Abstract
Background
Thoracic aortic aneurysm (TAA) outcomes are worse in women than men, although reasons for sex differences are unknown. Because faster TAA growth is a risk factor for acute aortic syndromes, we sought to determine the role of sex and aneurysm etiology on TAA growth.
Methods and Results
Eighty‐two consecutive unoperated subjects with TAA who had serial aneurysm measurements were recruited. In multivariable linear regression the association of female sex with aneurysm growth rate was assessed after adjustment for potential confounders. We also tested the interaction term sex×aneurysm etiology in the prediction of TAA growth. Seventy‐four percent of subjects were men; mean±SD age was 62.4±11.9 years in men and 67.7±10.7 years in women (P=0.06). Forty‐seven (57%) subjects had degenerative TAAs, and the remainder had heritable TAAs. Absolute baseline aneurysm size and follow‐up time were not different between men and women. Aneurysm growth rate was 1.19±1.15 mm/y in women and 0.59±0.66 mm/y in men (P=0.02). Female sex remained significantly associated with greater aneurysm growth in multivariable analyses (β±SE: 0.35±0.12, P=0.005). In addition, female sex was associated with faster TAA growth only among those with degenerative TAA (β±SE: 0.33±0.08, P=0.0002) and not among those with heritable TAA (P=0.79), with a significant sex×etiology interaction (P=0.001).
Conclusions
TAA growth rates are greater in women than men, and this difference is specific to women with degenerative TAAs. Our findings may explain sex differences in TAA outcomes and provide a foundation for future investigations of this topic.
Keywords: aneurysm, aorta, hypertension, thoracic aortic aneurysm, women
Subject Categories: Etiology, Women, Risk Factors, Aneurysm, Aortic Dissection
Introduction
Thoracic aortic aneurysms (TAAs) are considered “silent killers” because they seldom produce symptoms but are associated with high morbidity and mortality.1 As many as 22% of people who suffer an acute aortic syndrome die at home before receiving medical attention,2, 3 and among those who reach the hospital alive, 34% die within the first 30 days.2 Despite these somber statistics, TAA remains significantly understudied when compared to other cardiovascular or systemic diseases. Therefore, there is a need to enhance our understanding of the pathophysiology of TAA in order to improve clinical care and outcomes of this deadly disease.
Over the past few decades natural history studies have taught us about the epidemiology and outcomes of TAA.4, 5, 6, 7 Importantly, although TAAs are more prevalent in men,2 its prognosis is worse in women. Women with TAA are more likely to have an aortic dissection or rupture,1, 8 especially at smaller aneurysm sizes,9 and are more likely to die than men with TAA.9, 10 These findings persist despite indexing aneurysm size to body size,11 suggesting that the mechanism leading to adverse TAA‐related outcomes in women may be independent of aneurysm dimensions. Despite these known sex differences, reasons for worse TAA outcomes in women remain poorly understood and underexplored.
In regard to TAA outcomes, the growth rate of the aneurysm is a relevant parameter for risk assessment and monitoring. Faster aneurysm growth is an established risk factor for dissection/rupture,12, 13, 14 and for this reason guidelines recommend prophylactic surgical repair when aneurysm growth is greater than 0.5 cm/y.15, 16 However, there are no separate criteria based on sex, and whether TAA growth rates differ between men and women is unknown. Thus, in light of the reported worse TAA outcomes in women, we hypothesized that aneurysm growth rates would be greater in women than men. To address this hypothesis, we conducted a retrospective study of subjects with TAAs with different etiologies with the objective of documenting and comparing aneurysm growth rates between men and women. In addition, we sought to determine whether etiology of the aneurysm plays a role in the association of sex with aneurysm growth.
Methods
Study Participants and Assessment of Baseline Characteristics
Study participants consisted of unoperated men and women with TAA recruited from the University of Ottawa Heart Institute's Aorta Clinic. Those with ≥2 previous aneurysm measurements ≥6 months apart were considered eligible. Exclusion criteria included a previous history of aortic dissection, rupture, intramural hematoma, or surgery. The study was approved by University of Ottawa Heart Institute's Research Ethics Board, and participants gave informed consent.
Between February 2014 and February 2016, 82 consecutive participants were enrolled. At study enrollment, participants filled out a standard questionnaire about personal and family history and medication use, gave a blood sample, and had anthropometric data collected. Hypertension was defined by physician diagnosis and/or current treatment with antihypertensives. Diabetes mellitus was defined by physician diagnosis and/or use of insulin or oral hypoglycemic medications. Dyslipidemia was defined by physician diagnosis and/or use of lipid‐lowering medications. Smoking was defined as having smoked more than 100 cigarettes in the past. Weight was measured in kilograms using an electronic scale, and height was measured in meters by a stadiometer. BMI was calculated as weight (kg) divided by the square of height (m2). Body surface area (BSA) was calculated using the Gehan method.17 Blood pressure was measured using a sphygmomanometer 3 times, with 2‐minute intervals between readings, and their average was used for analyses. Mean arterial pressure (MAP) was calculated as 2/3 of the systolic blood pressure +1/3 of the diastolic blood pressure.
