After the first 6 weeks of BWL, participants completed an assessment that included weight measurements only. Final weight assessments were conducted after week 18 of BWL (Figure 2). Participants were paid $25 for completing the assessments at the end of the 4‐week intervention and the end of the 18‐week BWL programme (total = $50).
Figure 2.
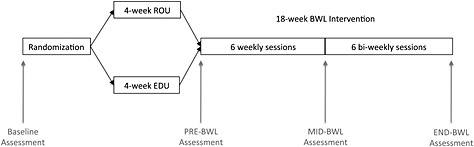
Study design: following baseline assessments participants were randomized to either the 4‐week ROU intervention or 4 weeks of the EDU control group sessions. They were assessed at the end of the 4 weeks, and then all participants began an 18‐week BWL intervention that met weekly for six sessions (with assessments after week 6), and then biweekly for six sessions (with assessments at the end of the 18‐week programme).
Clinical intervention
ROU (weeks 1–4)
Participants in the ROU group practiced establishing and following routines around two specific behaviours associated with weight regulation – namely, only eating at five pre‐specified times each day (three meals and two snacks) and following a consistent bedtime and wake time schedule to achieve 8 h of sleep per night. Beginning at week 1 of the intervention, the ROU group worked with interventionists to develop a schedule for eating episodes such that they never went for more than four waking hours without eating. During group sessions, ROU participants scheduled their eating episodes for each day of the following week in food diaries. A consistent sleep schedule was developed for each participant beginning at week 2 of the intervention. Given constraints of weekday schedules (i.e. needing to wake at a certain time for work), typical weekday wake times were maintained, and bedtimes were adjusted to promote 8 h time in bed each night. Further, given normal variability in weekday and weekend sleep schedules, participants were allowed to delay bedtimes and wake times by up to 1 hour on weekends as long as the 8‐h time in bed schedule was maintained. Participants planned ahead when they would go to bed and recorded their actual bedtime and wake time in their diary. Self‐regulation skills (goal setting, self‐monitoring, self‐evaluation and self‐reinforcement) were taught to help participants increase these two behaviours and inhibit incompatible behaviours (e.g. unplanned eating episodes or staying up late). The ROU group was instructed to continue adhering to these routines throughout the 18‐week BWL programme.
Education control (weeks 1–4)
Participants in the EDU group received educational sessions on the prevalence and health consequences of obesity, the importance of fruit and vegetable intake, and common myths about diet and exercise. No behaviour modification strategies were taught or promoted during the 4‐week period in the EDU group.
Behavioural weight loss
After the initial 4‐week programme, all participants received an 18‐week (12‐session) group BWL intervention that involved 6 weeks of weekly meetings followed by 12 weeks of biweekly meetings incorporating diet, exercise and behavioural therapy. All participants were placed on a standard caloric and fat restricted diet (e.g. 1,200–1,500 kcal day depending on initial weight, with ≤30% calories from fat) and were encouraged to increase their physical activity gradually to at least 200 min week (using activities similar in intensity to brisk walking and bouts of at least 10 min). They received a fat/calorie guidebook and a diary to record all the food they consumed and their physical activity. Clinicians continually reviewed participant diaries in order to provide patients with written feedback about calorie and fat goals and their eating and exercise behaviour. Prior to each group session, each participant was weighed privately with an interventionist. In addition to information on nutrition and physical activity, specific behavioural strategies addressed during the programme included self‐monitoring, goal setting, stimulus control and problem solving. During the BWL programme, participants in the ROU arm also received regular reminders to keep to their consistent 8‐h per night sleep schedule and to eat regularly according to their pre‐specified schedule.
