Abstract
Background
Wave reflections, which are increased in patients with heart failure with preserved ejection fraction, impair diastolic function and promote pathologic myocardial remodeling. Organic nitrates reduce wave reflections acutely, but whether this is sustained chronically or affected by hydralazine coadministration is unknown.
Methods and Results
We randomized 44 patients with heart failure with preserved ejection fraction in a double‐blinded fashion to isosorbide dinitrate (ISDN; n=13), ISDN+hydralazine (ISDN+hydral; n=15), or placebo (n=16) for 6 months. The primary end point was the change in reflection magnitude (RM; assessed with arterial tonometry and Doppler echocardiography). Secondary end points included change in left ventricular mass and fibrosis, measured with cardiac magnetic resonance imaging, and the 6‐minute walk distance. ISDN reduced aortic characteristic impedance (mean baseline=0.15 [95% CI, 0.14–0.17], 3 months=0.11 [95% CI, 0.10–0.13], 6 months=0.10 [95% CI, 0.08–0.12] mm Hg/mL per second; P=0.003) and forward wave amplitude (Pf, mean baseline=54.8 [95% CI, 47.6–62.0], 3 months=42.2 [95% CI, 33.2–51.3]; 6 months=37.0 [95% CI, 27.2–46.8] mm Hg, P=0.04), but had no effect on RM (P=0.64), left ventricular mass (P=0.33), or fibrosis (P=0.63). ISDN+hydral increased RM (mean baseline=0.39 [95% CI, 0.35–0.43]; 3 months=0.31 [95% CI, 0.25–0.36]; 6 months=0.44 [95% CI, 0.37–0.51], P=0.03), reduced 6‐minute walk distance (mean baseline=343.3 [95% CI, 319.2–367.4]; 6 months=277.0 [95% CI, 242.7–311.4] meters, P=0.022), and increased native myocardial T1 (mean baseline=1016.2 [95% CI, 1002.7–1029.7]; 6 months=1054.5 [95% CI, 1036.5–1072.3], P=0.021). A high proportion of patients experienced adverse events with active therapy (ISDN=61.5%, ISDN+hydral=60.0%; placebo=12.5%; P=0.007).
Conclusions
ISDN, with or without hydralazine, does not exert beneficial effects on RM, left ventricular remodeling, or submaximal exercise and is poorly tolerated. ISDN+hydral appears to have deleterious effects on RM, myocardial remodeling, and submaximal exercise. Our findings do not support the routine use of these vasodilators in patients with heart failure with preserved ejection fraction.
Clinical Trial Registration
URL: www.clinicaltrials.gov. Unique identifier: NCT01516346.
Keywords: heart failure, hemodynamics, magnetic resonance imaging, remodeling heart failure, vascular biology, vascular stiffness, vasodilators
Subject Categories: Heart Failure, Remodeling
Introduction
Heart failure with preserved ejection fraction (HFpEF) is an epidemic condition for which the underlying mechanisms are incompletely understood. Epidemiologic data demonstrate a greater prevalence of hypertension, advanced age, and vascular risk factors such as renal dysfunction and diabetes, in patients with HFpEF,1, 2, 3, 4 all of which increase vascular stiffness and pulsatile loading on the left ventricle.5 Observational data demonstrate increased pulsatile load and wave reflections in patients with HFpEF,6, 7, 8, 9 which correlate with decreased exercise capacity, a fundamental feature of the disease.9, 10
Wave reflections, arising from the gradual increase in impedance along the arterial tree and from discrete sites of impedance mismatch, augment late systolic load on the left ventricle.11, 12 Late systolic load increases left ventricular (LV) mass and fibrosis and worsens diastolic function in animal models.13, 14 Late systolic load is also associated with increased LV mass and geometry15 and impaired systolic and diastolic function in humans.16, 17, 18 Furthermore, the reduction in reflection magnitude (RM) correlates with reductions in LV mass during antihypertensive therapy.19
Organic nitrates have been shown to blunt wave reflections in short‐term studies,20, 21, 22, 23 although tolerance remains a concern with chronic administration.24 However, therapy with hydralazine has been shown to attenuate this tolerance.24, 25, 26 Whether organic nitrates can modulate wave reflections chronically and whether this effect is modulated by hydralazine is unknown. Furthermore, the effects of long‐term nitrate therapy on arterial hemodynamics, LV remodeling, diastolic function, and exercise capacity in patients with HFpEF are unknown. We designed the current pilot trial to test whether the organic nitrate isosorbide dinitrate (ISDN), with or without hydralazine, blunts wave reflections, thereby leading to improvements in myocardial structure and function.
Methods
Inclusion/Exclusion Criteria
Inclusion criteria included symptomatic heart failure with a preserved ejection fraction (LV ejection fraction >50%), in addition to at least one of the following: (1) prior hospitalization for decompensated heart failure; (2) acute treatment for heart failure requiring intravenous diuretics or hemofiltration; (3) echocardiographic evidence for elevated filling pressures27; (4) chronic treatment with a loop diuretic for control of symptoms; (5) or an elevated N‐terminal pro‐brain natriuretic peptide (NT‐pro‐BNP) level. Patients were required to be on stable medical therapy for the past month. Exclusion criteria included any rhythm other than sinus with native conduction; noncardiac conditions that significantly limited exercise (orthopedic or neuromuscular); known hypertrophic, infiltrative, or inflammatory cardiomyopathy; pericardial disease; significant pulmonary disease; primary pulmonary arteriopathy; acute coronary syndrome or coronary revascularization within the past 60 days; clinically significant perfusion defects on stress imaging without subsequent revascularization; significant valvular disease (eg, moderate or greater mitral regurgitation or aortic stenosis); uncontrolled hypertension (systolic blood pressure >180 mm Hg or diastolic blood pressure >100 mm Hg); prior reduced LV ejection fraction <50%; hemoglobin <10 g/dL; current therapy with organic nitrates or hydralazine; and elevations on liver function test results. Additional exclusion criteria for the cardiac magnetic resonance imaging (MRI) included impaired renal function precluding the administration of gadolinium (estimated glomerular filtration rate <30 mL/min per 1.73 m2) and significant claustrophobia.
Study Design
This was a randomized double‐blinded pilot clinical trial of ISDN (40 mg 3 times daily), ISDN plus hydralazine (ISDN 40 mg+hydral 75 mg, 3 times daily), or placebo (PB). The doses selected were based on A‐HeFT (the African‐American Heart Failure Trial), which demonstrated a benefit with these doses of ISDN+hydral in black patients with HFrEF.28 We tested the hypothesis that chronic administration of ISDN, with or without hydralazine, would blunt wave reflections. Our primary end point was the change in RM, assessed 6 months after study initiation. Key secondary end points included change in LV mass and diffuse interstitial myocardial fibrosis measured by MRI, change in 6‐minute walk (6MW) distance, change in diastolic function, change in NT‐pro‐BNP, and change in quality of life assessed by the Kansas City Cardiomyopathy Questionnaire. This protocol was approved by the institutional review boards of the Philadelphia Veterans Affairs Hospital and the Hospital of the University of Pennsylvania. All patients provided written informed consent. The trial was registered at ClinicalTrials.gov ( www.ClinicalTrials.gov, NCT01516346).
