Summary
Objective
To describe differences in weight loss (WL) trajectory patterns at a publicly funded clinical weight management centre.
Methods
Groups with differences in the attainment of a 5% total body WL and percentage WL patterns over time were identified in 7,121 patients who attended a physician lead multi‐disciplinary clinical lifestyle weight management that predominantly focused on education and diet counselling. Resultant health differences were examined.
Results
Patients had 3.2 ± 6.3%WL with 35% of patients achieving and maintaining a 5%WL. Half of these patients achieved the 5%WL within 6 months, while the other half had a more gradual approach. Another 10% achieved 5%WL, but regained weight after 6 months. There were seven distinct WL patterns identified: LargeWL (Mean WL: 21.2 ± 8.1%; Probability of group membership (PGM): 2.4%), ModerateWL (15.1 ± 5.1%WL; 5.4%PGM), SlowWL (6.7 ± 3.2%WL; 20.1%PGM) and MinimalWL (2.4 ± 2.2%WL; 34.6%PGM), WL Regain (9.4 ± 3.5%WL; 8.2%PGM), Weight Stable (1.2 ± 3.2%WL; 28.5%PGM) and Weight Gain (18.4 ± 11.2%WG; 0.8%PGM) groups. Improvements in blood pressure, lipids and glucose were generally related to the magnitude of WL achieved more than the pattern or speed of WL.
Conclusions
There are large differences in the absolute WL attained and the pattern of WL during a publicly funded weight management program. Changes in clinical health markers appear to be more strongly related with the absolute WL attained as opposed to patterns of weight change. © 2016 The Authors. Obesity Science & Practice published by John Wiley & Sons Ltd, World Obesity and The Obesity Society.
Keywords: Cardiovascular risk factors, lifestyle intervention, obesity, weight management
Introduction
Obesity is associated with increased morbidity and mortality, and it is well established that even a modest weight loss (WL) of 5% is associated with health benefits 1, 2, 3. Lifestyle is a cornerstone of weight management and is reported to be associated with a 5–10% WL over 6–12 months 4, but differences in the pattern of WL and longer term weight maintenance are most commonly described as the difference between two time points, and studies rarely report beyond four time points. This means that differences in how individuals lose weight and the prevalence of different WL patterns are not egularly described. Currently, the few studies that examine WL trajectories use treatment 5, 6 or outcome based groupings 7, 8. However, these approaches assume that all individuals who receive a given treatment have a similar efficacy or that those who had a similar program effectiveness arrive at their outcome through a similar pattern. Latent class trajectory analysis allows for the identification of multiple distinct longitudinal WL patterns, without a priori assumptions about how individuals should be grouped. This novel approach would allow for different groups of individuals who attain similar weight outcomes through different patterns of weight change to be identified. This may be important given the disputed health effects of weight cycling. However, we are unaware of a study that has used latent trajectory analyses to examine differences in WL patterns.
The purpose of the present study is to describe differences in WL trajectory patterns over 7.5 years of weight management at a publicly funded adult clinical weight management centre. In addition, demographic factors that are related to differences in these WL patterns and resultant health changes will be examined.
Materials and methods
Study population
The study population included 7,121 overweight and obese adults who attended the Wharton Medical Clinic (WMC) between July 2008 and Dec 2014. Data used was obtained from electronic medical files. Participants were excluded if they only attended the clinic once, and thus did not have WL data. All participants gave their written consent for the use of their medical data for research purposes and were informed that their decision to participate would not influence their medical treatment at WMC. Participants did not receive any form of stipends. All methods were approved by the York University Research Ethics Board (Ethics Certificate #: 2013 – 123).
The WMC has been described previously in detail 9, 10, but briefly, it is a physician referral‐based clinic designed to educate patients about weight management and enable patients to implement strategies to improve their health. The program is delivered by a team of physicians, dieticians, behavioural therapists and exercise specialists. Patients attend the clinic on a monthly basis or as needed to have individual meetings with bariatric educators to discuss personalized weight management strategies, dietary plans and physical activity options.
Nutrition intervention
All patients have a metabolic rate assessment via indirect calorimeter and prediction using the Mifflin St. Joer equation 11 to assist in determining appropriate caloric targets for their meal plan. Patients are prescribed a 500 kcal daily deficit to attain a gradual WL of 1–2 lbs/week. Meal plans range from approximately 1,200 to 2,500 kcal per day. Patients are encouraged to eat six times daily, with three meals and three snacks. Macronutrient content of the meals follows the Health Canada and American Dietitians Association recommendations for protein (20 – 30%), fat (20 – 30%) and carbohydrates (40 – 60%). Meal plans are adjusted based on medical comorbidities, including renal failure.
