Abstract
Background
Studies exploring the association between insulin resistance (IR) and cardiovascular disease in blacks have not been conclusive, especially for coronary heart disease (CHD). The McAuley index and homeostasis model assessment of IR (HOMA‐IR) perform differently in predicting cardiovascular disease. We investigated this association in the Jackson Heart Study, a large longitudinal cohort of blacks.
Methods and Results
IR was estimated for 3565 participants without diabetes mellitus and cardiovascular disease at baseline using the McAuley index and HOMA‐IR, and their associations with incident CHD and stroke (composite outcome) were compared. A lower McAuley index and higher HOMA‐IR are indicative of IR. Cox regression analysis was used to estimate adjusted hazard ratios for incident CHD and/or stroke. There were 158 events (89 CHD‐only, 58 stroke‐only, and 11 CHD/stroke) over a median follow‐up of 8.4 years. After adjustment for demographic factors, the risk of the composite outcome decreased with each SD increase in the McAuley index (hazard ratio 0.80; 95% CI: 0.67–0.96), with no attenuation after further accounting for CHD and stroke risk factors. When considered individually, McAuley index and HOMA‐IR were associated with CHD (hazard ratio 0.71, 95% CI: 0.55–0.92 and hazard ratio 1.33, 95% CI: 1.03–1.72, respectively), but not stroke risk. The logHOMA‐IR and CHD association was present in men, but not in women (P interaction=0.01).
Conclusions
Both HOMA‐IR and the McAuley index demonstrate strong associations with CHD but not stroke risk in blacks. The logHOMA‐IR and CHD association was present in men, but not in women.
Keywords: blacks, cardiovascular disease risk factors, cerebrovascular disease/stroke, coronary heart disease, epidemiology, insulin resistance, myocardial infarction, stroke
Subject Categories: Epidemiology, Cardiovascular Disease, Risk Factors, Cerebrovascular Disease/Stroke
Introduction
The role of insulin resistance, as measured by fasting insulin or the homeostasis model assessment of insulin resistance (HOMA‐IR), in the occurrence of cardiovascular disease (CVD), mainly coronary heart disease (CHD) and stroke, has been studied in nonblack populations.1, 2, 3, 4, 5 In a meta‐analysis including 65 studies with 516 325 participants and 13 474 cases of CVD, HOMA‐IR was associated with incident CHD, stroke, and CVD (composite of stroke, heart failure, angina pectoris, myocardial infarction, and sudden cardiac death).6 However, no race‐stratified analyses were done. Few studies have examined the impact of IR on CVD in blacks and the limited data suggest the association may vary by race/ethnicity, particularly for stroke. In the Atherosclerosis Risk in Communities (ARIC) study, fasting insulin was associated with incident stroke in whites but not in blacks (P interaction=0.036).7 In the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study, HOMA‐IR was associated with stroke risk in whites, but not in blacks.8 However, the authors did not find a significant race interaction. Similarly, no effect modification by race was observed in the association between HOMA‐IR and incident ischemic stroke in the Northern Manhattan Study (NOMAS).9 All previous studies including blacks have used either fasting insulin or HOMA‐IR as surrogate markers of IR.
The McAuley index of insulin sensitivity, based on weighted combinations of fasting insulin and fasting triglycerides,10 may perform better than HOMA‐IR and fasting insulin in predicting CVD risk in blacks. In particular, in blacks, triglyceride levels below the current metabolic syndrome threshold (110–149 mg/dL) are associated with IR estimated by the “gold standard” hyperinsulinemic euglycemic clamp,11 leading us to hypothesize that this index, which incorporates fasting triglycerides, may be a better marker of IR in this population, and in predicting CVD risk. In fact, the McAuley index performed better than other surrogate measures (including fasting insulin, HOMA‐IR, insulin‐to‐glucose ratio, and the Bennett index) in predicting IR (defined as a euglycemic clamp insulin sensitivity index ≤6.3 M·mU−1·L−1) in 178 adults from New Zealand without diabetes mellitus, with a specificity of 0.91 and a sensitivity of 0.75.10
The aim for the present study was 2‐fold: (1) to examine the associations between IR and incident CHD and/or stroke in blacks; and (2) to compare HOMA‐IR and the McAuley index in their associations with CHD and stroke. We used data from the Jackson Heart Study (JHS), a large prospective cohort of middle‐aged blacks.
Methods
Study Sample
The JHS enrolled 5306 participants aged 21 to 94 years at the time of the baseline assessment (2000–2004) from the Jackson, Mississippi metropolitan area (Hinds, Madison, and Rankin counties). Participants were recruited from 4 different sources: (1) previous participants in the ARIC study (30% of JHS cohort); (2) family members of participants (28%); (3) random selection from the 3 counties (17%); and (4) community volunteers (25%). The goal of the study was to examine factors that influence the development of CVD in black men and women to learn how to prevent this group of diseases in this population. Two subsequent in‐person follow‐up visits have been completed since baseline (2005–2008 and 2009–2013). Details on the study have been published elsewhere.12, 13
At baseline, we excluded participants with prevalent CHD or stroke (n=597), those with missing data to estimate HOMA‐IR and the McAuley index (insulin, glucose, and triglycerides, n=403), and those with type 2 diabetes mellitus (n=736), as surrogate markers for IR perform poorly in those with type 2 diabetes mellitus.14, 15 Our final sample was 3565 participants. The University of Mississippi Medical Center institutional review board approved the Jackson Heart Study, and all participants gave written informed consent.
