Abstract
Background
Racial‐ethnic disparities in acute stroke care can contribute to inequality in stroke outcomes. We examined race‐ethnic disparities in acute stroke performance metrics in a voluntary stroke registry among Florida and Puerto Rico Get With the Guidelines‐Stroke hospitals.
Methods and Results
Seventy‐five sites in the Florida Puerto Rico Stroke Registry (66 Florida and 9 Puerto Rico) recorded 58 864 ischemic stroke cases (2010–2014). Logistic regression models examined racial‐ethnic differences in acute stroke performance measures and defect‐free care (intravenous tissue plasminogen activator treatment, in‐hospital antithrombotic therapy, deep vein thrombosis prophylaxis, discharge antithrombotic therapy, appropriate anticoagulation therapy, statin use, smoking cessation counseling) and temporal trends. Among ischemic stroke cases, 63% were non‐Hispanic white (NHW), 18% were non‐Hispanic black (NHB), 14% were Hispanic living in Florida, and 6% were Hispanic living in Puerto Rico. NHW patients were the oldest, followed by Hispanics, and NHBs. Defect‐free care was greatest among NHBs (81%), followed by NHWs (79%) and Florida Hispanics (79%), then Puerto Rico Hispanics (57%) (P<0.0001). Puerto Rico Hispanics were less likely than Florida whites to meet any stroke care performance metric other than anticoagulation. Defect‐free care improved for all groups during 2010–2014, but the disparity in Puerto Rico persisted (2010: NHWs=63%, NHBs=65%, Florida Hispanics=59%, Puerto Rico Hispanics=31%; 2014: NHWs=93%, NHBs=94%, Florida Hispanics=94%, Puerto Rico Hispanics=63%).
Conclusions
Racial‐ethnic/geographic disparities were observed for acute stroke care performance metrics. Adoption of a quality improvement program improved stroke care from 2010 to 2014 in Puerto Rico and all Florida racial‐ethnic groups. However, stroke care quality delivered in Puerto Rico is lower than in Florida. Sustained support of evidence‐based acute stroke quality improvement programs is required to improve stroke care and minimize racial‐ethnic disparities, particularly in resource‐strained Puerto Rico.
Keywords: cerebrovascular disease, disparities, ethnicity, race
Subject Categories: Race and Ethnicity, Epidemiology, Cerebrovascular Disease/Stroke, Treatment
Introduction
Stroke is the fifth leading cause of death in the United States, the second leading cause of adult death worldwide, and the main cause of adult disability.1, 2 Racial‐ethnic disparities in acute stroke care have been documented in a statement from the American Heart Association/American Stroke Association (AHA/ASA),3 and represent a recognized research priority by the National Institute of Neurological Disorders and Stroke (NINDS). These disparities can contribute to inequality in outcomes after acute stroke. Disparities exist in stroke mortality, morbidity, prevalence of biological and environmental risk factors, healthcare access, and quality of care. Incidence data from NOMAS (the Northern Manhattan Study) has demonstrated racial‐ethnic differences in stroke incidence, with blacks and Hispanics having more than a 2‐fold increase in annual stroke incidence compared with whites.4, 5 Some studies have documented disparities in access to acute stroke care, activation of 911 services, delayed arrival to the emergency department, longer waiting times, and treatment delays in thrombolysis.3 The aging and rapid growth of the Hispanic population and growing socioeconomic crisis in Puerto Rico (PR) may increase the public health impact of stroke and widen stroke disparities.
The AHA/ASA Get With the Guidelines‐Stroke (GWTG‐S) program was developed to improve acute stroke performance by implementing evidence‐based care. National GWTG data have provided important insights into racial‐ethnic disparities in stroke care, showing poorer acute stroke care in blacks.6 The Florida‐Puerto Rico (FL‐PR) Stroke Registry is a voluntary stroke registry consisting of data already collected using the GWTG‐S system as well as additional data (eg, ethnicity, language, education, 30‐day follow‐up) among hospitals from diverse and less investigated US regions. The aims of this study were to identify racial‐ethnic and geographic disparities in acute stroke performance metrics, overall rates of “defect‐free care” (DFC; compliance with all eligible quality metrics), and evaluate temporal trends in disparities in DFC with a particular focus on PR.
Methods
Study Population
In March 2015, the FL‐PR Stroke Registry was comprised of 75 hospitals: 66 in FL and 9 in PR. The Registry includes retrospective and prospective hospital‐collected GWTG‐S data on patients with a primary diagnosis of ischemic stroke, transient ischemic attack, subarachnoid hemorrhage, intracerebral hemorrhage, and stroke not otherwise specified. In total, 88 978 cases were enrolled in the FL‐PR Stroke Registry from January 2010 through December 2014, including 58 864 cases with a primary diagnosis of ischemic stroke. Data were collected for the original purpose of inclusion in the GWTG‐S program––a voluntary, ongoing, national registry with the goal of improving stroke and cardiovascular disease care––and additional questions were added for those hospitals participating in the FL‐PR Stroke Registry, including further questions regarding ethnicity, language, education, and 30‐day outcomes. The University of Miami's institutional review board approved this study. Each participating center received institutional ethics approval to enroll cases in the FL‐PR Stroke Registry without requiring individual patient consent under the common rule or a waiver of authorization and exemption from subsequent review by their institutional review board.