Activity recommendations
All patients are advised to follow the Canadian Physical Activity Guidelines recommendations of 150 min of physical activity per week. Slow and incremental activity is suggested, and activity is adjusted based on comorbid conditions, including osteoarthritis and muscular disorder.
Mental health counselling
Patients with mental health conditions, eating disorders and emotional eating behaviours are counselled on these conditions. Referrals to psychiatry and eating disorder programs are also given to patients requiring these interventions.
Medications and surgical interventions
Patients who fit the criteria for medication or surgical intervention for obesity are given information and recommendations for these interventions. The WMC prescribes the medications and provides follow‐up care for these patients. The WMC refers and prepares patients for bariatric surgery and follows them post‐operatively.
Follow‐up and maintenance
All patients are followed for as long as they choose to receive care. There is no discharge from the clinic and long‐term maintenance and management of relapse care are provided for all patients. Maintenance involves nutrition, activity, counselling and medical and surgical support as needed.
Patients also undergo a standard battery of clinical tests including standard blood measures, resting metabolic rate, anthropometrics and cardiac testing. Patients meet with physicians to discuss medication options, interest in bariatric surgery and any obesity related comorbidities. If indicated, patients are referred for additional tests, to other medical professionals. Monthly group workshops are also offered on various weight management topics administered by MDs, dietitians, behavioural therapists and exercise specialists. The clinic operates within the Ontario Health Insurance Plan, and all services are provided at no charge to the patient.
Body weight (BW) was measured by staff at each patient visit on a calibrated MedWeight, MS‐2510 Digital High Capacity Platform Scales (Itin Scale Co, Inc., NY). Percent weight reduction was calculated as [(initial BW − final observed BW) / initial BW] × 100%. Clinically significant WL was defined as a minimum of 5% reduction in initial BW. Blood pressure (BP) was assessed at most patient visits. Fasting glucose, triglycerides (TG), low‐density lipoprotein (LDL) and high‐density lipoprotein (HDL) were assessed using standard clinical methods in a subsample of participants (n = 2,847 to 4,696). Patients self‐identified their ethnicity as White, Black, South Asian, East Asian or Other, and were categorized as White or Other for the purposes of these analyses.
Statistical analyses
A mixed‐model, semi‐parametric group‐based modelling technique was used to explore group differences in the shape of the trajectory of weight changes using a customized SAS macro (PROC TRAJ) 12. This allows for the identification of multiple distinct pathways or patterns, while accounting for unobserved heterogeneity in the data 13. All weight changes trajectories were modelled as a function of treatment time to identify: (i) the probability of losing at least 5% of their initial body weight and (ii) percent WL. Similar findings were observed with absolute WL, and so only percent WL values are reported here. The Bayesian Information Criterion (BIC), posterior probabilities and descriptive statistics were used to evaluate the fit of the most parsimonious model 12, 14. Models were chosen based on the lowest BIC or when there was no new meaningful trajectory patterns identified. Once all participants had been assigned to a trajectory group, group‐level profiles, age, sex and initial body mass index (BMI) were examined as independent group predictors relative to the referent group (Group 1). Simple group differences in the patient characteristics at baseline were determined using anova and chi‐square tests with Bonferroni post hoc tests.
Because metabolic variables were not always obtained at the onset of treatment, values at the last available measure were used to impute missing baseline values (50 imputations) (Supplementary Table). Individuals with no recorded values were excluded from each respective analysis. Proc Mianalyze was used with the imputed data to determine differences in metabolic changes between trajectory groups with adjustment for age, sex, initial BMI (or weight change), treatment time, medication use (yes/no) and the respective baseline metabolic value. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was established at P < 0.05.
Results
Within the entire cohort, patients were 51.1 ± 13.0 years of age, had a BMI of 40.3 ± 7.7 kg/m2, with 2.3 ± 1.3 health risk factors at baseline. Patients attended the clinic for an average 12.0 ± 14.6 months with 50% of patients attending the clinic for >6 months, 25% of patients attending >14 months and 10% of patients attending >33 months. Patients had 7 ± 6 visits (range 2 to 53 visits) with 50% of patients attended at least once per month and had an average WL of 3.6 ± 7.7 kg (3.2 ± 6.3%).
When examining trajectory WL patterns for the likelihood of achieving a 5%WL, the four group model emerged as the most parsimonious. The groups were descriptively named with the probability of group membership as follows: (i) noWL (54.4 ± 0.9%—i.e. patients at the clinic have a 54% chance of being in this group); (ii) Slow Weight Loser (WgtLoser—17.7 ± 0.8%); (iii) Fast WgtLoser (18.1 ± 0.7%); and (iv) WgtCycler (9.8 ± 0.7%) (Figure 1A). Participant characteristics for the four group model are presented in Table 1.