After randomization, patients were started on half of the intended target dose. After 1 week, patients were reassessed, and the doses of study medications were increased unless orthostatic hypotension or other limiting side effect was present. Study medications were continued for 6 months. The first patient was enrolled in March 2012, and the final study visit was conducted in December 2015.
Cardiac MRI—LV Structure and Function
Participants underwent a cardiac MRI examination at baseline and at 6 months to assess LV structure and function using a 1.5 Tesla (T) whole‐body MRI scanner (Avanto or Espree, Siemens Healthcare, Malvern, Pennsylvania) equipped with a phased‐array cardiac coil. LV volumes and ejection fractions were determined using balanced steady‐state free‐precession cine imaging. Typical parameters were as follows: repetition time=2.6 ms; echo time=1.3 ms; phases=30; slice thickness=8 mm; bandwidth=898 Hz/pixel, flip angle=70°, field of view=300 to 340 mm2, matrix size=192×192; and parallel imaging factor=2. LV short‐axis stack cine images were manually traced at end‐diastole and end‐systole using CMR42 software (Circle CVI, Calgary, Alberta, Canada). LV mass was computed as the difference between epicardial and endocardial volumes, multiplied by myocardial density, and was measured at end‐diastole and end‐systole with the results averaged. LV mass was normalized for height in meters raised to the power of 1.7.29 Stroke volume was computed as the difference between the end‐diastolic and end‐systolic volumes. End‐diastolic volume (EDV) and stroke volume were indexed to body surface area.
We used a modified Look‐Locker inversion recovery (MOLLI) sequence to assess T1 times prior to and following the intravenous administration of gadolinium contrast (gadopentetate dimeglumine, 0.15 mmol/kg or equivalent) in a midventricular short‐axis slice.30, 31 Scan parameters for MOLLI were: field of view=340 mm2; matrix size=144×192; slice thickness=6 mm; repetition time=2.4 ms; echo time=1.18 ms; flip angle=30°; bandwidth=1000 Hz/pixel; and parallel imaging=2. Myocardial T1 measurements were performed before and at several time points (≈5, 10, 15, and 20–40 minutes) after gadolinium administration. MOLLI was performed with a 5‐3‐3 schema (2 inversions, 5 TIs after inversion 1, 3 T1 recovery heartbeats, and 3 TIs after inversion 2). All available blood and myocardial T1 measurements were used to compute lambda (λ, the myocardium‐blood partition coefficient) as the slope of the myocardial 1/T1 over the blood 1/T1 change, via linear regression.31 The percent of myocardial tissue comprised by the extracellular space (extracellular volume fraction [ECV], %)=λ×(1−hematocrit). As heart rate correction did not appreciably affect the results, only the noncorrected values are presented.
Echocardiography and Arterial Tonometry
Echocardiography with arterial tonometry was performed at baseline, after 3 months, and at the final 6‐month visit. Echocardiography was performed using a Vivid e9 or Vivid I machine (General Electric, Fairfield, CT). Diastolic function was assessed according to American Society of Echocardiography criteria27 Each metric was quantified in triplicate with average values presented. Left atrial volume was quantified using the area‐length method and indexed to body surface area (left atrial volume index).32 Volumetric flow was quantified using pulse‐wave Doppler measurements from the left ventricular outflow tract in the 5‐chamber view and the left ventricular outflow tract cross‐sectional area computed from its diameter measured in the parasternal long‐axis view.
Applanation tonometry was performed at the carotid, radial, and femoral arteries using a high‐fidelity tonometer (Millar Instruments, Houston, TX), with a single‐lead ECG used as a fiducial point. Surface measurements were obtained from the sternal notch to the site of interrogation at the carotid and femoral arteries to compute pulse wave velocity. Radial tonometry was calibrated using the brachial systolic blood pressure and diastolic blood pressure, obtained using a validated oscillometric device (Omron HEM‐705CP, Omron Corp, Kyoto, Japan or Accutorr Plus, Datascope Corp., Paramus, NJ). Mean arterial pressure was computed as the mean pressure from the radial pressure waveform. Carotid tonometry, calibrated using mean arterial pressure and diastolic blood pressure, was used to estimate central pressures. Tonometric signals were processed using Sphygmocor software (AtCor Medical, Australia).
Central Arterial Hemodynamics
Custom‐designed software was programmed using MATLAB (R2014b, MathWorks, Natick, MA) to derive input impedance, as previously described (Figure 1).33 In brief, central pressure measurements were ensemble‐averaged and time‐aligned with left ventricular outflow tract flow such that the upstroke of pressure and flow occurred simultaneously, peak flow was coincident with the first systolic peak or inflection point in the pressure waveform, and flow ceased at the dicrotic notch. Characteristic impedance (Zc) was quantified in the frequency domain as the average modulus at higher frequencies. Total vascular resistance was quantified as the ratio of mean pressure to mean flow. Total arterial compliance was determined using the pulse pressure method.33 Linear wave separation was performed to obtain the amplitude of the forward (Pf) and backward (Pb) pressure waves. RM was defined as the ratio of Pb to Pf (Pb/Pf).
Figure 1.
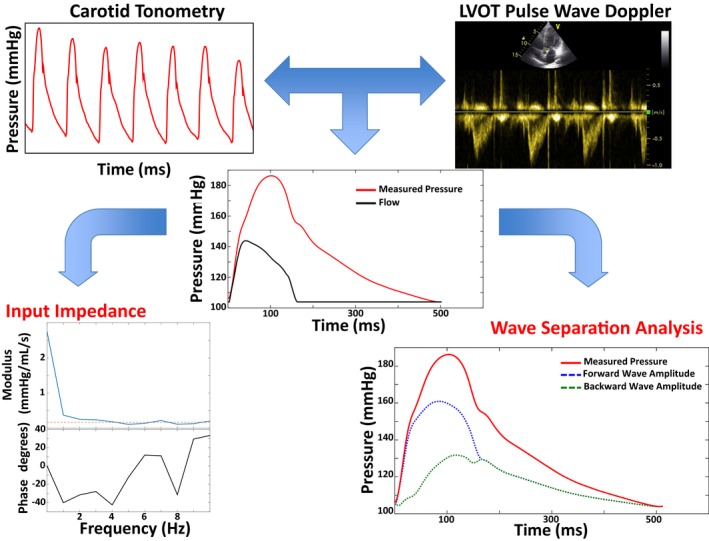
Arterial tonometry and flow methods for input impedance and wave separation analysis. Carotid tonometry (top left) and pulsed wave Doppler (top right) are used to obtain signal‐averaged pressure and flow waveforms (middle panel). Aortic root characteristic impedance (Zc) is computed in the frequency domain as the mean value of the modulus of higher harmonics (bottom left panel, dashed line). In the middle panel, the flow waveform is displayed in the pressure axis as the flow×Zc. This can be seen as the minimum pulse pressure required to eject the observed flow across the local aortic root impedance, in the complete absence of wave reflections. Additional pressure is related to wave reflections arising from more distal segments. The bottom right panel displays separation of the measured pressure wave into forward (Pf, blue) and backward components (Pb, green) components; the reflection magnitude is computed as the ratio of Pb/Pf. LVOT indicates left ventricular outfow tract.