Data Collection
Healthcare providers in GWTG are trained to utilize patient‐specific guideline information and have immediate access to clinical decision support through the AHA's Patient Management Tool, an online, interactive assessment and reporting system. Stroke patients eligible for the GWTG‐S registry are drawn from those admitted for acute stroke. Trained hospital personnel screen acute stroke admissions through prospective clinical identification, retrospective identification using International Classification of Diseases‐9th Revision discharge codes, or a combination. Extracted data include demographics, mode of arrival, time from symptom onset to hospital arrival, health insurance information, medical history, brain imaging, in‐hospital treatment and events (including timing of intravenous [IV] tissue plasminogen activator [tPA] treatment and contraindications), discharge treatment and counseling, mortality, and discharge destination. Specific data on quality performance measures are collected regarding eligibility for IV recombinant tPA, stroke onset and arrival times, deep vein thrombosis (DVT) treatments, dysphagia screening, antithrombotic use, and statin use. High data quality in the GWTG database is maintained through careful training for chart abstractors, standardized coding instructions, limitations of data fields to realistic entries, audit trails, and required site data quality reports. Data on hospital‐level characteristics (ie, bed size, academic status, stroke center certification) were obtained from the Agency for Health Care Administration in FL hospitals and the American Hospital Registry for Puerto Rico Hospitals, as well as a self‐reported hospital characteristics survey completed by all hospitals participating in the FL‐PR Stroke Registry.
Primary Outcome Measures
The stroke care measures of interest are the key performance indicators essential for improving the quality of care and outcome of all patients with ischemic stroke: (1) IV tPA administered to eligible patients who arrived at the hospital within 2 hours of symptom onset and received treatment within 3 hours of symptom onset; (2) antithrombotic therapy by the end of hospital day 2; (3) DVT prophylaxis by the end of hospital day 2 for nonambulatory patients; (4) discharged on antithrombotic therapy; (5) appropriate anticoagulation therapy for atrial fibrillation/flutter; (6) discharged on statin medication for patients with low‐density lipoprotein ≥100 or taking lipid‐lowering agents before admission or with unmeasured low‐density lipoprotein in the previous 30 days; (7) counseling or medication for smoking cessation; and (8) the composite variable of DFC, which is an indicator among those eligible for a specific metric of whether all appropriate interventions listed above in items 1 to 7 are performed properly.
Statistical Analysis
We report the frequencies of performance indicators and DFC for the population of participants with ischemic stroke in the FL‐PR Stroke Registry by race‐ethnicity (white, non‐Hispanic; black, non‐Hispanic; Hispanic) and geographic region (FL and PR) overall and across calendar years (2010–2014). Non‐Hispanic white (NHW) was used as the reference group among those in FL, and PR Hispanics were compared with FL NHWs and with Hispanics living in FL. To account for the clustering within hospitals, multilevel logistic regression models were used when examining racial‐ethnic and temporal differences in stroke performance indicators, with generalized estimating equations. Multilevel logistic regression was used to examine whether any observed racial‐ethnic disparities persist after controlling for potential prespecified explanatory factors. Model 1 was adjusted for age, model 2 was adjusted for individual characteristics (age, health insurance status, hospital mode of arrival) and hospital level characteristics (years in the GWTG‐S program, academic hospitals versus not, number of beds), and model 3 included variables from model 2 with additional adjustments for patient National Institutes of Health stroke scale (NIHSS) score and vascular risk factors (current smoker, hypertension, diabetes, dyslipidemia, and medical history of atrial fibrillation, coronary artery disease/prior myocardial infarction, previous stroke/transient ischemic attack). For any covariates with missingness, missing values of the categorical covariates (including medical insurance, mode of arrival, and NIHSS score) were imputed with multiple imputation by discriminant function method (10 imputations), allowing us to maximize the sample size for each analysis.
We examined whether racial‐ethnic disparities in DFC changed from 2010 to 2014, controlling for age, sex, smoking, diabetes, atrial fibrillation, coronary artery disease, mode of arrival, academic status, number of beds, and years in the GWTG‐S program. Effect modification by time was examined by including interaction terms between year and race‐ethnicity in models.
Results
The study population consisted of 58 864 cases with the primary diagnosis of acute ischemic stroke (50% women; 63% NHWs living in FL, 18% non‐Hispanic blacks [NHBs] living in FL, 14% Hispanics living in FL, 6% Hispanics living in PR). Table 1 shows the demographic and clinical characteristics of the patients overall and stratified by race‐ethnicity. NHW patients were oldest, followed by Hispanics, and NHB patients were youngest. Hispanics in FL and PR were less likely to be current smokers. PR Hispanics were most likely to have hypertension and diabetes, followed by NHBs, yet PR Hispanics were least likely to have dyslipidemia. A previous diagnosis of atrial fibrillation was most prevalent among NHWs and least prevalent among NHBs and PR Hispanics. Coronary artery disease or previous myocardial infarction was also observed less commonly among NHB patients, but was most prevalent among both NHWs and PR Hispanics. Less than half of patients arrived by EMS in all groups. NHBs and Hispanics living in PR were least likely to arrive to the hospital within 2 hours from symptom onset to be eligible for IV tPA treatment within 3 hours.
Table 1.
