Summary
Objective
Providing effective dietary counselling so that pregnancy weight gain remains within the 2009 Institute of Medicine (IOM) guidelines requires accurate maternal energy intake measures. Current practice is based on self‐reported intake that has been demonstrated unreliable. This study applies an objective calculation of energy intake from a validated mathematical model to identify characteristics of individuals more likely to misreport during pregnancy.
Methods
A validated maternal energy balance equation was used to calculate energy intake from gestational weight gain in 1,368 subjects. The difference between self‐reported and model‐predicted energy intake was tested for demographics, economic status, education level and maternal health status.
Results
A weight gain of 15.2 kg resulted in model‐predicted intake during pregnancy of 2,882.97 ± 135.71 kcal day−1, which differed from self‐reported intake of 2,180.5 ± 856.0 kcal day−1. The achieved weight gain exceeded the IOM guidelines; however, the model predicted weight gain from self‐reported energy intake was below IOM guidelines. Higher income (p = 0.004), education (p = 0.003), birth weight (p = 0.017), gestational diabetes (p = 0.008) and pre‐existing diabetes (p < 0.001) were associated with under‐reported energy intake. More children living at home (p = 0.001) were associated with more accurate self‐reported intake.
Conclusions
When assessing self‐reported energy intake in pregnancy studies, birth weight, gestational diabetes status, pre‐existing diabetes, higher income and education predict higher under‐reporting. Clinicians providing dietary treatment recommendations during pregnancy should be aware that individuals with pre‐existing diabetes and gestational diabetes mellitus are more likely to misreport their intake. Additionally, the systems model approach can be applied early in intervention to objectively monitor dietary compliance to treatment recommendations.
Keywords: Differential equation, maternal energy intake, mathematical model, pregnancy weight gain
Introduction
The 2009 Institute of Medicine (IOM) Pregnancy Weight Gain Guidelines (1) have generated increased attention for managing weight gain during pregnancy (2, 3, 4, 5). Energy intake is a controllable variable that influences changes in weight during pregnancy. Therefore, many interventions for pregnant women prescribe energy intake recommendations. In order to determine adherence to a prescribed dietary intervention in pregnancy, energy intake is most commonly evaluated from a self‐report instrument (2, 3, 4, 5). Unfortunately, self‐reported energy intake has been repeatedly demonstrated to be unreliable and, therefore, is an invalid tool to evaluate adherence to dietary recommendations (6, 7).
Validated dynamic differential equations based on the energy balance model have been successfully applied to determine energy intake in non‐pregnant adults during weight loss and weight gain (8, 9, 10, 11). Here, for the first time, we apply a systems model based on the first law of thermodynamics for gestational weight gain (12) to estimate energy intake in over 1,300 pregnant women. The model was derived using first principles and the best available energy balance data measured in a large cohort of pregnant women (13). The difference between dynamic model‐predicted energy intake and self‐report was defined as the degree of misreport. Using this measure of misreport, we identify demographic characteristics in individuals more likely to misreport during pregnancy. These conclusions can be used to improve patient guidance and adherence to lifestyle interventions during pregnancy.
Methods
Study subjects
This is a post hoc analysis of a study in which the original aim was to identify predictors for preterm delivery. The study was composed of four data collection phases. Data collected in the final two phases, where energy intake was assessed, were used for the current analysis. Women enrolled before 20 weeks of gestation that were seeking services from prenatal clinics in a southern US academic medical centre between January 2001 and June 2005 were eligible for enrollment. The dataset used for this study contained demographic variables required to simulate the dynamic energy balance model, which are age, height and pre‐pregnancy weight (12), self‐reported energy intake derived from food frequency questionnaires administered between gestational weeks 24 and 29 recorded in kcal day−1 and potential predictors of dietary misreport, household income, level of education, number of living children at home, maternal age, parity and maternal health status.