Additional Measurements Performed at Baseline and at 6 Months
The Kansas City Cardiomyopathy Questionnaire was administered at the baseline and 6‐month visits.34 Basic laboratory tests, including NT‐pro‐BNP levels (upper limit of normal=125 pg/mL), were also performed. Patients performed a 6MW test using the standard protocol.35 The Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation was used to estimate glomerular filtration rate.36
Statistical Methods
Baseline characteristics are presented as mean (percentage) or median (interquartile range [IQR]), as appropriate. Mixed‐effects models were generated using the xtreg command in STATA (Stata/SE version 13.1, StataCorp, College Station, TX), which incorporates the correlation between repeated measurements in the same individual. No assumption of linearity was made, and all available observations were used to estimate treatment effects. An overall P<0.05 was taken to be significant for each model, with post hoc comparisons between visits subsequently performed. Study end points are presented in the tables as marginal means estimated from the mixed models with 95% CIs. Due to the small number of patients who completed this pilot study, only intragroup comparisons were performed. Paired t tests using only data from the baseline and final visits were also performed for select end points. Given the small sample sizes, the signed rank test was additionally performed to demonstrate general agreement. Our study had 80% power to detect a within‐group minimal change of at least 14.4 g/m1.7 in LV mass and 0.10 in RM. Formal between‐group comparisons were not performed because of the risk of type II error.
Results
The flow of patients through the study is shown in Figure 2. A total of 53 patients consented to the study. Of these 53 patients, 9 withdrew before receiving study medications; thus, 44 (83%) patients were randomized and started the study medications: 13 patients were randomized to ISDN, 15 to ISDN+hydral, and 16 to PB. Of these individuals, 30 (68%) provided 3‐month central arterial hemodynamics data (ISDN=9, ISDN+hydral=9, PB=12) and 27 (61%) provided 6‐month data (ISDN=7, ISDN+hydral=9, PB=11). Demographic, echocardiographic, and cardiac MRI data are shown in Table 1. The median age of patients was 62 (IQR 59–68) years. The majority of patients were obese (81.8%), hypertensive (90.9%), and had a history of diabetes (61.4%). A total of 31 (70.5%) patients had an elevated NT‐pro‐BNP level, and 40.9% had an estimated glomerular filtration rate <60 mL/min per 1.73 m2. Median E/septal e′ ratio was 12.8 (IQR 10.2–15.2), and the median left atrial volume index was 29.9 (IQR 25.6–38.3) mL/m2. Aside from the study intervention, there were no significant differences in medication usage between the groups (P>0.10 for all). Compliance was assessed via pill counts. In the 27 patients who completed the study, compliance rates were: ISDN: 91.8%; ISDN+hydral: 90.2%; and PB: 94.8%. The mean daily dose of ISDN in the ISDN only group was 111.4±22.7 mg. The mean ISDN and hydralazine doses in the combination group were 100.0±30.0 mg of ISDN and 187.5±56.3 mg of hydralazine. There were no differences in demographic characteristics between those who did versus those who did not complete the study (data not shown).
Figure 2.
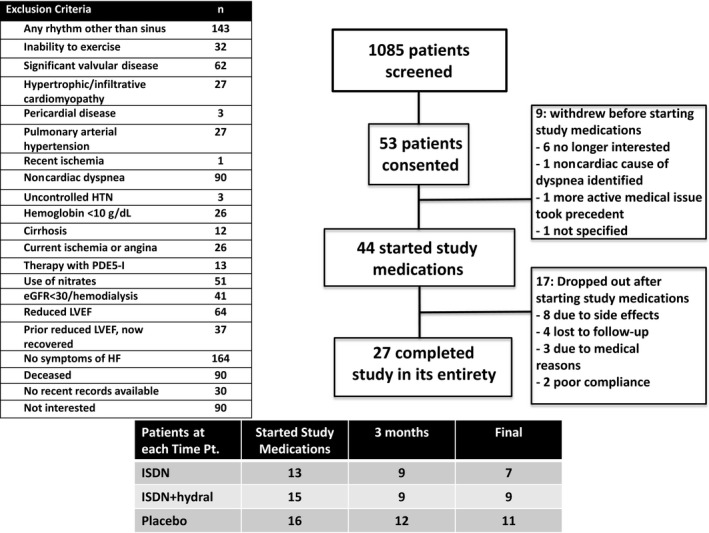
CONSORT diagram and flow of patients through each study visit. eGFR indicates estimated glomerular filtration rate; HF, heart failure; HTN, hypertension; hydral, hydralazine; ISDN, isosorbide dinitrate; LVEF, left ventricular ejection fraction; PDE5‐I, phosphodiesterase type 5 inhibitor.
Table 1.
