Abstract
Background
Prolonged intensive care unit length of stay (prICULOS) following cardiac surgery (CS) in older adults is increasingly common but rehospitalization characteristics and outcomes are understudied. We sought to describe the rehospitalization characteristics and subsequent non‐institutionalized survival of prICULOS (ICULOS ≥5 days) patients and identify modifiable risk factors to decrease 30‐day rehospitalization.
Methods and Results
Consecutive patients from January 1, 2000 to December 31, 2011 were analyzed utilizing linked clinical and administrative databases. Logistic regression was used to identify risk factors associated with 30‐day rehospitalization. Out of 9210 consecutive patients discharged from the hospital alive, 596 (6.5%) experienced prICULOS. Cumulative incidence of rehospitalization for the prICULOS cohort at 30 and 365 days was 17.5% and 45.6% versus 11.4% and 28.1% for non‐prICULOS (P<0.01). Over 40% of rehospitalizations for the entire cohort occurred within 30 days of discharge costing over $12 million. The most common reasons for rehospitalization were heart failure (in prICULOS) and infection (in non‐prICULOS). Rehospitalization within 30 days was associated with a 2.29‐fold risk of poor 1‐year noninstitutionalized survival for the entire cohort. Potentially modifiable factors affecting 30‐day rehospitalization included lack of physician visits within 30 days of discharge (odds ratio 2.11; P=0.01), and preoperative anxiety diagnosis (odds ratio 2.20; P=0.01).
Conclusions
PrICULOS patients have high rates of rehospitalization that is associated with an increased rate of poor noninstitutionalized survival. Addressing modifiable risk factors including early postdischarge access to physician services, as well as access to mental health services may improve patient outcomes.
Keywords: follow‐up study, intensive care unit, mortality, rehospitalization, surgery
Subject Categories: Cardiovascular Surgery, Mortality/Survival, Health Services, Quality and Outcomes
Introduction
Complex cardiac surgical procedures are increasingly being offered to older adult patients with more comorbidities, resulting in an increasing proportion of patients needing prolonged intensive care unit length of stay (prICULOS).1, 2 A recent study demonstrated a 57% increase in the number of patients needing prICULOS over a decade where the “functional survival” of these patients, defined as alive and not institutionalized, was 74% at 1 year.1 It is known from the noncardiac surgery ICU literature that the 30‐day and 1‐year post ICU discharge hospital readmission rates (not necessarily with prICULOS) are high at 16% and 41%, respectively.3, 4 There is less data specific to cardiac surgery ICU patients. With the potential increasing number of patients requiring and surviving prICULOS after cardiac surgery, significant economic and resource burdens related to rehospitalization may be anticipated for the future.
Early rehospitalization (within 30 days of discharge), considered a metric of quality of care, is estimated to cost the United States Medicare Program $26 billion per year.5, 6 In 2012, the Hospital Readmissions Reduction Program was set up in the United States in an effort to decrease rehospitalization by financially penalizing hospitals that had observed early rehospitalization rates above expected for specific diagnoses.6, 7, 8, 9, 10, 11, 12 However, some have questioned whether early readmissions are due to poor predischarge hospital care as opposed to pre‐existing patient factors (eg, socioeconomic) that are not directly in the hospital healthcare team's control.10, 13 Because of the costs and financial penalties associated with early rehospitalization, there has been significant interest in understanding the predictors and consequences of early rehospitalization following hospital discharge and determining modifiable factors that decrease the need for rehospitalization.7, 8, 9, 10, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36
Given the lack of information examining rehospitalization in cardiac surgery patients who have had prICULOS and have survived, the objectives of the study were the following: (1) to describe the rehospitalization characteristics and outcomes of cardiac surgery patients having suffered prICULOS during their initial cardiac surgery admission; and (2) to determine modifiable risk factors for early rehospitalization (within 30 days of discharge home).
Materials and Methods
This was a single‐region, retrospective database study. Approval was obtained from the local research ethics board, and the Health Information Privacy Committee. Informed consent was waived for this retrospective, de‐identified database study.
Databases
The Manitoba Centre for Health Policy at the University of Manitoba's Faculty of Medicine in Winnipeg, Manitoba, Canada, houses several provincial clinical and administrative databases within the Population Health Research Data Repository. The clinical data for this study were from the Winnipeg Regional Health Authority ICU database and the Manitoba Cardiac Surgical Database as previously published.1 Separation Abstracts data provided information about an individual's initial cardiac surgery hospitalization and any subsequent rehospitalization. Medical Claims (Physician Billings) data determined the degree of interaction a patient had with his/her physician following discharge. Long Term Care and Vital Statistics data provided an individual's functional status (alive and noninstitutionalized). Social Assistance data provided information on individuals requiring any income assistance before or after cardiac surgery. Publicly available Statistics Canada data provided neighborhood‐level income data across the entire province of Manitoba. Each neighborhood was assigned into a provincial income quintile. Specifics of definitions used are provided in Data S1. The databases have been validated and used in a number of studies.1, 37, 38
Patient Population
The study population included all surviving adults undergoing cardiac surgery from a single healthcare region (which is funded by a universal public healthcare system) with a catchment area of ≈1 million people who were discharged from the hospital alive between January 1, 2000 and December 31, 2011. The provincial databases only capture (complete) long‐term data on Manitoba patients, which comprised 90% of the patients that were operated on and represent the patients analyzed in this study. Patients requiring extracorporeal membrane oxygenation for postcardiotomy indications were included. Extracorporeal membrane oxygenation for any other indication was excluded from the analysis. The prICULOS cohort was defined as patients requiring 5 or more consecutive days in the ICU following their index cardiac surgery. The prICULOS population was compared to patients not needing prICULOS (ie, non‐prICULOS). Patient outcomes examined were “functional survival” defined as alive and not institutionalized within 1 year postdischarge.
Hospitalization Costs
The costs of hospital readmissions were calculated utilizing the Resource Intensity Weight and Cost per Weighted Case variables from the Canadian Institute for Health Information. The Resource Intensity Weight is an estimate of the quantity of resources utilized in a given hospitalization relative to a standard inpatient hospital visit in Manitoba. This Resource Intensity Weight was assigned to each patient in our study. The Resource Intensity Weight was then multiplied by the annual Cost per Weighted Case, which is calculated annually in Manitoba to obtain an estimate of the cost for each unique hospitalization for each patient.39, 40, 41 All costs quoted in this study are expressed in 2013/2014 Canadian dollars.