Collection of Study Data
JHS participants provided information on demographic, socioeconomic, and lifestyle variables, as well as medication use. Clinic interviews were conducted by certified technicians and nurses. Weight was measured to the nearest 0.1 kg and height to the nearest 1 cm. Body mass index (BMI) was calculated as weight divided by the square of the height. Waist circumference was measured to the nearest 1 cm as the average of 2 readings at the umbilicus with the patient upright. Two resting systolic and diastolic blood pressures were measured at 5‐minute intervals and the average was used for the current analysis. Hypertension was defined as blood pressure ≥140/90 mm Hg or use of blood pressure–lowering medication. Current smoking was defined as self‐reported cigarette smoking over the past 12 months. Current alcohol drinking was defined as alcohol drinking in the past year. Physical activity was defined according to the American Heart Association categorization as poor health (0 minute of moderate and vigorous activity), intermediate health (>0 minute but <150 minutes of moderate activity, >0 minute but <75 minutes of vigorous activity, or >0 minute but <150 minutes of combined moderate and vigorous activity), and ideal health (≥150 minutes of moderate activity, ≥75 minutes of vigorous activity, or ≥150 minutes of combined moderate and vigorous activity).16 Education level was characterized as having at least a college education or not.
Blood samples were collected using standard procedures and analyzed at a central laboratory (University of Minnesota).12, 13 Fasting glucose, insulin, and lipids were measured on a Vitros 950 or 250, Ortho‐Clinical Diagnostics analyzer (Raritan, NJ) in accordance with the College of American Pathologists Proficiency Testing Program.17 A high‐performance liquid chromatography system (Tosoh Corporation, Tokyo, Japan) was used to measure glycosylated hemoglobin A1c concentrations. Type 2 diabetes mellitus status was defined according to the 2010 American Diabetes Association guidelines18: (1) physician diagnosis of the condition, (2) use of diabetes mellitus medication, (3) fasting blood glucose ≥126 mg/dL, and/or (4) glycosylated hemoglobin ≥6.5%.
Measurement of Insulin Resistance
Insulin resistance was assessed using 2 indices as follows: HOMA‐IR=[glucose (nmol/L)×insulin (μU/mL)/22.5],19 and the McAuley index=exp [2.63–0.28 ln(insulin in mU/L)−0.31 ln(triglycerides in mmol/L)].10 A lower McAuley index and higher HOMA‐IR are indicative of IR.
Ascertainment of CHD and Stroke
All CHD and stroke events were ascertained and adjudicated from baseline to December 31, 2012. Events were identified by passive community surveillance by contacting participants annually, and also by querying all hospitalizations and death records in the prior year. Disease‐specific classifications of hospitalized and fatal CHD and stroke were completed by review and adjudicated by 2 independent physician reviewers.20 Any disagreements by the first 2 reviewers were adjudicated by a third physician reviewer. The method of ascertainment of events for the JHS followed the protocol developed by the ARIC study investigators.21, 22
A CHD event was defined as a probable or definite myocardial infarction (combinations of chest pain, cardiac enzyme levels, and ECG changes), fatal CHD (cause of death from death certificate, chest pain symptoms), and/or a cardiac procedure (angiography or any revascularization procedure). A stroke event was defined as a definite or probable stroke (sudden or rapid onset of neurologic symptoms lasting for 24 hours or more, or leading to death) on neuroimaging studies or autopsy.
Follow‐up time for incident CHD and stroke events was defined as the time from their baseline examination (visit 1) until the incident event. The end of follow‐up for those who remained free of CHD and stroke was as follows: (1) December 31, 2012, (2) date of last contact for those lost to follow‐up (n=283), or (3) date of death (n=256), whichever occurred first.
Statistical Analysis
HOMA‐IR was log‐transformed for all analyses. Unadjusted differences in baseline characteristics were assessed across tertiles of logHOMA‐IR and the McAuley index, using a 1‐way ANOVA (for continuous variables) and χ2 test (for categorical variables). Pearson correlation coefficients were estimated between the IR measures (logHOMA‐IR and McAuley index) and covariates (age, blood pressure, high‐density lipoprotein [HDL] cholesterol, low‐density lipoprotein–cholesterol, BMI, waist circumference, aldosterone, adiponectin, and high‐sensitivity C‐reactive protein).
Cox regression was used to estimate hazard ratios (HR) and 95% CI for incident events. HRs were estimated per unit SD increase in logHOMA‐IR and McAuley index. The primary outcome was a composite of CHD and stroke. Both events were also analyzed individually in separate regression models. Covariates were adjusted in sequential models: model 1 was adjusted for age, sex, education, physical activity, and smoking status; model 2: model 1 plus BMI; model 3: model 2 plus HDL, systolic blood pressure, and hypertension status. The proportional hazard assumption was assessed by using log (−log survival) plots for categorical variables and testing time‐dependent covariates for continuous variables. Effect modification by sex was assessed by fitting regression models with logHOMA‐IR×sex and McAuley index×sex interaction terms. For logHOMA‐IR, interaction tests for the composite outcome and CHD models were significant, and thus our analyses are stratified by sex. The association between IR and ischemic stroke appears to differ from that with intracerebral hemorrhage,8 so we performed a sensitivity analysis including only cases of ischemic stroke. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC). Statistical significance was inferred at 2‐sided P<0.05.