TAA etiology was categorized as heritable (hTAA) or degenerative (dTAA). hTAA was defined as being associated with heritable conditions such as bicuspid aortic valve, Marfan syndrome, familial thoracic aorta aneurysm, and dissections or other genetic syndromes known to be associated with TAA. A TAA was considered degenerative if hypertension and/or other atherosclerotic risk factors were present in the absence of known congenital or genetic factors as defined in hTAA above.
Assessment of Aneurysm Size and Growth Rates
All imaging studies had been previously performed in our Institution and were available for retrospective assessment. Imaging modalities assessed included echocardiography, computed tomography (CT), and magnetic resonance imaging (MRI). Maximum thoracic aorta size was measured by an imaging cardiologist (T.C.) in the first available and most recent imaging studies according to published guidelines.18 Whenever possible, the same imaging modality was used to compute aneurysm growth (concordant imaging modality). When the initial imaging modality was not subsequently repeated, measurement of the most recent aneurysm size was performed using a different imaging modality. Aneurysm growth rate was calculated as the absolute growth of the aneurysm divided by the total follow‐up time (mm/y). In addition, because aneurysm size may vary with body size, we also calculated aneurysm growth rates using indexed new and baseline aneurysm sizes [aneurysm size/BSA, (mm/m2)/y].
Statistical Analyses
Continuous variables were reported as mean±standard deviation (SD), and nominal variables were reported as total number and percentages. Differences between men and women were compared using a t test for normally distributed continuous variables, Wilcoxon rank‐sum test for skewed variables and chi‐squared test for nominal variables.
A sample equal to 10% of the original sample size was randomly selected for blinded repeated measures of baseline and follow‐up aneurysm size in order to assess intraobserver variability.
The association of female sex with aneurysm growth rate was assessed with multivariable linear regression models adjusted for parameters that are available for clinical decision making in this population: age, BSA, MAP, baseline aneurysm size, aneurysm etiology, follow‐up time, antihypertensive use, and history of hypertension, diabetes mellitus, dyslipidemia, and smoking. In addition, to determine whether aneurysm etiology modifies the associations of sex with aneurysm size, we included an interaction term for sex×aneurysm etiology in the models. If the interaction term was significant, analyses were repeated after stratifying the sample for aneurysm etiology.
Fast aneurysm growth was defined as aneurysm growth greater than the median value in the sample. We then performed multivariable logistic regression models, adjusted for the aforementioned covariates, to determine the independent association of female sex with fast aneurysm growth. C‐statistics for models with and without female sex were computed.
All analyses were performed using JMP version 12 (SAS Institute, Cary, NC). A 2‐sided P≤0.05 was considered statistically significant.
Results
Participant characteristics are summarized in Table 1. Seventy‐four percent of participants were men, consistent with the known higher prevalence of TAA in men.2 The average age was 62.4±11.9 years in men and 67.7±11.8 years in women (P=0.06). The prevalence of cardiovascular risk factors was similar between men and women. Forty‐seven (57%) subjects had dTAAs; the remainder had hTAAs. The proportion of dTAA to hTAA was similar in men and women (P=0.57). All subjects had aneurysms of the aortic root and/or ascending aorta. Twenty‐four subjects (29%) had maximal aneurysm dilation at the aortic root, and 58 (71%) at the ascending aorta. Absolute aneurysm size was not different between men and women, although indexed aneurysm size was greater in women than men. CT was the most commonly used modality at baseline in men and women. Aneurysm size was assessed using the same imaging modality in 57% of the participants, which was not different between men and women (P=0.57).
Table 1.