Measures
ROU intervention adherence
To verify adherence to the targeted routines, self‐reported times for eating and sleeping from participant diaries were used to assess ‘on‐schedule’ and ‘off‐schedule’ episodes for each behaviour. ‘On‐schedule’ eating was defined as consuming a meal or snack within 15 min of the planned time, and eating at all other time points was thus considered ‘off schedule’. Similarly, self‐reported bedtimes and wake times within 15 min of each participant's planned schedule were considered ‘on schedule’ and any deviation beyond that was deemed ‘off schedule’. Adherence to the sleep schedule was also assessed objectively and via self‐report by determining changes in sleep duration over the initial four‐week period. For a one‐week period at baseline and the end of the 4‐week ROU or EDU intervention, participants wore a BodySense Armband (BodyMedia, Inc., Pittsburgh, PA), and kept a sleep diary (i.e., they self‐reported their sleep duration for that same time period). At the end of the week, the armband data were downloaded and reviewed for completeness with the corresponding self‐report diary. Sleep duration was calculated based on BodyMedia Inc. software.
Outcomes assessments
All participants completed assessments at baseline, after the initial 4 weeks of ROU or EDU intervention, after the first 6 weeks (intensive phase) of the BWL programme, and at the conclusion of the 18‐week BWL treatment. These assessments were conducted by staff members blinded to the participants' treatment condition. At these assessments, weight was measured with Tanita digital scales (TANITA Corporation of America, Inc., Arlington Heights, IL) with the participant in street clothes, without shoes. Height, used for determining BMI, was measured with a wall‐mounted stadiometer.
Statistical analysis
To assess adherence within the ROU intervention, the number of on‐schedule and off‐schedule eating episodes and on‐schedule sleep times were compared from the initial week of the target behaviour change (week 1 for eating and week 2 for sleep) to the final week of ROU (week 4). In addition, the ROU and EDU groups were compared on changes in weight, and objective and self‐reported sleep assessed at baseline and the end of the 4‐week intervention. To assess weight loss during the BWL intervention, we compared changes in weight for ROU and EDU from the start of the weight loss intervention to both the end of the 6‐week intensive phase and the end of the full 18‐week BWL programme. The weight at the start of BWL was carried forward as the final assessment weight for non‐completers.
Descriptive statistics were generated for all variables, including means and standard deviations for continuous variables and percentages for categorical variables. Independent T‐tests were conducted to investigate potential differences in each outcome measure between the ROU and EDU arms, and paired samples T‐tests were conducted to assess change within group.
Results
Baseline characteristics
At baseline, the two groups did not differ on age (t(23) = 1.1, p = 0.31), gender (X 2 = 0.1, p = 0.75), baseline BMI (t(23) = 0.72, p = 0.48), self‐reported sleep (t(23) = 0.26, p = 0.80) or objectively measured sleep (t(23) = 1.58, p = 0.13) (Table 1). There was a non‐significant trend for a group difference in race/ethnicity (X 2 = 3.15, p = 0.08).
Table 1.
Baseline characteristics (mean ± standard deviation)
| Education control | Routine intervention | T‐value | p‐value | |
|---|---|---|---|---|
| N | 13 | 12 | ||
| Gender | 69% female | 75% female | 0.1 (X 2) | 0.75 |
| Age | 50.4 (±11.4) | 54.5 (±7.6) | 0.31 | 0.76 |
| Race/ethnicity | 77% White | 100% White | 3.15 (X 2) | 0.08 |
| Baseline body mass index | 34.0 (±3.7) | 32.8 (±4.6) | 0.72 | 0.48 |
| Baseline sleep hours (armband) | 6.7 (±1.0) | 6.8 (±0.7) | 1.58 | 0.13 |
Adherence and outcomes during initial 4‐week intervention
All participants in both arms of the study attended 100% of the treatment sessions during weeks 1–4. Weight changes in the two groups during the first 4 weeks of either ROU or EDU were minimal and did not differ from each other (percent weight change ROU: +0.12 ± 2.7% [+0.2 ± 1.4 kg]; EDU: +1.1 ± 1.3% [+1.1 ± 2.4 kg]; p = 0.28). The percentage of reported ‘on‐schedule’ eating episodes increased significantly from week 1 (when the individual's schedule of eating episodes was developed) to week 4 (mean week 1 = 59.3% and mean week 4 = 79.6%; t(11) = 4.02, p = 0.002). Similarly, the mean number of reported ‘off‐schedule’ eating episodes decreased over time (mean week 1 = 13.3 ± 5.9 and mean week 4 = 7.2 ± 5.9; t(11) = 3.31, p = 0.007). However, the percentage of reported ‘on‐schedule’ bed times did not significantly change from week 2 (when the individual's sleep schedule was developed) to week 4 (mean week 2 = 65.3% and week 4 = 60.6%; p = 0.74). In terms of sleep duration, objectively measured sleep time did not change significantly over the 4 weeks in either ROU (6.8 ± 0.5 h per night at baseline to 7.0 ± 0.7 h per night; t(11) = 0.99, p = 0.35) or EDU (mean = 6.3 ± 1.0 at baseline to 6.3 ± 0.9 h per night, p = 0.98). By contrast, self‐reported sleep duration increased from 7.02 ± 1.27 h at baseline to 7.63 ± 0.78 in the ROU group (p = 0.04), but there was no change in self‐reported sleep duration for the EDU group (p = 0.79).