Data Analysis
Continuous variables were compared using a t test (for parametric data) or Mann–Whitney test (for nonparametric data), and categorical variables were compared using a chi‐square or Fisher exact test where appropriate. Rates of rehospitalization were calculated using cumulative incidence curves with a competing risk of death prior to rehospitalization for up to 1 year postdischarge and the values were compared between non‐prICULOS and prICULOS patients using Gray's test.42
In addition, a multivariable logistic regression model was developed to further characterize the factors associated with poor 1‐year functional survival as well as readmission to the hospital within 30 days of hospital discharge for the entire study cohort as well as just the prICULOS cohort. All variables presented in Table 1 taken from both clinical and administrative data sources were considered for the final model. A stepwise selection method was used that considered the Score test criteria with a P<0.05 for entry of variables into the model and the Wald test criteria with a P>0.05 for removal of selected variables. Model fit was assessed with the Hosmer‐Lemeshow test. Model discrimination was assessed by calculating the area under the receiver operating characteristic curve. Statistical analysis was undertaken using SAS software, Version 9.3 of the SAS System for Windows (copyright ©2011 SAS Institute Inc, Cary, NC).
Table 1.
Selected Characteristics of Patients Rehospitalized Within 30 Days vs Not Rehospitalized Following PrICULOS After Cardiac Surgery
| Variable | Not Rehospitalized in 30 Days (N=487) | Rehospitalized in 30 Days (N=109) | P Value |
|---|---|---|---|
| Preoperative variables (initial cardiac surgery admission) | |||
| Age, y | 72 (62–77) | 72 (60–77) | 0.83 |
| Female | 183 (37.6%) | 34 (31.2%) | 0.21 |
| Cerebrovascular disease | 72 (14.8%) | 15 (13.8%) | 0.78 |
| Diabetes mellitus | 146 (30.0%) | 33 (30.3%) | 0.95 |
| Chronic obstructive pulmonary disease | 68 (14.0%) | 18 (16.5%) | 0.49 |
| Previous myocardial infarction | 230 (47.2%) | 53 (48.6%) | 0.79 |
| Congestive heart failure | 88 (18.1%) | 25 (22.9%) | 0.24 |
| History of arrhythmia | 106 (21.8%) | 32 (29.4%) | 0.09 |
| Peripheral vascular disease | 124 (25.5%) | 25 (22.9%) | 0.58 |
| Renal insufficiency (creatinine ≥1.8 mg/dL) | 55 (11.3%) | 22 (20.2%) | 0.01a |
| Any previous anxiety condition | 46 (9.5%) | 19 (17.4%) | 0.02a |
| Social assistance prior to surgery | 18 (3.7%) | 6 (5.5%) | 0.42 |
| Intraoperative variables (initial cardiac surgery admission) | |||
| CABG | 221 (45.4%) | 39 (35.8%) | 0.30 |
| Single non‐CABG | 92 (18.9%) | 25 (22.9%) | |
| Two procedures | 137 (28.1%) | 37 (33.9%) | |
| Three procedures | 37 (7.6%) | 8 (7.3%) | |
| Elective surgery | 355 (72.9%) | 82 (75.2%) | 0.62 |
| Postoperative variables (initial cardiac surgery admission) | |||
| Cerebral vascular accident | 47 (9.7%) | 6 (5.5%) | 0.17 |
| Cardiogenic shock | 102 (20.9%) | 22 (20.2%) | 0.86 |
| Congestive heart failure | 63 (12.9%) | 17 (15.6%) | 0.46 |
| Pericardial tamponade | 19 (3.9%) | 6 (5.5%) | 0.43 |
| Red blood cells transfused (units) | 3 (2–6) | 4 (2–7) | 0.14 |
| Acute renal insufficiency (creatinine ≥1.8 mg/dL) | 60 (12.3%) | 10 (9.2%) | 0.36 |
| Acute renal failure (dialysis) | 57 (11.7%) | 22 (20.2%) | 0.02a |
| Days on mechanical ventilation | 5 (3–8) | 6 (4–9) | 0.25 |
| Intensive care unit length of stay (days) | 7.08 (5.89–10.95) | 7.07 (5.94–11.11) | 0.68 |
| Total hospital length of stay (days) | 28 (17–51) | 28 (22–47) | 0.44 |
| Postdischarge variables | |||
| Physician visits within 30 days of discharge | |||
| No visits | 55 (11.3%) | 20 (18.4%) | 0.02a |
| 1 to 4 visits | 331 (68.0%) | 59 (54.1%) | |
| 5 or more visits | 101 (20.7%) | 30 (27.5%) | |
| Social assistance required after surgery | 21 (4.3%) | 7 (6.4%) | 0.35 |
Statistically significant.
Categorical variables expressed as N (%) and compared using chi‐square test; continuous variables expressed as median (interquartile range) and compared using Mann–Whitney test. Variables not statistically significant between the groups and thus not listed include the following: APACHE score, hypertension, cardiogenic shock preoperatively, cardiac arrest preoperatively, any previous mental health condition, income quintile, urban residence, plasma transfused, platelets transfused, nosocomial pneumonia, and arrhythmia postoperatively. CABG indicates coronary artery bypass graft; ICU, intensive care unit; PrICULOS, prolonged intensive care unit length of stay.
“Modifiable” variables were defined as variables where one could intervene such as optimizing diabetes treatment or facilitating access to postdischarge services, as opposed to “nonmodifiable” variables such as age, sex, or established comorbidities such as dialysis‐dependent renal failure.
Results
Comparison of Rehospitalized Versus Nonrehospitalized PrICULOS Patients
During the study period, 9210 Manitoban cardiac surgery patients were discharged from the hospital alive and were still alive at 30 days postdischarge. Of these, 596 (6.5%) experienced prICULOS. PrICULOS patients needing rehospitalization within 30 days (versus those not rehospitalized) tended to have higher rates of preoperative renal insufficiency, preoperative history of an anxiety disorder, postoperative renal failure requiring dialysis, and no physician visits or 5 or more physician visits (as compared to 1–4 physician visits) within the first 30 days postdischarge (Table 1).
Rehospitalization Rates and Description of Rehospitalized Patients
The 30‐ and 365‐day cumulative incidence rehospitalization rates for the entire cohort were 11.7% and 29.2%, respectively. The cumulative incidence rehospitalization rates for the prICULOS cohort were nearly double those of the non‐prICULOS cohort (17.5% and 45.6%, respectively, versus 11.4% and 28.1%, respectively—P<0.001; Figure 1).
Figure 1.
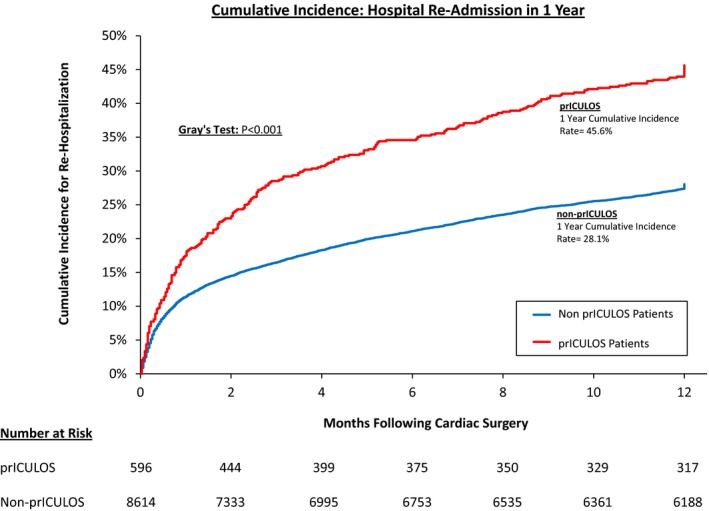
Patients with prolonged intensive care unit length of stay (prICULOS) had significantly higher rehospitalization rates compared to patients with nonprolonged ICU length of stay.