Participant Characteristics
| Variable | All (n=82) | Men (n=61) | Women (n=21) |
|---|---|---|---|
| Age, y | 63.8±11.8 | 62.4±11.9 | 67.7±10.7 |
| Height, m | 173.8±8.7 | 176.2±7.1 | 166.6±8.9a |
| Weight, kg | 86.6±17.9 | 89.6±17.5 | 78.1±16.9a |
| BSA, m2 | 2.0±0.2 | 2.1±0.2 | 1.9±0.2a |
| BMI, kg/m2 | 28.6±5.2 | 28.7±4.7 | 28.2±6.7 |
| Hypertension, n (%) | 40 (49%) | 31 (51%) | 9 (43%) |
| Diabetes mellitus, n (%) | 4 (5%) | 3 (5%) | 1 (5%) |
| Dyslipidemia, n (%) | 36 (44%) | 31 (51%) | 5 (24%)a |
| Smoking, n (%) | 48 (58%) | 34 (56%) | 14 (67%) |
| β‐Blocker use, n (%) | 26 (32%) | 20 (32%) | 6 (29%) |
| Aneurysm location (root/ascending aorta) | 24/58 | 24/37 | 0/21a |
| dTAA/hTAA | 47/35 | 36/25 | 11/10 |
| Baseline imaging modality | |||
| Echocardiogram, n (%) | 16 (20%) | 12 (20%) | 4 (19%) |
| CT, n (%) | 58 (70%) | 41 (67%) | 17 (81%) |
| MRI, n (%) | 8 (10%) | 8 (13%) | 0a |
| Concordant imaging modality, n (%) | 47 (57%) | 36 (59%) | 11 (52%) |
| Baseline aneurysm size, mm | 45.6±4.3 | 45.8±4.0 | 45.1±5.1 |
| Indexed baseline aneurysm size, mm/m2 | 22.9±3.0 | 22.4±2.6 | 24.5±3.5a |
| Follow‐up time, y | 3.1±2.8 | 2.9±2.5 | 3.7±3.5 |
| Most recent aneurysm size, mm | 47.2±4.1 | 46.9±3.8 | 48.1±4.6 |
| Indexed most recent aneurysm size, mm/m2 | 23.8±3.0 | 23.0±2.6 | 26.1±3.0a |
| TAA growth rate, mm/y | 0.74±0.85 | 0.59±0.66 | 1.19±1.15a |
| Indexed TAA growth rate, (mm/m2)/y | 0.38±0.45 | 0.29±0.32 | 0.65±0.65a |
Concordant imaging modality was defined as the same imaging modality used in the first and last imaging studies. BMI indicates body mass index; BSA, body surface area; CT, computed tomography; dTAA, degenerative thoracic aortic aneurysm; hTAA, heritable thoracic aortic aneurysm; MRI, magnetic resonance imaging; TAA, thoracic aortic aneurysm.
P≤0.05 when compared to men.
Repeatability analyses revealed that there was excellent intraobserver agreement (see Figure 1). For baseline aneurysm size, Spearman correlation coefficient=0.98, P<0.0001. The Bland‐Altman plot revealed that the mean difference was 0.34 mm (95% confidence interval [CI]: −0.32, 1.00 mm), and that the bias was not different from zero (P=0.26). For follow‐up aneurysm size, Spearman correlation coefficient=0.98, P<0.0001. The Bland‐Altman plot revealed that the mean difference was −0.31 mm (95% CI: −1.12, 0.51 mm), and the bias was not different from zero (P=0.40).
Figure 1.
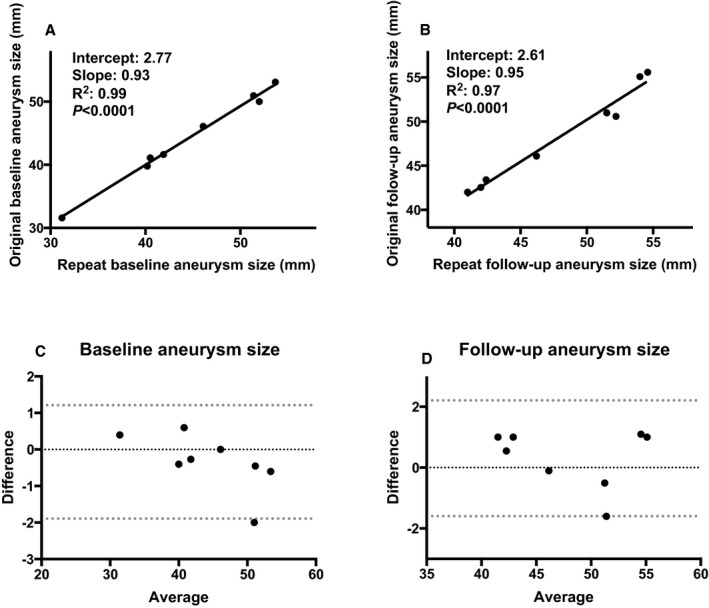
Intraobserver agreement analyses (n=8). A, Linear regression for baseline aneurysm size. There was excellent correlation between the original and repeated measurements. B, Linear regression for follow‐up aneurysm size. There was excellent correlation between the original and repeated measurements. C, Bland‐Altman plot for baseline aneurysm size. The bias was not different from zero (P=0.26). D, Bland‐Altman plot for follow‐up aneurysm size. The bias was not different from zero (P=0.40).