Application of dynamic energy balance model to calculate energy intake
A systems model that predicts pregnancy weight gain in response to changes in energy intake and physical activity was previously developed and employed for this study (12). The model requires input of age, pre‐pregnancy weight and height. A weight graph trajectory is generated after entry of trimester‐specific energy intake. The systems model is a nonlinear piecewise defined differential equation that was programmed into freely available java® software http://www.pbrc.edu/research‐and‐faculty/calculators/gestational‐weight‐gain/ (12). Because the systems model connects pregnancy intake to pregnancy weight gain, energy intake can be determined from knowledge of weight gain (Figure 1). For this analysis, energy intake was adjusted until the predicted pregnancy weight gain matched the reported pregnancy weight gain. The energy intake was held constant over the three trimesters because trimester‐specific maternal weight was not available. As a result, the model‐predicted energy intake represents an average gestational energy intake in kcal day−1 for each individual participant.
Figure 1.
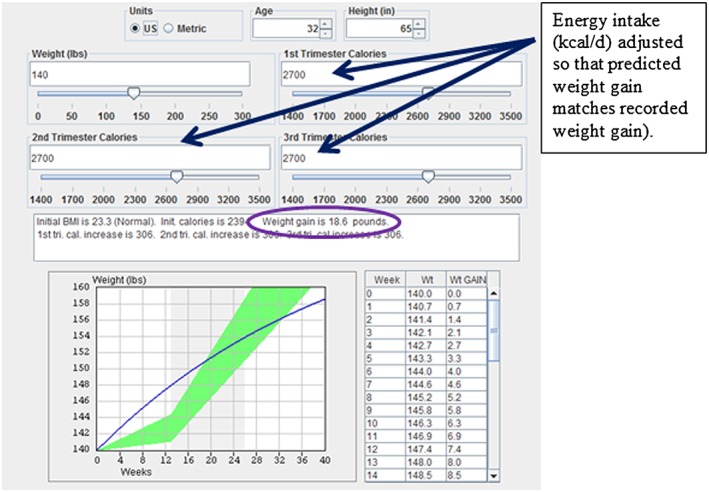
Screen shot of calculator that houses the gestational weight gain dynamic model used to determine energy intake from gestational weight gain. Energy intake was adjusted so that predicted weight gain matched recorded weight gain in the database for the individual (circled).
Calculation of misreported energy intake
Misreported energy intake was calculated as the difference between the model‐predicted energy intake and self‐reported energy intake from the food frequency questionnaire.
Statistical analysis
Weight regulation treatment often requires dietary intake prescriptions; thereby, knowledge of what individual characteristics will predict misreporting dietary intake is important. To this end, logistic regression in spss (IBM, Armonk, NY, 2012, USA) was applied to determine which measurable individual pregnancy variables predict higher degrees of misreported energy intake. Specifically, the variables pre‐pregnancy body mass index (BMI) in kg m−2, baby's gender, gestational age in weeks at delivery, baby's birth weight, preterm or term birth, percent of 2001 poverty level, children currently living at home, employment status, maternal race, maternal age, maternal education level, number of previous known pregnancies, number of previous live births, number of previous known miscarriages, number of previous induced abortions, number of previous stillbirths, number of previous known preterm births, number of previous births that were small for gestational age, maternal height (cm), maternal pre‐pregnancy weight (kg), gestational weight gain (kg), gained above, below or within the IOM recommended guidelines, diagnosed with gestational diabetes, diagnosed with pregnancy induced hypertension, diagnosed with pre‐eclampsia, diagnosed with chronic hypertension and maternal pre‐existing diabetes were tested as predictors for misreported energy intake.
Results
Study subjects
The original cohort was composed of 2,006 pregnant women. Because individual measures of age, height, pre‐pregnancy weight and self‐reported energy intake are required to simulate the model and calculate misreport, only those subjects that contained all data simultaneously were retained for this analysis. Dynamic models require input of accurate individual baseline data to provide an individual prediction necessitating restriction versus any attempt to impute missing data. This final dataset included 1,368 subjects.
Subjects were aged 29.4 ± 5.5 years, with pre‐pregnancy BMI 25.6 ± 6.8 kg m−2 and 39% classified overweight and obese with BMI >25 kg m−2 (Table 1). Average gestational weight gain was 15.2 ± 6.0 kg. Self‐reported energy intake was 2180.5 ± 856.0 kcal day−1. Ninety‐six percent of subjects exceeded the IOM recommended weight gain limits. Higher variation in self‐reported energy intake was observed in the overweight and obese BMI classifications (overweight: 2,235.0 ± 1,040.1 kcal day−1, obese: 2,314.4 ± 1,214.4 kcal day−1) evidenced by higher standard deviation (Table 1).