Weight change during behavioural weight loss intervention
As shown in Figure 3, the ROU group lost significantly less weight than the EDU group during the first 6 weeks of the BWL programme (percentage change = −2.5 ± 2.5% [−2.3 ± 2.5 kg] in ROU vs. −4.8 ± 2.6% [−4.6 ± 2.6 kg] in EDU; t(23) = 2.3, p = 0.029). There were no significant differences between groups over the full 18‐week programme (percent change ROU = −3.4 ± 4.7% [−3.2 ± 4.6 kg], EDU = −6.4 ± 6.0% [−5.8 ± 5.7 kg]; t(23) = 1.4, p = 0.18).
Figure 3.
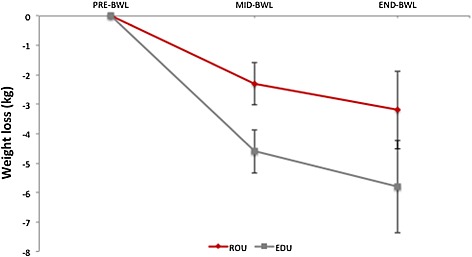
Mean weight loss (kg) in ROU (red) and EDU (grey) groups during the behavioural weight loss intervention. Error bars represent standard error of the mean.
Discussion
The hypothesis that working on establishing healthy eating and sleep routines prior to the start of a BWL programme would improve weight loss was not supported. In fact, in this pilot study, those in the routine intervention lost less weight during the first 6 weeks of the BWL programme than those in the educational control group and did not differ significantly in weight loss at the end of the 18‐week programme. Although the difference between groups at the conclusion of treatment was not statistically significant with the relatively small sample, this finding represents a medium size effect (d = .56) with the routine intervention group losing less weight than EDU overall.
There are several possible explanations for our observed findings. First, providing only 4 weeks for establishment of consistent eating and sleep routines may have been too short for participants to master the changes prescribed (especially in sleep, which was introduced in week 2). Thus, when they started the weight loss programme, these participants may have been continuing to work on establishing their behavioural routines, while simultaneously taking on the multiple ‘new’ goals of the BWL programme.
Behaviourists have long known that attempts to change too many behaviours at once may backfire 36. Basic principles of behaviour modification suggest that making smaller changes gradually may lead to greater long‐term success rather than taking on larger, more abrupt changes. Moreover, while in some cases working on multiple behaviours simultaneously may be beneficial (e.g. smoking cessation and physical activity or diet and physical activity) 37, 38, 39, this is not always the case, and it may depend on the targeted behaviours themselves and the demands of the changes required 40. Prochaska et al. (2008) note that although multiple health behaviour change interventions in individuals may provide a larger impact on overall health, there is a necessary increase in demands and complexity of the intervention, and efforts to make concurrent changes in multiple behaviours may be overwhelming and lead to poor adherence. They suggest sequencing behaviour change goals (as opposed to asking participants to work on more than one change at a time) or assessing participants' readiness to change in each behaviour and matching the strategies accordingly 41. Although standard BWL programmes target eating and exercise behaviours together, additionally requiring specific changes to eating and sleep schedules may have negatively influenced participant attitudes and adherence to BWL goals and may have impacted self‐efficacy among ROU participants.