Figure 1.
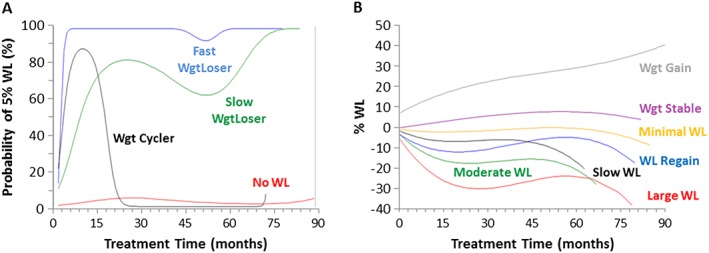
Trajectory patterns for achieving a 5%WL (A) and %WL (B) over treatment time. WL = weight loss.
Table 1.
Participant characteristics in trajectory groups for attaining a 5% weight loss
| Group | No WL | Slow WgtLoser | Fast WgtLoser | Wgt Cycler |
|---|---|---|---|---|
| Probability of group membership | 54.4 ± 0.9% | 17.7 ± 0.8% | 18.1 ± 0.7% | 9.8 ± 0.7% |
| Age (years) | 49.9 ± 13.0 b , c , d | 52.9 ± 12.8a, d | 53.0 ± 12.7a, d | 55.6 ± 12.8 a , b , c |
| Sex (%male) | 24.9 | 28.3 | 27.5 | 26.4 |
| Ethnicity (%White) | 82.7 b , c , d | 88.3a | 90.7a | 90.2a |
| BMI (kg/m2) | 40.1 ± 7.6b | 41.3 ± 8.1a | 40.5 ± 7.8 | 40.5 ± 7.8 |
| Health risk factors (#) | 2.3 ± 1.3 | 2.4 ± 1.4 | 2.3 ± 1.4 | 2.5 ± 1.2 |
| Weight change (kg) | −0.4 ± 4.3 b , c , d | −7.7 ± 7.1 a , c , d | −12.6 ± 8.9a, b, d | −3.2 ± 6.1 a , b , c |
| Weight change (%) | −0.3 ± 3.9 b , c , d | −6.6 ± 5.3 a , c , d | −10.9 ± 5.9a, b, d | −2.9 ± 5.2 a , b , c |
| Treatment time (months) | 9 ± 13 b , c , d | 22 ± 18 a , c | 13 ± 14 a , b , d | 23 ± 17 a , c |
Values are mean ± SD or %.
= Significant difference from noWL (P < 0.05).
= Significant difference from Slow WgtLoser (P < 0.05).
= Significant difference from Fast WgtLoser (P < 0.05).
= Significant difference from Wgt Cycler (P < 0.05).
In general, patients in the noWL group were younger and were less likely to be White than Slow WgtLoser, Fast WgtLoser and WgtCycler (P < 0.05), but with minimal differences in initial BMI, sex or metabolic status. Slow and Fast WgtLoser groups were able to achieve 6.6% and 10.9% WL respectively. Similar to Fast WgtLoser, a large proportion of WgtCycler (~90%; Figure 1A) was able to achieve a 5% WL, but then regained weight after 6 months. Interestingly, Slow WgtLoser and WgtCycler attended the clinic for a longer period of time as compared to the noWL and Fast WgtLoser groups. Age was an independent predictor of group membership, while BMI was only an independent predictor of being in the WgtLoser groups as compared to noWL. Sex was not predictive of group membership.
The noWL group tended to have subtly worse lipid profiles, higher fasting glucose and lower BP than the other groups at the onset of treatment (data not shown). The changes in BP, lipids and glucose for each 5% WL trajectory group adjusted for age, sex, initial BMI, treatment time, medication use and the respective baseline metabolic value are shown in Figure 2. Slow and Fast WgtLoser had superior BP and TG improvements as compared to noWL. Fast WgtLoser also had significantly greater improvements in glucose compared with noWL, with no significant between group differences in LDL and HDL. Although Fast WgtLoser tended to have greater improvements in health than Slow WgtLoser this did not reach statistical significance, except for systolic BP (SBP) (P = 0.01). However, after adjustment for differences in WL, there were no differences in any of the metabolic improvements between Slow and Fast WgtLoser groups (P > 0.05).
Figure 2.