The 30‐day rehospitalization rate based on initial operative procedure type for the entire cohort was 11.0% for isolated coronary artery bypass graft (CABG), 13.1% for isolated valve, 16.7% for CABG+Valve, 13.2% for aortic procedure, and 12.5% for “other.” “Other” included all procedures that were not isolated CABG, CABG+Valve, or aortic cases and included procedures such as ventricular septal defect repair, resection of infracted myocardium, pericardectomy, and infected graft resection. The procedure with the highest 30‐day rehospitalization rate in the non‐prICULOS cohort was CABG+Valve with a rate of 15.9%; however, the procedure with the highest 30‐day rehospitalization rate in the prICULOS cohort was “other” at a rate of 27.6%.
The median (interquartile range) time to first rehospitalization for patients readmitted within 1 year of discharge was 51 (11–170) days for non‐prICULOS patients, which was similar to 52 (16–150) days for prICULOS patients.
Of all patients rehospitalized within 1 year of discharge, 996/2416 (41.2%) of the non‐prICULOS patients and 109/272 (40.1%) of the prICULOS patients were rehospitalized within 30 days of discharge (Figure 2). The median hospital LOS for the first rehospitalization in these patients was 4 (interquartile range: 2–8) days for non‐prICULOS patients versus a median of 6 (interquartile range: 3–12) days for prICULOS patients (P<0.001). The total number of first‐time rehospitalizations occurring within 30 days of discharge for the entire cohort was 1105, which equated to 12 602 inpatient days (10 707 days for non‐prICULOS patients and 1895 for prICULOS patients) (Table 2). The average cost of a hospital readmission occurring within the first 30 days postdischarge from cardiac surgery was $13 960 CDN ($10 738 USD using exchange rate of $1.3 CDN/USD) for the prICULOS patient cohort and $10 100 CDN ($7769 USD) for the non‐prICULOS cohort. Using an average value of $12 000 CDN per rehospitalization for the entire cohort and 1105 rehospitalizations in the first 30 days, gives a total cost of over $13 million dollars CDN (≈$10 million USD) just for patients readmitted within the first 30 days postdischarge home.
Figure 2.

Most rehospitalizations occur early postdischarge, with ≈60% of rehospitalizations for both prolonged intensive care unit length of stay (prICULOS) and non‐prICULOS patients occurring within 90 days from discharge home.
Table 2.
Total Inpatient Days for Patients Rehospitalized
| Hospital Readmission | Total Number of First | Number of Inpatient Days | ||
|---|---|---|---|---|
| Non‐prICULOS | PrICULOS | Total Inpatient | ||
| Date Occurring in | Rehospitalizations in Specific Time Period | Patients (N=2416) | Patients (N=272) | Days (N=2688) |
| 0 to 30 days postdischarge home | 1105 | 10 707 | 1895 | 12 602 |
| 31 to 90 days postdischarge home | 513 | 7857 | 1822 | 9679 |
| 91 to 180 days postdischarge home | 444 | 7630 | 1489 | 9119 |
| 181 to 270 days postdischarge home | 359 | 7216 | 1273 | 8489 |
| 271 to 365 days postdischarge home | 267 | 6330 | 1174 | 7504 |
| Totals | 2688 | 39 740 | 7653 | 47 393 |
PrICULOS indicates prolonged intensive care unit length of stay.
Heart failure (ICD 9 code 428 and ICD 10 code I50) was the most common reason for rehospitalization in prICULOS patients for those rehospitalized within 30 days from discharge home, accounting for 20.5% of the “most responsible diagnosis” at time of readmission (Table 3). Conversely, “Complications of Procedures” (ICD 9 code 998 and ICD 10 code T81) was the most common reason for rehospitalization for non‐prICULOS patients for rehospitalization within 30 days, accounting for 12.8% of the “most responsible diagnosis” at time of readmission. Further exploration into the “Complications of Procedures” diagnosis code revealed that postoperative infections were the most prevalent complication within this code, occurring in ≈80% of cases. Atrial fibrillation and pleural effusion were also common reasons for rehospitalization in the non‐prICULOS cohort, occurring 6% to 8% of the time (Table 3).
Table 3.
Top 4 “Most Responsible” Diagnosis for Rehospitalization for Various Time Periods (With Frequency of the Diagnosis)
| Entire Population (N=1105) | Non‐PrICULOS Cohort (N=996) | PrICULOS Cohort (N=109) |
|---|---|---|
| (a) Within 30 Days of Discharge Home | ||
| Complications of proceduresa—12.2% | Complications of proceduresa—12.8% | Heart failure—20.5% |
| Heart failure—10.8% | Heart failure—9.7% | Convalescence—6.1% |
| Pleural effusion—6.7% | Pleural effusion—7.0% | Complications of proceduresb—6.1% |
| Atrial fibrillation—5.7% | Atrial fibrillation—6.0% | b |
| Entire Population (N=2688) | Non‐PrICULOS Cohort (N=2416) | PrICULOS Cohort (N=272) |
|---|---|---|
| (b) Within 365 Days of Discharge Home | ||
| Heart failure—9.8% | Heart failure—8.7% | Heart failure—17.6% |
| Complications of proceduresa—5.6% | Complications of proceduresa—5.9% | Complications of proceduresa—3.9% |
| Atrial fibrillation—4.0% | Atrial fibrillation—4.2% | Convalescence—3.8% |
| Pleural effusion—3.2% | Pleural effusion—3.4% | Chronic ischemic heart disease—2.7% |
ICD 9/10 Codes for Various Diagnosis: Heart Failure: ICD 9 code 428 and ICD 10 code I50, Atrial fibrillation: ICD 9 code 427 and ICD 10 code I48, Pleural Effusion: ICD 9 code 511 and ICD 10 code J90; Complications of Procedures: ICD 9 code 998 and ICD 10 code T81; Convalescence: ICD 9 code V66 and ICD 10 code Z54; Chronic Ischemic Heart Disease: ICD 9 code 414 and ICD 10 code I25. PrICULOS indicates prolonged intensive care unit length of stay.
Most commonly postoperative infection.
Not reportable as count <6.
