Summary
Objectives
The purpose of this paper is to measure the change in body weight after a 6‐month telephone‐based weight loss intervention in overweight and obese subjects with idiopathic intracranial hypertension (IIH) and mild visual loss randomized to receive either acetazolamide or placebo.
Methods
One hundred sixty‐five subjects with IIH, aged 29.1 ± 7.5 (mean ± SD) and BMI 39.9 + 8.3 kg/m2, enrolled at 38 academic and private practice sites in North America, participated in this trial. This was a randomized, double‐masked, placebo‐controlled trial of acetazolamide in subjects with IIH and mild visual loss. All participants received a reduced‐sodium, weight‐reduction diet and a 6‐month telephone‐based weight loss intervention. Six‐month changes from baseline in body weight, perimetric mean deviation as assessed by automated perimetry and quality of life using the National Eye Institute Visual Function Questionnaire 25 and the 36‐item Short Form Health Survey were measured.
Results
Mean percent weight change at 6 months was −5.9% ± 6.7% of initial body weight overall, −3.5% ± 5.9% in the placebo group and −7.8% ± 6.8% in the acetazolamide group. Weight change was not associated with changes in either mean deviation or quality of life scores.
Conclusion
Patients with IIH and mild visual loss assigned to either acetazolamide or placebo, all of whom received a 6‐month telephone‐based weight loss intervention, lost an average of 5.9% of initial body weight, consistent with NHLBI guidelines of 5% to 10% of body weight loss for clinically significant health benefit.
Keywords: Adults, clinical research, idiopathic intracranial hypertension, telephone‐based weight loss programme, weight loss
Introduction
Idiopathic intracranial hypertension (IIH) is a disorder of elevated intracranial pressure of unknown cause 1 characterized by headache, pulsatile tinnitus and papilledema 2, 3. Approximately 90% of patients with IIH are overweight or obese women of childbearing age 4. In 2007 overweight and obese IIH patients had a very high hospital admission rate of 38% (perhaps because of poor treatment options), with total hospital costs per IIH admission four times greater than non‐IIH admissions, and total economic costs exceeding $444 million. Despite the rising rate of IIH as a result of the rising rates of obesity and the morbidity and economic costs associated with the condition, IIH remains largely unrecognized in the obesity field.
There are several theories regarding the relationship of weight and IIH. While obesity is strongly associated with IIH, the causal relationship is not known. Whether it is obesity per se, a disorder of sodium metabolism, a factor related to increased food intake such as increased intake of vitamin A, or an abnormality of an obesity hormone, is unclear.
A second theory is that alveolar hypoventilation leads to retention of carbon dioxide, resulting in increased intracranial pressure 5. Finally, some authors have suggested that extraovarian conversion of androstenedione to oestrogen by adipocytes leads to increased intracranial pressure 6.
Patients with IIH usually have severe and constant headaches and pulsatile tinnitus. The latter symptom is a type of ear noise, also known as pulse synchronous tinnitus, because it is often in rhythm with the heartbeat and can be experienced as a thumping or whooshing sound thought to be due to turbulent flow through a stenosed lateral venous sinus 7. Another symptom in patients with IIH is binocular double vision, usually caused by unilateral or bilateral abducens (sixth cranial) nerve paresis. The main morbidity of IIH, however, is permanent visual loss from chronic papilledema – swelling of the optic nerve head caused by increased intracranial pressure 8; thus, it is imperative to intervene in a timely fashion before vision is irreversibly lost. Treatments that have been used are all intended to lower intracranial pressure and include weight loss programmes, bariatric surgery, medications such as acetazolamide and topiramate that reduce the production of cerebrospinal fluid (CSF), repeated lumbar punctures with removal of CSF, optic nerve sheath fenestration to allow CSF that is surrounding and compressing the optic nerve to escape into the orbit and procedures that divert CSF to another location such as the abdominal cavity. Until recently 6, however, none had been consistently effective in preserving or improving vision in a randomized trial.
Three small prospective studies suggest that IIH can be treated successfully with weight loss. Newborg 9 treated nine patients with a dietary regimen of 400 to 1000 kcal/d. Subjects lost 27% of their initial body weight and papilledema resolved completely in all. Sinclair et al. 10 treated 25 women for 3 months with a very low‐calorie, liquid diet. Subjects lost an average of 15.3% of body weight, and all three objective measures of papilledema (optic disc elevation, optic nerve diameter and retinal nerve fibre layer thickness) improved. Sugerman et al. 11 demonstrated in eight morbidly obese women complete resolution of papilledema and resolution or marked reduction of headache after Roux‐en‐Y gastric bypass surgery.