The average follow‐up time was 3.1±2.8 years, which was similar between men and women (P=0.61). Median (interquartile range) aneurysm growth rate in the whole sample was 0.38 (0.13‐1.09) mm/y. TAA growth rates were over twice as fast in women as in men (Table 1 and Figure 2). Sex differences in aneurysm growth rates persisted once indexed aneurysm sizes were used to compute growth rates (Table 1). Results of the multivariable linear regression model to predict absolute aneurysm growth rate are depicted in Table 2. Of note, baseline aneurysm size and etiology were not independently associated with rate of growth. We found that women had an average 0.35 mm/y greater aneurysm growth rate than men after adjustment for potential confounders (Table 2). The only other independent predictor of aneurysm growth rate was follow‐up time: for each 1‐year increase in follow‐up time, there was a lower 0.12 mm/y TAA growth, which likely reflects the fact that indolent aneurysms can be observed for longer periods of time, whereas aggressive aneurysms are surgically corrected. When we used indexed aneurysm growth rate as the dependent variable instead, female sex remained independently associated with greater aneurysm growth (Table 3), with an average 0.21 (mm/m2)/y greater indexed TAA growth rate than men. As in the aforementioned model, the only other independent predictor of indexed aneurysm growth rate was follow‐up time: for each 1 year increase in follow‐up time, there was a lower 0.06 (mm/m2)/y TAA growth. Inferences remained unchanged whether models were unadjusted, age‐adjusted, or adjusted only for variables univariately associated with the dependent variable (analyses not shown).
Figure 2.
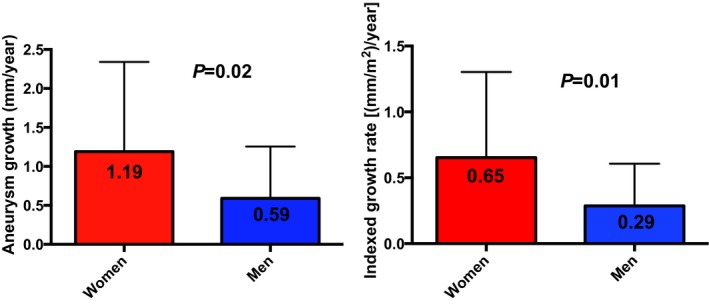
Aneurysm growth rates in men and women. Absolute (left) and indexed (right) aneurysm growth rates were over twice as fast in women as in men.
Table 2.
Multivariable Linear Regression Models to Predict Absolute Aneurysm Growth Rate (mm/y)
| Variable | β±SE | P Value |
|---|---|---|
| Female sex | 0.35±0.12 | 0.005 |
| Age, y | 0.009±0.01 | 0.40 |
| BSA, m2 | 0.18±0.53 | 0.74 |
| Mean arterial pressure, mm Hg | 0.006±0.009 | 0.53 |
| Aneurysm etiology (dTAA) | 0.02±0.13 | 0.89 |
| Baseline aneurysm size, mm | −0.03±0.02 | 0.14 |
| Follow‐up time, y | −0.12±0.04 | 0.001 |
| Hypertension | 0.09±0.16 | 0.58 |
| Antihypertensive use | 0.06±0.15 | 0.68 |
| Diabetes mellitus | −0.07±0.22 | 0.76 |
| Dyslipidemia | −0.02±0.10 | 0.83 |
| Smoking | 0.08±0.10 | 0.42 |
BSA indicates body surface area; dTAA, degenerative thoracic aortic aneurysm.
Table 3.
Multivariable Linear Regression Model to Predict Indexed Aneurysm Growth Rate [(mm/m2)/y]
| Variable | β±SE | P Value |
|---|---|---|
| Female sex | 0.21±0.06 | 0.001 |
| Age, y | 0.004±0.006 | 0.45 |
| Mean arterial pressure, mm Hg | 0.002±0.005 | 0.62 |
| Aneurysm etiology (dTAA) | 0.04±0.06 | 0.52 |
| Indexed baseline aneurysm size, mm/m2 | −0.006±0.02 | 0.73 |
| Follow‐up time, y | −0.06±0.02 | 0.003 |
| Hypertension | 0.04±0.08 | 0.65 |
| Antihypertensive use | 0.02±0.08 | 0.75 |
| Diabetes mellitus | −0.08±0.11 | 0.47 |
| Dyslipidemia | −0.02±0.05 | 0.69 |
| Smoking | 0.03±0.05 | 0.59 |
dTAA indicates degenerative thoracic aortic aneurysm.
The sex×aneurysm etiology interaction term was significant (P=0.003). Thus, we repeated the models after stratifying by aneurysm etiology. Baseline aneurysm size was similar in dTAA and hTAA subjects (45.5±4.7 and 45.8±3.7 mm, P=0.78, respectively, for absolute size and 23.2±3.3 and 22.6±2.6 (mm/m2)/y, P=0.36, respectively, for indexed size). Aneurysm growth rates in men and women with dTAA and hTAA are shown in Figure 3. Aneurysm growth was over 3 times greater in women than men with dTAA (1.74±1.17 vs 0.55±0.59, P=0.0009). However, among hTAA subjects, growth rates were similar in men and women (0.65±0.76 in men, 0.58±0.79 in women, P= 0.83). Results of etiology‐specific multivariable models are shown in Table 4. We found that female sex was only independently associated with faster aneurysm growth among subjects with dTAA.