An alternative explanation for the observed effects may be that the level of self‐control required to change eating and sleep routines, in addition to the self‐control demands of decreasing caloric intake and increasing physical activity, surpassed participants' capabilities. An extensive body of work focusing on self‐control has characterized this ability as a ‘limited resource’. This model posits that as self‐control is used, there will be less available for subsequent tasks; this state of ‘ego depletion’ increases the likelihood that the individual will fail when exertion of self‐control is needed again 42. Applying this model to the current study, our routine‐based intervention was designed to increase self‐control capacity in two ways. First, the routine‐based intervention addressed some of the physiological underpinnings that can deplete self‐control, namely sleep deprivation and low blood glucose levels 43, 44, and should, as a result, have led to improvements in self‐control. Second, the routines intervention provided opportunities to practice self‐control. There is evidence suggesting that self‐control abilities may be strengthened, as in a muscle, with repeated practice of small acts of self‐control 45. Previous studies show that practicing self‐regulatory exercises for 2 weeks that included tracking food eaten, working on improving mood, or improving posture, strengthened self‐control and led to less ego depletion 45. Similarly, a 4‐month financial monitoring programme has been shown to increase self‐regulatory capacity compared with a no‐treatment control group 46. Based on these studies, we hypothesized that the routines intervention used in our pilot would likewise provide a chance to practice self‐regulation and consequently might enhance self‐control capacity. However, in the current study, the acts of self‐control required for changing eating and sleep schedules in the routine intervention may have been too difficult, or, perhaps, not practiced for enough time and therefore may have depleted, rather than enhanced, individuals' self‐control abilities. In accordance with this, many ROU participants reported being overwhelmed by the behaviour changes required to follow the sleep and eating routines in addition to the demands of BWL.
There are some limitations to this study that must be considered. Because this was a pilot randomized controlled trial, the sample size is relatively small, and a larger cohort may have yielded differing results. Furthermore, as noted earlier, it is possible that the 4‐week timeframe for the routine intervention was not adequate to allow for full mastery of the behavioural routines. Although self‐reported eating behaviours improved, participants did not make significant changes in their sleep. Other studies in which certain behaviours are targeted prior to the start of a weight loss programme have had a longer timeframe for practicing target behaviours (8 weeks vs. the 4 weeks used in the current study) 47. Thus, a lengthened ROU intervention may have been more effective in the establishment of the desired routines. Additionally, it is not known whether the sleep and/or eating routine behaviours were maintained during the BWL; thus, the present study cannot discern how adherence to sleep and eating schedule routines during actual BWL may impact weight loss. Finally, a design in which participants were randomly assigned to change only their eating routines, only their sleep routines, or both, or a design in which participants addressed these two behaviours in sequence rather than in parallel may have yielded different results.
Despite limitations, this study provides important insight into attempts at health behaviour change. A novel intervention targeting two potentially critical behaviours related to successful weight control, regular eating and consistent sleep, was developed. Participants reported modifying eating and sleep duration; however, self‐reported sleep consistency did not improve, and objective measures of sleep did not change. Although the routine intervention did not ultimately improve weight losses, further study with a longer period for establishing the routines or focusing on eating and sleep routines independently is needed to determine whether establishing healthy routines can improve subsequent weight loss outcomes.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by a U01 (5U01CA150387‐05) (PI: RRW) from the National Cancer Institute of the National Institute of Health.
Demos, K. E. , Leahey, T. M. , Hart, C. N. , Trautvetter, J. , Coward, P. R. , Duszlak, J. , and Wing, R. R. (2015) A pilot randomized controlled trial testing the effects of a routine‐based intervention on outcomes in a behavioural weight loss programme. Obesity Science & Practice, 1: 110–118. doi: 10.1002/osp4.16.
Funding: The funding source for this study is a grant from the National Institutes of Health (NIH) National Cancer Institute (5U01CA150387) awarded to RRW. Additional funding to support Dr. Demos comes from an NIH National Institute of Diabetes and Digestive and Kidney Diseases grant (5K01090445) awarded to KED.