Results
Baseline Characteristics of Participants
Of the 3565 participants included in our analyses (mean age of 53 years), 1292 (36.2%) were male, 921 (25.8%) were hypertensive, 445 (12.5%) were current smokers, and 2153 (60.4%) had at least some college education. Table 1 displays the baseline characteristics of participants by tertiles of HOMA‐IR and the McAuley index. The most insulin‐resistant participants were those in the third tertile of HOMA‐IR and first tertile of the McAuley index. These participants were more obese, had larger waist circumference, higher low‐density lipoprotein–cholesterol, lower HDL‐cholesterol, higher mean glycosylated hemoglobin, greater levels of systemic inflammation (measured by high‐sensitivity C‐reactive protein), and a higher prevalence of hypertension.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants by Tertiles of HOMA‐IR and the McAuley Index
| Tertiles of HOMA‐IR | Tertiles of McAuley Index | |||||
|---|---|---|---|---|---|---|
| <2.4 (N=1387) | 2.4 to 3.8 (N=1385) | >3.8 (N=1387) | <6.1 (N=1386) | 6.1 to 7.4 (N=1386) | >7.4 (N=1387) | |
| Age, y | 53.3±13.2 | 54.1±13.1 | 54.2±12.0§ | 54.7±11.9 | 54.8±12.8 | 52.1±13.4 |
| Women, n (%) | 826 (59.5) | 920 (66.4) | 925 (66.7) | 868 (62.6) | 923 (66.6) | 880 (63.4)§ |
| Systolic BP, mm Hg | 123.7±18.4 | 126.1±17.8 | 128.0±17.7 | 128.3±17.4 | 126.7±18.0 | 122.9±18.3 |
| Diastolic BP, mm Hg | 78.6±10.5 | 79.2±10.1 | 80.1±10.4‡ | 80.4±10.3 | 79.2±10.3 | 78.3±10.3 |
| BMI, kg/m2 | 27.9±5.8 | 31.7±6.5 | 35.2±7.4 | 34.2±6.9 | 31.9±7.2 | 28.7±6.6 |
| Waist circumference, cm | 90.4±12.5 | 100.0±13.9 | 109.1±15.9 | 107.4±15.3 | 100.6±15.0 | 91.5±13.8 |
| LDL‐cholesterol, mg/dL | 123.7±35.5 | 129.5±35.0 | 128.8±38.3 | 131.9±39.9 | 130.7±35.0 | 119.5±32.7 |
| HDL‐cholesterol, mg/dL | 56.7±16.0 | 51.6±13.3 | 47.7±12.9 | 45.9±12.3 | 52.2±13.3 | 58.0±15.3 |
| HbA1c, % | 5.4±0.5 | 5.6±0.7 | 6.3±1.4 | 6.1±1.2 | 5.8±1.0 | 5.5±0.7 |
| At least college education, n (%) | 851 (61.6) | 820 (59.5) | 785 (56.8)‡ | 807 (58.4) | 780 (56.4) | 869 (63.0)† |
| Hypertension status, n (%) | 342 (24.7) | 339 (24.9) | 422 (30.4)* | 425 (30.7) | 358 (25.9) | 320 (23.1) |
| Incident composite events, n (%) | 61 (4.4) | 76 (5.5) | 83 (5.9)§ | 87 (6.3) | 72 (5.2) | 61 (4.4)§ |
| Aldosterone∥, ng/dL | 3.7 (3.8) | 4.2 (4.1) | 5.4 (5.5) | 5.2 (5.5) | 4.3 (4.2) | 3.6 (3.8) |
| Adiponectin∥, μg/mL | 5.7 (5.1) | 4.4 (3.8) | 3.1 (2.7) | 3.1 (2.7) | 4.1 (3.5) | 5.8 (5.3) |
| Hs‐CRP∥, mg/L | 1.6 (3.2) | 2.6 (4.5) | 3.7 (5.5) | 3.4 (5.2) | 2.83 (5.9) | 1.6 (3.3) |
| eGFR, mL/min | 88.4±16.2 | 86.7±16.4 | 86.7±18.1‡ | 86.0±17.7 | 86.3±16.8 | 89.5±16.1 |
| Cigarette smoking, n (%) | 208 (15.1) | 158 (11.5) | 135 (9.8) | 176 (12.8) | 142 (10.3) | 183 (13.3)‡ |
| Alcohol consumption, n (%) | 725 (52.6) | 677 (49.1) | 594 (43.0) | 632 (45.8) | 659 (47.7) | 705 (51.2)‡ |
| Physical activity, n (%) | ||||||
| AHA I | 593 (42.8) | 625 (45.2) | 724 (52.2) | 695 (50.1) | 659 (47.6) | 588 (42.4)* |
| AHA II | 452 (32.6) | 469 (33.9) | 446 (32.2) | 446 (32.2) | 438 (31.6) | 483 (34.9) |
| AHA III | 341 (24.6) | 288 (20.8) | 217 (15.6) | 245 (17.7) | 287 (20.7) | 314 (22.7) |
| Diabetes mellitus status, n (%) | 41 (3.0) | 123 (8.9) | 430 (31.0) | 335 (24.2) | 180 (13.0) | 79 (5.7) |
Data are presented as mean±SD or numbers (percentage). AHA indicates American Heart Association; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; Hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein.
All P<0.0001, except for *P<0.001, † P<0.01, † P<0.05, and §not significant.