Retrospective studies also report an association between weight loss and papilledema improvement. Johnson et al. 12 observed that of 15 patients with papilledema treated with weight loss, acetazolamide, or both, 10 (66.7%) had resolution and one had improvement of papilledema during the 24‐week study period. Patients whose papilledema completely resolved had a mean loss of 6.2% of body weight. Four acetazolamide‐treated patients who did not lose weight had no improvement in papilledema, whereas one patient who did not take acetazolamide but did lose weight had complete resolution of papilledema. Kupersmith et al. 13, in a chart review of 58 patients who did not undergo early IIH surgical intervention and had adequate documentation of visual status, papilledema and weight at the baseline evaluation and at 6 months or longer, found that mean time in months to improve one grade for papilledema and visual field in one eye was 4.0 versus 6.7 (p = 0.013) and 4.6 versus 12.2 (p = 0.032), respectively, for the 38 patients with weight loss compared with the 20 patients with no weight loss. Papilledema resolved in 28 of 38 patients with weight loss (mean, 7.6 months) and 8 of 20 without weight loss (mean, 10.2 months; p = 0.352). The authors concluded that weight reduction was associated with more rapid recovery of both papilledema and visual field dysfunction in patients with IIH compared with those who did not lose weight.
We have completed the Idiopathic Intracranial Hypertension Treatment Trial, a multicenter randomized, double‐masked, placebo‐controlled study of acetazolamide‐plus‐diet versus placebo‐plus‐diet in 165 subjects with IIH with mild visual loss 6. Subjects on acetazolamide lost more weight over 6 months (mean −7.9 kg) than those on placebo (mean −3.7 kg; treatment effect −4.1 kg, 95% CI −6.3 to −1.8 kg, p < 0.001), and we found statistically significant acetazolamide‐associated improvement in visual field function, CSF pressure, papilledema grade and quality of life measures. In a mediation analysis, the total effect of acetazolamide on perimetric mean deviation (PMD) in the study eye was estimated to be 0.75 dB (95% CI 0.06 to 1.44 dB, p = 0.03), with the direct effect being 0.72 dB (95% CI 0.02 to 1.42 dB, p = 0.04) and the indirect effect (that mediated through the effect of acetazolamide on weight) being only 0.03 dB (95% CI −0.10 to 0.16 dB, p = 0.64).
The New York Obesity Nutrition Research Center (NYONRC) was charged with designing a telephone‐based weight loss intervention for this multicenter trial with a goal of loss of 6.0% of initial body weight over 6 months. This value has been shown to be associated with improvement in IIH, although with limited data.
The purpose of this paper is to describe the design and present the outcomes of the weight loss intervention and the associations between change in body weight and changes in quality of life.
Methods
The trial was developed by the Neuro‐Ophthalmology Research Disease Investigator Consortium (NORDIC) and was funded by the National Eye Institute (NEI). A detailed description of the overall trial design, and specifically the ophthalmology intervention and outcomes, has been published 6. The current paper describes specifically and in detail the telephone‐based weight loss intervention and weight loss outcomes. This includes a description of the subjects and weight loss coaches; specifics of the educational component including the nutrition, exercise and behaviour intervention; details of the education materials; logistics and outline of the phone counselling sessions; flow of the protocol and statistical analyses.
Design
This was a multicenter randomized, double‐masked, placebo‐controlled trial in which participants were randomly assigned to receive acetazolamide (up to 4 g/d or their maximum tolerated dosage) or matching placebo. In addition, all participants received a weight loss intervention described in the next sections. The study was approved by the Institutional Review Board at each participating site, and individual informed consent was obtained.
Outcomes
Subjects reported to their local study site at baseline and at months 1, 2, 3, 4, 5 and 6 for measurement of body weight, blood pressure, blood work (e.g. electrolytes and complete blood count), complete ocular examination including visual field testing and ophthalmoscopy and other protocol‐specified medical testing. The primary outcome variable was the change in PMD, a measure of global visual field loss, from baseline to month 6 in the most affected eye at baseline (study eye), as assessed by automated perimetry using a Humphrey Field Analyzer 5. Secondary outcome variables included 6‐month changes in papilledema grade, the NEI Visual Function Questionnaire 25 (NEI‐VFQ‐25) 14, the 36‐Item Short Form Health Survey (SF‐36) 15 and body weight.
Subjects
One hundred sixty‐five subjects with IIH were enrolled at 38 academic and private practice sites in North America from March 2010 to November 2012. Subjects aged 18–60 years were eligible if they had IIH characterized by reproducible mild visual loss and bilateral papilledema and had not been treated for the condition.