One‐Year Outcomes of Patients Post Rehospitalization
Patients who were rehospitalized within 30 days from discharge had increased mortality within 1 year of readmission compared to patients who were not rehospitalized for both non‐prICULOS patients as well as prICULOS patients. However, the mortality rate in rehospitalized prICULOS patients (15.6%) was double the mortality rate of rehospitalized non‐prICULOS patients (7.6%) (Figure 3). Most patients in both groups who died did so while in the hospital (59% in non‐prICULOS and prICULOS cohorts) and the most common primary causes of death were chronic ischemic heart disease, ST elevation and non‐ST elevation myocardial infarction as well as stroke. In addition, for those discharged post rehospitalization, 9/8614 (0.1%) of non‐prICULOS patients and 10/596 (1.7%) of prICULOS patients were discharged to a personal care home (P<0.01) after their rehospitalization in the first year after discharge from the index cardiac surgery hospitalization.
Figure 3.
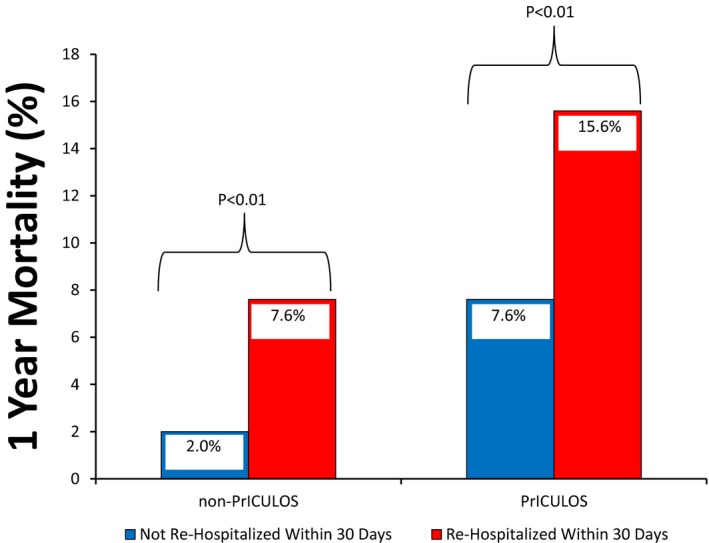
Rehospitalization within 30 days of discharge home was associated with an increased risk of mortality within 1 year of rehospitalization. PrICULOS indicates prolonged intensive care unit length of stay.
To examine whether rehospitalization was an independent factor associated with poor functional survival (dead or institutionalized), a multivariate logistic regression analysis was performed, and this confirmed that rehospitalization within 30 days of discharge was indeed independently associated (odds ratio [OR] 2.29, P<0.001) with poor functional survival at 1 year (Table 4).
Table 4.
Multivariable Logistic Regression Model for Factors Associated With Poor Functional Survival (Dead or Institutionalized) at 1 Year for All Patients After Discharge From Hospital After Cardiac Surgery (N=9210)
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age ≥80 years old | 1.89 | 1.35 to 2.64 | <0.01a |
| Chronic obstructive pulmonary disease | 1.47 | 1.05 to 2.05 | 0.03a |
| Arrhythmia | 1.52 | 1.14 to 2.02 | <0.01a |
| Diabetes mellitus | 1.61 | 1.24 to 2.07 | <0.01a |
| Peripheral vascular disease | 1.66 | 1.28 to 2.16 | <0.01a |
| Preoperative renal insufficiency (creatinine >1.8 mg/dL) | 1.80 | 1.27 to 2.55 | <0.01a |
| Preoperative renal failure (dialysis) | 3.29 | 1.82 to 5.94 | <0.0001a |
| Single non‐CABG vs isolated CABG | 1.55 | 1.11 to 2.16 | 0.01a |
| Two procedures vs isolated CABG | 2.16 | 1.61 to 2.89 | <0.0001a |
| Three procedures vs isolated CABG | 1.72 | 0.92 to 3.21 | 0.09 |
| Other respiratory problems postoperatively | 3.14 | 1.31 to 7.51 | 0.01a |
| Pericardial tamponade postoperatively | 2.42 | 1.11 to 5.31 | 0.03a |
| Ejection fraction grade out of 4 (per increase of 1 grade) | 1.44 | 1.25 to 1.67 | <0.0001a |
| Total days on mechanical ventilation (per day) | 1.07 | 1.02 to 1.14 | 0.01a |
| Total hospital length of stay (per day) | 1.02 | 1.01 to 1.02 | <0.0001a |
| Rehospitalization within 30 days | 2.29a | 1.76 to 2.99a | <0.001a |
| No physician visits vs 1 to 4 physician visits in 30 days postdischarge | 4.69a | 3.53 to 6.23a | <0.0001a |
| 5+ physician visits vs 1 to 4 physician visits in 30 days postdischarge | 1.26 | 0.91 to 1.75 | 0.17 |
Area under receiver operating characteristic curve=0.848 (0.827–0.869); Hosmer‐Lemeshow P value=0.30.
CABG indicates coronary artery bypass graft.
Statistically significant.
Curiously, when looking at all patients discharged home regardless of ICULOS or rehospitalization (Table 4), no physician visits was also associated with poor functional survival at 1 year (OR 4.69; P<0.0001); and for just prICULOS patients, preoperative need for social assistance (OR 11.2, P<0.0001) and no physician visits was also associated with poor functional survival (OR 5.15; P<0.0001) at 1 year. Interestingly, prICULOS as an independent variable was not associated with poor functional survival (OR=0.70, 95% CI: 0.42–1.15, P=0.16) likely because of other variables being highly correlated with having prICULOS and already being present in the model.
Predictors of Early Rehospitalization
Examining the entire cohort of patients regardless of ICULOS showed 16 different variables to be associated with increased risk of rehospitalization (Table 5). PrICULOS was not independently associated with rehospitalization (OR=1.00, 95% CI: 0.77–1.28, P=0.97), because of other variables being highly correlated with having prICULOS and already being present in the model. Some of the 16 variables were nonmodifiable (eg, age, sex), others were comorbidities that could possibly be modified (such as chronic obstructive pulmonary disease, and diabetes); however, there were a number of modifiable psychosocial variables as well such as preoperative mental health status, social assistance need, household income status, and access to physician services within 30 days of discharge. Examining just the prICULOS cohort (Table 6) again demonstrated preoperative anxiety disorders and no physician visits as modifiable risk factors.
Table 5.