Patient and Hospital‐Level Characteristics of Patients With Ischemic Stroke in the FL‐PR Stroke Registry Cohort, 2010–2014
| Clinical Characteristics | All (N=58 864) | FL Non‐Hispanic White (n=36 844) | FL Non‐Hispanic Black (n=10 536) | FL Hispanic (n=8207) | PR Hispanic (n=3277) | P Value |
|---|---|---|---|---|---|---|
| Age, mean±SD, y | 70.8±14.4 | 72.9±14.0 | 63.6±13.9 | 70.9±14.2 | 70.0±13.8 | <0.0001 |
| Sex, % | ||||||
| Male | 50.3 | 50.5 | 48.8 | 51.1 | 51.9 | 0.0154 |
| Female | 49.7 | 49.5 | 51.2 | 48.9 | 48.1 | |
| Vascular risk factor, % | ||||||
| Hypertension | 65.9 | 65.4 | 70.3 | 55.6 | 83.4 | <0.0001 |
| Diabetes mellitus | 28.9 | 24.8 | 37.6 | 27.9 | 49.6 | <0.0001 |
| Dyslipidemia | 32.3 | 41.7 | 32.4 | 28.8 | 24.7 | <0.0001 |
| Current smoker | 16.8 | 17.3 | 19.6 | 14.2 | 8.5 | <0.0001 |
| Medical history, % | ||||||
| Atrial fibrillation | 17.4 | 21.3 | 8.3 | 14.8 | 8.9 | <0.0001 |
| Coronary artery disease/prior myocardial infarction | 22.4 | 24.9 | 15.5 | 18.7 | 25.3 | <0.0001 |
| Previous stroke/TIA | 25.8 | 26.0 | 29.3 | 21.9 | 21.5 | <0.0001 |
| Medical insurance, % | <0.0001 | |||||
| Privatea | 36.7 | 43.1 | 28.9 | 20.2 | 31.0 | |
| Medicare | 33.0 | 33.5 | 30.5 | 38.5 | 21.9 | |
| Medicaidb | 11.5 | 8.0 | 23.7 | 15.5 | 1.7 | |
| Unknown | 18.8 | 15.4 | 16.9 | 25.9 | 45.4 | |
| Mode of arrival emergency medical services | <0.0001 | |||||
| Yes | 48.7 | 52.0 | 47.6 | 51.6 | 47.7 | |
| No | 44.1 | 41.0 | 43.4 | 37.7 | 31.6 | |
| Missing | 7.2 | 7.0 | 9.0 | 10.7 | 20.6 | |
| Arrival times | <0.0001 | |||||
| <2 h | 20.3 | 21.7 | 15.8 | 21.7 | 16.1 | |
| <2 to 3.5 h | 6.1 | 6.4 | 4.8 | 6.3 | 7.6 | |
| <3.5 to 4.5 h | 3.0 | 3.0 | 2.7 | 3.0 | 2.8 | |
| <4.5 to 6 h | 3.3 | 3.4 | 3.0 | 3.2 | 3.1 | |
| >6 h | 16.5 | 16.0 | 16.9 | 17.4 | 18.3 | |
| Undocumented/unknown | 50.8 | 49.5 | 56.8 | 48.7 | 52.1 | |
| NIHSS score, % | <0.0001 | |||||
| ≤5 | 36.9 | 38.6 | 37.8 | 32.3 | 26.8 | |
| 6 to 15 | 18.6 | 18.2 | 18.9 | 16.0 | 28.9 | |
| ≥16 | 11.2 | 11.5 | 8.8 | 10.5 | 16.8 | |
| Missing | 33.3 | 31.7 | 34.5 | 41.2 | 27.5 | |
| Hospital characteristics | ||||||
| No. of beds, mean±SD | 555.6±316.5 | 540.7±308.2 | 641.5±330.4 | 600.8±337.1 | 333.0±127.0 | <0.0001 |
| Academic hospital | 30.8 | 28.5 | 33.0 | 32.3 | 13.3 | <0.0001 |
| Years in GWTG, mean±SD | 7.9±2.3 | 8.2±2.2 | 8.1±2.1 | 7.2±2.2 | 5.5±1.7 | <0.0001 |
FL indicates Florida; GWTG, Get With the Guidelines; NIHSS, National Institutes of Health Stroke Scale; PR, Puerto Rico; TIA, transient ischemic attack.
Includes private insurance, Veterans Affairs, and other.
Includes Medicaid, self‐pay, and no insurance.
Among the prespecified acute stroke performance targets, blacks who smoked were less likely to receive smoking cessation counseling versus whites (Table 2). In the fully adjusted models, blacks were more likely to receive VTE prophylaxis, antithrombotics at discharge, and DFC overall versus whites. There were no differences in care observed for Hispanics living in FL versus whites. Covariates predictive of DFC included male sex, health insurance, arrival by EMS, low NIHSS score, and dyslipidemia.
Table 2.
Acute Stroke Care Metrics Among Different Racial‐Ethnic Subgroups in Florida and Puerto Rico, 2010–2014
| AOR (95% CI) | Florida | Puerto Rico | ||
|---|---|---|---|---|
| Non‐Hispanic White | Non‐Hispanic Black | Hispanic | Hispanic | |
| IV tPA, arrive by 2 hours and treat by 3 hours | ||||
| No. eligible | 2540 | 500 | 552 | 286 |
| Treated, % | 89 | 87 | 91 | 78 |
| AOR (95% CI) Model 1a | Ref | 0.96 (0.75–1.22) | 1.20 (0.90–1.58) | 0.22 (0.07–0.73) |
| AOR (95% CI) Model 2b | 0.92 (0.66–1.28) | 1.30 (0.90–1.89) | 0.72 (0.24–2.13) | |
| AOR (95% CI) Model 3c | 0.89 (0.63–1.26) | 1.30 (0.85–2.00) | 0.60 (0.21–1.72) | |
| Early antithrombotic use | ||||
| No. eligible | 24 813 | 7465 | 5488 | 2103 |
| Treated, % | 96 | 95 | 96 | 93 |
| AOR (95% CI) Model 1 | Ref | 0.91 (0.80–1.03) | 0.96 (0.73–1.27) | 0.13 (0.05–0.32) |
| AOR (95% CI) Model 2 | 0.94 (0.79–1.12) | 0.98 (0.69–1.38) | 0.37 (0.13–1.06) | |
| AOR (95% CI) Model 3 | 0.98 (0.84–1.14) | 1.01 (0.75–1.36) | 0.24 (0.10–0.55) | |
| Antithrombotics at discharge | ||||
| No. eligible | 25 689 | 7654 | 5621 | 2095 |
| Treated, % | 98 | 97 | 97 | 94 |
| AOR (95% CI) Model 1 | Ref | 0.92 (0.77–1.10) | 1.04 (0.60–1.80) | 0.10 (0.04–0.24) |
| AOR (95% CI) Model 2 | 0.97 (0.80–1.18) | 1.07 (0.67–1.69) | 0.20 (0.09–0.47) | |
| AOR (95% CI) Model 3 | 1.10 (1.02–1.18) | 1.08 (0.96–1.21) | 0.29 (0.12–0.74) | |
| Anticoagulation for atrial fibrillation | ||||
| No. eligible | 4765 | 601 | 793 | 275 |
| Treated, % | 96 | 97 | 94 | 90 |
| AOR (95% CI) Model 1 | Ref | 1.09 (0.76–1.56) | 0.93 (0.66–1.33) | 0.36 (0.11–1.14) |