∥Data are median (interquartile range).
Correlation Analysis Between IR Measures and Covariates
There was a strong correlation between HOMA‐IR and the McAuley index (Pearson r=−0.80). Compared to HOMA‐IR, the McAuley index was more strongly correlated with age (r=−0.09 versus 0.01), systolic blood pressure (r=0.12 versus 0.07), and kidney function (r for estimated glomerular filtration rate=0.12 versus −0.06) (Table 2). On the other hand, HOMA‐IR was better correlated with clinical measures of obesity: BMI (r=0.43 versus −0.32) and waist circumference (r=0.47 versus −0.41). The strengths of the correlations of both HOMA‐IR and the McAuley index with log(aldosterone), log(adiponectin), and log(high‐sensitivity C‐reactive protein) were comparable.
Table 2.
Pearson Correlation Coefficients Between Insulin Resistance Indices and Covariates
| Log HOMA‐IR | McAuley Index | |||
|---|---|---|---|---|
| r | P Value | R | P Value | |
| Age | 0.01 | 0.55 | −0.09 | <0.0001 |
| Systolic blood pressure | 0.07 | <0.0001 | −0.12 | <0.0001 |
| Diastolic blood pressure | 0.05 | 0.002 | −0.08 | <0.0001 |
| Body mass index | 0.43 | <0.0001 | −0.32 | <0.0001 |
| Waist circumference | 0.47 | <0.0001 | −0.41 | <0.0001 |
| LDL‐cholesterol | 0.07 | <0.0001 | −0.17 | <0.0001 |
| HDL‐cholesterol | −0.25 | <0.0001 | 0.36 | <0.0001 |
| Total cholesterol | 0.06 | 0.001 | −0.21 | <0.0001 |
| HbA1c | 0.27 | <0.0001 | −0.25 | <0.0001 |
| Log aldosterone | 0.22 | <0.0001 | −0.22 | <0.0001 |
| Log adiponectin | −0.35 | <0.0001 | 0.38 | <0.0001 |
| Log HsCRP | 0.27 | <0.0001 | −0.27 | <0.0001 |
| eGFR | −0.06 | <0.001 | 0.12 | <0.0001 |
eGFR indicates estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein; HsCRP, high‐sensitivity C‐reactive protein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein.
Incident Cardiovascular Events
There were 158 incident events (89 CHD‐only, 58 stroke‐only, and 11 CHD/stroke) over a median follow‐up of 8.4 years (range 0–11.2 years). Overall, the crude rate of the composite outcome was 5.4 events per 1000 person‐years. Rates for the composite outcome (CHD+stroke), for CHD and for stroke varied across tertiles of HOMA‐IR and the McAuley index (Figure 1). For the McAuley index, there was a distinct pattern of decreasing rates from the first to the third tertile for the composite outcome and CHD, but not for stroke. In comparison, there was no evident linear pattern for HOMA‐IR. Participants in the second tertile of HOMA‐IR had the highest rates for all events (composite, CHD, and stroke). Overall, the cumulative incidence of CHD was slightly higher than the cumulative incidence of stroke in the sample. However, there were no sex differences in cumulative incidence for the composite outcome, CHD, or stroke (Figure 2).
Figure 1.
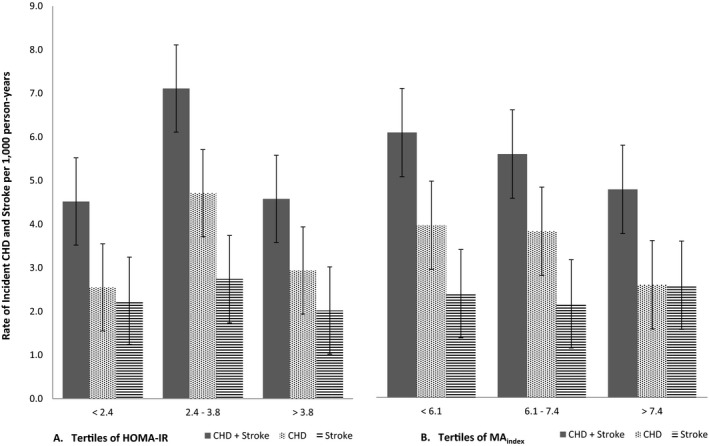
Rates of incident coronary heart disease and stroke. A, By tertiles of HOMA‐IR. B, By tertiles of the McAuley index. Numbers on the bars represent the actual rates of events (per 1000 person‐years). CHD indicates coronary heart disease; HOMA‐IR, homeostasis model assessment for insulin resistance; MA index, McAuley index.
Figure 2.