Multivariable Logistic Regression Model for Factors Associated With Rehospitalization Within 30 Days of Discharge for All Patients After Discharge From Hospital After Cardiac Surgery (N=9210)
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age (per year of age) | 1.01 | 1.00 to 1.02 | <0.01a |
| Female | 1.13 | 1.05 to 1.21 | <0.01a |
| Cerebrovascular disease | 1.31 | 1.07 to 1.59 | 0.01a |
| Chronic obstructive pulmonary disease | 1.41 | 1.15 to 1.74 | <0.01a |
| Hypertension | 1.18 | 1.10 to 1.27 | 0.03a |
| Arrhythmia | 1.55 | 1.31 to 1.83 | <0.0001a |
| Diabetes mellitus | 1.26 | 1.09 to 1.46 | <0.01a |
| Preoperative renal insufficiency (creatinine >1.8 mg/dL) | 1.75 | 1.4 0 to 2.20 | <0.0001a |
| Preoperative renal failure (dialysis) | 2.22 | 1.48 to 3.33 | <0.01a |
| Any preoperative mental health condition | 1.24 | 1.05 to 1.46 | 0.01a |
| Social assistance requirement before surgery | 1.48 | 1.09 to 2.03 | 0.01a |
| Income quintile 2 vs 1 | 0.94 | 0.78 to 1.15 | 0.56 |
| Income quintile 3 vs 1 | 0.84 | 0.68 to 1.03 | 0.09 |
| Income quintile 4 vs 1 | 0.81 | 0.66 to 0.98 | 0.03a |
| Income quintile 5 vs 1 | 0.62 | 0.50 to 0.78 | <0.0001a |
| Income quintile not found vs 1 | 0.71 | 0.50 to 1.00 | 0.05a |
| Urban residence vs rural residence | 0.59 | 0.52 to 0.68 | <0.0001a |
| Cardiopulmonary bypass time (per min) | 1.00 | 1.00 to 1.00 | 0.02a |
| Red blood cell transfusion (per unit) | 1.06 | 1.03 to 1.08 | <0.0001a |
| No physician visits vs 1 to 4 physician visits in 30 days postdischarge | 1.91 | 1.56 to 2.33 | <0.0001a |
| 5+ physician visits vs 1 to 4 physician visits in 30 days postdischarge | 1.49 | 1.25 to 1.78 | <0.0001a |
Area under receiver operating characteristic curve=0.670 (0.653–0.686); Hosmer‐Lemeshow P value=0.19.
Statistically significant.
Table 6.
Multivariable Logistic Regression Model for Factors Associated With Rehospitalization Within 30 Days of Discharge From Hospital for Cardiac Surgery Patients Who Had PrICULOS (N=596)
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Preoperative renal insufficiency (creatinine >1.8 mg/dL) | 2.02 | 1.15 to 3.55 | 0.01a |
| Any preoperative anxiety diagnosis | 2.20 | 1.21 to 3.98 | 0.01a |
| Red blood cell transfusion (per unit) | 1.04 | 1.01 to 1.07 | 0.02a |
| No physician visits vs 1 to 4 physician visits in 30 days postdischarge | 2.11 | 1.17 to 3.83 | 0.01a |
| 5+ physician visits vs 1 to 4 physician visits in 30 days postdischarge | 1.50 | 0.90 to 2.50 | 0.12 |
Area under receiver operating characteristic curve=0.645 (0.586–0.705); Hosmer‐Lemeshow P value=0.83. PrICULOS indicates prolonged intensive care unit length of stay.
Statistically significant.
Discussion
In this analysis, we sought to understand the impact of rehospitalization in a vulnerable group of patients, namely, those who required prICULOS following cardiac surgery. PrICULOS was defined as ≥5 days in our study, as that would allow one to study the “sick” cardiac surgery ICU patient (as opposed to the patient just requiring 48 hours of inotrope/vasopressor for low cardiac output or vasoplegia) but still allow enough sample size to perform meaningful statistical analysis. PrICULOS patients had high rehospitalization rates, with almost 20% of rehospitalizations occurring within 30 days and almost 50% of rehospitalizations occurring within 1 year of discharge. It is possible that prICULOS patients had increased rehospitalization rates compared to non‐prICULOS patients because the prICULOS patients developed complications that would increase the probability of readmission such as heart failure, renal dysfunction, and infection. We also found that rehospitalization was costly, with the total number of rehospitalizations within the first 30 days postdischarge costing over $13 million CDN (≈$10 million USD) in a population of just 1 million people. Acknowledging the inherent flaws of simple extrapolation, this would translate to direct healthcare costs of $3.9 billion over the study period in the United States, assuming an average population of 303 million in the United States. We also found that rehospitalization within 30 days of discharge from the hospital was associated with a doubling risk of poor functional survival (dead or institutionalized) within 1 year of rehospitalization for prICULOS patients. Importantly, that was associated with almost a quadrupling of risk in non‐prICULOS patients, the etiology of which needs further investigation. Furthermore, at present, about 10% of patients need prICULOS after cardiac surgery but this number is over 50% higher than a decade ago.1, 2 With high rehospitalization rates and an expected increasing number of cardiac surgery patients expected to have prICULOS over the next decades, it is likely that there will be significant economic implications for policy makers, healthcare systems, and society. To our knowledge, this is the first report enumerating the current clinical and financial impact of treatment related to this specific group of patients.
The high rehospitalization rate could point to quality of care issues at our institution. Firstly, looking at our entire population shows rehospitalization rates similar to those published in the literature. Our 30‐day rehospitalization rate for a mixture of cardiac surgery operations for the entire patient population (not just prICULOS patients) postdischarge was 11.7%. This is in keeping with the published literature, which generally consists of large studies (thousands of patients) from multiple hospitals in the United States where 30‐day rehospitalization rates (mostly post CABG) average about 5% to 12% with the range being 0% to 80%.10, 19, 20, 21, 23, 24, 29 Our 90‐day rehospitalization rate of 17.2% (Figure 1) is also comparable to the published results from Iribarne et al,19 who reported 65‐day rehospitalization rates (from index operation) among 10 centers with 5158 adult cardiac surgical patients at 18.7%. Our observed 30‐day rehospitalization rates based on procedure type for the entire cohort were 11.0% for CABG, 13.1% for isolated valve, and 16.7% for CABG+Valve. This is comparable to a previous report published by Iribarne et al,19 where rehospitalization rates were 14.9% for isolated CABG, 18.3% for isolated valve, and 25% for CABG+Valve. Hence, our rehospitalization rates overall are similar to the published literature.
Secondly, the probability that the high rehospitalization rate was secondary to early discharge from the hospital was low. From our previous analysis,1 our hospital length of stay after the index operation was a median of 24.0 days, with 14.3% needing transfer to a community hospital requiring an additional 21.5 days median stay for prICULOS patients. The equivalent numbers for non‐prICULOS patients was 8.0 days median hospital LOS, where 4.2% of them were transferred for further care of 12.0 days (median). In addition, the median time to first rehospitalization was 51 to 52 days for non‐prICULOS and prICULOS patients, respectively, and only ≈40% of patients were rehospitalized within the first 30 days postdischarge—both of which suggest early discharge was likely not the reason for the high rate of rehospitalization. Our times to rehospitalization and percentage of patients rehospitalized for the entire cohort of patients are consistent with the published literature, where 60% to 80% of cardiac surgery patients are rehospitalized within 30 days of discharge with the median time to rehospitalization varying between 5 and 20 days.10, 19, 20, 23 This again supports the fact that the high rehospitalization rates of prICULOS patients were not attributable to early discharge of these patients.