Baseline Demographic, Medication, Laboratory, and Imaging Data
| Variable | All Participants (N=44) | ISDN (n=13) | ISDN+Hydral (n=15) | Placebo (n=16) |
|---|---|---|---|---|
| Age, median (IQR), y | 62 (59–68) | 61 (56–65) | 60 (55–66) | 66.5 (59.5–72) |
| Male, No. (%) | 31 (70.5) | 8 (61.5) | 11 (73.3) | 12 (75) |
| Race, No. (%) | ||||
| Black | 27 (61.4) | 8 (61.5) | 10 (66.7) | 9 (56.3) |
| White | 16 (36.4) | 4 (30.8) | 5 (33.3) | 7 (43.8) |
| Other | 1 (2.3) | 1 (7.7) | 0 (0) | 0 (0) |
| Body mass index (kg/m2), mean (SD) | 36.7 (6.2) | 35.7 (6.7) | 38.2 (5.1) | 36.2 (6.8) |
| Obese, No. (%) | 36 (81.8) | 10 (76.9) | 13 (86.7) | 13 (81.3) |
| Hypertension, No. (%) | 40 (90.9) | 13 (100) | 14 (93.3) | 13 (81.3) |
| Hyperlipidemia, No. (%) | 36 (81.8) | 10 (76.9) | 12 (80.0) | 14 (87.5) |
| Coronary artery disease, No. (%) | 16 (36.4) | 4 (30.8) | 6 (40.0) | 6 (37.5) |
| History of atrial fibrillation/flutter, No. (%) | 5 (11.4) | 0 (0) | 3 (20.0) | 2 (12.5) |
| Diabetes, No. (%) | 27 (61.4) | 11 (84.6) | 6 (40.0) | 10 (62.5) |
| Obstructive sleep apnea, No. (%) | 24 (54.6) | 6 (46.2) | 10 (66.7) | 8 (50.0) |
| Medical therapy | ||||
| β‐Blockers, No. (%) | 26 (59.1) | 6 (46.2) | 9 (60.0) | 11 (68.8) |
| Aspirin, No. (%) | 30 (68.2) | 10 (76.9) | 9 (60.0) | 11 (68.8) |
| ACEI/ARB, No. (%) | 29 (65.9) | 7 (53.9) | 10 (66.7) | 12 (75.0) |
| Loop diuretics, No. (%) | 24 (54.6) | 6 (46.2) | 8 (53.3) | 10 (62.5) |
| Mineralocorticoid receptor antagonists, No. (%) | 2 (4.6) | 1 (7.7) | 1 (6.7) | 0 (0) |
| Calcium channel blockers, No. (%) | 19 (43.2) | 5 (38.5) | 6 (40.0) | 8 (50.0) |
| Thiazide diuretics, No. (%) | 16 (36.4) | 7 (53.9) | 6 (40.0) | 3 (18.8) |
| Statins, No. (%) | 29 (65.9) | 10 (76.9) | 8 (53.3) | 11 (68.8) |
| Baseline laboratories | ||||
| Hematocrit, mean (SD), % | 38.5 (4.9) | 39.3 (3.7) | 38.6 (4.0) | 37.8 (6.4) |
| eGFR (mL/min per 1.73 m2), mean (SD) | 70.3 (26.7) | 77.1 (27.1) | 70.8 (32.1) | 64.4 (21.0) |
| eGFR <60 mL/min per 1.73 m2, No. (%) | 18 (40.9) | 5 (38.5) | 7 (46.7) | 6 (37.5) |
| NT‐pro‐BNP, median (IQR), pg/mL | 233.0 (90.5–527.0) | 154 (88–280) | 210 (118–1190) | 326 (83.3–720.3) |
| Elevated NT‐pro‐BNP, No. (%) | 31 (70.5) | 9 (69.2) | 11 (73.3) | 11 (68.8) |
| Baseline cMRI data | ||||
| LV mass, median (IQR), g | 170.0 (132.9–200.5) | 183.3 (136.3–215.5) | 168.8 (131.9–198.5) | 159.5 (126.6–215.0) |
| Indexed LV mass, median (IQR), g/m1.7 | 64.2 (56.6, 81.2) | 68.0 (58.3, 87.2) | 60.2 (57.2, 73.9) | 63.6 (53.1, 80.7) |
| LVEDV, mean (SD), mL | 171.9 (43.8) | 159.1 (39.3) | 176.8 (38.9) | 179.5 (51.6) |
| Indexed LVEDV, mean (SD), mL/m2 | 74.9 (15.2) | 71.2 (12.8) | 74.0 (13.6) | 79.2 (18.4) |
| LV ejection fraction, median (IQR), % | 59.4 (55.1–65.5) | 64.1 (57.8–65.9) | 56.9 (52.9–67.4) | 59.3 (55.5–65.0) |
| LVECV fraction, mean (SD), % | 28.9 (6.6) | 28.3 (4.5) | 28.6 (8.0) | 29.6 (6.8) |
| Native myocardial T1, mean (SD), s | 1013.2 (52.0) | 1017.1 (41.3) | 1002.0 (43.2) | 1019.9 (64.5) |
| Baseline echocardiographic data | ||||
| Mitral E velocity, mean (SD), cm/s | 81.0 (23.8) | 69.4 (21.0) | 82.2 (26.7) | 89.8 (20.1) |
| Mitral A velocity, mean (SD), cm/s | 77.1 (22.6) | 76.7 (19.7) | 76.9 (25.2) | 77.7 (23.8) |
| Mitral E/A ratio, median (IQR) | 1.01 (0.83–1.30) | 0.83 (0.75–1.08) | 1.01 (0.89–1.25) | 1.16 (0.88–1.54) |
| Mitral septal tissue (e′) velocity, mean (SD), cm/s | 6.3 (2.1) | 6.0 (1.6) | 6.3 (2.3) | 6.6 (2.3) |
| Mitral E/e′, median (IQR) | 12.8 (10.2–15.2) | 11.0 (9.8–12.8) | 12.4 (10.2–15.2) | 13.5 (11.3–17.3) |
| Left atrial volume index, median (IQR), mL/m2 | 29.9 (25.6–38.3) | 29.0 (26.0–34.1) | 34.3 (22.7–43.5) | 31.0 (27.9–35.4) |
Elevated N‐terminal pro‐brain natriuretic peptide (NT‐pro‐BNP) defined as >125 pg/mL. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; cMRI, cardiac magnetic resonance imaging; eGFR, estimated glomerular filtration rate; Hydral, hydralazine; IQR, interquartile range; ISDN, isosorbide dinitrate; LV, left ventricular; LVECV, left ventricular extracellular volume fraction; LVEDV, left ventricular end‐diastolic volume.
Arterial Hemodynamics
Treatment with ISDN did not reduce brachial systolic blood pressure (P=0.09), yet tended to reduce central systolic blood pressure (visit 2 versus visit 1: −27.6 [95% CI −54.0 to −1.3]; visit 3 versus visit 1: −30.4 [95% CI −58.2 to −2.6] mm Hg; overall P=0.051; Table 2), although this reduction did not reach statistical significance. There were no significant changes in brachial or central blood pressures with ISDN+hydral or PB. Heart rate and augmentation index were not significantly altered by any study medication (data not shown).
Table 2.