Figure 3.
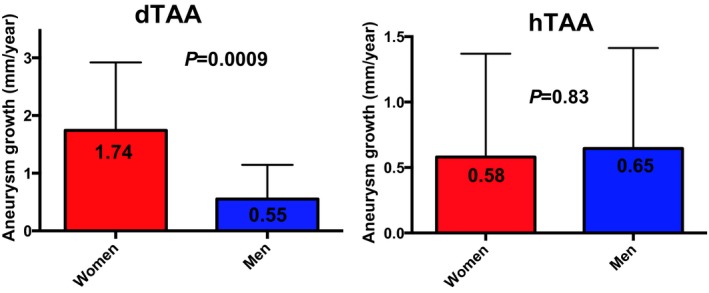
Aneurysm growth rates in men and women based on aneurysm etiology. dTAA indicates degenerative thoracic aortic aneurysm; hTAA, heritable thoracic aortic aneurysm. Among subjects with dTAA, aneurysm growth rates were over 3 times greater in women than in men. However, in subjects with hTAA, aneurysm growth was similar in men and women.
Table 4.
Results of Multivariable Linear Regression Models Stratified by Aneurysm Etiology — Indexed Aneurysm Growth Rate [(mm/m2)/y] as Dependent Variable
| Variable | dTAA (n = 47) (β±SE) | hTAA (n = 35) (β±SE) |
|---|---|---|
| Female sex |
0.33±0.08 P = 0.0002 |
0.03±0.10 P = 0.79 |
| Age, y |
0.01±0.008 P = 0.17 |
0.0005±0.01 P = 0.96 |
| Mean arterial pressure, mm Hg |
−0.005±0.007 P = 0.51 |
0.004±0.007 P = 0.56 |
| Indexed baseline aneurysm size, mm/m2 |
−0.03±0.02 P = 0.21 |
0.007±0.04 P = 0.86 |
| Follow‐up time, y |
−0.06±0.03 P = 0.02 |
−0.04±0.03 P = 0.24 |
| Hypertension |
−0.05±0.12 P = 0.68 |
0.13±0.14 P = 0.33 |
| Antihypertensive use |
0.14±0.12 P = 0.27 |
−0.08±0.10 P = 0.41 |
| Diabetes mellitus |
−0.04±0.13 P = 0.72 |
−0.18±0.24 P = 0.47 |
| Dyslipidemia |
−0.08±0.07 P = 0.26 |
0.009±0.09 P = 0.92 |
| Smoking |
0.01±0.02 P = 0.85 |
0.08±0.08 P = 0.33 |
In sensitivity analyses we separately evaluated subjects who had had concordant and discordant imaging modalities and found that inferences remained unchanged: the independent association of female sex with faster aneurysm growth among dTAA subjects remained significant among those who had concordant (β±SE 0.21±0.10, P=0.05) and discordant (1.09±0.32, P=0.01) imaging modalities between the baseline and the follow‐up study. No other clinical variables were independently associated with aneurysm growth rate in dTAA subjects with concordant or discordant imaging.
Lastly, the multivariable logistic regression model showed that women had 6.3‐fold greater odds of having fast aneurysm growth than men (95% CI: 1.4, 33.8. P=0.01). The C‐statistic for the multivariable model that did not include female sex was 0.78. By adding female sex to the model, the C‐statistic increased to 0.82 (P=0.01).
Discussion
To better understand potential reasons for the sex differences in TAA‐related outcomes, we assessed the effect of female sex on TAA growth. To the best of our knowledge, this is the first study to assess sex differences in TAA growth rates. Our findings confirm our hypothesis that TAA grows faster in women despite adjustment for body size and other relevant parameters used for clinical assessment. Furthermore, we found that sex differences in aneurysm growth are only present in subjects with dTAA and that growth rates in hTAA subjects are similar between men and women. Our study brings novel insights into sex‐specific TAA behavior and provides a potential explanation for the higher risk of TAA dissection, rupture, and death in women.
Sex Differences in Aneurysm Outcomes and Growth
We found that although absolute aneurysm size was similar between men and women, baseline indexed aneurysm size was greater in women, highlighting the need to correct aneurysm size to body size when clinically assessing TAA patients. Importantly, we demonstrated that women experienced greater aneurysm growth rates than men despite adjustment for baseline aneurysm size and other clinically relevant covariates.