Lastly, we examined whether there was something unique about the “most responsible reason” for rehospitalization in the prICULOS patients to see if this explained the high rate of rehospitalization. In the literature, the top 3 reasons for rehospitalization post cardiac surgery (not specifically related to ICULOS) are heart failure (13–25% of cases), infection (10–25% of cases), and arrhythmias (13–25% of cases).1 Our results were similar, with heart failure being the cause ≈20% of the time and the main reason for rehospitalization in prICULOS survivors; whereas complications of procedures (mostly postoperative infection) were the cause ≈12% of the time and the main reason for rehospitalization in the non‐prICULOS cohort early after discharge. Our rate of readmission for atrial fibrillation (which is only in the non‐prICULOS cohort) occurred only 4% to 6% of the time, which is lower than what has been previously reported.2 However, the prICULOS patients did have convalescence (which is a specific ICD 9/10 diagnostic code) as the most responsible reason for rehospitalization 4% to 6% of the time within the first 30 days of discharge. Though it is possible that rehospitalization with a most responsible diagnosis of convalescence may be interpreted as being discharged from the hospital too early, this is unlikely to be the case the majority of the time (though we do recognize that there is the possibility that occasional patients may have been discharged too early). We have comprehensive biweekly multidisciplinary meetings involving clinicians, physiotherapists, occupational therapists, social work, and home care personnel who review each patient on the ward to plan an appropriate and safe discharge. The fact that convalescence is an important reason for rehospitalization and psychological/outpatient physician access factors are significant in our multivariable model may suggest a link between the 2. Because of privacy restrictions on being able to report data for any sample size <6 per category, we were unable to report on secondary diagnosis for the convalescence diagnosis as these were all counts <6 per category to better determine the reason for need for convalescence.
Examining our models that predict rehospitalization for the entire cohort (Table 5) shows a number of risk factors relating to comorbidity, which would generally be considered to be nonmodifiable such as age, sex, and established comorbidities such as cerebrovascular disease and preoperative renal failure. In addition, some potentially modifiable variables such as hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and arrhythmias, which may have the capacity for optimization prior to surgery, also were significant in the model. Finally, psychosocial variables such as mental health issues, access to physician services early postischarge, and socioeconomic factors were also associated with early rehospitalization. These may represent potentially underrecognized modifiable, nonmedical risk factors when it comes to rehospitalization. Even though the prICULOS cohort (Table 6) has a reduced sample size resulting in a smaller number of covariates being selected in the final model, it is particularly interesting that preoperative anxiety disorder and access to physician services within 30 days of discharge are significant in the model. Once again, potentially modifiable risk factors for rehospitalization outside of “classical comorbid medical conditions” seem to be present in this specific cohort. With minimal access to physician services, it stands to reason that ongoing management of manageable comorbidities would degrade, resulting in rehospitalization. Our findings are corroborated by others who have also found that patients with lower education levels, lower income, and increased distance from the hospital/rural location had a higher risk of rehospitalization, though the patient population studied was different from ours.9, 21, 25, 29, 31, 43 These hypothesis‐generating findings need corroboration with prospective study to determine whether manipulating these variables affects outcomes. If indeed psychosocial and physician access postdischarge are major obstacles for patients and the only viable option for the patient becomes repeated inpatient treatment, then perhaps this would need to be accounted for before hospitals (in the United States) are financially penalized for rehospitalization. Of note, the specific dichotomous variable, prICULOS, did not come out independently associated with either poor functional survival or rehospitalization. This is likely explained by the fact that other variables, which are correlated with prICULOS (eg, age, certain comorbidities, days on mechanical ventilation), were already in the model.
There have been a number of studies done to try to prevent rehospitalization in medical and surgical patients. As heart failure is a very common reason for rehospitalization, attempts have been made to try to detect when a patient is decompensating (with remote electronic monitoring technologies) and institute outpatient therapy (eg, increase diuretics or afterload reduction) before they get severe enough to need rehospitalization. The results have been variable and not all institutions have access to these advanced technologies.44, 45 There have also been studies done (mostly in noncardiac surgery patients and none in cardiac surgery patients with prICULOS) trying to address the psychosocial and healthcare access issues patients have to see if this can decrease rehospitalization, but again the results have been variable.26, 33, 34, 35, 36 One of the problems has been the cost and logistics to instituting the interventions, and thus better processes of care need to be established.34, 46
Another very important finding identified in this analysis was that rehospitalization was independently associated with poor 1‐year functional survival (dead or institutionalized), being 15.6% in the prICULOS cohort and 7.6% in the non‐prICULOS cohort. In both cohorts, this risk of death or institutionalization was over 2‐fold higher compared to nonrehospitalized patients (Figure 3 and Table 4). While similar to previous findings in general cardiac surgery and other patient groups that looked at death rates post rehospitalization, length of time from discharge to rehospitalization and death, and rehospitalization hospital type and outcome,15, 16, 17 this reporting with respect to rehospitalization in prICULOS patients is rather novel and emphasizes the importance of identifying reasons for rehospitalization to prevent its occurrence.
Analysis of the potential reasons for poor functional survival at 1 year post discharge from initial cardiac surgery (Table 4) reveals that the patients were older with multiple comorbidities including cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, and most importantly renal dysfunction. The primary causes of death were most commonly chronic ischemic heart disease, new myocardial infarction, and stroke. Note: We only collected the primary cause of death recorded and not all the causes (which may have included renal failure, chronic obstructive pulmonary disease, and such diagnoses). It is logical that patients with more comorbidities were more likely to be readmitted and suffer worse long‐term outcomes, often succumbing to cardiovascular diseases. Nonetheless, readmission itself was independently predictive of poor functional survival and possibly overlooked psychosocial and medical access issues were predictive of the need for readmission. Intervention with medical and other community supports to reduce hospital readmission after cardiac surgery, particularly in prICULOS patients, presents a potential opportunity to improve postdischarge care and reduce the tremendous cost of rehospitalization.
Limitations
Limitations to our study include those typical of a retrospective, administrative database study including biases and incorrect data collection; however, these databases have been validated and used in many previously published studies.1, 4, 38 Secondly, we do not have access in the databases to information about quality of life, cognitive impairment, or frailty, which would be valuable. However, we feel that using functional survival (which takes into account institutionalization) is a reasonable surrogate for these missing variables, which we are endeavoring to incorporate in the future. Thirdly, we did not collect all the causes (if multiple) for each rehospitalization or death but just collected what was recorded as the primary cause. It is possible that we may have missed important information by this method. Lastly, the area under the receiver operating characteristic curve for our rehospitalization models were not very high, being in the 0.65 to 0.67 range (Tables 5 and 6). This, however, is consistent with the literature where various models to predict rehospitalization in various populations give c‐statistic ranges between 0.56 and 0.83, with the majority being in the 0.55 to 0.65 range.47 One of the reasons the c‐stat is so low in many studies is because models generally do not include variables that are likely important in determining rehospitalization such as social support/quality of life/activities of daily independence, ethnicity, and similar factors. Future studies should try to include these variables.