Central Arterial Hemodynamics
| Marginal Means (95% CI) | ISDN | ISDN+Hydral | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=13) | 3 Months (n=8) | Final (n=7) | P Value | Baseline (n=14) | 3 Months (n=7) | Final (n=5) | P Value | Baseline (n=15) | 3 Months (n=11) | Final (n=10) | P Value | |
| Brachial SBP, mm Hg | 146.0 (134.8–157.3) | 129.2 (115.1–143.2) | 123.9 (108.7–139.1) | 0.09 | 126.8 (117.9–135.7) | 126.0 (115.0–137.1) | 118.2 (105.5–130.9) | 0.56 | 138.0 (130.5–145.5) | 137.5 (128.5–146.4) | 143.0 (134.0–151.9) | 0.64 |
| Central SBP, mm Hg | 141.3 (126.9–155.7) | 113.7 (95.7–131.7) | 110.9 (91.4–130.4) | 0.051 | 122.6 (110.4–134.8) | 122.1 (105.4–138.8) | 115.3 (95.1–135.5) | 0.83 | 128.4 (121.1–135.8) | 130.1 (121.8–138.3) | 133.5 (124.8–142.3) | 0.70 |
| Central DBP, mm Hg | 79.2 (71.4–87.1) | 70.7 (60.9–80.5) | 72.1 (61.5–82.8) | 0.41 | 73.2 (68.3–78.2) | 72.4 (65.6–79.2) | 72.9 (64.8–81.1) | 0.98 | 69.3 (64.4–74.2) | 74.2 (68.6–79.7) | 77.1 (71.2–82.9) | 0.18 |
| Central MAP | 102.6 (93.4–111.7) | 88.4 (77.0–99.9) | 87.4 (75.0–99.8) | 0.13 | 92.7 (85.9–99.6) | 92.0 (82.5–101.4) | 91.6 (80.2–103.0) | 0.99 | 93.3 (88.2–98.5) | 95.2 (89.4–101.0) | 98.7 (92.5–104.8) | 0.47 |
| TVR, mm Hg/mL per second | 1.30 (1.09–1.52) | 1.06 (0.79–1.34) | 1.12 (0.83–1.42) | 0.42 | 1.18 (0.97–1.38) | 0.98 (0.70–1.27) | 0.94 (0.60–1.28) | 0.48 | 1.06 (0.95–1.18) | 1.09 (0.96–1.21) | 1.09 (0.95–1.22) | 0.96 |
| TAC, mL/m mHg | 1.01 (0.84–1.18) | 1.51a (1.30–1.73) | 1.38b (1.15–1.61) | 0.01 | 1.52 (1.22–1.82) | 1.33 (0.92–1.74) | 1.50 (1.01–1.99) | 0.76 | 0.99 (0.85–1.13) | 1.16 (1.00–1.32) | 1.08 (0.92–1.25) | 0.34 |
| Zc, mm Hg/mL per s | 0.15 (0.14–0.17) | 0.11a (0.10–0.13) | 0.10b (0.08–0.12) | 0.003 | 0.16 (0.09–0.22) | 0.09 (0.00–0.18) | 0.06 (−0.05 to 0.17) | 0.34 | 0.16 (0.13–0.18) | 0.13 (0.10–0.15) | 0.12 (0.09–0.15) | 0.18 |
| RM | 0.39 (0.35–0.42) | 0.41 (0.37–0.46) | 0.38 (0.33–0.43) | 0.64 | 0.39 (0.35–0.43) | 0.31 (0.25–0.36) | 0.44c (0.37–0.51) | 0.03 | 0.36 (0.30–0.41) | 0.34 (0.28–0.39) | 0.37 (0.31–0.43) | 0.75 |
| Pf, mm Hg | 54.8 (47.6–62.0) | 42.2 (33.2–51.3) | 37.0b (27.2–46.8) | 0.04 | 47.9 (38.6–57.2) | 42.4 (29.6–55.1) | 36.7 (21.3–52.0) | 0.53 | 55.0 (48.0–62.0) | 49.6 (41.7–57.5) | 50.8 (42.4–59.1) | 0.61 |
| Pb, mm Hg | 21.1 (17.4–24.7) | 17.7 (13.1–22.3) | 13.0 (8.1–18.0) | 0.08 | 18.9 (13.5–24.4) | 12.3 (4.8–19.7) | 16.0 (7.1–25.0) | 0.44 | 19.2 (16.3–22.1) | 16.5 (13.2–19.8) | 17.2 (13.8–20.7) | 0.50 |
DBP indicates diastolic blood pressure; Hydral, hydralazine; ISDN, isosorbide dinitrate; MAP, mean arterial pressure; Pb, magnitude of the backward wave; Pf, magnitude of the forward wave; RM, reflection magnitude; SBP, systolic blood pressure; TAC, total arterial compliance; TVR, total vascular resistance; Zc, characteristic impedance of the ascending aorta.
P<0.05 between baseline and the 3‐month visit.
P<0.05 between baseline and the final visits.
P<0.05 between the 3‐month and final visits.
ISDN did not reduce RM, the primary end point of the study (P=0.64; Table 2). In contrast, ISDN reduced aortic Zc (P=0.003), reduced Pf (P=0.04), and increased total arterial compliance (P=0.01). Combination therapy with ISDN+hydral increased RM between the 3‐ and 6‐month visits (P=0.012). No changes in arterial hemodynamic parameters were demonstrated in the PB group (Figure 3).
Figure 3.
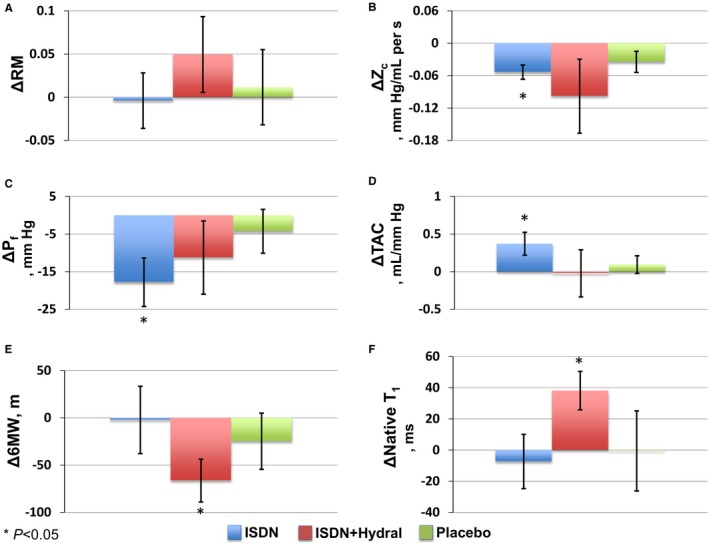
Changes in end points as compared with baseline (marginal mean differences with standard error presented). A, Reflection magnitude (RM). B, Characteristic impedance (Zc). C, Forward wave magnitude (Pf). D, Total arterial compliance (TAC). E, Six‐minute walk (6MW) distance. F, Native T1 myocardial relaxation time. Hydral indicates hydralazine; ISDN, isosorbide dinitrate.
LV Mass and Fibrosis
There was no change in LV mass in any of the trial arms (Table 3). Similarly, there were no changes in ECV, assessed following the administration of gadolinium, in any of the trial arms. Combination therapy with ISDN+hydral increased the native T1 relaxation time (P=0.021).
Table 3.