Conflict of interest: None
ClinicalTrials.gov Identifier: NCT01428687
References
- 1. O'Carroll RE, Chambers JA, Dennis M, Sudlow C, Johnston M. Improving medication adherence in stroke survivors: mediators and moderators of treatment effects. Health Psychol. 2014; 33: 1241–1250. doi: 10.1037/hea0000082. PubMed PMID: 25020155. [DOI] [PubMed] [Google Scholar]
- 2. Chambers JA, O'Carroll RE, Hamilton B, et al. Adherence to medication in stroke survivors: a qualitative comparison of low and high adherers. Br J Health Psychol 2011; 16: 592–609. doi: 10.1348/2044-8287.002000. PubMed PMID: 21199537. [DOI] [PubMed] [Google Scholar]
- 3. Karner A, Tingstrom P, Abrandt‐Dahlgren M, Bergdahl B. Incentives for lifestyle changes in patients with coronary heart disease. J Adv Nurs 2005; 51: 261–275. doi: 10.1111/j.1365-2648.2005.03467.x. PubMed PMID: 16033594. [DOI] [PubMed] [Google Scholar]
- 4. Karatsoreos IN. Effects of circadian disruption on mental and physical health. Curr Neurol Neurosci Rep 2012; 12: 218–225. doi: 10.1007/s11910-012-0252-0. PubMed PMID: 22322663. [DOI] [PubMed] [Google Scholar]
- 5. Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett 2008; 582: 142–151. doi: 10.1016/j.febslet.2007.06.079. PubMed PMID: 17707819. [DOI] [PubMed] [Google Scholar]
- 6. Anderson SE, Whitaker RC. Household routines and obesity in US preschool‐aged children. Pediatrics 2010; 125: 420–428. doi: 10.1542/peds.2009-0417. PubMed PMID: 20142280. [DOI] [PubMed] [Google Scholar]
- 7. Haines J, McDonald J, O'Brien A, et al. Healthy habits, happy homes: randomized trial to improve household routines for obesity prevention among preschool‐aged children. JAMA Pediatrics 2013; 167: 1072–1079. doi: 10.1001/jamapediatrics.2013.2356. PubMed PMID: 24019074. [DOI] [PubMed] [Google Scholar]
- 8. Mistry KB, Minkovitz CS, Strobino DM, Borzekowski DL. Children's television exposure and behavioral and social outcomes at 5.5 years: does timing of exposure matter? Pediatrics 2007; 120: 762–769. doi: 10.1542/peds.2006-3573. PubMed PMID: 17908763. [DOI] [PubMed] [Google Scholar]
- 9. Gregory AM, Van der Ende J, Willis TA, Verhulst FC. Parent‐reported sleep problems during development and self‐reported anxiety/depression, attention problems, and aggressive behavior later in life. Arch Pediatr Adolesc Med 2008; 162: 330–335. doi: 10.1001/archpedi.162.4.330. PubMed PMID: 18391141. [DOI] [PubMed] [Google Scholar]
- 10. Jones BL, Fiese BH, Team SK. Parent routines, child routines, and family demographics associated with obesity in parents and preschool‐aged children. Frontiers in Psychology 2014; 5: 374. doi: 10.3389/fpsyg.2014.00374. PubMed PMID: 24808883; PMCID: 4010746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011; 19: 1374–1381. doi: 10.1038/oby.2011.100. PubMed PMID: 21527892. [DOI] [PubMed] [Google Scholar]
- 12. Garaulet M, Gomez‐Abellan P. Timing of food intake and obesity: a novel association. Physiol Behav 2014; 134: 44–50. doi: 10.1016/j.physbeh.2014.01.001. PubMed PMID: 24467926. [DOI] [PubMed] [Google Scholar]
- 13. Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res 2014; 34: 930–935. doi: 10.1016/j.nutres.2014.09.010. PubMed PMID: 25439026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leidy HJ, Campbell WW. The effect of eating frequency on appetite control and food intake: brief synopsis of controlled feeding studies. J Nutr 2011; 141: 154–157. doi: 10.3945/jn.109.114389. PubMed PMID: 21123467. [DOI] [PubMed] [Google Scholar]