Epidemiology and natural history studies of TAA demonstrate that women have a 40% increase in the risk of mortality10 and a 3‐fold increase in the risk of TAA dissection or rupture compared to men.8 In addition, there is evidence that acute aortic syndromes may occur in women despite a relatively small aneurysm size. In a study of 613 subjects with TAA, the proportion of women was much greater among those who had an aortic dissection at aortic diameters <3.5 cm (51% women) than among those who had an aortic dissection at aortic diameters >3.5 cm (29% women).19 These data confirm the worse TAA‐related outcomes in women and indicate that aneurysm size alone does not capture the complexity of aneurysm‐related risk in women. However, the mechanisms for these sex differences remain unclear. Based on our findings, we can postulate that faster TAA growth in women may at least partially explain the sex differences in TAA outcomes.
The epidemiology of TAA is opposite to that of another vascular disease: pulmonary arterial hypertension (PAH). TAA is more common in men but has poorer outcomes in women; PAH is more common in women but has worse prognosis in men. Interestingly, men with PAH are older than women,20 whereas the opposite is true for TAA.21 The older age of men with PAH and women with TAA may partially explain the poorer outcomes. Alternatively, if there are any biological factors that protect men from PAH and women from TAA (thus justifying the lower prevalence of disease), perhaps men who develop PAH and women who develop TAA have more extensive arterial abnormalities and/or comorbidities to explain the poorer outcomes. These examples serve to illustrate the complexity and heterogeneity of arterial diseases and the need for sex‐specific research to understand them.
Because sex‐based literature on TAA growth is lacking, we must compare our findings to studies of aneurysms of other arterial beds. In this context, growth and rupture rates of abdominal aortic aneurysms (AAAs) are also greater in women than men. AAA rupture risk has been shown to be 4‐fold higher in women than men,22 and AAA growth rates are also greater in women (3.7 mm/y vs 2.0 mm/y in 1 study,23 and 2.4 mm/y vs 1.7 mm/y in another study).24 Similarly, in the cerebral circulation, female sex is associated with greater incidence25 and growth26 of intracranial aneurysms, which is in agreement with the AAA literature and with the findings from the present study. Thus, our findings add to the growing body of evidence showing that aneurysm behavior differs based on sex and raise a question about potential biological and biomechanical differences in the arteries of men and women.
To determine why aneurysms may grow faster in women, we must seek potential molecular and biomechanical differences in men's and women's aortas. Matrix metalloproteinases (MMPs) are enzymes capable of causing adverse arterial wall remodeling and have been shown to predispose to aortic aneurysm formation and dissection.27, 28, 29 Notably, women with dTAA have greater concentration of MMP‐2 and MMP‐9 and lower concentrations of inhibitory enzymes TIMP‐1 and TIMP‐2 than men, highlighting greater extracellular matrix remodeling in women's aortas.30 This results in greater aortic wall stiffness.30 In addition, MMP‐2 and MMP‐9 inhibit Ca2+ entry for smooth muscle contraction, promoting aortic dilation and inhibiting muscle contraction.31 Further, in patients with AAA, an increase in estrogen receptor α in the aortic wall has an inverse association with MMP activity and aneurysm growth rate,32 suggesting a beneficial effect of female reproductive hormones on aneurysm biology. This is relevant to the present study, as most of the female participants were postmenopausal. However, further biomechanical, histological, and molecular studies are needed in order to identify structural differences in men's and women's TAAs, especially in the setting of dTAA, that may ultimately predispose women to progressive aneurysm enlargement, dissection, and rupture.
Role of Aneurysm Etiology on Sex Differences in Aneurysm Growth
Arterial hypertension plays a major role in TAA formation and aneurysm growth. By increasing shear stress on the aortic wall, hypertension is the principal risk factor predisposing to TAAs and aortic dissection in the community. A recent epidemiologic study of over 30 000 subjects followed for 16 years showed that hypertension is present in 76% of all people with TAA and in 86% of people who subsequently develop an aortic dissection.33 In this study, hypertension was associated with 2.6 times greater odds of future aortic dissection and conferred a population‐attributable risk of 54%. Therefore, hypertension plays a major role in aneurysm‐related risk. Importantly, hypertension is among the chronic conditions that are more prevalent in older women than men.34 These data motivated our hypothesis that the etiology of the aneurysm would modify the association of female sex with aneurysm growth because the majority of dTAA patients are hypertensive. Indeed, in this cohort of predominantly older individuals, we found that TAA growth was only greater in women than men with dTAA, and that aneurysm growth was similar in men and women with hTAA. Notably, hTAA subjects were also younger (55±10 vs 69±10 years, P<0.0001) and had lower prevalence of hypertension (26% vs 66%, P=0.0002) than dTAA subjects.
In cohorts of similar age to the present one, we have previously shown that arterial hemodynamic load is greater in women than men.35 Therefore, it is possible that greater hemodynamic load in older women with dTAA may increase shear stress on the aneurysm wall, promoting aneurysm expansion. Alternatively, it is also possible that there are fundamental histological and/or molecular differences in the aneurysm walls of women and men with dTAA, whereas in hTAA wall pathology may be uniform between sexes. Our study provides a solid foundation that motivates further studies of aneurysm pathology and mechanobiology in men and women with dTAA and hTAA, in order to disentangle the complex links among sex, aneurysm etiology, and aneurysm behavior.