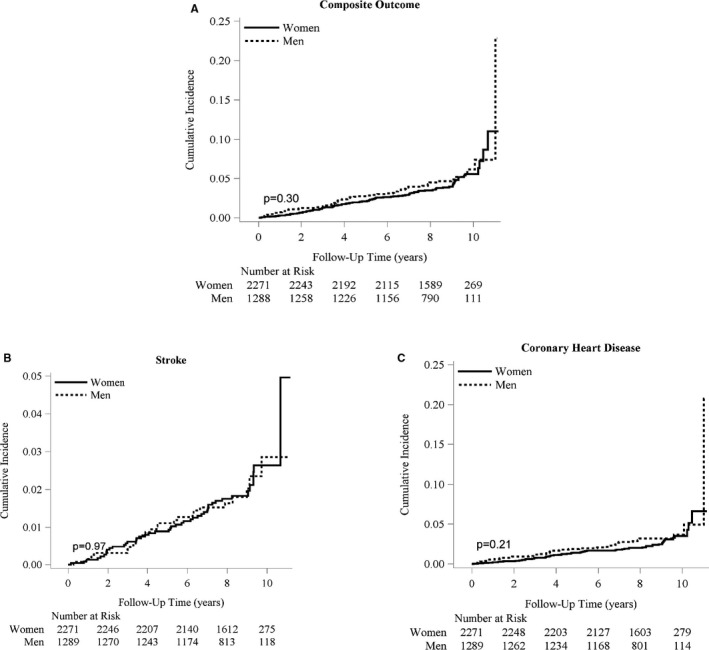
Cumulative incidence functions for men and women. A, For the incident composite outcome of CHD and stroke. B, For incident stroke. C, For incident coronary heart disease. The solid line represents the cumulative incidence function curve for women, and the dashed line represents that for men. CHD indicates coronary heart disease.
Association Between the HOMA‐IR, McAuley Indices, and Incident Events
There was a significant effect modification by sex for the HOMA‐IR index (P interaction=0.01 and 0.02 for the composite outcome and CHD, respectively), but not for the McAuley index (P interaction=0.18, 0.27, and 0.28 for the composite outcome, CHD, and stroke, respectively). For comparison, we stratified analyses for both HOMA‐IR and the McAuley index by sex, except for stroke where we had very few events (Table 3).
Table 3.
Hazard Ratios (95% CI) Per Unit SD Increase in HOMA‐IR and McAuley Index for the Composite Outcome (CHD+Stroke), and Individual Outcomes (CHD and Stroke)
| Models | McAuley Index | HOMA‐IR Index | ||||
|---|---|---|---|---|---|---|
| All (N=3565) | Males (N=1292) | Females (N=2273) | All (N=3565) | Males (N=1292) | Females (N=2273) | |
| Hazard Ratios (95% CI) | ||||||
| CHD+stroke | ||||||
| (nevents=158) | (nevents=62) | (nevents=96) | (nevents=158) | (nevents=62) | (nevents=96) | |
| Model 1 | 0.80 (0.67–0.96)* | 0.70 (0.52–0.94)* | 0.89 (0.71–1.12) † | 1.16 (0.97–1.39) † | 1.56 (1.15–2.11) ‡ | 0.96 (0.76–1.21) † |
| Model 2 | 0.76 (0.63–0.92) ‡ | 0.70 (0.52–0.96)* | 0.82 (0.64–1.05) † | 1.24 (1.02–1.59)* | 1.61 (1.15–2.24) ‡ | 1.05 (0.82–1.35) † |
| Model 3 | 0.80 (0.65–0.98)* | 0.72 (0.51–1.00) § | 0.88 (0.67–1.15) † | 1.20 (0.98–1.47) † | 1.58 (1.13–2.20) ‡ | 1.02 (0.78–1.32) † |
| CHD | ||||||
| (nevents=100) | (nevents=41) | (nevents=59) | (nevents=100) | (nevents=41) | (nevents=59) | |
| Model 1 | 0.73 (0.58–0.92) ‡ | 0.63 (0.43–0.92)* | 0.81 (0.60–1.10) † | 1.24 (0.99–1.56) † | 1.72 (1.20–2.47) ‡ | 1.03 (0.77–1.37) † |
| Model 2 | 0.67 (0.53–0.86) ‡ | 0.62 (0.42–0.92)* | 0.72 (0.52–0.99)* | 1.39 (1.09–1.79) ‡ | 1.83 (1.25–2.70) ‡ | 1.18 (0.86–1.62) † |
| Model 3 | 0.71 (0.55–0.92) ‡ | 0.65 (0.42–1.01) § | 0.75 (0.53–1.06) † | 1.33 (1.03–1.72)* | 1.75 (1.18–2.61) ‡ | 1.14 (0.82–1.59) † |
| Stroke | ||||||
| (nevents=69) | (nevents=69) | |||||
| Model 1 | 0.96 (0.74–1.23) † | 1.09 (0.83–1.42) † | ||||
| Model 2 | 0.95 (0.73–1.23) † | 1.12 (0.83–1.50) † | ||||
| Model 3 | 0.96 (0.74–1.25) † | 1.12 (0.84–1.50) † | ||||
For HOMA‐IR, P interaction by sex=0.01 (composite outcome) and 0.02 (CHD); for McAuley index, P interaction by sex=0.18 (composite outcome), 0.27 (CHD) and 0.28 (stroke). For the composite outcome and CHD: Model 1: adjusted for age, sex, education, physical activity, and smoking. Model 2: model 1 plus BMI. Model 3: model 2 plus HDL, systolic BP, and hypertension status. Stratified analyses are not adjusted for sex in model 1. For stroke analysis, Model 1 was adjusted for age, sex, and smoking. Model 2: model 1 plus BMI. Model 3: model 2 plus systolic BP. BMI indicates body mass index; BP, blood pressure; CHD, coronary heart disease; HOMA‐IR, homeostasis model assessment for insulin resistance.
*P<0.05.
†Not significant.
‡ P<0.01.
§ P=0.05.