Weight loss coaches
Our counsellors included dietitians, certified life coaches and social workers experienced with weight loss. They were hired locally and trained for the study by one of the investigators (B. K.) to whom they reported. They also received additional training and counsel at least once a month during the study.
Materials
Educational materials that were previously developed by two of the authors (B. K., R. W.) for the standard NYONRC 52‐week outpatient weight loss programme were modified for telephone counselling and subjects with low vision and possible low reading skills. A workbook was produced and distributed to subjects after randomization. Content of the workbook is described below.
Nutrition treatment
Each subject was prescribed a 500‐ to 1000‐cal deficit food plan to yield 1–2 lb of weight loss per week after weight loss coaches calculated their caloric needs 16. The goal was a balanced diet containing about 20% of calories from protein (lean sources), 25–30% from fat (mono‐ and polyunsaturated sources) and the remainder from carbohydrate (high fibre sources). Subjects were encouraged to use food journals and resources such as online Websites and apps that use the USDA Nutrient Database 17. Subjects were instructed on the use of meal replacements (shakes, bars and frozen meals) and given the option of a partial meal replacement plan 18. Food plans were tailored to meet subjects' preferences and reviewed during phone sessions. Subjects were taught how to restrict their sodium intake. Nutrition topics included weight loss science, portion sizes, food‐label reading, volumizing (large volume of food with low energy density), fibre content, eating out, sodium, alcohol, macronutrients and micronutrients. The single subject who was not overweight (BMI 24.9) received the entire intervention with the exception of caloric restriction.
Exercise
Each subject was encouraged to increase physical activity to reach a goal of 30–60 min of moderate‐intensity exercise five or more days/week or 20–60 min of vigorous‐intensity exercise five or more times/week 19. Exercise topics included aerobic and resistance exercise, determining exercise intensity (heart rate and Borg Scale of Perceived Exertion 20), increasing activities of daily living, goal setting, stretching, maintaining motivation, working out at a gym or at home and walking tips and programmes. Subjects received a pedometer (Yamax SW‐200, Yamax Inc., San Antonio, TX, USA) and were encouraged to wear it daily. Subjects were instructed to provide WLCs with weekly pedometer step counts and number of days the pedometer was worn so that the average number of steps per day could be calculated.
Behaviour treatment
Cognitive behavioural therapy was the foundation of the behaviour intervention. It involves identifying and challenging maladaptive thinking to change behaviour (cognitive restructuring) 21. Behaviour topics included goal setting, relapse prevention, motivation, assertiveness, problem solving, stress management, managing cravings and urges, sleep habits, social support and lifestyle balance.
Counselling sessions
Phone counselling sessions during the first 6 months were 30 min in duration after an initial 1‐h session. The session format was (i) completion of a case report form; (ii) homework review; (iii) check‐in for any issue that occurred during the week that might have distracted the subject from focusing on the phone session content; (iv) a new topic and (v) homework assignment. The case report form was a standardized form to collect self‐reported data including body weight and weighing frequency, compliance with the food plan and sodium restriction, pedometer steps, exercise goals and homework compliance. The WLC entered the data into an online database.
The WLCs had the discretion to teach a topic from the workbook in any order that they determined would best meet the subject's needs with the stipulation that the first seven topics be taught in the order in which they appeared in the workbook. Thus, although it was mandatory for coaches to cover all the material in the workbook during the trial, the order of topics after the first 7 weeks was left to their clinical judgement.
During months 6–12, phone counselling sessions included a review of material presented during the first 6 months. Discussions revolved primarily around whether the subject was losing, gaining or maintaining weight, stress management, relapse prevention, exercise and diet.
Flow
Subjects were assigned a WLC by the NYONRC Study Coordinator within 1 week of randomization and spoke with the coordinator by telephone for a 60‐min initial session. During this session subjects were introduced to the weight loss treatment protocol, nutrition and exercise intervention and the workbook. All WLCs followed a standardized interview. Telephone appointments were set up at the end of the initial session.
Phone counselling schedule
Months 1 to 6: After the initial 1‐h phone session with the WLC, 30‐min phone sessions were conducted weekly.
Months 7 to 12: Thirty‐minute phone sessions were conducted every other week for 2 months, then once a month.
Telephone call logistics
The WLCs called the subjects, and if they reached voicemail, they would leave a message as to when they would call back. Subjects without telephones (n = 4) were provided with pre‐paid cell phones.
Statistical analysis
Regression and correlation analyses were used to examine the relationships between baseline characteristics and 6‐month weight change, weight changes between the first 3 months and between the second 3 months of follow‐up, weight change and changes in PMD and quality of life scores at 6 months, and weight change and change in waist circumference at 6 months.