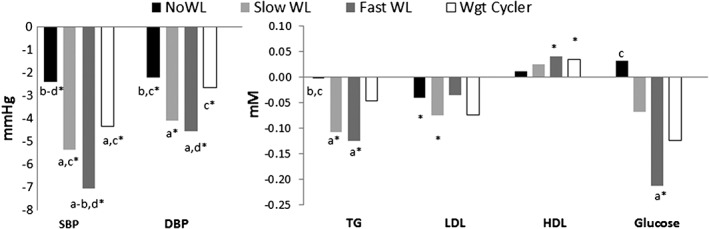
Differences in metabolic changes for trajectory groups achieving a 5% weight loss.Adjusted for age, sex, BMI, treatment time, medication use and the respective baseline metabolic value.*within group change (P<0.05)a = versus noWL (P<0.05)b = versus Slow WgtLoserc = versus Fast WgtLoserd = versus Wgt Cycler
When examining trajectory patterns for percent weight change, the seven group model emerged as the most parsimonious: (i) LargeWL (representing 2.4 ± 0.2% of the sample); (ii) ModerateWL (5.4 ± 0.4%); (iii) WLRegain (8.2 ± 0.6%); (iv) SlowWL (20.1 ± 0.9%); (v) MinimalWL (34.6 ± 1.2%); (vi) WgtStable (28.5 ± 1.1%); and (vii) WgtGain (0.8 ± 0.1%) (Figure 1B). Individuals in the WL groups were younger than the WLRegain group, but older than those in WgtStable and WgtGain groups (Table 2). WgtGain had the lowest initial BMI, the lowest proportion of White individuals and the longest treatment time (27 months), while LargeWL had the highest initial BMI, the highest proportion of White individuals, and a relatively shorter treatment time (13 months). BMI and male sex were all positively and independently associated with being in the LargeWL group. Age was inconsistently associated with group membership.
Table 2.
Participant characteristics in trajectory groups for % weight change
| Group | Large WL | Moderate WL | Regain WL | Slow WL | Minimal WL | Wgt stable | Wgt gain |
|---|---|---|---|---|---|---|---|
| Group membership probability (%) | 2.4 ± 0.2% | 5.4 ± 0.4% | 8.2 ± 0.6% | 20.1 ± 0.9% | 34.6 ± 1.2% | 28.5 ± 1.1% | 0.8 ± 0.1% |
| n | 165 | 291 | 481 | 1,131 | 2,625 | 2367 | 54 |
| Age (years) | 50.6 ± 12.5 c , e | 52.7 ± 13.6 c , e , f | 56.3 ± 11.8 a , b , d , f , g | 53.0 ± 12.7 c , e , f | 55.2 ± 11.5 a , b , d , e , f , g | 44.4 ± 12.3 a , b , c , d , e , f | 48.3 ± 14.4 c , d , e |
| Sex (%male) | 41.8 b , d , e , f , g | 18.6 a , c , e | 35.8 b , d , f | 19.4 a , c , e | 32.7 a , b , d , f | 19.0 a , c , e | 24.1a |
| Ethnicity (%White) | 92.2 e , f , g | 89.5f, g | 88.7f | 90.2 e , f , g | 86.0 a , d , f | 80.3 a , b , c , d , e | 79.2 a , b , d |
| BMI (kg/m2) | 43.9 ± 9.3 b , c , d , e , f , g | 40.4 ± 7.7a, g | 40.5 ± 7.8 a , g | 40.5 ± 7.6 a , g | 40.6 ± 7.9 a , f , g | 39.7 ± 7.4 a , e , g | 35.5 ± 7.7 a , b , c , d , e , f |
| Health risk factors (#) | 2.3 ± 1.3 | 2.3 ± 1.3 | 2.5 ± 1.2 | 2.4 ± 1.4 | 2.5 ± 1.3g | 2.1 ± 1.3f | 1.9 ± 0.9 |
| Weight change (kg) | −27.0 ± 15.5 b , c , d , e , f , g | −17.0 ± 7.8 a , c , d , e , f , g | −10.7 ± 4.9 a , b , d , e , f , g | −7.5 ± 4.2 a , b , c , e , f , g | −2.7 ± 2.7 a , b , c , d , f , g | 1.4 ± 3.8 a , b , c , d , e , g | 18.1 ± 11.6 a , b , c , d , e , f |
| Weight change (%) | −21.2 ± 8.1 b , c , d , e , f , g | −15.1 ± 5.1 a , c , d , e , f , g | −9.4 ± 3.5 a , b , d , e , f , g | −6.7 ± 3.2 a , b , c , e , f , g | −2.4 ± 2.2 a , b , c , d , f , g | 1.2 ± 3.2 a , b , c , d , e , g | 18.4 ± 11.2 a , b , c , d , e , f |
| Treatment time (mo) | 13 ± 14b, c, f, g | 19 ± 17 d , e , f , g | 20 ± 18 a , b , d , e , f , g | 14 ± 13 a , b , c , e , f , g | 11 ± 14 a , b , c , d , f , g | 10 ± 13 a , b , c , d , e , g | 27 ± 24 a , b , c , d , e , f |
Values are mean ± SD or %.