For HOMA‐IR, after accounting for demographic factors (age, education, and smoking) and physical activity, each unit SD increase in the index was associated with an increased risk of the composite outcome (CHD+stroke) in men (HR 1.56, 95% CI: 1.15–2.11). Further accounting for BMI, HDL, systolic blood pressure, and hypertension status did not attenuate the risk (HR 1.58, 95% CI: 1.13–2.20). This association was nonsignificant in women (HR 1.02, 95% CI: 0.78–1.32) (Table 3). The association between HOMA‐IR and the composite outcome in men was primarily driven by CHD. After complete adjustment, HOMA‐IR was associated with an increased risk of CHD in men (HR 1.75, 95% CI: 1.18–2.61), but not in women (HR 1.14, 95% CI: 0.82–1.59). The association between HOMA‐IR and stroke was not significant, even in the model minimally adjusted for demographic factors only (HR 1.09, 95% CI: 0.83–1.42).
In the overall sample, in the model adjusted for demographic factors and physical activity, each unit SD increase in the McAuley index was associated with a reduction in the risk of the composite outcome (HR 0.80, 95% CI: 0.67–0.96) (Table 3). Further adjustments for BMI, HDL, systolic blood pressure, and hypertension status did not attenuate the association (HR 0.80, 95% CI: 0.65–0.98). When the individual outcomes were considered, there was a significant association for CHD but not for stroke. For the CHD analysis including the overall sample, each unit SD increase in the McAuley index was associated with a risk reduction after adjusting for demographic factors (HR 0.73, 95% CI: 0.58–0.92). This association persisted and remained significant after complete adjustment (HR 0.71, 95% CI: 0.55–0.92). The association between the McAuley index and stroke was not significant even in the model minimally adjusted for demographic factors only (HR 0.96, 95% CI: 0.74–1.23).
When modeled in tertiles, there was an apparent association between HOMA‐IR and incident CHD in the second and third tertiles for men, and in the second tertile for women (data not shown). No associations between HOMA‐IR and incident stroke, as well as between the McAuley index and incident CHD or stroke, were observed. However, because there were very few events in each tertile (<38), the results are nonconclusive and should be interpreted with caution.
Over 85.5% of the sample had incident ischemic stroke (59/69) events. In sensitivity analysis including only participants with ischemic stroke, though the strengths of the associations between HOMA‐IR, McAuley index, and incident stroke increased, these associations, however, remained nonsignificant (Table S1).
When 10‐year risk prediction was assessed for the composite outcome and CHD, the McAuley performed comparably to HOMA‐IR: for the CHD outcome (c‐statistic for HOMA‐IR: 0.748, McAuley: 0.759), and for the composite outcome (c‐statistic for HOMA‐IR: 0.755, McAuley: 0.756).
Discussion
To our knowledge, this study is the first to prospectively assess the effect of IR on the occurrence of CHD in an entirely black cohort. In this large community‐based cohort, we found that IR (measured by the McAuley Index and HOMA‐IR) was more strongly associated with CHD, but not with stroke. The relation between IR (as measured by HOMA‐IR) and CHD was present among men, but absent in women. Our findings also suggest that the association between the McAuley index and incident CHD may be more linear than that between HOMA‐IR and CHD, with the association between HOMA‐IR and CHD assuming an inverse U‐shape. However, despite these differences, the McAuley index performed comparably to HOMA‐IR.
Our findings of a positive association between IR (as measured by HOMA‐IR) and CHD or an inverse association between insulin sensitivity (as measured by the McAuley index) and CHD are consistent with previous studies in predominantly white populations.6, 7, 8, 9 Prior studies that investigated sex differences in the association between IR and CVD found varying effects depending on which measure was used; the association was stronger in men than in women using HOMA‐IR, but the opposite was found using fasting insulin and fasting glucose.6
A number of studies have reported no association between IR and stroke in blacks, primarily using HOMA‐IR and fasting insulin.7, 8 Howard et al, using data from the REGARDS study, reported a significant association between HOMA‐IR and ischemic stroke risk in whites, but not in blacks, though no significant race interaction was found.8 However, the relationship between HOMA‐IR and intracerebral hemorrhage was different; there was a trend towards a decrease in risk of intracerebral hemorrhage in whites (HR 0.61, 95% CI: 0.35–1.04) and an increase in risk in blacks (HR 1.20, 95% CI: 0.60–2.39). In the ARIC study, an association was reported between fasting insulin and stroke in whites, but not blacks.7 Although the direction of the association between HOMA‐IR and stroke in our study was similar to previous studies,7, 8, 9 we may have had limited power to explore these relationships by stroke subtypes. It is possible that because of the association between IR and pro‐atherogenic factors, it may therefore be more likely linked to ischemic stroke attributable to small or large vessel atherosclerosis than to intracerebral hemorrhage.
Another important finding in our study was the lack of association between IR (as measured by HOMA‐IR) and CHD among black women. Results from prior studies have been conflicting. While some studies have reported no association between IR and CHD,23, 24 as well as IR and stroke9 in women, other studies have reported a positive and significantly higher risk of CVD among women than among men, independent of differences in major CVD risk factors between both sexes.25, 26, 27, 28, 29 The latter studies, however, were carried out in people with diabetes mellitus. The association between IR and incident CVD events may therefore differ among people with and without diabetes mellitus. Reasons for the sex‐specific effect modification observed for HOMA‐IR, but not for the McAuley index, in our study remain unclear. It could be explained by sex differences in body fat distribution and insulin action,30 or by a lower incidence of myocardial infarction in black women compared to black men.31 In the Rotterdam community in the Netherlands, it was shown that middle‐aged men were more likely to develop CHD as a first CVD event compared to middle‐aged women.32 This could be the case among blacks as well. Alternatively, residual confounding may mask a potential relationship in women. The observed sex difference warrants further investigation.