Conclusions and Future Studies
Patients with prICULOS post cardiac surgery have high rates of rehospitalization, which is costly and associated with significantly worse functional survival at 1 year. Three potentially modifiable psychosocial risk factors to prevent rehospitalization in this cohort include provision of access to physicians, and mental health and social services. Development and evolution of dedicated discharge clinics for prICULOS patients using multidisciplinary teams to facilitate access to comprehensive health and other services may represent the next logical step in the quest to improve patient outcomes and minimize the associated extreme costs.
Sources of Funding
This work was funded by University of Manitoba Department of Surgery Research Grants.
Disclosures
Dr R.C. Arora has an unrestricted educational grant from Pfizer Canada Inc. The other authors have no disclosures.
Supporting information
Data S1: Definitions and ICD codes used for variables collected
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository under project #2013‐21 (HIPC#2013/14‐08). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. Data used in this study are from the Population Health Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba and were derived from data provided by Manitoba Health, the Winnipeg Regional Health Authority, and Manitoba Jobs and the Economy. The authors also acknowledge Manitoba Vital Statistics Agency. Mr Brett M. Hiebert, BSc (Statistics), MSc (Community Health Sciences) is a statistician with the Cardiac Sciences Program.
(J Am Heart Assoc. 2017;6:e004072. DOI: 10.1161/JAHA.116.004072.)
Notes
References
- 1. Manji RA, Arora RC, Singal RK, Hiebert B, Moon MC, Freed DH, Menkis AH. Long term outcome and predictors of non‐institutionalized survival after prolonged ICU stay post cardiac surgery. Ann Thorac Surg. 2016;101:56–63. [DOI] [PubMed] [Google Scholar]
- 2. Deschka H, Schreier R, El‐Ayoubi L, Erler S, Müller D, Alken A, Wimmer‐Greinecker G. Prolonged intensive care treatment of octogenarians after cardiac surgery: a reasonable economic burden? Interact Cardiovasc Thorac Surg. 2013;17:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hua M, Gong MN, Brady J, Wunsch H. Early and late unplanned rehospitalizations for survivors of critical illness. Crit Care Med. 2015;43:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garland A, Olafson K, Ramsey CD, Yogendran M, Fransoo R. A population‐based observational study of intensive care unit‐related outcomes with emphasis on post‐hospital outcomes. Ann Am Thorac Soc. 2015;12:202–208. [DOI] [PubMed] [Google Scholar]
- 5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 6. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131:1796–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MV, Tsai TC, Ko CY, Bilimoria KY. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313:483–495. [DOI] [PubMed] [Google Scholar]
- 8. Morris MS, Deierhoi RJ, Richman JS, Altom LK, Hawn MT. The relationship between timing of surgical complications and hospital readmission. JAMA Surg. 2014;149:348–354. [DOI] [PubMed] [Google Scholar]
- 9. Ferraris VA, Ferraris SP, Harmon RC, Evans BD. Risk factors for early hospital readmission after cardiac operations. J Thorac Cardiovasc Surg. 2001;122:278–286. [DOI] [PubMed] [Google Scholar]
- 10. Lancey R, Kurlansky P, Argenziano M, Coady M, Dunton R, Greelish J, Nast E, Robbins SG, Scribani M, Tingley J, Williams T, Zapolansky A, Smith C. Uniform standards do not apply to readmission following coronary artery bypass surgery: a multi‐institutional study. J Thorac Cardiovasc Surg. 2015;149:850–857. [DOI] [PubMed] [Google Scholar]
- 11. Glance LG, Kellermann AL, Osler TM, Li Y, Mukamel DB, Lustik SJ, Eaton MP, Dick AW. Hospital readmission after noncardiac surgery: the role of major complications. JAMA Surg. 2014;149:439–445. [DOI] [PubMed] [Google Scholar]
- 12. Lahey SJ, Campos CT, Jennings B, Pawlow P, Stokes T, Levitsky S. Hospital readmission after cardiac surgery. Does “fast track” cardiac surgery result in cost saving or cost shifting? Circulation. 1998;98:II35–II40. [PubMed] [Google Scholar]
- 13. Parina RP, Chang DC, Rose JA, Talamini MA. Is a low readmission rate indicative of a good hospital? J Am Coll Surg. 2015;220:169–176. [DOI] [PubMed] [Google Scholar]
- 14. Lum HD, Studenski SA, Degenholtz HB, Hardy SE. Early hospital readmission is a predictor of one‐year mortality in community‐dwelling older Medicare beneficiaries. J Gen Intern Med. 2012;27:1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzalez AA, Abdelsattar ZM, Dimick JB, Dev S, Birkmeyer JD, Ghaferi AA. Time‐to‐readmission and mortality after high‐risk surgery. Ann Surg. 2015;262:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brooke BS, Goodney PP, Kraiss LW, Gottlieb DJ, Samore MH, Finlayson SR. Readmission destination and risk of mortality after major surgery: an observational cohort study. Lancet. 2015;386:884–895. doi: 10.1016/S0140‐6736(15)60087‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee R, Homer N, Andrei AC, McGee EC, Malaisrie SC, Kansal P, McCarthy PM. Early readmission for congestive heart failure predicts late mortality after cardiac surgery. J Thorac Cardiovasc Surg. 2012;144:671–676. [DOI] [PubMed] [Google Scholar]
- 18. Celkan MA, Ustunsoy H, Daglar B, Kazaz H, Kocoglu H. Readmission and mortality in patients undergoing off‐pump coronary artery bypass surgery with fast‐track recovery protocol. Heart Vessels. 2005;20:251–255. [DOI] [PubMed] [Google Scholar]
- 19. Iribarne A, Chang H, Alexander JH, Gillinov AM, Moquete E, Puskas JD, Bagiella E, Acker MA, Mayer ML, Ferguson TB, Burks S, Perrault LP, Welsh S, Johnston KC, Murphy M, DeRose JJ, Neill A, Dobrev E, Baio KT, Taddei‐Peters W, Moskowitz AJ, O'Gara PT. Readmissions after cardiac surgery: experience of the National Institutes of Health/Canadian Institutes of Health research cardiothoracic surgical trials network. Ann Thorac Surg. 2014;98:1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Price JD, Romeiser JL, Gnerre JM, Shroyer AL, Rosengart TK. Risk analysis for readmission after coronary artery bypass surgery: developing a strategy to reduce readmissions. J Am Coll Surg. 2013;216:412–419. [DOI] [PubMed] [Google Scholar]