= Significant difference from Large WL (P < 0.05).
= Significant difference from Moderate WL (P < 0.05).
= Significant difference from Regain WL (P < 0.05).
= Significant difference from Slow WL (P < 0.05).
= Significant difference from Minimal WL (P < 0.05).
= Significant difference from Wgt Stable (P < 0.05).
= Significant difference from Wgt Gain (P < 0.05).
WgtStable and WgtGain groups tended to have slightly better baseline BP, but with no consistent pattern of group differences for the lipid or glucose variables (data not shown). BP improvements were greater in groups with greater overall WL adjusted for age, sex, initial BMI, treatment time, medication use and the respective baseline metabolic value (Figure 3A). TG and glucose improvements also tended to be greater in the WL groups than WgtStable, with minimal differences in LDL and HDL (Figure 3B). Adjustment for final WL attained instead of initial BMI abolished most of the significant group differences in metabolic changes (data not shown).
Figure 3.
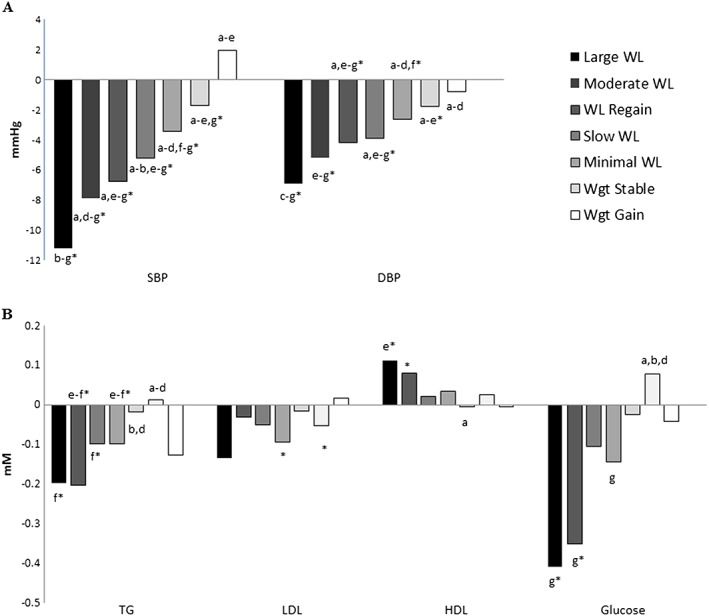
Differences in metabolic changes for percent weight loss trajectory groups.Adjusted for age, sex, BMI, treatment time, medication use and the respective baseline metabolic value.*within group change (P<0.05)a = versus LargeWL (P<0.05)b = versus ModerateWLc = versus WL Regaind = versus SlowWLe = versus MinimalWLf = versus WgtStableg = versus WgtGain
Discussion
Our study expands on traditional examinations of WL, by illustrating not only the patterns of weight change associated with attending a publicly funded clinical weight management program, but also how commonly these weight change patterns occur and the metabolic changes that are associated with these weight change patterns. We observe that there are large variations in the absolute WL, how quickly that WL is attained, and whether or not that WL is maintained over time. However, health changes appear to be more strongly related with the absolute WL attained as opposed to patterns of weight change.
Current weight management guidelines stress the importance of attaining a 5% WL to achieve health benefits 1. Indeed, the Fast and Slow WgtLoser trajectory groups had a large proportion of patients who attained 5%WL, and had significant improvements in several health factors. Although Fast WgtLoser tended to have larger health improvements than Slow WgtLoser, this only reached statistical significance for SBP. We are only aware of a lone study in 10 postmenopausal women that has examined the effect of WL rate on health risk factors 15. That study did not observe any differences in health change between fast and slow WL, but may have been underpowered. However, in accordance with others 16, faster WL is associated with greater WL overall as compared to slower rates of WL, but whether the rate of WL results in differential effects on health warrants further investigation. Interestingly, the currently recommended 1–2 lb/wk WL was created to reduce the risk for gallstone formation and not because of associations with cardiovascular or diabetes health outcomes 17.
Unexpectedly, NoWL and WgtCycler groups also had significant improvements in BP despite not achieving the 5%WL target by the end of the study. We and others 18, 19 have previously demonstrated that clinically relevant health improvements can occur even in the absence of WL. This may be associated with improvement in the quality of diet 20, 21, 22 and incorporation of daily physical activity 23, both of which are associated with health benefits independent of weight change 24. Further, this may be reflective of medication changes, and although we adjusted for medication use, this may not have fully accounted for its effects. Nevertheless, this research together with previous literature suggests that health and obesity may not necessarily track together, and that achieving the 5%WL goal may not necessarily be required for health benefits.