- 15. Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal‐weight, middle‐aged adults. Am J Clin Nutr. 2007; 85: 981–988. PubMed PMID: 17413096; PMCID: 2645638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaix A, Zarrinpar A, Miu P, Panda S. Time‐restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 2014; 20: 991–1005. doi: 10.1016/j.cmet.2014.11.001. PubMed PMID: 25470547; PMCID: 4255155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hatori M, Vollmers C, Zarrinpar A, et al. Time‐restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high‐fat diet. Cell Metab 2012; 15: 848‐860. doi: 10.1016/j.cmet.2012.04.019. PubMed PMID: 22608008; PMCID: 3491655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farshchi HR, Taylor MA, Macdonald IA. Regular meal frequency creates more appropriate insulin sensitivity and lipid profiles compared with irregular meal frequency in healthy lean women. Eur J Clin Nutr. 2004; 58: 1071–1077. doi: 10.1038/sj.ejcn.1601935. PubMed PMID: 15220950. [DOI] [PubMed] [Google Scholar]
- 19. Farshchi HR, Taylor MA, Macdonald IA. Decreased thermic effect of food after an irregular compared with a regular meal pattern in healthy lean women. Int J Obes Relat Metab Disord. 2004; 28: 653–660. doi: 10.1038/sj.ijo.0802616. PubMed PMID: 15085170. [DOI] [PubMed] [Google Scholar]
- 20. Farshchi HR, Taylor MA, Macdonald IA. Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. Am J Clin Nutr 2005; 81: 16–24. PubMed PMID: 15640455. [DOI] [PubMed] [Google Scholar]
- 21. Carlson O, Martin B, Stote KS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal‐weight middle‐aged men and women. Metabolism 2007; 56: 1729–1734. doi: 10.1016/j.metabol.2007.07.018. PubMed PMID: 17998028; PMCID: PMC2121099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 2004; 1: e62. doi: 10.1371/journal.pmed.0010062. PubMed PMID: 15602591; PMCID: 535701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005; 99: 2008–2019. doi: 10.1152/japplphysiol.00660.2005. PubMed PMID: 16227462. [DOI] [PubMed] [Google Scholar]
- 24. Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 2007; 15: 253–261. doi: 10.1038/oby.2007.512. PubMed PMID: 17228054. [DOI] [PubMed] [Google Scholar]
- 25. Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinology / Eur Fed Endocrine Societies 2008; 159: S59–66. doi: 10.1530/EJE-08-0298. PubMed PMID: 18719052; PMCID: 2755992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magee CA, Iverson DC, Huang XF, Caputi P. A link between chronic sleep restriction and obesity: methodological considerations. Public Health 2008; 122: 1373‐1381. doi: 10.1016/j.puhe.2008.05.010. PubMed PMID: 18722633. [DOI] [PubMed] [Google Scholar]
- 27. Spiegel K, Leproult R, L'Hermite‐Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004; 89: 5762–5771. doi: 10.1210/jc.2004-1003. PubMed PMID: 15531540. [DOI] [PubMed] [Google Scholar]
- 28. Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141: 846–850. PubMed PMID: 15583226. [DOI] [PubMed] [Google Scholar]
- 29. Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 2009; 5: 253–261. doi: 10.1038/nrendo.2009.23. PubMed PMID: 19444258; PMCID: PMC4457292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He F, Bixler EO, Berg A, et al. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Med 2015; 16: 856–861. doi: 10.1016/j.sleep.2015.03.004. PubMed PMID: 26002758; PMCID: PMC4466046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kjeldsen JS, Hjorth MF, Andersen R, et al. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes (Lond). 2014; 38: 32–39. doi: 10.1038/ijo.2013.147. PubMed PMID: 23924757. [DOI] [PubMed] [Google Scholar]