| AOR (95% CI) Model 2 | 1.07 (0.68–1.68) | 0.90 (0.61–1.34) | 0.72 (0.26–2.02) | |
| AOR (95% CI) Model 3 | 1.06 (0.67–1.66) | 0.88 (0.59–1.30) | 0.67 (0.24–1.85) | |
| Smoking cessation counseling | ||||
| No. eligible | 4712 | 1524 | 869 | 190 |
| Treated, % | 97 | 96 | 95 | 93 |
| AOR (95% CI) Model 1 | Ref | 0.67 (0.55–0.82) | 0.86 (0.50–1.46) | 0.23 (0.06–0.92) |
| AOR (95% CI) Model 2 | 0.69 (0.55–0.86) | 0.89 (0.54–1.48) | 0.44 (0.12–1.60) | |
| AOR (95% CI) Model 3 | 0.68 (0.54–0.86) | 0.91 (0.54–1.53) | 0.44 (0.12–1.61) | |
| LDL ≥100 mg/dL or ND—Statin | ||||
| No. eligible | 19 965 | 5917 | 4428 | 1596 |
| Treated, % | 92 | 93 | 93 | 83 |
| AOR (95% CI) Model 1 | Ref | 1.02 (0.92–1.14) | 1.14 (0.86–1.51) | 0.15 (0.07–0.31) |
| AOR (95% CI) Model 2 | 1.06 (0.96–1.18) | 1.16 (0.87–1.54) | 0.19 (0.09–0.38) | |
| AOR (95% CI) Model 3 | 1.10 (0.99–1.22) | 1.19 (0.89–1.58) | 0.23 (0.12–0.47) | |
| VTE prophylaxis | ||||
| No. eligible | 29 315 | 8394 | 6416 | 2527 |
| Treated, % | 85 | 87 | 86 | 67 |
| AOR (95% CI) Model 1 | Ref | 1.06 (0.99–1.14) | 1.05 (0.95–1.16) | 0.24 (0.13–0.46) |
| AOR (95% CI) Model 2d | 1.08 (1.00–1.16) | 1.07 (0.95–1.20) | 0.25 (0.10–0.66) | |
| AOR (95% CI) Model 3 | 1.10 (1.02–1.18) | 1.08 (0.96–1.21) | 0.29 (0.12–0.74) | |
| Defect‐free care | ||||
| No. eligible | 29 865 | 8538 | 6529 | 2588 |
| Treated, % | 79 | 81 | 79 | 57 |
| AOR (95% CI) Model 1 | Ref | 1.10 (1.03–1.17) | 1.02 (0.96–1.09) | 0.35 (0.32–0.38) |
| AOR (95% CI) Model 2 | 1.06 (1.00–1.13) | 1.05 (0.95–1.16) | 0.26 (0.12–0.56) | |
| AOR (95% CI) Model 3 | 1.07 (1.00–1.14) | 1.06 (0.95–1.18) | 0.27 (0.12–0.58) | |
AOR indicates adjusted odds ratio; IV tPA, intravenous tissue plasminogen activator; LDL, low‐density lipoprotein; ND, not documented; VTE, venous thromboembolism.
Model 1 adjusted for age and correlation within and between hospitals.
Model 2 additionally adjusted for age, health insurance status, mode of arrival and hospital‐level factors including number of beds, years in Get With the Guidelines‐Stroke, and academic vs not.
Model 3 includes variables in model 2 and patient National Institutes of Health Stroke Scale score, current smoker, hypertension, diabetes, dyslipidemia, and medical history of atrial fibrillation, coronary artery disease/prior myocardial infarction, and previous stroke/transient ischemic attack.
In order for this model to converge, health insurance status was not included as a covariate.
PR Hispanics were less likely than whites to receive overall DFC. PR Hispanics were less likely than FL whites to meet any of the stroke care performance metrics other than anticoagulation for atrial fibrillation. After adjustment, the disparities observed for PR Hispanics persisted for early antithrombotics, antithrombotics at discharge, statin therapy, VTE prophylaxis, and DFC. Compared with FL Hispanics, PR Hispanics were still less likely to receive these individual stroke care measures and DFC (Table 3).
Table 3.
Acute Stroke Care Metrics in PR Hispanics Versus FL Hispanics
| AOR (95% CI) | PR Hispanic vs FL Hispanic (Reference) |
|---|---|
| IV tPA, arrive by 2 hours and treat by 3 hours | |
| AOR (95% CI) Model 1a | 0.19 (0.06–0.62) |
| AOR (95% CI) Model 2b | 0.55 (0.18–1.67) |
| AOR (95% CI) Model 3c | 0.46 (0.16–1.38) |
| Early antithrombotic use | |
| AOR (95% CI) Model 1 | 0.14 (0.06–0.33) |
| AOR (95% CI) Model 2 | 0.37 (0.12–1.14) |
| AOR (95% CI) Model 3 | 0.24 (0.10–0.59) |
| Antithrombotics at discharge | |
| AOR (95% CI) Model 1 | 0.10 (0.04–0.22) |
| AOR (95% CI) Model 2 | 0.19 (0.07–0.49) |
| AOR (95% CI) Model 3 | 0.27 (0.11–0.70) |
| Anticoagulation for atrial fibrillation | |
| AOR (95% CI) Model 1 | 0.39 (0.12–1.27) |
| AOR (95% CI) Model 2 | 0.81 (0.27–2.41) |
| AOR (95% CI) Model 3 | 0.76 (0.26–2.26) |
| Smoking cessation counseling | |
| AOR (95% CI) Model 1 | 0.26 (0.06–1.21) |
| AOR (95% CI) Model 2 | 0.49 (0.12–2.05) |
| AOR (95% CI) Model 3 | 0.49 (0.12–2.07) |
| LDL ≥100 mg/dL or ND—statin | |
| AOR (95% CI) Model 1 | 0.13 (0.06–0.28) |
| AOR (95% CI) Model 2 | 0.16 (0.08–0.35) |
| AOR (95% CI) Model 3 | 0.20 (0.09–0.41) |
| VTE prophylaxis | |
| AOR (95% CI) Model 1 | 0.23 (0.12–0.44) |
| AOR (95% CI) Model 2d | 0.24 (0.09–0.62) |
| AOR (95% CI) Model 3 | 0.27 (0.11–0.70) |
| Defect‐free care | |
| AOR (95% CI) Model 1 | 0.22 (0.12–0.45) |
| AOR (95% CI) Model 2 | 0.25 (0.11–0.54) |
| AOR (95% CI) Model 3 | 0.25 (0.12–0.55) |
AOR indicates adjusted odds ratio; FL, Florida; IV tPA, intravenous tissue plasminogen activator; LDL, low‐density lipoprotein; ND, not documented; PR, Puerto Rico; VTE, venous thromboembolism.