The short and long‐term effectiveness of weight management programs have been previously documented 25, 26, 27, 28, 29. Most studies use the pragmatic approach in examining the mean absolute changes, with a small subset examining changes at a few intermediate time points during the intervention. Latent trajectory analyses cluster groups of individuals with similar patterns of weight change, with the flexibility to model both linear and non‐linear changes over time 12, 14. This is only possible with larger datasets with multiple measures over time, and can be used to assess differences in the pattern of weight changes that may be missed when only examining change scores, or WL trajectories that use a priori groups 5, 6, 7, 8. For example, the entire sample of patients as a whole lost 3.6 kg or 3.2% of their initial body weight; however, after separating the patients into the distinct clusters, several patterns of weight change emerged with several different weight outcomes. In fact, only 35% of the population had a WL in this 3% range, while approximately 8% of the study sample (Moderate and Large WL groups) had mean weight reductions of 15–21%. However, when these groups are followed over time, the mean WL attained for individuals that remain in treatment after 2 years is approximately 20 and 30% respectively (Figure 1B), values that are comparable to bariatric surgery 30.
Very few lifestyle interventions have been able to induce a WL of greater than 15% without the use of very low calorie diets, and none over such a long follow‐up 31. This is important as health improvements based on lifestyle interventions are largely related to the magnitude of lipid and BP improvements 32. Impressively, BP reductions observed in Moderate and Large WL groups are in par with those observed with several types of hypertension medications 33, 34, 35, 36. However, improvements in fasting glucose were inferior to that typically seen with diabetes medications, but are likely in part because of basement effects in patients without diabetes 37, 38. It is unclear what factors distinguish this unique group of obese patients, and future research may focus on genetic, behavioural and environmental factors.
We also observed that 28% of patients (WLRegain and SlowWL) attained a 7.5 to 10.7%WL which is comparable with lifestyle weight management interventions reported in the literature 4. SlowWL had steady WL, while WLRegain had a bout of WL that was followed by weight regain and another WL. Thus, depending on the time point in question, the WLRegain group had a mean WL ranging between 5 and 10%. Although, WLRegain had greater mean WL than SlowWL, both groups had similar final WL attainments using the trajectory modelling. This may explain in part why there were no differences in metabolic changes between groups. Weight cycling has inconsistently reported to be associated with deleterious changes in metabolic health independent of body weight 39. The degree of weight regain observed here is smaller than what is typically observed in studies that end treatments after a predetermined length 31, suggesting that sustained care is associated with better maintenance of WL outcomes. This may indicate that obesity management should be reframed to be a chronic lifelong therapy as opposed to a finite program of predetermined length. Under the Transtheoretical Model 40, relapse is thought to be an inevitable part of any behaviour modification and should be planned for in the treatment strategy. Given our obesogenic environment, expecting that 6–12 month interventions will undo a lifetime of practices and that these newly minted behaviours will persist lifelong is likely unrealistic, and is reflected in the poor weight maintenance seen in studies that adopt this finite treatment model 31.
The remaining 28.5% of the patient cohort were weight stable and a small minority of patients (0.8%) experienced a very significant weight gain. It is unclear why these patients gained weight, but they tended to be younger, have a lower BMI, were more likely to be non‐White and had the longest mean treatment time of all trajectory groups. This may indicate that patients were struggling with other issues that may have interfered with their WL goal or were simply non‐compliant. Indeed, there are several conditions and pharmacological treatments that result in weight gain, such as certain medications for mental disorders 41, diabetes medications 42 and anti‐hypertensives 43. Further, there may be a number of factors that could impair their ability to comply to treatment such as stress, finances, work, family or health. Alternatively, patients could be in the ‘Contemplation’ or even ‘Precontemplation’ stages of the Transtheoretical Model, and may be present at the clinic simply because they were referred by their physician or other external reasons, but are not ready to make the behaviour changes promoted at the clinic. For these patients, the reasons ‘why’ the individual should adopt behaviour change should be stressed until they are ready to engage. Interestingly, despite the large weight gain, there were very few negative metabolic changes observed, although we may have been underpowered despite our large study sample as this group was less than 1% of the patient population (n = 54).
Strengths and limitations of the current study warrant mention. First, although weight changes and BP were assessed frequently throughout the intervention, lipids and glucose were not, and thus we were less certain about the true changes in these factors as compared to body weight and BP. Second, although treatment follow‐up extended over 7.5 years, the average follow‐up lengths were generally less than 2 years, and the weight and health changes after leaving the clinic are unclear. We are unsure if these observations seen here would hold true with other methods of WL such as surgery or medications. Further, there are many factors that can influence health independent of WL that may have obscured our ability to examine the independent effect of WL patterns on health. Our study sample consisted of patients from a publicly funded weight management clinic that was predominately middle aged women of White ethnicity, and the applicability of these findings to other demographics is unclear. Finally, as this was mainly individualized clinical weight management care, we cannot say for certain what specific intervention components or external factors lead to the weight or health changes observed.