Results
Table 1 provides a summary of subject characteristics at baseline by treatment group. One hundred sixty‐one subjects (97.6%) were female and 4 (2.4%) were male. The mean age was 29.0 ± 7.4 years ranging from 18 to 52 years. The mean BMI was 39.9 ± 8.3 kg/m2, ranging from 24.9 to 71.1 kg/m2.
Table 1.
Subject characteristics at baseline
| Variable | Acetazolamide (n = 86) | Placebo (n = 79) |
|---|---|---|
| Age, mean (SD), years | 28.2 (6.9) | 30.0 (8.0) |
| Female sex (n, %) | 84 (97.7) | 77 (97.5) |
| Race (n, %) | ||
| Caucasian | 54 (62.8) | 54 (68.4) |
| African‐American | 25 (29.1) | 16 (20.3) |
| Mixed/other | 4 (4.6) | 2 (2.5) |
| Not reported | 3 (3.5) | 7 (8.9) |
| Weight, mean (SD), kg | 108.1 (25.6) | 107.3 (24.5) |
| Body mass index, mean (SD), kg/m2 | 40.0 (8.5) | 39.9 (8.1) |
| Minimum | 24.9 | 26.9 |
| Median | 39.5 | 38.6 |
| Maximum | 61.4 | 71.1 |
Attrition from the trial was 23.6% (n = 39) at 6 months. Thirty‐two were early withdrawals (including two pregnancies), and seven were treatment failures based on specific visual field criteria 7. Because of delays in scheduling premature withdrawal visits, data on weight at month 6 were actually collected from 128 subjects rather than 126 subjects.
Mean weight loss was 3.5% and 7.0% at 6 and 12 months, respectively, for the placebo group; 7.8% and 8.1% for the acetazolamide group and 5.9% and 7.6% for both groups combined (Table 2). Overall, 80% of subjects (n = 99) lost weight, 20% (n = 25) gained weight and two subjects had no change in weight (Figure 1). Sixteen subjects (28%) in the placebo group and nine subjects (13%) in the acetazolamide group gained weight. The precise mechanism for the effect of acetazolamide on weight loss is unknown; however, adverse events in the trial that occurred significantly more frequently in the acetazolamide group that might have lead to a reduction in calorie intake included dysgeusia (a salty, rancid or metallic taste sensation that persists in the mouth), nausea, dyspepsia, vomiting and diarrhoea.
Table 2.
Absolute and percent weight changes at months 6 and 12 by treatment group
| N | Mean | SD | Min | Median | Max | ||
|---|---|---|---|---|---|---|---|
| Weight (kg) change month 6 | Treatment | 57 | −3.7 | 6.1 | −19.3 | −2.4 | 5.9 |
| Placebo | |||||||
| Acetazolamide | 71 | −7.9 | 7.0 | −23.1 | −6.7 | 3.9 | |
| All | 128 | −6.1 | 6.9 | −23.1 | −4.9 | 5.9 | |
| % Weight (kg) change month 6 | Treatment | 57 | −3.5 | 5.9 | −20.0 | −2.0 | 6.0 |
| Placebo | |||||||
| Acetazolamide | 71 | −7.8 | 6.8 | −22.0 | −7.0 | 5.0 | |
| All | 128 | −5.9 | 6.7 | −22.0 | −5.0 | 6.0 | |
| Weight (kg) change month 12 | Treatment | 48 | −7.4 | 8.4 | −27.2 | −7.4 | 12.5 |
| Placebo | |||||||
| Acetazolamide | 51 | −8.5 | 8.8 | −35.4 | −6.5 | 5.9 | |
| All | 99 | −8.0 | 8.6 | −35.4 | −6.8 | 12.5 | |
| % Weight (kg) change month 12 | Treatment | 48 | −7.0 | 7.9 | −26.0 | −7.0 | 15.0 |
| Placebo | |||||||
| Acetazolamide | 51 | −8.1 | 8.2 | −30.0 | −7.0 | 4.0 | |
| All | 99 | −7.6 | 8.1 | −30.0 | −7.0 | 15.0 | |
Figure 1.