The McAuley index seems appealing as a measure of insulin sensitivity/resistance in blacks because it includes triglyceride levels, which have been shown to significantly correlate (stronger among men than women) with the hyperinsulinemic euglycemic clamp‐derived M‐value in middle‐aged nondiabetic black adults, even at levels below the current metabolic syndrome threshold (110–149 mg/dL).11 This index performed better than other surrogates (including fasting insulin, HOMA‐IR, insulin‐to‐glucose ratio, and the Bennett index) in predicting IR in normoglycemic individuals in New Zealand, with a specificity of 0.91 and a sensitivity of 0.75.10 In addition, there is evidence from a number of studies that increased triglyceride levels are an additional causal risk factor for CVD.33, 34, 35 Taken together, these findings suggest that the McAuley index may be superior to other steady‐state measures such as HOMA‐IR in predicting CHD, and also in assessing IR among blacks, but this index has not been validated in this population.
Insulin resistance can promote the development of atherosclerosis, and thus CHD through a number of mechanisms. First, IR leads to increased levels of glucose and insulin, which have been shown to be pro‐atherogenic.36, 37 Second, IR can promote atherosclerosis through mechanisms involving dyslipidemia, hypertension, endothelial dysfunction, and systemic inflammation.37, 38
Our study has a number of strengths. We used different IR indices in an entirely black cohort to assess the relationship between IR and CVD. These indices were derived from fasting measures of glucose, insulin, and triglycerides among participants free of CVD or diabetes mellitus at baseline. The JHS is a large cohort of blacks with a well‐characterized sample, and presented a unique opportunity to address our main objectives. Also, all CHD and stroke events were robustly ascertained through adjudication by a committee. There are, however, some limitations. First, we used fasting surrogate measures of IR that have not been extensively validated in the black population. In 1 study, the correlation between HOMA‐IR and the hyperinsulinemic euglycemic clamp (the “gold standard” measure of IR) in blacks was modest (r=−0.42 overall; r=−0.54 in women and r=−0.34 in men). Also, these fasting indices measure primarily hepatic IR and not peripheral IR. It is possible that part of the inconsistent findings between IR and CVD reported in many studies is attributable to the limitations of fasting measures in identifying individuals with peripheral IR. Second, we had limited power to more extensively explore the subtypes of stroke. Third, although our data suggest that the McAuley index may better capture the influence of IR in the occurrence of CHD among blacks than HOMA‐IR, these results require confirmation in additional cohorts, especially as this index has not been widely validated against the hyperinsulinemic euglycemic clamp in blacks. Finally, family relatives made up about 26% of our final sample. However, family connection data are currently unavailable. As such we were unable to account for familial correlations in our analysis, which could potentially inflate our Type I error.
Conclusion
Our study highlights the important role played by IR, measured using HOMA‐IR and the McAuley index, in the development of CVD, particularly CHD among blacks, independent of traditional CVD risk factors. Our findings also suggest that the association between IR (as measured by HOMA‐IR) and CHD may be more important in black men than in women. We provide a first assessment of the effect of the McAuley index in CHD risk in blacks, and suggest that this index may be used interchangeably with HOMA‐IR to assess the influence of IR in the occurrence of CHD in this population. Our findings certainly require replication in other black cohorts.
Implications
Given the disproportionately high burden of cardiometabolic risk factors in blacks, identifying individuals at a high risk of CVD in this population is of utmost importance. Our findings confirm reports in prior studies, which have shown that IR may not be an important factor in the occurrence of stroke in this population. On the other hand, the fact that IR was strongly associated with the occurrence of CHD, independent of traditional CVD risk factors, suggests that this factor should be taken into account when evaluating an individual's risk of CHD. Further studies are needed in this population to replicate our findings of an association between IR and CHD, and to ultimately inform clinical care.
Sources of Funding
JHS Diabetes and Obesity Working Group supported by 1 R01 HL117285‐01 from National Heart, Lung, and Blood Institute. The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Disclosures
None.
Supporting information
Table S1. Sensitivity Analysis Displaying Hazard Ratios (95% CI) Per Unit SD Increase in HOMA‐IR and McAuley Index for Ischemic Stroke Events Only
Acknowledgments
The authors thank the participants and data collection staff of the Jackson Heart Study for their important contributions.
(J Am Heart Assoc. 2017;6:e004229. DOI: 10.1161/JAHA.116.004229.)
An abstract of this manuscript was presented as a poster during the American Heart Association EPI/Lifestyle Scientific Sessions, March 1–4, 2016 in Phoenix, AZ.