Model 1 adjusted for age and correlation within and between hospitals.
Model 2 additionally adjusted for age, health insurance status, mode of arrival and hospital‐level factors including number of beds, years in Get With the Guidelines‐Stroke, and academic vs not.
Model 3 includes variables in model 2 and patient National Institutes of Health Stroke Scale score, current smoker, hypertension, diabetes, dyslipidemia, and medical history of atrial fibrillation, coronary artery disease/prior myocardial infarction, and previous stroke/transient ischemic attack.
In order for this model to converge, health insurance status was not included as a covariate.
Significant effect modification by year (2010–2014) was evident for the association between race‐ethnicity and all stroke care metrics other than smoking cessation counseling. Therefore, Table 4 presents analyses restricted to years 2010 and 2014 for each of the stroke care metrics, as well as across all 5 years for overall DFC. Within FL, temporal trends showed improved performance across all stroke care metrics from 2010 to 2014 for all racial‐ethnic subgroups, other than smoking cessation counseling, which remained high over time. The percentage of patients receiving DFC also rose over time (Table 4, Figure). Lower rates of early antithrombotic use, antithrombotics at discharge, and smoking cessation counseling among blacks compared with whites in 2010 were no longer disparate by 2014. In 2014, Hispanics living in FL were more likely than whites to receive IV tPA within 3 hours.
Table 4.
Acute Stroke Performance Metrics Among Different Racial‐Ethnic Groups Over Time, 2010–2014
| Percentage, AOR (95% CI)a | ||||
|---|---|---|---|---|
| FL Non‐Hispanic White | FL Non‐Hispanic Black | FL Hispanic | PR Hispanic | |
| IV tPA, arrive by 2 hours and treat by 3 hours | Ref | |||
| 2010 | 80.0 | 72.3, 0.59 (0.34–1.02) | 85.2, 1.21 (0.66–2.22) | 63.9, 1.64 (0.59–4.54) |
| 2014 | 93.7 | 96.0, 1.93 (0.93–4.03) | 97.0, 2.05 (1.14–3.68) | 66.7, 0.24 (0.06–0.90)b |
| Early antithrombotic use | Ref | |||
| 2010 | 95.6 | 93.0, 0.73 (0.60–0.90) | 91.8, 0.85 (0.60–1.19) | 94.1, 1.11 (051–2.45) |
| 2014 | 97.9 | 97.6, 0.98 (072–1.34) | 97.4, 0.96 (0.67–1.38) | 84.6, 0.11 (0.06–0.20)b |
| Antithrombotics at discharge | Ref | |||
| 2010 | 96.3 | 94.7, 0.83 (0.71–0.98) | 94.8, 0.85 (0.62–1.15) | 94.0, 0.26 (0.08–0.82)b |
| 2014 | 99.2 | 99.3, 1.19 (0.64–2.21) | 99.1, 0.85 (0.49–1.47) | 93.2, 0.20 (0.11–0.39)b |
| Anticoagulation for atrial fibrillation | Ref | |||
| 2010 | 94.0 | 95.1, 1.42 (0.57–3.58) | 90.5, 1.09 (0.46–2.60) | 94.4, 1.75 (0.56–5.49) |
| 2014 | 97.1 | 96.5, 0.75 (0.33–1.72) | 97.8, 1.60 (0.65–3.94) | 92.6, 0.72 (0.26–1.94) |
| Smoking cessation counseling | Ref | |||
| 2010 | 98.4 | 96.9, 0.48 (0.29–0.79) | 96.3, 0.75 (0.19–2.88) | 95.5, 0.71 (0.18–2.81) |
| 2014 | 97.9 | 96.8, 0.70 (0.44–1.12) | 96.9, 0.91 (0.38–2.17) | 95.2, 0.47 (0.08–2.84) |
| LDL ≥100 mg/dL or ND—Statin | Ref | |||
| 2010 | 87.8 | 87.9, 0.97 (0.85–1.11) | 86.0, 1.03 (0.84–1.26) | 78.0, 0.64 (0.23–1.75) |
| 2014 | 97.3 | 97.9, 1.22 (0.86–1.73) | 98.1, 1.13 (0.73–1.75) | 83.4, 0.16 (0.09–0.29)b |
| VTE prophylaxis | Ref | |||
| 2010 | 70.0 | 73.2, 0.98 (0.90–1.06) | 67.9, 1.08 (0.98–1.20) | 34.6, 0.58 (0.12–2.75) |
| 2014 | 97.3 | 98.1, 1.11 (0.83–1.48) | 98.0, 1.16 (0.83–1.62) | 78.7, 0.13 (0.07–0.25)b |
| Defect‐free care | Ref | |||
| 2010 | 63.1 | 65.2, 0.97 (0.89–1.05) | 58.7, 1.10 (0.99–1.23) | 30.8, 0.69 (0.18–2.73) |
| 2011 | 67.6 | 70.9, 1.02 (0.96–1.09) | 66.5, 0.96 (0.84–1.09) | 39.5, 0.63 (0.16–2.58) |
| 2012 | 83.4 | 83.3, 0.99 (0.86–1.14) | 84.1, 0.97 (0.80–1.18) | 62.8, 0.29 (0.12–0.69)b |
| 2013 | 91.1 | 91.7, 1.10 (0.92–1.32) | 92.1, 1.12 (0.96–1.30) | 76.6, 0.36 (0.19–0.68)b |
| 2014 | 92.9 | 93.7, 1.10 (0.90–1.34) | 93.5, 1.11 (0.86–1.43) | 63.1, 0.13 (0.07–0.25)b |
AOR indicates adjusted odds ratio; IV tPA, intravenous tissue plasminogen activator; LDL, low‐density lipoprotein; ND, not documented; VTE, venous thromboembolism.