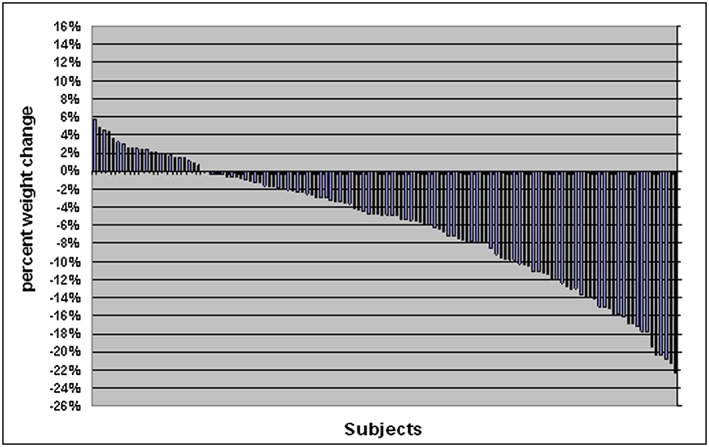
Percent weight change at 6 months by subject (n = 128)
The mean weight change during the first 3 months of follow‐up was −4.5 kg overall (−2.4 kg in the placebo group and −6.3 kg in the acetazolamide group). During the second 3 months, the mean weight change was only −1.6 kg overall (−1.4 kg in the placebo group and −1.7 kg in the acetazolamide group). The weight change during the first 3 months, however, was associated with the weight change during the second 3 months (overall: r = 0.27, p = 0.002; placebo: r = 0.29, p = 0.03; acetazolamide: r = 0.28, p = 0.02).
Six‐month change in PMD in the study eye was not associated with either absolute weight change (r = 0.10, p = 0.26) or percent weight change (r = 0.11, p = 0.20).
The mean change in BMI was −3.0 kg/m2 in the acetazolamide group, −1.3 kg/m2 in the placebo group and −2.2 kg/m2 overall (Table 3). BMI at baseline was not associated with either absolute weight change (r = −0.03, p = 0.70) or percent weight change (r = 0.10, p = 0.24). Similarly, age was not associated with either absolute weight change (r = −0.07, p = 0.46) or percent weight change (r = 0.002, p = 0.99). Mean weight change was not significantly different between Caucasian (−6.1 ± 7.1 kg) and African‐American (−6.3 ± 6.4 kg) subjects (p = 0.17).
Table 3.
Change in BMI from baseline to month 6
| Treatment | N | Mean | SD | Min | Median | Max | p‐value |
|---|---|---|---|---|---|---|---|
| Placebo | 57 | −1.3 | 2.3 | −6.7 | −0.8 | 2.2 | <0.0001 |
| Acetazolamide | 71 | −3.0 | 2.6 | −9.4 | −2.6 | 1.5 | <0.0001 |
| Total | 128 | −2.2 | 2.6 | −9.4 | −1.9 | 2.2 | <0.0001 |
Mean waist circumference changes at 6 months were −4.9 ± 6.4 cm in the placebo group and −7.7 ± 7.4 cm in the acetazolamide group (p < 0.0001 for the change within each group).
Correlations between percent weight change at 6 months and changes in scores of the NEI‐VFQ‐25, its l0‐item neuro‐ophthalmic supplement and the SF‐36 summary and subscale scores at 6 months are given in Table 4. There were no statistically significant correlations between weight change and any of the quality of life scores.
Table 4.
Correlations between percent weight change and changes in scores of the NEI Visual Function Questionnaire 25 [NEI‐VFQ‐25], its l0‐item neuro‐ophthalmic supplement and the 36‐Item Short Form Health Survey [SF‐36] at month 6
| Label | N | Correlation | p‐value |
|---|---|---|---|
| VFQ‐25 total score | 122 | 0.05 | 0.61 |
| VFQ‐25 10‐item supplement score | 119 | −0.03 | 0.76 |
| SF‐36 physical functioning subscale | 121 | −0.10 | 0.29 |
| SF‐36 role‐physical subscale | 121 | 0.05 | 0.66 |
| SF‐36 bodily pain subscale | 121 | 0.07 | 0.47 |
| SF‐36 general health subscale | 121 | −0.20 | 0.04 |
| SF‐36 vitality subscale | 121 | 0.10 | 0.28 |
| SF‐36 social functioning subscale | 121 | −0.0 | 0.80 |
| SF‐36 role‐emotional subscale | 120 | 0.12 | 0.19 |
| SF‐36 mental health subscale | 121 | 0.06 | 0.51 |
| SF‐36 physical component summary | 120 | −0.09 | 0.34 |
| SF‐36 mental component summary | 120 | 0.11 | 0.23 |
Discussion
Idiopathic intracranial hypertension is a relatively rare condition with approximately 90% of patients being overweight or obese women of childbearing age. The main morbidity of IIH is permanent vision loss, with the annual economic costs estimated at greater than $444 million. However, the literature on weight loss and IIH is scarce. We identified only three small prospective studies worthy of mention suggesting that weight loss can improve IIH and two retrospective studies that showed an association between weight loss and IIH improvement.