MRI Quantitative Assessment of LV Mass, Volume, and Fibrosis
| Marginal Means (95% CI) | ISDN | ISDN+Hydral | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=12) | Final (n=7) | P Value | Baseline (n=13) | Final (n=8) | P Value | Baseline (n=16) | Final (n=11) | P Value | |
| iEDV, mL/m2 | 70.9 (66.5–75.3) | 60.2 (54.3–66.2) | 0.037 | 71.5 (67.6–75.4) | 79.5 (74.8–84.1) | 0.052 | 78.6 (73.0–84.3) | 63.8 (56.6–71.0) | 0.037 |
| iStroke volume, mL/m2 | 44.4 (42.2–46.6) | 38.9 (36.0–41.8) | 0.029 | 41.3 (39.4–43.2) | 49.9 (47.6–52.2) | 0.002 | 47.6 (43.6–51.5) | 38.4 (33.4–43.4) | 0.054 |
| LV mass, g/m1.7 | 73.9 (67.9–79.9) | 68.2 (60.0–76.3) | 0.33 | 67.4 (63.6–71.2) | 66.2 (61.4–71.1) | 0.74 | 68.0 (64.6–71.5) | 67.2 (62.8–71.5) | 0.80 |
| ECV (%) | 27.5 (24.6–30.5) | 29.0 (25.0–33.0) | 0.63 | 29.9 (28.4–31.3) | 31.3 (29.2–33.4) | 0.34 | 29.5 (27.0–32.0) | 29.5 (26.4–32.6) | >0.99 |
| Native T1, ms | 1016.0 (995.9–1036.0) | 1008.6 (984.0–1033.3) | 0.69 | 1016.2 (1002.7–1029.7) | 1054.4 (1036.5–1072.3) | 0.021 | 1017.5 (986.9–1048.1) | 1016.9 (979.4–1054.4) | 0.98 |
ECV indicates extracellular volume fraction; iEDV, end‐diastolic volume indexed to body surface area; Hydral, hydralazine; ISDN, isosorbide dinitrate; iStroke, stroke volume indexed to body surface area; LV, left ventricular; MRI, magnetic resonance imaging.
Other LV Geometric Measures
A reduction in EDV was observed in the ISDN (P=0.037) and PB (P=0.037) arms (Table 3). This occurred in concert with reductions in stroke volume for ISDN (P=0.029) and a trend towards reduced stroke volume in PB (P=0.054). In contrast, combination therapy with ISDN+hydral tended to increase EDV (P=0.052) and significantly increased stroke volume (P=0.002).
Additional Assessments
NT‐pro‐BNP levels, mitral E/septal e′ ratio, left atrial volume index (Table 4), and the overall summary score for the Kansas City Cardiomyopathy Questionnaire (Table 5) did not change in any of the trial arms. The 6MW distance was unchanged in both the ISDN and PB groups; however, 6MW distance worsened in the ISDN+hydral arm (baseline 343.3 [95% CI, 319.2–367.4]; final 277.0 [95% CI, 242.7–311.4] meters; P=0.022).
Table 4.
Echocardiographic Indices of Diastolic Function
| Marginal Means (95% CI) | ISDN | ISDN+Hydral | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=13) | 3 Months (n=8) | Final (n=7) | P Value | Baseline (n=15) | 3 Months (n=8) | Final (n=9) | P Value | Baseline (n=15) | 3 Months (n=11) | Final (n=10) | P Value | |
| E, cm/s | 69.6 (62.7–76.6) | 64.0 (55.2–72.8) | 62.0 (52.6–71.5) | 0.45 | 83.4 (77.1–89.7) | 80.4 (71.9–88.9) | 89.7 (81.8–97.7) | 0.29 | 92.3 (87.5–97.2) | 83.6a (78.2–89.0) | 77.4b (71.7–83.2) | 0.006 |
| A, cm/s | 74.4 (69.8–78.9) | 70.5 (64.8–76.2) | 71.1 (64.9–77.2) | 0.57 | 77.7 (73.1–82.2) | 80.6 (74.3–86.8) | 80.4 (74.1–86.6) | 0.72 | 78.0 (71.9–84.0) | 78.6 (71.7–85.4) | 78.3 (71.0–85.5) | 0.99 |
| E/A ratio | 0.98 (0.83–1.14) | 0.92 (0.73–1.11) | 0.93 (0.72–1.14) | 0.89 | 1.16 (1.08–1.24) | 1.11 (1.01–1.22) | 1.18 (1.08–1.29) | 0.66 | 1.27 (1.15–1.40) | 1.16 (1.02–1.30) | 1.03 (0.89–1.18) | 0.09 |
| Septal e′, cm/s | 6.0 (5.0–6.9) | 6.2 (5.0–7.3) | 6.8 (5.5–8.0) | 0.60 | 6.1 (5.1–7.1) | 6.7 (5.5–8.0) | 7.3 (6.1–8.6) | 0.35 | 6.7 (5.9–7.6) | 6.7 (5.7–7.7) | 6.5 (5.4–7.6) | 0.96 |
| E/Septal e′ ratio | 11.7 (10.2–13.2) | 11.9 (10.0–13.8) | 10.3 (8.2–12.3) | 0.47 | 15.2 (14.0–16.4) | 13.9 (12.3–15.5) | 14.6 (13.1–16.1) | 0.46 | 15.3 (13.6–17.0) | 14.3 (12.3–16.3) | 12.9 (10.6–15.1) | 0.30 |
| LAVI, mL/m2 | 28.2 (24.4–31.9) | 27.2 (21.3–33.1) | 26.1 (20.5–31.7) | 0.85 | 35.2 (29.9–40.4) | 35.5 (28.6–42.5) | 40.3 (33.8–46.8) | 0.47 | 33.9 (29.4–38.5) | 34.2 (28.3–40.0) | 34.7 (28.9–40.6) | 0.98 |
Hydral indicates hydralazine; ISDN, isosorbide dinitrate; LAVI, left atrial volume index.
P<0.05 between baseline and the 3‐month visit.
P<0.05 between baseline and the final visits.
Table 5.