- 21. Maniar HS, Bell JM, Moon MR, Meyers BF, Marsala J, Lawton JS, Damiano RJ Jr. Prospective evaluation of patients readmitted after cardiac surgery: analysis of outcomes and identification of risk factors. J Thorac Cardiovasc Surg. 2014;147:1013–1018. [DOI] [PubMed] [Google Scholar]
- 22. Glebova NO, Hicks CW, Taylor R, Tosoian JJ, Orion KC, Arnaoutakis KD, Arnaoutakis GJ, Black JH III. Readmissions after complex aneurysm repair are frequent, costly, and primarily at nonindex hospitals. J Vasc Surg. 2014;60:1429–1437. [DOI] [PubMed] [Google Scholar]
- 23. Brown JR, Parikh CR, Ross CS, Kramer RS, Magnus PC, Chaisson K, Boss RA Jr, Helm RE, Horton SR, Hofmaster P, Desaulniers H, Blajda P, Westbrook BM, Duquette D, LeBlond K, Quinn RD, Jones C, DiScipio AW, Malenka DJ; Northern New England Cardiovascular Disease Study Group . Impact of perioperative acute kidney injury as a severity index for thirty‐day readmission after cardiac surgery. Ann Thorac Surg. 2014;97:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hannan EL, Racz MJ, Walford G, Ryan TJ, Isom OW, Bennett E, Jones RH. Predictors of readmission for complications of coronary artery bypass graft surgery. JAMA. 2003;290:773–780. [DOI] [PubMed] [Google Scholar]
- 25. Chen JC, Shaw JD, Ma Y, Rhoads KF. The role of the hospital and health care system characteristics in readmissions after major surgery in California. Surgery. 2016;159:381–388. doi: 10.1016/j.surg.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 26. Hall MH, Esposito RA, Pekmezaris R, Lesser M, Moravick D, Jahn L, Blenderman R, Akerman M, Nouryan CN, Hartman AR. Cardiac surgery nurse practitioner home visits prevent coronary artery bypass graft readmissions. Ann Thorac Surg. 2014;97:1488–1493. [DOI] [PubMed] [Google Scholar]
- 27. Shehata N, Forster A, Li L, Rothwell DM, Mazer CD, Naglie G, Fowler R, Tu JV, Rubens FD, Hawken S, Wilson K. Does anemia impact hospital readmissions after coronary artery bypass surgery? Transfusion. 2013;53:1688–1697. [DOI] [PubMed] [Google Scholar]
- 28. Brown JR, Landis RC, Chaisson K, Ross CS, Dacey LJ, Boss RA Jr, Helm RE, Horton SR, Hofmaster P, Jones C, Desaulniers H, Westbrook BM, Duquette D, Leblond K, Quinn RD, Magnus PC, Malenka DJ, Discipio AW. Preoperative white blood cell count and risk of 30‐day readmission after cardiac surgery. Int J Inflam. 2013;2013:781024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Z, Armstrong EJ, Parker JP, Danielsen B, Romano PS. Hospital variation in readmission after coronary artery bypass surgery in California. Circ Cardiovasc Qual Outcomes. 2012;5:729–737. [DOI] [PubMed] [Google Scholar]
- 30. D'Agostino RS, Jacobson J, Clarkson M, Svensson LG, Williamson C, Shahian DM. Readmission after cardiac operations: prevalence, patterns, and predisposing factors. J Thorac Cardiovasc Surg. 1999;118:823–832. [DOI] [PubMed] [Google Scholar]
- 31. Zitser‐Gurevich Y, Simchen E, Galai N, Braun D. Prediction of readmissions after CABG using detailed follow‐up data: the Israeli CABG Study (ISCAB). Med Care. 1999;37:625–636. [DOI] [PubMed] [Google Scholar]
- 32. Shahian DM, He X, O'Brien SM, Grover FL, Jacobs JP, Edwards FH, Welke KF, Suter LG, Drye E, Shewan CM, Han L, Peterson E. Development of a clinical registry‐based 30‐day readmission measure for coronary artery bypass grafting surgery. Circulation. 2014;130:399–409. [DOI] [PubMed] [Google Scholar]
- 33. Bradley EH, Curry L, Horwitz LI, Sipsma H, Wang Y, Walsh MN, Goldmann D, White N, Piña IL, Krumholz HM. Hospital strategies associated with 30‐day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brooke BS, Stone DH, Cronenwett JL, Nolan B, DeMartino RR, MacKenzie TA, Goodman DC, Goodney PP. Early primary care provider follow‐up and readmission after high‐risk surgery. JAMA Surg. 2014;149:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179–185. [DOI] [PubMed] [Google Scholar]
- 36. Stewart S, Horowitz JD. Home‐based intervention in congestive heart failure: long‐term implications on readmission and survival. Circulation. 2002;105:2861–2866. [DOI] [PubMed] [Google Scholar]
- 37. Garland A, Fransoo R, Olafson K, Ramsey C, Yogendran M, Chateau D, McGowan K. The Epidemiology and Outcomes of Critical Illness in Manitoba. Winnipeg, MB: Manitoba Centre for Health Policy; 2012. [Google Scholar]
- 38. Lix L, Yogendran M, Burchill C, Metge C, McKeen N, Moore D, Bond R. Defining and Validating Chronic Diseases: An Administrative Data Approach. Winnipeg, MB: Manitoba Centre for Health Policy; 2006. [Google Scholar]
- 39. Finlayson G, Jacobs P, Watson D, Bogdanovic B. A Comparison of Preliminary and Adjusted Cost per Weighted Case Determinations for Manitoba Hospitals: Impact for Evaluation and Report Cards. Winnipeg, MB: Manitoba Centre for Health Policy; 2001. [Google Scholar]
- 40. Finlayson G, Reimer J, Dahl M, Stargardter M, McGowan K. The Direct Cost of Hospitalizations in Manitoba, 2005/06. Winnipeg, MB: Manitoba Centre for Health Policy; 2009. [Google Scholar]
- 41. Canadian Institute for Health Information . Resource Indicators: DAD Resource Intensity Weights and Expected Length of Stay. Available at: https://www.cihi.ca/en/data-and-standards/standards/case-mix/resource-indicators-dad-resource-intensity-weights-and. Accessed November 20, 2015.
- 42. Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559–565. [DOI] [PubMed] [Google Scholar]
- 43. Acher AW, LeCaire TJ, Hundt AS, Greenberg CC, Carayon P, Kind AJ, Weber SM. Using human factors and systems engineering to evaluate readmission after complex surgery. J Am Coll Surg. 2015;221:810–820. doi:10.1016/j.jamcollsurg.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD; Telemedical Interventional Monitoring in Heart Failure Investigators . Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123:1873–1880. [DOI] [PubMed] [Google Scholar]
- 45. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944. [DOI] [PubMed] [Google Scholar]
- 46. Curtis JP. Baby or bathwater? Early follow‐up after hospital discharge. Circulation. 2013;128:1177–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, Kripalani S. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Definitions and ICD codes used for variables collected