Table 1.
Weight gain, model‐determined energy intake, self‐reported energy intake and percent of women gaining outside of the IOM guidelines in each BMI classification
| Weight gain (kg) | Model EI (kcal day−1) | Self‐reported EI (kcal day−1) | % exceeding IOM GWG guidelines | |
|---|---|---|---|---|
| Underweight (N = 55) | 15.3 ± 4.2 | 2,862.9 ± 131.5 | 2,169.6 ± 688.4 | 100 |
| Normal weight (N = 775) | 16.2 ± 4.9 | 2,883.0 ± 135.7 | 2,101.1 ± 758.3 | 100 |
| Overweight (N = 259) | 16.0 ± 6.5 | 2,845.2 ± 173.4 | 2,235.0 ± 1,040.1 | 97 |
| Obese (N = 279) | 11.8 ± 7.2 | 2,754.2 ± 183.5 | 2,314.4 ± 1,214.4 | 87 |
| Total (N = 1,368) | 15.2 ± 6.0 | 2,848.7 ± 161.7 | 2,180.5 ± 926.6 | 96 |
BMI, body mass index; EI, energy intake; GWG, gestational weight gain; IOM, Institute of Medicine.
Actual energy intake determined by the systems model
The average actual energy intake was 2,882.97 ± 135.71 kcal day−1. There was no association (R 2 = 0.004, p = 0.43) between actual energy intake and self‐reported energy intake (Figure 2).
Figure 2.
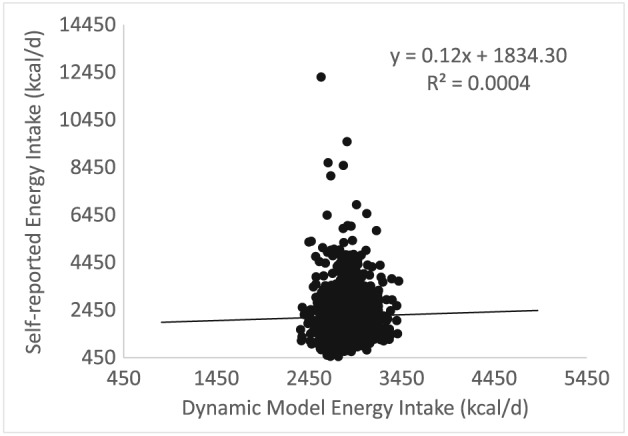
Self‐reported energy intake (kcal day−1) versus model‐predicted energy intake (kcal day−1). There is no significant correlation between self‐reported intake and model‐predicted intake (kcal day−1).
Predictors of misreported energy intake
A summary of variable coefficients, standard error and p value for all model covariates appear in Table 2. The overall adjusted R 2 for the logistic regression model predicting misreport was 0.14. There were three types of covariates that were significant predictors of misreport. These were covariates associated with pregnancy complications or pre‐existing complications, covariates that reflect socioeconomic status and covariates involved with body weight. From the complications category, pre‐existing diabetes (β = 602.453. p < 0.001) and having gestational diabetes (β = 332.26, p = 0.008) were positively associated with higher degrees of under‐reporting. Previous preterm birth was inversely correlated to misreporting (β = −>234.586, 0.012). Under the category of socioeconomic factors, higher maternal education level (β = 35.101, p = 0.003) and income levels reflected as the percent above poverty level (β = 0.424, p = 0.004) were positively associated with higher degrees of under‐reporting. On the other hand, a higher number of children living at home were associated with improved reporting (β = −193.923, p = 0.001). Pre‐pregnancy BMI (β = 93.686, p = 0.013), the baby's weight (β = 0.104, p = 0.017) and gestational weight gain (β = 10.086, p = 0.026) were positively associated with higher degrees of under‐reporting. Pre‐pregnancy weight was inversely correlated to misreporting (β = −38.762, p = 0.005).
Table 2.