The model is multilevel adjusted for hospital‐level random effect and age, sex, health insurance status, mode of arrival, academic status, number of beds, and years in Get With the Guidelines.
P<0.05 Puerto Rico (PR) Hispanics vs Florida (FL) Hispanics.
Figure 1.
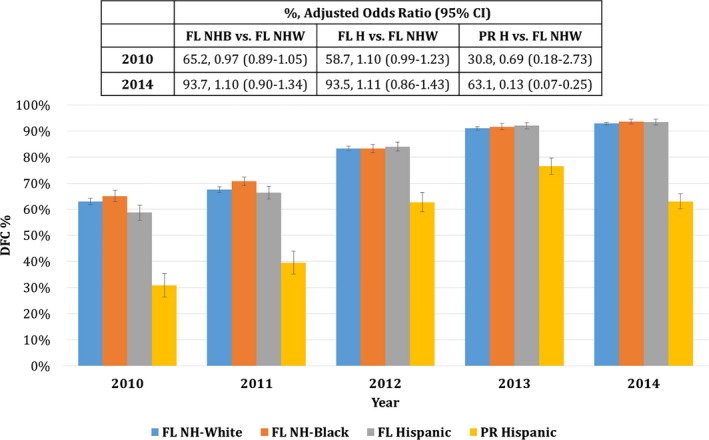
Defect‐free care (DFC) over time across race‐ethnicity, 2010–2014. Blue=non‐Hispanic white (NHW); orange=non‐Hispanic black (NHB); gray=Florida (FL) Hispanic (H); yellow=Puerto Rico (PR) Hispanic.
For PR, the rate of DFC improved from 2010 to 2013, with a slight dip from 2013 to 2014 (Figure). Despite rises in the absolute rate of DFC among PR Hispanics, in 2012–2014 the rate of DFC was significantly lower in PR versus FL whites and FL Hispanics. In addition, the use of antithrombotics and anticoagulants did not improve in PR from 2010 to 2014.
Discussion
In the FL‐PR Stroke Registry, racial‐ethnic and geographic disparities were observed in stroke DFC overall and in all acute stroke care performance metrics other than anticoagulation for atrial fibrillation. These disparities indicated that whites, blacks, and Hispanics living in FL received superior stroke care compared with PR Hispanics. PR Hispanics were less likely to receive DFC compared with FL whites and FL Hispanics. This was driven by differences in early antithrombotic use and antithrombotic use at discharge, statin therapy, and VTE prophylaxis. Although stroke care improved over time in PR, as well as in all racial‐ethnic groups in FL from 2010 to 2014, the disparities observed for PR Hispanics compared with FL whites and FL Hispanics persisted.
It is encouraging to note that DFC was high in the non‐white populations in FL. DFC was similar in FL Hispanics and in fact more common among FL blacks as compared with whites in our registry, even after multivariable adjustment. Although the difference for blacks reached statistical significance, the absolute difference was quite small (81% in blacks versus 79% in whites), and therefore the clinical relevance is limited. The higher rate of DFC among blacks appeared to be driven by VTE prophylaxis, better low‐density lipoprotein levels/statin therapy, and early antithrombotics. Further exploration in future studies is warranted.
Stroke care performance for all racial‐ethnic groups has improved during the past 5 years in FL and PR. Although blacks in FL arrived to the hospital later than whites and were less likely to receive smoking cessation counseling, they were more likely to receive overall DFC compared with whites among these experienced GWTG‐S centers. By 2014 in FL, racial‐ethnic disparities in the major acute stroke performance were minimal to none. However, despite absolute improvements in DFC over time, disparities in stroke care for PR Hispanics increased over time relative to NHWs.
As the gap in stroke care between FL and PR widens, the urgent need for programs and initiatives to improve acute stroke care grows in the setting of an economic crisis in PR. PR is undergoing the worst fiscal and economic crisis in the country's history ($72 billion debt) translating to cutbacks in medical services, delays in provider payments, and a major physician exodus leading to deterioration in patient care.7 While ≈60% of the island's population receives care through Medicare or Medicaid, reimbursement rates are 70% lower compared with that of any other state on the mainland. Despite the implementation of the AHA GWTG‐S program in 15 hospitals in PR, there are shrinking resources to support the needs of their high‐risk population, with only one certified primary stroke center. PR is unique in that its population has declined by 5% during the past decade, with more than 50 000 residents leaving the US territory each year.8 This exodus, which is expected to continue gradually through 2050, is mainly due to the emigration of younger citizens secondary to economic decline and has subsequently accelerated the aging of the population.9 In addition, it is estimated that at least one physician leaves the island each day, which has detrimental effects on health care, particularly affecting the aging population. A decade‐long recession, exodus of physicians, and far lower Medicare and Medicaid reimbursements compared with other states have created an economic and healthcare crisis, which can only lead to further disparities in care and outcomes overall. There are also several hospital‐level factors that may contribute to the inadequate stroke care in PR. Compared with FL hospitals in the registry, PR hospitals were smaller, had fewer stroke neurologists, and were less likely to have a stroke unit or stroke team, were less likely to achieve stroke center certification, and were less likely to have implemented acute stroke care practices (eg, designated stroke team, stroke care protocols, and availability of neurological services).