Neuro‐ophthalmologists who treat this condition are aware that weight loss can improve IIH, but they are not trained to help patients lose weight, and there are few, if any, resources for them to refer patients to for weight loss and especially weight loss programmes that can address some of the specific weight loss challenges for the IIH population, e.g. low socio‐economic status, low‐vision, side effects of IIH medications and the hardships of invasive medical procedures and surgery. In light of this, we designed a telephone‐based weight loss intervention that was provided to all participants in the Idiopathic Intracranial Hypertension Treatment Trial. The weight loss objective was a mean weight loss of 6.0% after 6 months, a value that has been suggested to be associated with improvement in IIH, albeit with limited data. Because of the relative rarity of the condition we needed to recruit from 38 sites across the USA and Canada to adequately power the trial, and because of this the only practical intervention was telephone‐based. We came close to achieving the 6‐month weight loss goal of 6.0% (5.9%) of initial body weight.
Percent weight change from baseline to month 3 correlated positively with percent weight change from months 3 to 6 in both the placebo and acetazolamide groups. The finding of initial weight loss as a predictor of subsequent weight loss is consistent with data from our own outpatient weight loss programme as well as those of others 22.
Mean waist circumference decreased significantly in both the placebo and acetazolamide groups; however, the mean values at 6 months did not fall within the NIH/NHLBI recommended range of less than 101.6 cm (40 in.) for men and 88.9 cm (35 in.) for women.
Overall, 20.0% of subjects gained weight during the trial. This is consistent with results from other weight loss studies 23, 24. We were surprised, however, that more subjects did not lose weight because these subjects knew that weight loss could potentially resolve the disorder that could cause them permanent vision loss or even blindness.
Attrition was 23.6% (126 completers out of 165 randomized). Thirty‐two subjects (19.0%) withdrew prematurely, whereas seven (4.0%) experienced treatment failure (see CONSORT Diagram in 6 for attrition details). We are pleased with this result because attrition tends to be high in weight loss programmes, even with face‐to‐face contact 25, 26, 27.
Initial BMI was not a predictor of weight loss. This is consistent with the results of other phone intervention studies 28 and unpublished data from our own weight loss programme. Age and race were also not predictors of weight loss, with no significant differences in weight loss between African‐American and Caucasian subjects despite evidence to the contrary 29, 30. Racial weight loss differences have been attributed to behavioural, sociocultural and biological factors. We have no reason to believe that weight change would differ between African‐Americans with and without IIH who are overweight.
We were surprised that there were no significant correlations between percent weight change at 6 months and changes in scores of the physical component of the SF‐36 because previous studies have shown that weight loss is associated with improvement in physical function in obese individuals 31, 32, 33. It may be that the SF‐36 is not sensitive enough to detect changes in quality of life due specifically to weight loss. For example, in unpublished data from our own outpatient weight loss programme, where the mean weight loss after 52 weeks is 9.1% of initial body in individuals with a mean starting BMI of 43.1, we measure weight‐related quality of life using the Impact of Weight on Quality of Life questionnaire 34, and our results show that quality of life related specifically to body weight is positively associated with weight loss (R 2 = 0.222, p = .008, n = 148). It may also be that the negative effects of IIH‐related vision disturbances surpass the general benefits of weight loss. And finally, it may simply be that the magnitude of weight loss in this population was, on average, not high enough to yield significant improvements in physical function independent of vision impairment. The National Eye Institute Visual Function Questionnaire 25 measures vision‐related QOL only, items such as night vision, driving, reading and watching movies and television, and therefore was not associated with body weight QOL.
The phone intervention posed special challenges, including scheduling times to speak (all of our WLCs were employed professionals), different time zones, cultural food preferences, regional accents making calls occasionally difficult, loss of the importance of face‐to‐face interactions in establishing a relationship with a subject and ensuring that subjects had sufficient time to focus on the phone sessions (subjects were frequently at home alone with small children – 97.6% of participants were women with mean age 29.1 years).
We chose a telephone‐based intervention because subjects were spread across North America. We were confident that we could achieve the weight loss goal of 6.0% of initial body weight in 6 months because telephone‐based weight loss interventions are consistently associated with weight loss. For example, Sherwood et al. 35 found that subjects participating in a 20‐session telephone intervention lost at least 5% of their initial body weight at 6 months, which is consistent with our results. In a comparison of conference calls to face‐to‐face counselling in 295 overweight and obese men and women, Donnelly et al. 36 found that weight change was −13.4 + 6.7% and −12.3 + 7.0%, for FTF and phone counselling, and importantly, the cost per phone session was $44.07 ± 18.33 in the FTF group and $22.47 ± 13.69 in the phone group, and $1985.05 in the FTF group and $1195.47 in the phone group over 18 months. Group phone counselling may be even more cost‐effective than one‐on‐one phone counselling. In a study of 34 obese rural women, the cost for group versus one‐on‐one phone counselling was $714 and $1029, respectively, while weight loss was robust; 62% of subjects in the group condition and 50% in the individual condition lost at least 10% of their initial body weight 37. The cost for individual phone counselling per subject in our study was $780 for 6 months and $1020 for 12 months. We would consider group calls in the future based on the literature.