Relationship between dietary misreporting in pregnant women and maternal and infant outcomes
| Coefficients | |||
|---|---|---|---|
| Model covariates | Unstandardized coefficients | Significance | |
| B | Standard error | p value | |
| Pre‐existing diabetes | 602.453 | 131.728 | p < 0.001 |
| Children currently living at home | −143.923 | 42.762 | 0.001 |
| Maternal level of education | 35.101 | 11.689 | 0.003 |
| Percent of 2001 poverty level | 0.424 | 0.147 | 0.004 |
| Pre‐pregnancy weight | −38.762 | 13.664 | 0.005 |
| Gestational diabetes | 332.26 | 124.923 | 0.008 |
| Previous low birth weight | −234.586 | 92.811 | 0.012 |
| Maternal pre‐pregnancy BMI (kg m−2) | 93.686 | 37.553 | 0.013 |
| Infant birth weight | 0.104 | 0.043 | 0.017 |
| Gestational weight gain | 10.086 | 4.525 | 0.026 |
| Maternal height | 18.454 | 12.218 | 0.131 |
| Preeclampsia | −170.05 | 114.556 | 0.138 |
| (Constant) | −3,181.29 | 2,172.3 | 0.143 |
| Maternal race | −9.281 | 8.084 | 0.251 |
| Previous live births | 109.811 | 106.046 | 0.301 |
| Pregnancy‐induced hypertension | 100.414 | 97.89 | 0.305 |
| Maternal age | 5.028 | 5.705 | 0.378 |
| GWG above the IOM guidelines | 114.509 | 143.633 | 0.425 |
| Previous stillbirths | −99.064 | 138.272 | 0.474 |
| Previous induced abortions | 57.661 | 102.785 | 0.575 |
| Previous miscarriages | 52.015 | 96.43 | 0.59 |
| Gestational age at delivery | −8.929 | 19.559 | 0.648 |
| Chronic hypertension | −36.866 | 103.239 | 0.721 |
| Classified with BMI over 25 | −22.851 | 75.261 | 0.761 |
| Preterm birth | 18.096 | 106.999 | 0.866 |
| Number of previously known pregnancies | −3.827 | 92.821 | 0.967 |
| Previous preterm | −1.134 | 75.526 | 0.988 |
| Infant gender | 0.532 | 46.51 | 0.991 |
BMI, body mass index; GWG, gestational weight gain; IOM, Institute of Medicine.
Discussion
Studies have found managing maternal energy intake to be an effective intervention approach to keep weight gain within the 2009 IOM gestational weight gain recommendations (1). Although objective energy intake measurements are necessary to counsel women, safe and effective approaches to determine energy intake during pregnancy remain elusive. To overcome these obstacles, we applied a validated systems model (12) based on the first law of thermodynamics to calculate energy intake from gestational weight gain. The model‐determined energy intake was then applied to evaluate the validity of self‐reported intake during pregnancy.
On average, pregnant women in the study under‐reported energy intake. There was no correlation between self‐reported intake and model‐predicted intake. This is corroborated by the literature that demonstrates low correlation (R 2 ≤ 0.10) between self‐reported and objectively measured energy intake in non‐pregnancy (14). Higher pre‐pregnancy BMI, infant birth weight, household income and maternal level of education were associated with dietary under‐reporting. This is surprising because populations with higher income and education levels are less likely to under‐report in non‐pregnant populations (15). We hypothesize that during pregnancy there is strong incentive for women to practice healthy behaviours. As a result, selection of undesirable or unhealthy foods on the food frequency questionnaire may be omitted by pregnant women (16). Women with higher socioeconomic and education status with better access to prenatal care may have an increased understanding of which foods are appropriate during pregnancy, feel more social pressure to conform to ideals (17) and may be more likely to not report high‐calorie foods consumed.
Additionally, we found that women with pre‐existing diabetes or gestational diabetes pregnancy were more likely to under‐report energy intake. This is particularly concerning because diet is important for controlling diabetes (18).
Finally, women with more children living at home were more likely to accurately report energy intake. We speculate that additional children living in the home could result in more meals prepared and consumed at home, which could enhance the capacity of the mother to recall food intake on food frequency questionnaire. Furthermore, because misreporting was lower in women with a lower level of household income, we also speculate that these households are likely to receive nutritional support through supplemental nutritional programmes. This supports our idea that these women are preparing more meals at home, allowing for a closer approximation of intake against the food frequency questionnaire which could improve the capacity for women to more accurately recall food intake as these programmes provide more intensive discussions around diet quality and nutrition.