PR faces challenges in delivering the stroke standards of care required to achieve stroke center designation. Compared with the United States, PR has less than half the number of emergency physicians and neurosurgeons.10 PR hospitals also had a shorter duration of participation in GWTG‐S as compared with FL hospitals overall, and now some PR hospitals are withdrawing because they lack the staff and funding to continue data collection. Time in GWTG‐S is associated with increased odds of receiving each predefined stroke performance measure, although the benefit is not equally distributed. The greatest rates of improvement were seen in larger hospitals with more bed capacity, those with larger annual stroke discharge rates, and those identified as teaching hospitals.11
Although not the focus of the current study, the higher NIHSS score observed among PR Hispanics is noteworthy. Our data are unable to elucidate whether this is due to strokes being more clinically severe among PR Hispanics or whether this is due to delays in hospital arrival, differences in the familiarity with and recognition of stroke symptoms among people in PR, or a higher threshold of severity before patients in PR will seek medical attention at a hospital.
This study was motivated by previous evidence of race‐ethnic differences in stroke incidence, mortality, and care. The AHA/ASA summarized the importance of racial‐ethnic disparities in stroke care.3 Our study fills an important gap in stroke research, as the AHA/ASA emphasized the need for more statewide or regional stroke registries that track stroke care data and statistics on quality performance metrics. The NINDS also recently emphasized their commitment to supporting much‐needed outcomes research that identifies sources and causes of race/ethnic disparities in the treatment of neurological diseases. This is important for the transition to a value‐based healthcare system in the United States. This study represents the first stroke registry in FL and PR to focus on racial‐ethnic disparities in acute stroke care. FL and PR are ideal regions for a stroke registry with this focus. Demographers speculate that FL will have a Hispanic majority by 2035. However, the current study is not the first regional or statewide stroke registry. The Centers for Disease Control and Prevention funded 7 state health departments to implement stroke care improvement registries through the Paul Coverdell National Acute Stroke Registry.
Study Strengths
A strength of our study is the use of existing well‐established standardized GWTG‐S data. The GWTG‐S program includes data from >3 million stroke admissions from >2600 US hospitals. Our regional data together with national GWTG‐S data demonstrate that adherence to evidence‐based guidelines and quality improvement programs may effectively minimize and eliminate acute stroke disparities.6 Improved quality of stroke care over time underscores the importance of prolonged participation in GWTG‐S, and differences observed between FL and PR may be driven in part by the number of years in GWTG‐S. In fact, the dip in DFC in PR in 2014 may be attributable to the addition of new, less experienced hospitals to the GWTG‐S program.
Among a nationwide sample of GWTG‐S hospitals evaluated from 2003 to 2008, black patients had less adequate performance across multiple patient care measures including receiving IV thrombolysis, DVT prophylaxis, smoking cessation therapy, discharge antithrombotics, anticoagulants for atrial fibrillation, lipid therapy, and of dying in‐hospital.12 With the exception of smoking cessation therapy, these findings were not replicated in our study, possibly due to temporal improvements in acute stroke care among blacks in recent years. Although overall Hispanic DFC in the national sample was similar to whites, there was less adequate performance for antithrombotics and smoking cessation.12 Regional differences were not evaluated in that study. Similar to our findings, temporal trends documented significantly improved quality of care in all racial‐ethnic subgroups.
Identification of disparities is the first step in eradicating them. Our results highlight the need for physician training, better organization of hospital care resources for acute stroke, and programs aimed at reducing disparities in stroke care. Evidence‐based quality improvement programs can help create learning environments for healthcare professionals and ensure best practices are applied uniformly to all patients and help eliminate variations in care and disparities.
Our results may not apply to other regions of the United States. Studies have suggested generalizability of the GWTG registry overall, but the current study includes a subset of GWTG‐S–participating hospitals in FL and PR and may not represent stroke care metrics and disparities across race‐ethnic groups for the entirety of FL and PR. Results comparing ischemic stroke patients 65 years and older enrolled in GWTG‐S and Medicare patients with ischemic stroke have generally shown reasonable comparability.13 GWTG hospitals are more likely to be larger, urban, teaching centers. Data suggest that the GWTG‐S admissions for Medicare patients older than 65 years are generally representative of national samples. FL centers participating in our FL‐PR Stroke Registry had similar performance results compared with all FL and national GWTG‐S samples. However, the excellent performance of GWTG‐S across multiple stroke measures may not be representative of non–GWTG‐S centers. We restricted our analyses to commonly collected prespecified GWTG‐S performance metrics. In addition, an important limitation was the high amount of missingness for some covariates, most notably the stroke severity variable NIHSS score. In order to maintain the sample size and limit bias, a multiple imputation approach was used.
It is crucial to interpret the results of this study in the context of the large sample size and many outcomes and comparisons examined. As mentioned previously, with a very large sample size, statistically significant differences may not be large enough to be clinically relevant, and the large number of comparisons increases the opportunity for significant differences observed due to chance alone.
Several studies, led by key FL‐PR Stroke Registry collaborators focusing on stroke and cardiovascular disease in PR have been published in recent years, demonstrating the burden of stroke in PR and the challenges in acute care.14, 15, 16 Most recently (2015), it was shown that stroke was the 5th leading cause of death and first cause of long‐term disability in PR, with ischemic stroke representing 75% of all stroke types on the island.16 Hypertension and diabetes rates in PR patients were substantially higher compared with studies that included national US cohorts. However, our study provides novel insight into the differences in acute stroke care between FL and PR, as we are aware of no other study that has examined this comparison. It represents the first regional stroke registry for FL and PR, focused on racial‐ethnic and geographic disparities in acute stroke care. Significant differences in performance in some acute stroke care practices were observed between FL and PR; however, improvements and reductions in disparities were observed the longer hospitals participated in the hospital quality improvement GWTG‐S program. Specific interventions are needed to address smoking cessation counseling among blacks in FL.