Limitations
Our study has limitations. First, this is a retrospective study of living and unoperated subjects. Thus, our study cannot directly determine whether faster aneurysm growth rates contribute to greater risk of acute aortic syndromes and mortality in women. Second, not all participants had aneurysm size measured with the same imaging technique. However, previous studies have shown excellent concordance in aortic measurements across imaging modalities (r=0.90‐0.97),36 and the proportion of subjects having follow‐up with different imaging modalities was not different between men and women. Moreover, in sensitivity analyses we demonstrated that results were unchanged when subjects with concordant and discordant imaging were analyzed separately, confirming the robustness of our findings. Third, we did not have data on MMPs or other molecular, histological, or hemodynamic factors that may underlie the associations found. Last, most of the women included in this study were older and postmenopausal; thus, additional studies are needed to determine aneurysm growth rates in younger women and compare it to growth rates of similarly aged men.
Conclusions
Women with dTAA have greater aneurysm growth rates than men, independently of body size or other clinical variables, whereas aneurysm growth rates are similar between men and women with hTAA. Our novel observations corroborate similar findings in the AAA and intracranial aneurysm literature and provide a potential explanation for the worse TAA‐related outcomes observed in women. Further, results from our study motivate future investigations aimed at assessing potential molecular, hormonal, and biomechanical bases for faster aneurysm growth and acute aortic syndromes in women.
Sources of Funding
This work was supported by an Emergent Research Leaders Initiative (ERLI) grant from a partnership between the Heart and Stroke Foundation of Canada and the Canadian Vascular Network, awarded to Dr Coutinho. Dr Coutinho is a Career Investigator supported by a Clinician Scientist Phase I Award from the Heart and Stroke Foundation of Ontario.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e003792. DOI: 10.1161/JAHA.116.003792.)
References
- 1. Juvonen T, Ergin MA, Galla JD, Lansman SL, Nguyen KH, McCullough JN, Levy D, de Asla RA, Bodian CA, Griepp RB. Prospective study of the natural history of thoracic aortic aneurysms. Ann Thorac Surg. 1997;63:1533–1545. [DOI] [PubMed] [Google Scholar]
- 2. Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population‐based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. [DOI] [PubMed] [Google Scholar]
- 3. Meszaros I, Morocz J, Szlavi J, Schmidt J, Tornoci L, Nagy L, Szep L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–1278. [DOI] [PubMed] [Google Scholar]
- 4. Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg. 2012;56:565–571. [DOI] [PubMed] [Google Scholar]
- 5. Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–S1880; discussion S1892‐S1898. [DOI] [PubMed] [Google Scholar]
- 6. Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM Jr, Elefteriades JA, Coady MA. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. 2007;83:1338–1344. [DOI] [PubMed] [Google Scholar]
- 7. Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, Elefteriades JA. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–491; discussion 489‐491. [DOI] [PubMed] [Google Scholar]
- 8. Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27; discussion 27‐28. [DOI] [PubMed] [Google Scholar]
- 9. Pape LA, Tsai TT, Isselbacher EM, Oh JK, O'Gara PT, Evangelista A, Fattori R, Meinhardt G, Trimarchi S, Bossone E, Suzuki T, Cooper JV, Froehlich JB, Nienaber CA, Eagle KA; International Registry of Acute Aortic Dissection (IRAD) Investigators . Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116:1120–1127. [DOI] [PubMed] [Google Scholar]
- 10. Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, Cooper JV, Januzzi JL, Ince H, Sechtem U, Bossone E, Fang J, Smith DE, Isselbacher EM, Pape LA, Eagle KA; International Registry of Acute Aortic Dissection . Gender‐related differences in acute aortic dissection. Circulation. 2004;109:3014–3021. [DOI] [PubMed] [Google Scholar]
- 11. Davies RR, Gallo A, Coady MA, Tellides G, Botta DM, Burke B, Coe MP, Kopf GS, Elefteriades JA. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169–177. [DOI] [PubMed] [Google Scholar]
- 12. Lobato AC, Puech‐Leao P. Predictive factors for rupture of thoracoabdominal aortic aneurysm. J Vasc Surg. 1998;27:446–453. [DOI] [PubMed] [Google Scholar]