Kansas City Cardiomyopathy Questionnaire Results
| Marginal Means (95% CI) | ISDN | ISDN+Hydral | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=11) | 3 Months (n=8) | Final (n=6) | P Value | Baseline (n=13) | 3 Months (n=8) | Final (n=9) | P Value | Baseline (n=16) | 3 Months (n=8) | Final (n=9) | P Value | |
| Physical Limitation Score | 49.6 (41.2–58.0) | 52.1 (42.9–61.3) | 65.9 (55.4–76.4) | 0.09 | 51.1 (42.4–59.7) | 49.4 (38.2–60.6) | 51.3 (41.3–61.3) | 0.97 | 55.5 (44.5–66.5) | 54.8 (39.4–70.1) | 59.0 (44.6–73.3) | 0.91 |
| Symptom Stability Score | 44.9 (31.8–57.9) | 54.3 (39.4–69.1) | 62.1 (44.6–79.6) | 0.35 | 51.1 (39.5–62.7) | 61.4 (46.3–76.5) | 46.7 (33.2–60.1) | 0.39 | 53.6 (44.3–62.9) | 54.3 (41.4–67.3) | 73.0 (60.9–85.1) | 0.06 |
| Total Symptom Score | 48.1 (38.1–58.1) | 57.0 (45.6–68.3) | 64.1 (50.7–77.5) | 0.23 | 58.2 (49.8–66.7) | 50.2 (39.3–61.1) | 50.1 (40.4–59.8) | 0.43 | 55.8 (44.2–67.5) | 60.0 (43.8–76.1) | 68.7 (53.6–83.8) | 0.46 |
| Self‐Efficacy Score | 67.2 (54.5–79.9) | 84.4 (70.0–98.8) | 78.9 (61.9–95.9) | 0.26 | 70.1 (57.5–82.7) | 58.4 (42.0–74.7) | 39.9b (25.4–54.5) | 0.03 | 62.0 (54.4–69.7) | 85.6a (74.2–96.9) | 78.7b (68.9–88.6) | 0.016 |
| Functional Status Score | 48.5 (41.6–55.4) | 54.4 (46.5–62.2) | 64.8 (55.5–74.0) | 0.06 | 54.6 (46.7–62.6) | 49.8 (39.4–60.2) | 50.7 (41.5–59.9) | 0.75 | 55.7 (45.5–65.9) | 57.4 (43.1–71.6) | 63.8 (50.5–77.1) | 0.65 |
| Overall Summary Score | 43.5 (35.3–51.6) | 50.6 (41.3–59.9) | 62.1 (51.1–73.1) | 0.07 | 49.1 (42.4–55.9) | 45.2 (36.4–54.0) | 44.9 (37.1–52.7) | 0.70 | 54.0 (45.3–62.7) | 57.6 (45.5–69.7) | 62.1 (50.8–73.4) | 0.58 |
Hydral indicates hydralazine; ISDN, isosorbide dinitrate.
P<0.05 between baseline and the 3‐month visit.
P<0.05 between baseline and the final visits.
Sensitivity Analyses
Paired analyses using only data from the baseline and final visits were also performed on select end points (Table 6). The results of these sensitivity analyses were consistent with the findings using the mixed models approach.
Table 6.
Paired Analyses of Select End Points Using Only Data From the Baseline and Final Visits
| Mean (SD) | ISDN (n=7) | ISDN+Hydral (n=7) | Placebo (n=6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Difference | P Value | Baseline | Final | Difference | P Value | Baseline | Final | Difference | P Value | |
| iLVEDV, mL/m2 | 70.4 (14.5) | 59.7 (15.4) | −10.6 (10.5) | 0.037 | 68.6 (11.4) | 76.6 (12.0) | 8.0 (8.7) | 0.052 | 80.7 (24.2) | 65.9 (14.8) | −14.8 (12.9) | 0.037 |
| iSV, mL/m2 | 42.7 (6.8) | 37.2 (8.3) | −5.5 (5.2) | 0.029 | 39.6 (4.7) | 48.2 (5.8) | 8.6 (4.3) | 0.002 | 50.6 (16.5) | 41.4 (10.8) | −9.2 (8.9) | 0.054 |
| Native T1, ms | 1011.0 (19.1) | 1003.7 (39.5) | −7.33 (42.6) | 0.69 | 1022.6 (47.0) | 1060.7 (56.1) | 38.1 (32.6) | 0.02 | 1013.9 (66.8) | 1013.4 (48.0) | −0.55 (85.2) | 0.98 |
| ISDN (n=7) | ISDN+Hydral (n=5) | Placebo (n=9) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Difference | P Value | Baseline | Final | Difference | P Value | Baseline | Final | Difference | P Value | |
| Brachial SBP, mm Hg | 148.4 (31.7) | 124.6 (16.6) | −23.9 (29.9) | 0.08 | 129.3 (14.7) | 119.9 (12.5) | −9.4 (25.4) | 0.36 | 135.8 (14.9) | 143.3 (15.8) | 7.6 (18.7) | 0.26 |
| Central SBP, mm Hg | 146.5 (40.8) | 113.7 (11.3) | −32.8 (38.8) | 0.07 | 122.7 (15.1) | 116.0 (12.2) | −6.6 (25.7) | 0.59 | 126.5 (13.6) | 133.6 (18.1) | 7.1 (19.0) | 0.30 |
| Compliance, mm Hg/mL | 1.02 (0.54) | 1.38 (0.50) | 0.36 (0.22) | 0.005 | 1.67 (1.00) | 1.67 (0.53) | 0.00 (0.72) | >0.99 | 1.01 (0.35) | 1.09 (0.34) | 0.08 (0.36) | 0.50 |
| Zc, mm Hg/mL per s | 0.16 (0.05) | 0.11 (0.05) | −0.05 (0.02) | <0.001 | 0.18 (0.22) | 0.08 (0.03) | −0.10 (0.22) | 0.36 | 0.15 (0.06) | 0.12 (0.06) | −0.03 (0.07) | 0.21 |
| Reflection magnitude | 0.41 (0.06) | 0.40 (0.09) | −0.02 (0.09) | 0.61 | 0.43 (0.05) | 0.48 (0.11) | 0.05 (0.10) | 0.32 | 0.37 (0.11) | 0.38 (0.13) | 0.01 (0.06) | 0.70 |
| Pb, mm Hg | 23.3 (11.3) | 13.9 (3.7) | −9.4 (8.8) | 0.03 | 20.1 (12.4) | 18.0 (8.0) | −2.1 (13.0) | 0.74 | 19.2 (6.0) | 17.5 (3.4) | −1.7 (6.5) | 0.44 |
| Pf, mm Hg | 56.7 (27.7) | 37.8 (14.9) | −18.9 (18.6) | 0.04 | 46.5 (27.3) | 37.0 (10.9) | −9.5 (25.3) | 0.45 | 52.4 (12.4) | 49.5 (16.9) | −2.9 (21.2) | 0.69 |
| ISDN (n=6) | ISDN+Hydral (n=8) | Placebo (n=8) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Difference | P Value | Baseline | Final | Difference | P Value | Baseline | Final | Difference | P Value | |
| 6MW, m | 379.6 (71.7) | 377.5 (99.0) | −2.2 (87.3) | 0.95 | 318.8 (128.7) | 252.5 (148.7) | −66.3 (64.1) | 0.02 | 348.8 (67.6) | 373.5 (46.5) | 24.7 (84.2) | 0.43 |
6MW indicates 6‐minute walk distance; Hydral, hydralazine; iLVEDV, indexed left ventricular end‐diastolic volume; ISDN, isosorbide dinitrate; iSV, indexed stroke volume; Pb, backward pressure waves; Pf, forward pressure waves; SBP, systolic blood pressure; Zc, characteristic impedance of ascending aorta.