In summary, we demonstrate that there are several different patterns of WL observed in patients attending a publicly funded weight management program. However, differences in health changes appear to be more closely related with the absolute WL achieved than the pattern of WL. Nevertheless, those with faster rates of WL tended to have greater WL overall and superior health improvements. Thus, future research may also wish to consider the speed and pattern of weight change when examining health consequences.
Conflict of interest
SW is the Medical Director; JLK held an industry grant by the Mitacs‐Accelerate internship program in partnership with the Wharton Medical Clinic. This study was supported by a grant from the Canadian Institutes of Health Research (#131594) to JLK. Funders had no role in this study.
Author roles
The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JLK wrote the manuscript and analysed the data. SW and JLK collected the data and SW revised the manuscript for important intellectual content. All authors contributed to the concept and design of the study, interpretation of the data and had approval of the final submitted version of the manuscript.
Supporting information
Supporting info item
Acknowledgements
The authors would like to gratefully acknowledge the patients who volunteered their medical data for the research study. The study could not have been completed without the administrative support of Kristin Serodio and the staff at each of the Wharton Medical Clinic‐Weight Management Centres, as well as the help of the York University volunteer students, Dr. Chris Ardern, PhD for reviewing the manuscript and Dr. Michael Rotondi, PhD for providing his statistical expertise.
Kuk, J. L. , and Wharton, S. (2016) Differences in weight change trajectory patterns in a publicly funded adult weight management centre. Obesity Science & Practice, 2: 215–223. doi: 10.1002/osp4.35.
References
- 1. Jensen MD, Ryan DH, Donato KA, et al. Executive summary: guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014; 22: S5–S39. [DOI] [PubMed] [Google Scholar]
- 2. Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 2007; 176: S1–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Practical Guide Identification , Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health: Bethesda, 2000. [Google Scholar]
- 4. Kushner RF, Ryan DH. Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. JAMA. 2014; 312: 943–952. [DOI] [PubMed] [Google Scholar]
- 5. Kullgren JT, Troxel AB, Loewenstein G, et al. Individual vs. group‐based incentives for weight loss: a randomized, controlled trial. Ann Intern Med 2013; 158: 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franz MJ, VanWormer JJ, Crain AL, et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc 2007; 107: 1755–1767. [DOI] [PubMed] [Google Scholar]
- 7. The Look ARG . Eight‐year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obes (Silver Spring, Md) 2014; 22: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neve M, Morgan PJ, Collins CE. Weight change in a commercial Web‐based weight loss program and its association with website use: cohort study. J Med Internet Res 2011; 13: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu RH, Wharton S, Sharma AM, Ardern CI, Kuk JL. Difference in weight loss based on ethnicity, age and comorbidity status in a publicly funded adult weight management centre: 1‐year results. Clin Obes 2013; 3: 21–31. [DOI] [PubMed] [Google Scholar]
- 10. Wharton S, VanderLelie S, Sharma AM, Sharma S, Kuk JL. Feasibility of an interdisciplinary program for obesity management in Canada. Can Fam Physician 2012; 58: e32–38. [PMC free article] [PubMed] [Google Scholar]
- 11. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990; 51: 241–247. [DOI] [PubMed] [Google Scholar]
- 12. Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Socio Methods Res 2001; 29: 374–393. [Google Scholar]
- 13. Dodge HH, Shen C, Ganguli M. Application of the pattern‐mixture latent trajectory model in an epidemiological study with non‐ignorable missingness. J Data Sci: JDS 2008; 6: 247–259. [PMC free article] [PubMed] [Google Scholar]
- 14. Jones BL, Nagin DS. Advances in group‐based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res 2007. 2007; 35: 542–571. [Google Scholar]
- 15. Sénéchal M, Arguin H, Bouchard DR, et al. Effects of rapid or slow weight loss on body composition and metabolic risk factors in obese postmenopausal women. A pilot study. Appetite 6// 2012; 58: 831–834. [DOI] [PubMed] [Google Scholar]
- 16. Nackers L, Ross K, Perri M. The association between rate of initial weight loss and long‐term success in obesity treatment: does slow and steady win the race? Int J Behav Med 2010/09/01 2010; 17: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weinsier RL, Wilson LJ, Lee J. Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med 2// 1995; 98: 115–117. [DOI] [PubMed] [Google Scholar]