- 13. Thompson AR, Cooper JA, Ashton HA, Hafez H. Growth rates of small abdominal aortic aneurysms correlate with clinical events. Br J Surg. 2010;97:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson SG, Brown LC, Sweeting MJ, Bown MJ, Kim LG, Glover MJ, Buxton MJ, Powell JT. Systematic review and meta‐analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost‐effectiveness. Health Technol Assess. 2013;17:1–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine . 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. [DOI] [PubMed] [Google Scholar]
- 16. Boodhwani M, Andelfinger G, Leipsic J, Lindsay T, McMurtry MS, Therrien J, Siu SC; Canadian Cardiovascular Society . Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can J Cardiol. 2014;30:577–589. [DOI] [PubMed] [Google Scholar]
- 17. Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54:225–235. [PubMed] [Google Scholar]
- 18. Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, Bolen MA, Connolly HM, Cuellar‐Calabria H, Czerny M, Devereux RB, Erbel RA, Fattori R, Isselbacher EM, Lindsay JM, McCulloch M, Michelena HI, Nienaber CA, Oh JK, Pepi M, Taylor AJ, Weinsaft JW, Zamorano JL, Dietz H, Eagle K, Elefteriades J, Jondeau G, Rousseau H, Schepens M. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2015;28:119–182. [DOI] [PubMed] [Google Scholar]
- 19. Trimarchi S, Jonker FH, Froehlich JB, Upchurch GR, Moll FL, Muhs BE, Rampoldi V, Patel HJ, Eagle KA; International Registry of Acute Aortic Dissection (IRAD) Investigators . Acute type B aortic dissection in the absence of aortic dilatation. J Vasc Surg. 2012;56:311–316. [DOI] [PubMed] [Google Scholar]
- 20. Manes A, Palazzini M, Dardi F, D'Adamo A, Rinaldi A, Galie N. [Female gender and pulmonary arterial hypertension: a complex relationship]. G Ital Cardiol. 2012;13:448–460. [DOI] [PubMed] [Google Scholar]
- 21. Grubb KJ, Kron IL. Sex and gender in thoracic aortic aneurysms and dissection. Semin Thorac Cardiovasc Surg. 2011;23:124–125. [DOI] [PubMed] [Google Scholar]
- 22. Sweeting MJ, Thompson SG, Brown LC, Powell JT; RESCAN Collaborators . Meta‐analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. [DOI] [PubMed] [Google Scholar]
- 23. Mofidi R, Goldie VJ, Kelman J, Dawson AR, Murie JA, Chalmers RT. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94:310–314. [DOI] [PubMed] [Google Scholar]
- 24. Solberg S, Singh K, Wilsgaard T, Jacobsen BK. Increased growth rate of abdominal aortic aneurysms in women. The Tromso study. Eur J Vasc Endovasc Surg. 2005;29:145–149. [DOI] [PubMed] [Google Scholar]
- 25. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta‐analysis. Lancet Neurol. 2011;10:626–636. [DOI] [PubMed] [Google Scholar]
- 26. Kubo Y, Koji T, Kashimura H, Otawara Y, Ogawa A, Ogasawara K. Female sex as a risk factor for the growth of asymptomatic unruptured cerebral saccular aneurysms in elderly patients. J Neurosurg. 2014;121:599–604. [DOI] [PubMed] [Google Scholar]
- 27. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 28. Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg. 2004;78:2106–2110; discussion 2110‐2111. [DOI] [PubMed] [Google Scholar]
- 29. Morris DR, Biros E, Cronin O, Kuivaniemi H, Golledge J. The association of genetic variants of matrix metalloproteinases with abdominal aortic aneurysm: a systematic review and meta‐analysis. Heart. 2014;100:295–302. [DOI] [PubMed] [Google Scholar]
- 30. Sokolis DP, Iliopoulos DC. Impaired mechanics and matrix metalloproteinases/inhibitors expression in female ascending thoracic aortic aneurysms. J Mech Behav Biomed Mater. 2014;34:154–164. [DOI] [PubMed] [Google Scholar]
- 31. Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase‐specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg. 2004;40:1001–1010. [DOI] [PubMed] [Google Scholar]
- 32. Yeap BB, Hyde Z, Norman PE, Chubb SA, Golledge J. Associations of total testosterone, sex hormone‐binding globulin, calculated free testosterone, and luteinizing hormone with prevalence of abdominal aortic aneurysm in older men. J Clin Endocrinol Metab. 2010;95:1123–1130. [DOI] [PubMed] [Google Scholar]
- 33. Landenhed M, Engström G, Gottsäter A, Caulfield MP, Hedblad B, Newton‐Cheh C, Melander O, Smith JG. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc. 2015;4:e001513 DOI: 10.1161/JAHA.114.001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 35. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dinsmore RE, Liberthson RR, Wismer GL, Miller SW, Liu P, Thompson R, McLoud TC, Marshall J, Saini S, Stratemeier EJ, Okada RD, Brady TJ. Magnetic resonance imaging of thoracic aortic aneurysms: comparison with other imaging methods. AJR Am J Roentgenol. 1986;146:309–314. [DOI] [PubMed] [Google Scholar]