Conclusions
Despite temporal improvements in acute stroke care across all racial‐ethnic groups, improvements for Hispanics living in PR are significantly lagging relative to all FL groups. The results underscore the need for increased healthcare resources in PR and continued monitoring of disparities in various metrics of stroke care. Importantly, our findings highlight the need for continued investments in evidence‐based stroke quality improvement programs to target areas where performance is suboptimal and to reduce racial‐ethnic disparities.
Appendix
FL‐PR CReSD Investigators and Collaborators
Indrani E. Acosta, MD; Peter Antevy, MD; Bhuvaneswari Dandapani, MD; Angel Davila, MD; Sandra Diaz‐Acosta; Kathy Fenelon; Antonio Gandia, MD; Juan A. Gonzalez‐Sanchez, MD; Ricardo Hanel, MD, PhD; Jonathan Harris, MD; Wayne Hodges, RN, PMD, SCRN; Dianne Foster, RN, MBA; Bruce Inverso; Carlos Luciano Roman, MD; Brijesh Mehta, MD; Julia Mora; Nils Mueller‐Kronast, MD; Terry Neill, MD; Joe Nelson, MD; Abiezer Rodriguez, MD; Julio Rodriguez‐Colon, MD; Charles Sand, MD; Rhoda Saunders, PhD; Jeffrey Walker, Dileep Yavagal, MD.
Sources of Funding
This work was funded by a National Institute of Neurological Disorders and Stroke Prevention Intervention Research Program cooperative grant (U54NS081763).
Disclosures
University of Miami receives grant support from Boehringer Ingelheim for Ralph Sacco's role as a member of the executive committee for a secondary stroke prevention trial with dabigatran versus aspirin.
(J Am Heart Assoc. 2017;6:e004073. DOI: 10.1161/JAHA.116.004073.)
The abstract of this work was previously presented at the International Stroke Conference, February 11–13, 2015, in Nashville, TN.
References
- 1. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker‐Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C; Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group . Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 3. Cruz‐Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, Howard G, Leira EC, Morgenstern LB, Ovbiagele B, Peterson E, Rosamond W, Trimble B, Valderrama AL; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Epidemiology and Prevention; Council on Quality of Care and Outcomes Research . Racial‐ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. [DOI] [PubMed] [Google Scholar]
- 4. Sacco RL, Boden‐Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. [DOI] [PubMed] [Google Scholar]
- 5. White H, Boden‐Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. [DOI] [PubMed] [Google Scholar]
- 6. Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, Zhao X, Peterson E, Fonarow GC. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. [DOI] [PubMed] [Google Scholar]
- 7. Understanding Puerto Rico's Healthcare Collapse . Puerto Rico Healthcare Crisis Coalition. Puerto Rico; June 21, 2016. Available at: https://morningconsult.com/opinions/understanding-puerto-ricos-healthcare-collapse/. Accessed September 6, 2016. [Google Scholar]
- 8. Hispanic Nativity Shift . Pew Research Center. Washington, DC; April 29, 2014. Available at: http://www.pewhispanic.org/2014/04/29/hispanic-nativity-shift/. Accessed March 14, 2016. [Google Scholar]
- 9. The Causes and Consequences of Puerto Rico's Declining Population. Federal Reserve Bank of New York; 2014. Available at: https://www.newyorkfed.org/medialibrary/media/research/current_issues/ci20-4.pdf. Accessed March 14, 2016.
- 10. America's Emergency Care Environment . A State‐by‐State Report Card. Available at: http://www.emreportcard.org/uploadedFiles/ACEP-ReportCard-10-22-08.pdf.pdf. Updated 2014. Accessed May 28, 2015. [DOI] [PubMed]
- 11. Schwamm LH, Fonarow GC, Reeves MJ, Pan W, Frankel MR, Smith EE, Ellrodt G, Cannon CP, Liang L, Peterson E, Labresh KA. Get With the Guidelines‐Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119:107–115. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . Use of a registry to improve acute stroke care—seven states, 2005–2009. MMWR Morb Mortal Wkly Rep. 2011;60:206–210. [PubMed] [Google Scholar]
- 13. Reeves MJ, Fonarow GC, Smith EE, Pan W, Olson D, Hernandez AF, Peterson ED, Schwamm LH. Representativeness of the Get With the Guidelines‐Stroke Registry: comparison of patient and hospital characteristics among Medicare beneficiaries hospitalized with ischemic stroke. Stroke. 2012;43:44–49. [DOI] [PubMed] [Google Scholar]
- 14. Zevallos JC, Gonzalez J, Santiago F, Rodriguez R, Rivera A, Garcia AM, Flecha F, Colón M, Yarzebski J. Pilot study of the characteristics of acute stroke events in patients discharged from the Carolina University Hospital, Puerto Rico in 2007. Bol Asoc Med P R. 2009;101:11–13. [PubMed] [Google Scholar]
- 15. Zevallos JC, Yarzebski J, Gonzalez JA, Banchs HL, Garcia‐Palmieri M, Mattei H, Ayala J, González M, Torres V, Ramos IN, Pericchi LR, Torres DA, González MC, Goldberg RJ. Incidence, in‐hospital case‐fatality rates, and management practices in Puerto Ricans hospitalized with acute myocardial infarction. P R Health Sci J. 2013;32:138–145. [PubMed] [Google Scholar]
- 16. Zevallos J, Santiago F, Gonzalez J, Rodriguez A, Pericchi L, Rodriguez‐Mercado R, Nobu U. Burden of stroke in Puerto Rico. Int J Stroke. 2015;10:117–119. [DOI] [PubMed] [Google Scholar]