Unlike most studies in which the weight loss protocol is standardized throughout the intervention, we permitted WLCs discretion in the order in which they taught workbook material after the first seven lessons. We did this because it is not possible to predict what topics will be relevant at a specific time in an individual's life. For instance, if a subject was facing an immediate family crisis, then the best intervention would most likely be stress management. WLCs reported that this approach was helpful because they observed an unusual amount of stress in this population; divorce, loss of a job in a poor economy, family illness and death, lower socioeconomic status, and in particular the recent diagnosis of a disease that can cause blindness or already living with vision impairment were all stressors that the WLCs believed contributed to attrition and difficulties in losing weight. As a result we recommend that life stress be measured in future IIH studies.
We believe there is merit to the idea of a national telephone‐based weight loss programme for IIH patients. We would not rule out the possibility of an Internet‐based programme as well because data show that online weight loss programmes are also effective, but this has not been demonstrated in IIH patients. A telephone‐based program such as the one in this trial for IIH patients could be funded through the NORDIC, and if further efficacy is proven, then more long‐term programming could be developed. This is a population of individuals who are in desperate need of weight loss, and there is evidence now that it can be carried out.
In summary, we showed that a telephone‐based weight loss intervention in individuals with IIH was associated with a 5.9% mean loss of initial body weight after 6 months, consistent with the IIH literature suggesting that 6.0% loss of body weight is associated with improvement of the condition, and also consistent with NHLBI guidelines of 5% to 10% of body weight loss for clinically significant health benefit. As a result, we believe that a low‐cost, telephone‐based intervention for individuals with a medical condition associated with blindness as the result of excess body weight is warranted and worthy of further investigation.
Conflict of interest
None of the authors declare any conflict of interest.
Funding
This work was supported by National Eye Institute grants 1U10EY017281‐01A1, DCBC 1U10EY017387‐01A1, ARRA for NORDIC 3U10EY017281‐01A1S1, DCBC 1U10EY017387‐01A1S1, NIH grants 2P30DK0126687, supplements for NORDIC 3U10EY017281‐01A1S2, and NIH grant U10 EY017281 for the New York Obesity Research Center.
Acknowledgements
We thank all of our weight loss coaches for their hard work and expertise in helping us reach the weight loss goal and all of our subjects for their commitment to completing the study.
Weil, R. , Kovacs, B. , Miller, N. , McDermott, M. P. , Wall, M. , Kupersmith, M. , Pi‐Sunyer, F. X. , and NORDIC Idiopathic Intracranial Hypertension Study Group (2016) A 6‐month telephone‐based weight loss intervention in overweight and obese subjects with idiopathic intracranial hypertension. Obesity Science & Practice, 2: 95–103. doi: 10.1002/osp4.34.