- 18. Liu RH, Wharton S, Sharma AM, Ardern CI, Kuk JL. Influence of a lifestyle‐based weight loss on the metabolic risk profile of metabolically normal and abnormal obese adults. Obesity 2013; 21: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 19. Ross R, Janiszewski PM. Is weight loss the optimal target for obesity‐related cardiovascular disease risk reduction? Can J Cardiol 2008; 24 (Suppl D): 25d–31d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwab U, Lauritzen L, Tholstrup T, et al. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res 2014; 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013; 88: 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lippi G, Cervellin G, Mattiuzzi C. Red meat, processed meat and the risk of venous thromboembolism: friend or foe? Thromb Res 2015; 136: 208–211. [DOI] [PubMed] [Google Scholar]
- 23. Physical Activity and Health: A Report of the Surgeon General. National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Department of Health and Human Services: Atlanta (GA), 1996. [Google Scholar]
- 24. Wylie‐Rosett J, Mossavar‐Rahmani Y, Gans K. Recent dietary guidelines to prevent and treat cardiovascular disease, diabetes, and obesity. Heart Dis 2002; 4: 220–230. [DOI] [PubMed] [Google Scholar]
- 25. Agborsangaya CB, Majumdar SR, Sharma AM, Gregg EW, Padwal RS. Multimorbidity in a prospective cohort: prevalence and associations with weight loss and health status in severely obese patients. Obesity 2015; 23: 707–712. [DOI] [PubMed] [Google Scholar]
- 26. Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight‐loss programs: an updated systematic review. Ann Intern Med. 2015; 162: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johns DJ, Hartmann‐Boyce J, Jebb SA, Aveyard P. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta‐analysis of direct comparisons. J Acad of Nutr Diet 2014; 114: 1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loveman E, Frampton GK, Shepherd J, et al. The clinical effectiveness and cost‐effectiveness of long‐term weight management schemes for adults: a systematic review. Health Technol Assess 2011; 15: 1–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lang A, Froelicher ES. Management of overweight and obesity in adults: behavioral intervention for long‐term weight loss and maintenance. Eur J Cardiovasc Nurs: journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology. 2006; 5: 102–114. [DOI] [PubMed] [Google Scholar]
- 30. Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non‐surgical treatment for obesity: a systematic review and meta‐analysis of randomised controlled trials. BMJ. 2013; 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dombrowski SU, Knittle K, Avenell A, Araújo‐Soares V, Sniehotta FF. Long term maintenance of weight loss with non‐surgical interventions in obese adults: systematic review and meta‐analyses of randomised controlled trials. BMJ: Br Med J 2014; 348: g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dattilo AM, Kris‐Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta‐analysis. Am J Clin Nutr 1992; 56: 320–328. [DOI] [PubMed] [Google Scholar]
- 33. Musini VM, Nazer M, Bassett K, Wright JM. Blood pressure‐lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst Rev (Online). 2014; 5: Cd003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta‐blockers for hypertension. Cochrane Database Syst Rev (Online) 2012; 11: Cd002003. [DOI] [PubMed] [Google Scholar]
- 35. Musini VM, Fortin PM, Bassett K, Wright JM. Blood pressure lowering efficacy of renin inhibitors for primary hypertension: a Cochrane systematic review. J Hum Hypertens 2009; 23: 495–502. [DOI] [PubMed] [Google Scholar]
- 36. Makani H, Bangalore S, Supariwala A, Romero J, Argulian E, Messerli FH. Antihypertensive efficacy of angiotensin receptor blockers as monotherapy as evaluated by ambulatory blood pressure monitoring: a meta‐analysis. Eur Heart J 2014; 35: 1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005; 65: 385–411. [DOI] [PubMed] [Google Scholar]
- 38. Waugh J, Keating GM, Plosker GL, Easthope S, Robinson DM. Pioglitazone: a review of its use in type 2 diabetes mellitus. Drugs 2006; 66: 85–109. [DOI] [PubMed] [Google Scholar]
- 39. Mehta T, Smith DL, Jr. , Muhammad J, Casazza K. Impact of weight cycling on risk of morbidity and mortality. Obes Rev 2014; 15: 870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zimmerman GL, Olsen CG, Bosworth MF. A ‘stages of change’ approach to helping patients change behavior. Am Fam Physician 2000; 61: 1409–1416. [PubMed] [Google Scholar]
- 41. Hasnain M, Vieweg WV. Weight considerations in psychotropic drug prescribing and switching. Postgrad Med 2013; 125: 117–129. [DOI] [PubMed] [Google Scholar]
- 42. Purnell TS, Joy S, Little E, Bridges JF, Maruthur N. Patient preferences for noninsulin diabetes medications: a systematic review. Diabetes Care 2014; 37: 2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uguz F, Sahingoz M, Gungor B, Aksoy F, Askin R. Weight gain and associated factors in patients using newer antidepressant drugs. Gen Hosp Psychiatry 2015; 37: 46–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