References
- 1. Folsom AR, Szklo M, Stevens J, Liao F, Smith R, Eckfeldt JH. A prospective study of coronary heart disease in relation to fasting insulin, glucose, and diabetes. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 1997;20:935–942. [DOI] [PubMed] [Google Scholar]
- 2. Hwang YC, Jee JH, Oh EY, Choi YH, Lee MS, Kim KW, Lee MK. Metabolic syndrome as a predictor of cardiovascular diseases and type 2 diabetes in Koreans. Int J Cardiol. 2009;134:313–321. [DOI] [PubMed] [Google Scholar]
- 3. Jeppesen J, Hansen TW, Torp‐Pedersen C, Madsbad S, Ibsen H, Jorgensen T, Fenger M. Relationship between common lipoprotein lipase gene sequence variants, hyperinsulinemia, and risk of ischemic heart disease: a population‐based study. Atherosclerosis. 2010;211:506–511. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura K, Sakurai M, Miura K, Morikawa Y, Ishizaki M, Yoshita K, Kido T, Naruse Y, Nakagawa H. Homeostasis model assessment of insulin resistance and the risk of cardiovascular events in middle‐aged non‐diabetic Japanese men. Diabetologia. 2010;53:1894–1902. [DOI] [PubMed] [Google Scholar]
- 5. Onat A, Hergenc G, Turkmen S, Yazici M, Sari I, Can G. Discordance between insulin resistance and metabolic syndrome: features and associated cardiovascular risk in adults with normal glucose regulation. Metabolism. 2006;55:445–452. [DOI] [PubMed] [Google Scholar]
- 6. Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta‐analysis. PLoS One. 2012;7:e52036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rasmussen‐Torvik LJ, Yatsuya H, Selvin E, Alonso A, Folsom AR. Demographic and cardiovascular risk factors modify association of fasting insulin with incident coronary heart disease and ischemic stroke (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2010;105:1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howard G, Wagenknecht LE, Kernan WN, Cushman M, Thacker EL, Judd SE, Howard VJ, Kissela BM. Racial differences in the association of insulin resistance with stroke risk: the reasons for geographic and racial differences in stroke (REGARDS) study. Stroke. 2014;45:2257–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rundek T, Gardener H, Xu Q, Goldberg RB, Wright CB, Boden‐Albala B, Disla N, Paik MC, Elkind MS, Sacco RL. Insulin resistance and risk of ischemic stroke among nondiabetic individuals from the northern Manhattan study. Arch Neurol. 2010;67:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis‐Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24:460–464. [DOI] [PubMed] [Google Scholar]
- 11. Stein E, Kushner H, Gidding S, Falkner B. Plasma lipid concentrations in nondiabetic African American adults: associations with insulin resistance and the metabolic syndrome. Metabolism. 2007;56:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA Jr. Recruiting African‐American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6‐18‐29. [PubMed] [Google Scholar]
- 13. Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6‐4‐17. [PubMed] [Google Scholar]
- 14. Howard G, Bergman R, Wagenknecht LE, Haffner SM, Savage PJ, Saad MF, Laws A, D'Agostino RB Jr. Ability of alternative indices of insulin sensitivity to predict cardiovascular risk: comparison with the “minimal model”. Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Ann Epidemiol. 1998;8:358–369. [DOI] [PubMed] [Google Scholar]
- 15. Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki‐Järvinen H, Van Haeften T, Renn W, Gerich J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301. [DOI] [PubMed] [Google Scholar]
- 16. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task F, Statistics C . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 17. Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. [DOI] [PubMed] [Google Scholar]
- 18. American Diabetes A . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 20. Keku E, Rosamond W, Taylor HA Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L, Sarpong D. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15:S6‐62‐70. [PubMed] [Google Scholar]
- 21. Rosamond WD, Folsom AR, Chambless LE, Wang C‐H, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 22. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 23. Welborn TA, Wearne K. Coronary heart disease incidence and cardiovascular mortality in Busselton with reference to glucose and insulin concentrations. Diabetes Care. 1979;2:154–160. [DOI] [PubMed] [Google Scholar]
- 24. Ducimetiere P, Eschwege E, Papoz L, Richard JL, Claude JR, Rosselin G. Relationship of plasma insulin levels to the incidence of myocardial infarction and coronary heart disease mortality in a middle‐aged population. Diabetologia. 1980;19:205–210. [DOI] [PubMed] [Google Scholar]
- 25. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta‐analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973–1980. [DOI] [PubMed] [Google Scholar]
- 26. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta‐analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551. [DOI] [PubMed] [Google Scholar]
- 27. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta‐analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanaya AM, Grady D, Barrett‐Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta‐analysis. Arch Intern Med. 2002;162:1737–1745. [DOI] [PubMed] [Google Scholar]
- 29. Emerging Risk Factors C , Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blaak E. Sex differences in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2008;11:500–504. [DOI] [PubMed] [Google Scholar]
- 31. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e60. [DOI] [PubMed] [Google Scholar]
- 32. Leening MJG, Ferket BS, Steyerberg EW, Kavousi M, Deckers JW, Nieboer D, Heeringa J, Portegies MLP, Hofman A, Ikram MA, Hunink MGM, Franco OH, Stricker BH, Witteman JCM, Roos‐Hesselink JW. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ. 2014;349:g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 34. Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther. 2014;141:358–367. [DOI] [PubMed] [Google Scholar]
- 35. Varbo A, Nordestgaard BG. Remnant cholesterol and ischemic heart disease. Curr Opin Lipidol. 2014;25:266–273. [DOI] [PubMed] [Google Scholar]
- 36. Yu Q, Gao F, Ma XL. Insulin says NO to cardiovascular disease. Cardiovasc Res. 2011;89:516–524. [DOI] [PubMed] [Google Scholar]
- 37. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sensitivity Analysis Displaying Hazard Ratios (95% CI) Per Unit SD Increase in HOMA‐IR and McAuley Index for Ischemic Stroke Events Only