- 32. Patel SR, Hayes AL, Blackwell T, et al. Osteoporotic fractures in M, Study of Osteoporotic Fractures Research G. The association between sleep patterns and obesity in older adults. Int J Obes (Lond) 2014; 38: 1159–1164. doi: 10.1038/ijo.2014.13. PubMed PMID: 24458262; PMCID: PMC4110191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta‐analysis. Sleep. 1996; 19: 318–326. PubMed PMID: 8776790. [DOI] [PubMed] [Google Scholar]
- 34. Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med : JCSM : Off Publ Am Acad Sleep Med 2007; 3: 519–528. PubMed PMID: 17803017; PMCID: 1978335. [PMC free article] [PubMed] [Google Scholar]
- 35. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight‐better sleep study: a pilot intervention in primary care. Am J Health Behav 2012; 36: 319–334. doi: 10.5993/AJHB.36.3.4. PubMed PMID: 22370434. [DOI] [PubMed] [Google Scholar]
- 36. Spiegler MD, Guevremont DC. Contemporary Behavior Therapy, 4th edn Wadsworth Thomson Learning: Belmont, CA, 2003. [Google Scholar]
- 37. Albrecht AE, Marcus BH, Roberts M, Forman DE, Parisi AF. Effect of smoking cessation on exercise performance in female smokers participating in exercise training. Am J Cardiol 1998; 82: 950–955. PubMed PMID: 9794350. [DOI] [PubMed] [Google Scholar]
- 38. Hyman DJ, Pavlik VN, Taylor WC, Goodrick GK, Moye L. Simultaneous vs sequential counseling for multiple behavior change. Arch Intern Med. 2007; 167: 1152–1158. doi: 10.1001/archinte.167.11.1152. PubMed PMID: 17563023. [DOI] [PubMed] [Google Scholar]
- 39. King AC, Castro CM, Buman MP, Hekler EB, Urizar GG, Jr. , Ahn DK. Behavioral impacts of sequentially versus simultaneously delivered dietary plus physical activity interventions: the CALM trial. Ann Behav Med 2013; 46: 157–168. doi: 10.1007/s12160-013-9501-y. PubMed PMID: 23609341; PMCID: 3755035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shumaker SA, Ockene JK, Reikert KA. The Handbook of Health Behavior Change, 3rd edn Springer Publishing Company: New York, 2008, p 856. [Google Scholar]
- 41. Prochaska JJ, Spring B, Nigg CR. Multiple health behavior change research: an introduction and overview. Prev Med 2008; 46: 181–188. doi: 10.1016/j.ypmed.2008.02.001. PubMed PMID: 18319098; PMCID: 2288583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muraven M, Baumeister RF. Self‐regulation and depletion of limited resources: does self‐control resemble a muscle? Psychol Bull. 2000; 126: 247–259. PubMed PMID: 10748642. [DOI] [PubMed] [Google Scholar]
- 43. Gailliot MT, Baumeister RF. The physiology of willpower: linking blood glucose to self‐control. Pers Soc Psychol Rev. 2007; 11: 303–327. doi: 10.1177/1088868307303030. PubMed PMID: 18453466. [DOI] [PubMed] [Google Scholar]
- 44. Gailliot MT, Baumeister RF, DeWall CN, et al. Self‐control relies on glucose as a limited energy source: willpower is more than a metaphor. J Pers Soc Psychol 2007; 92: 325–336. doi: 10.1037/0022-3514.92.2.325. PubMed PMID: 17279852. [DOI] [PubMed] [Google Scholar]
- 45. Muraven M, Baumeister RF, Tice DM. Longitudinal improvement of self‐regulation through practice: building self‐control strength through repeated exercise. J Soc Psychol. 1999; 139: 446–457. doi: 10.1080/00224549909598404. PubMed PMID: 10457761. [DOI] [PubMed] [Google Scholar]
- 46. Oaten M, Cheng K. Improvements in self‐control from financial monitoring. J Econ Psycholog 2007; 28: 487–501. [Google Scholar]
- 47. Kiernan M, Brown SD, Schoffman DE, et al. Promoting healthy weight with “stability skills first”: a randomized trial. J Consult Clin Psychol 2013; 81: 336–346. doi: 10.1037/a0030544. PubMed PMID: 23106759. [DOI] [PMC free article] [PubMed] [Google Scholar]