Although this is the first investigation into the evaluation of self‐report of dietary intake during pregnancy, the accuracy of self‐reported birth weights has been examined (19). Women with higher levels of education and higher parity were more likely to misreport their children's birth weight (19), consistent with our results.
This analysis of energy intake is limited because energy intake by self‐report was not available in each trimester. Additionally, we did not have a weight measurement for each trimester. Our study therefore relies on the assumption that weight gain in pregnancy is linear beyond the first trimester, consistent with the IOM recommendations. Importantly, the systems model allows for differential changes in energy intake in each trimester, which was taken as an average for this study. Additionally, the data were restricted to an academic medical centre in one specific US region. The evaluation of a larger population more representative of the USA is required to evaluate whether our conclusions hold on a larger scale.
Conclusions
Weight gain during pregnancy requires management of maternal energy intake, which is most typically measured through self‐report. Clinicians and healthcare providers should be aware of the tendency of pregnant women to under‐report energy intake during pregnancy, especially by women with higher income and education levels or a diagnosis of gestational or pre‐existing diabetes. Further the ability of the mother to prepare and consume meals at home could be an effective strategy to improve the accuracy of dietary recall and then to enhance diet quality in pregnancy. The systems model can be applied early in intervention to objectively monitor dietary compliance to treatment recommendations.
Conflict of Interest Statement
Drs Salafia and Thomas have a patent pending titled ‘System and method for predicting fetal and maternal health risks’.
Acknowledgement
This research was supported in part by grants from the National Institutes of Health to LMR (U01DK094418 and R01DK099175).
Author contributions
Redman, Salafia, and Thomas developed the study concept and design. Redman, Misra and Salafia reviewed the data required for model simulations and statistical analysis. Thomas drafted the manuscript. Islam, Armah, Kunnipparampil, Patel, Redman, Misra and Salafia critically revised the manuscript for important intellectual content. Bredlau performed the programming of the mathematical model into an accessible applet. Thomas carried out the statistical analysis. Islam, Armah, Kunnipparampil and Patel conducted the mathematical simulations.
Thomas, D. M. , Bredlau, C. , Islam, S. , Armah, K. A. , Kunnipparampil, J. , Patel, K. , Redman, L. M. , Misra, D. , and Salafia, C. (2016) Relationships between misreported energy intake and pregnancy in the pregnancy, infection and nutrition study: new insights from a dynamic energy balance model. Obesity Science & Practice, 2: 174–179. doi: 10.1002/osp4.29.
References
- 1. Rasmussen KM, Yaktine AL, Institute of Medicine (U.S.) . Committee to reexamine IOM pregnancy weight guidelines Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. xiv, pp. 854. [PubMed] [Google Scholar]
- 2. Vinter CA, Jensen DM, Ovesen P, Beck‐Nielsen H, Jorgensen JS. The LiP (lifestyle in pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 2011; 34: 2502–7. doi: 10.2337/dc11‐1150. PubMed PMID: 21972411; PMCID: 3220844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gresham E, Bisquera A, Byles JE, Hure AJ. Effects of dietary interventions on pregnancy outcomes: a systematic review and meta‐analysis. Matern Child Nutr 2014. doi: 10.1111/mcn.12142. PubMed PMID: 25048387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGowan CA, Walsh JM, Byrne J, Curran S, McAuliffe FM. The influence of a low glycemic index dietary intervention on maternal dietary intake, glycemic index and gestational weight gain during pregnancy: a randomized controlled trial. Nutr J 2013; 12: 140. doi: 10.1186/1475‐2891‐12‐140. PubMed PMID: 24175958; PMCID: 4176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hui AL, Back L, Ludwig S, Gardiner P , Sevenhuysen G, Dean HJ , Sellers E, McGavock J, Morris M, Jiang D, Shen GX. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre‐pregnancy body mass index in a randomized control trial. BMC Pregnancy Childbirth 2014; 14: 331. doi: 10.1186/1471‐2393‐14‐331. PubMed PMID: 25248797; PMCID: 4287470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schoeller DA, Thomas D, Archer E, Heymsfield SB, Blair SN, Goran MI, Hill JO, Atkinson RL, Corkey BE, Foreyt J, Dhurandhar NV, Kral JG, Hall KD, Hansen BC, Heitmann BL, Ravussin E, Allison DB. Self‐report‐based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr 2013; 97: 1413–5. doi: 10.3945/ajcn.113.062125. PubMed PMID: 23689494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sorensen TI, Speakman JR, Jeansonne M, Allison DB, the Energy Balance Measurement Working G . Energy balance measurement: when something is not better than nothing. Int J Obes (Lond) 2014. doi: 10.1038/ijo.2014.199. PubMed PMID: 25394308; PMCID: 4430460. [Google Scholar]
- 8. Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab 2010; 298: E449–66. doi: 10.1152/ajpendo.00559.2009. PubMed PMID: 19934407; PMCID: 2838532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. Am J Clin Nutr 2010; 92: 1326–31 PMCID: 2980958. Epub 2010/10/22. doi: ajcn.2010.29687 [pii] 10.3945/ajcn.2010.29687. PubMed PMID: 20962159; PMCID: 2980958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall KD, Chow CC. Estimating changes in free‐living energy intake and its confidence interval. Am J Clin Nutr 2011; 94: 66–74. doi: 10.3945/ajcn.111.014399. PubMed PMID: 21562087; PMCID: 3127505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas DM, Martin CK, Redman LM, Heymsfield SB, Lettieri S, Levine JA, Bouchard C, Schoeller DA. Effect of dietary adherence on the body weight plateau: a mathematical model incorporating intermittent compliance with energy intake prescription. Am J Clin Nutr 2014; 100: 787–95. doi: 10.3945/ajcn.113.079822. PubMed PMID: 25080458; PMCID: 4135489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas DM, Navarro‐Barrientos JE, Rivera DE, Heymsfield SB, Bredlau C, Redman LM, Martin CK, Lederman SA, Collins LM, Butte NF. Dynamic energy‐balance model predicting gestational weight gain. Am J Clin Nutr 2012; 95: 115–22. doi: 10.3945/ajcn.111.024307. PubMed PMID: 22170365; PMCID: 3238455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butte NF, Wong WW, Treuth MS, Ellis KJ, O'Brian Smith E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am J Clin Nutr 2004; 79: 1078–87. Epub 2004/05/26. PubMed PMID: 15159239. [DOI] [PubMed] [Google Scholar]
- 14. Allison D. Article recommendation: pooled results from 5 validation studies of dietary self‐report instruments using recovery biomarkers for energy and protein intake. F1000 Diabetes & Endocrinology, 2015.
- 15. Johansson G, Wikman A, Ahren AM, Hallmans G, Johansson I. Underreporting of energy intake in repeated 24‐hour recalls related to gender, age, weight status, day of interview, educational level, reported food intake, smoking habits and area of living. Public Health Nutr 2001; 4: 919–27. PubMed PMID: 11527517. [DOI] [PubMed] [Google Scholar]
- 16. Macdiarmid J, Blundell J. Assessing dietary intake: Who, what and why of under‐reporting. Nutr Res Rev 1998; 11: 231–53. doi: 10.1079/NRR19980017. PubMed PMID: 19094249. [DOI] [PubMed] [Google Scholar]
- 17. Simmons R. Odd girl out: the hidden culture of aggression in girls Completely Rev. and Updated, 1st Mariner Books Ed. New York: Mariner Books; 2011. xix, pp. 412. [Google Scholar]
- 18. Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, Kumanyika S. The look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006; 14: 737–52. Epub 2006/07/21. doi: 14/5/737 [pii] 10.1038/oby.2006.84. PubMed PMID: 16855180; PMCID: 2613279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andersson SW, Niklasson A, Lapidus L, Hallberg L, Bengtsson C, Hulthen L. Poor agreement between self‐reported birth weight and birth weight from original records in adult women. Am J Epidemiol 2000; 152: 609–16. PubMed PMID: 11032155. [DOI] [PubMed] [Google Scholar]