References
- 1. Thurtell MJ, Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri): recognition, treatment, and ongoing management. Curr Treat Options Neuro 2013. February; 15: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri: population studies in Iowa and Louisiana. Arch Neurol 1988; 45: 875–877. [DOI] [PubMed] [Google Scholar]
- 3. Daniels AB, Liu GT, Volpe NJ, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol 2007; 143: 635–641. [DOI] [PubMed] [Google Scholar]
- 4. Friesner D1, Rosenman R, Lobb BM, Tanne E. Idiopathic intracranial hypertension in the USA: the role of obesity in establishing prevalence and healthcare costs. Obes Rev 2011. May; 12: 372–380. [DOI] [PubMed] [Google Scholar]
- 5. Corbettj J, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow‐up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. ArchNeurol 1982; 39: 461–74. [DOI] [PubMed] [Google Scholar]
- 6. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee , Wall M, McDermott MP, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss – the idiopathic intracranial hypertension treatment trial. JAMA 2014; 311: 1641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 2003; 60: 1418–1424. [DOI] [PubMed] [Google Scholar]
- 8. Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain 1991; 114: 155–180. [PubMed] [Google Scholar]
- 9. Newborg B. Pseudotumor cerebri treated by rice/reduction diet. Arch Intern Med 1974; 133: 802–7. [PubMed] [Google Scholar]
- 10. Sinclair AJ1, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ 2010; 341: c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sugerman HJ, Felton WL 3rd, Salvant JB Jr, et al. Sismanis A, Kellum JM, Effects of surgically induced weight loss on idiopathic intracranial hypertension in morbid obesity. Neurology 1995; 45: 1655–9. [DOI] [PubMed] [Google Scholar]
- 12. Johnson L, Krohel G, Madsen R, March G. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri). Ophthalmology 1998; 12: 2313–2317. [DOI] [PubMed] [Google Scholar]
- 13. Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology 1998. Apr; 50: 1094–8. [DOI] [PubMed] [Google Scholar]
- 14. Mangione CM1, Lee PP, Gutierrez PR, et al. Development of the 25‐list‐item National Eye Institute Visual Function Questionnaire. Arch Ophthalmology 2001; 119: 1050–8. [DOI] [PubMed] [Google Scholar]
- 15. Maruish ME. (ed.). User's Manual for the SF‐36v2 Health Survey. Quality Metric Incorporated: Lincoln, RI, 2011. [Google Scholar]
- 16. Mifflin MD1, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990; 1: 241–7. [DOI] [PubMed] [Google Scholar]
- 17.http://ndb.nal.usda.gov/
- 18. Heymsfield SB1, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord 2003; 27: 537–49. [DOI] [PubMed] [Google Scholar]
- 19. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007; 39: 1423–1434. [DOI] [PubMed] [Google Scholar]
- 20. Borg GA. Perceived exertion. Exerc Sport Sci Rev 1974; 2: 131–53. [PubMed] [Google Scholar]
- 21. Fabricatore AN. Behavior therapy and cognitive‐behavioral therapy of obesity: is there a difference? J Am Diet Assoc 2007; 107: 92–99. [DOI] [PubMed] [Google Scholar]
- 22. Stotland SC, Larocque M. Early treatment response as a predictor of ongoing weight loss in obesity treatment. British Journal of Health Psychology 2005; 10: 601–614. [DOI] [PubMed] [Google Scholar]
- 23. Aronne LJ1, Halseth AE, Burns CM, et al. Miller S, Shen LZ, Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity 2010; 18: 1739–46. [DOI] [PubMed] [Google Scholar]
- 24. Teixeira PJ1, Palmeira AL, Branco TL, et al. Who will lose weight? A reexamination of predictors of weight loss in women. Int J Behav Nutr Phys Act 2004; 1: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Honas JJ, Early JL, Frederickson DD, O'Brien MS. Predictors of attrition in a large clinic‐based weight‐loss program. Obes Res 2003; 11: 888–894. [DOI] [PubMed] [Google Scholar]
- 26. Neve MJ, Collins CE, Morgan PJ. Dropout, nonusage attrition, and pretreatment predictors of nonusage attrition in a commercial Web‐based weight loss program. J Med Internet Res 2010; 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gill RS, Karmali S, Hadi G, Al‐Adra DP, Shi X, Birch DW. Predictors of attrition in a multidisciplinary adult weight management clinic. Can J Surg 2012; 55: 239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeffery RW, Sherwood NE, Brelje K, et al. Mail and phone interventions for weight loss in a managed care setting: weigh‐to‐be one‐year outcomes. Int I Obes Relat Metab Disord 2003; 27: 1584–1592. [DOI] [PubMed] [Google Scholar]
- 29. West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity 2008; 16: 1413–20. [DOI] [PubMed] [Google Scholar]
- 30. Kumanyika SK1, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight‐loss experience of black and white participants in NHLBI‐sponsored clinical trials. Am J Clin Nutr 1991; 53(6 Suppl): 1631S–1638S. [DOI] [PubMed] [Google Scholar]
- 31. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011; 364: 1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santanasto AJ1, Glynn NW, Newman MA, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. Journal of Obesity 2011; 2011: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity 2006; 14: 1219–30. [DOI] [PubMed] [Google Scholar]
- 34. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001 Feb; 9: 102–11. [DOI] [PubMed] [Google Scholar]
- 35. Sherwood NE1, Jeffery RW, Welsh EM, Vanwormer J, Hotop AM. The drop it at last study: six‐month results of a phone‐based weight loss trial. Am J Health Promot 2010; 24: 378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Donnelly JE, Goetz J, Gibson C, et al. Equivalent weight loss for weight management programs delivered by phone and clinic. Obesity 2013 Oct; 21: 1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Befort CA, Donnelly JE, Sullivan DK, Ellerbeck EF, Perri MG. Group versus individual phone‐based obesity treatment for rural women. Eat Behav 2010; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]