Adverse Events
Therapy with ISDN or ISDN+hydral was poorly tolerated, with significantly more patients experiencing adverse events in both active arms as compared with those taking PB (ISDN, n=8 [61.5%]; ISDN+hydral, n=9 [60.0%], PB, n=2 [12.5%]; P=0.007 [Table 7]). Common side effects in the active treatment arms were headache, dizziness/lightheadedness, hypotension, and orthostasis.
Table 7.
AEs in Patients Who Started Study Medications
| No. (%) | ISDN (n=13) | ISDN+Hydral (n=15) | Placebo (n=16) | P Value |
|---|---|---|---|---|
| Any AE | 8 (61.5) | 9 (60.0) | 2 (12.5) | 0.007 |
| Treatment‐related AE | 6 (46.2) | 6 (40.0) | 1 (6.3) | |
| Headache | 4 (30.8) | 2 (13.3) | 1 (6.3) | |
| GI symptoms | 0 (0) | 1 (6.7) | 0 (0) | |
| Dizziness/lightheadedness | 2 (15.4) | 2 (13.3) | 0 (0) | |
| Hypotension | 1 (7.7) | 3 (20.0) | 0 (0) | |
| Orthostasis | 1 (7.7) | 2 (13.3) | 0 (0) | |
| Fatigue | 0 (0) | 1 (6.7) | 0 (0) | |
| Reduced renal function | 1 (7.7) | 0 (0) | 0 (0) | |
| Discontinued study medications due to related AE | 4 (30.8) | 3 (20.0) | 1 (6.3) | |
| Treatment‐unrelated AE | 2 (15.4) | 6 (40.0) | 1 (6.3) | |
| GI symptoms | 1 (7.7) | 1 (6.7) | 1 (6.3) | |
| Reduced renal function | 0 (0) | 0 (0) | 1 (6.3) | |
| Retinal hemorrhage | 1 (7.7) | 0 (0) | 0 (0) | |
| Bacterial pneumonia | 0 (0) | 1 (6.7) | 0 (0) | |
| Atrial fibrillation | 0 (0) | 2 (13.3) | 0 (0) | |
| Atypical chest pain | 1 (7.7) | 1 (6.7) | 1 (6.3) | |
| Cauda equina syndrome | 0 (0) | 0 (0) | 1 (6.3) | |
| Cellulitis | 0 (0) | 1 (6.7) | 0 (0) | |
| Rhabdomyolysis | 0 (0) | 0 (0) | 1 (6.3) |
AE indicates adverse event; GI, gastrointestinal; Hydra, hydralazine; ISDN, isosorbide dinitrate.
Discussion
In this randomized pilot trial, we examined the impact of ISDN, ISDN+hydral, and PB on wave reflections, LV remodeling, 6MW distance, NT‐pro‐BNP, and quality of life. Contrary to our hypothesis, ISDN significantly reduced aortic Zc and Pf but did not reduce RM or improve LV remodeling. Moreover, combination therapy with ISDN+hydral led to an increase in RM, a decrease in 6MW distance, and adverse myocardial remodeling, as demonstrated by increased myocardial native T1 relaxation time. Importantly, ISDN and ISDN+hydral were poorly tolerated, with an increase in adverse events. Our study does not support the use of ISDN or ISDN+hydral in HFpEF.
With LV contraction, a pulse wave is generated that propagates down the arterial tree. When this wave encounters sites of impedance mismatch, such as at bifurcations, a portion of this pulse wave is reflected back towards the heart. Optimally timed wave reflections increase diastolic pressure and coronary perfusion, without exerting pronounced effects in central systolic pressure or left ventricular load. In individuals with increased vessel stiffness, the reflected wave arrives back at the heart earlier, increasing the mid‐to‐late systolic workload of the left ventricle. This increase in late‐systolic load has been shown to induce LV hypertrophy,13, 15 impair systolic16 and diastolic14, 16, 17 function, and increase myocardial fibrosis.13 Therapies that reduce RM are associated with regression in LV mass in hypertensive patients,19 suggesting that RM may be a potential therapeutic target in HFpEF patients, who are generally hypertensive, and exhibit prominent wave reflections.7, 8
In this study, ISDN reduced aortic Zc and Pf but did not reduce RM. The venodilating effect of ISDN, which would reduce preload and stroke volume, as well as the effects of this drug on aortic Zc, can explain the reduction in Pf, yet the neutral effects on RM bear additional mention. It is possible that chronic administration of organic nitrate led to increased oxidative stress and worsened endothelial dysfunction, reducing nitric oxide bioavailability37, 38 and mitigating any long‐term benefit on wave reflections. This mechanism could be particularly prominent in HFpEF patients, a population in which oxidative stress and decreased nitric oxide bioavailability have been demonstrated.39, 40 Second, the long‐term administration of ISDN could exert hemodynamic effects on multiple arterial segments, with differential impact on RM. ISDN reduced aortic Zc, which may have counteracted the vasodilatory effects on more distal vessels, such as the muscular arteries. Consequently, overall impedance matching may have been unchanged, leading to similar RM.41, 42 Finally, differences in the vasculature of HFpEF patients may have led to different responses than that which occurs in patients with hypertension or HFrEF.
An unexpected finding of our study was the increase in RM seen after 6 months of ISDN+hydral administration, along with reduced 6MW distances, increased EDV, and increased native T1 relaxation time. Prior work in a small number of HF patients found no change in sodium excretion following 3 days of hydralazine, although renin activity increased.43 In V‐HeFT II (the Vasodilator‐Heart Failure Trial), treatment with ISDN+hydral increased norepinephrine levels.44 More recently, hydralazine has been shown to activate preganglionic sympathetic neurons.45 Our findings of increased preload (suggesting volume retention), adverse interstitial remodeling, and reduced 6MW distances may be consistent with sympathetic nervous system activation.46, 47, 48 Other manifestations of sympathetic activation, such as tachycardia, could have been masked by the high utilization of concomitant cardiovascular medications, such as β‐blockers.
In our study, we found a significant increase in pregadolinium native T1 time in the ISDN+hydral group, in the absence of an increase in extracellular volume fraction, assessed following the administration of gadolinium. Several explanations may underlie this finding. First, ECV calculations reflect interstitial changes, given its reliance on gadolinium, an extracellular contrast agent.49 Native T1 signals, however, arise from the entirety of the myocardium, and thus reflect both the intracellular as well as the interstitial spaces.50 The increased native T1 time, in the absence of a change in ECV, may thus be a reflection of changes occurring at the intracellular level (cardiomyocytes, cardiac fibroblasts, smooth muscle cells, and endothelial cells), including increased edema or cardiomyocyte disarray.50, 51
Finally, treatment with ISDN, with or without hydralazine, was poorly tolerated in this population and led to frequent adverse events. Overall, our findings are consistent with those of NEAT‐HFpEF (Nitrate's Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction) trial, which showed that isosorbide mononitrate decreased physical activity on accelerometry and led to numerically greater adverse events as compared with PB.52 While patients in NEAT‐HFpEF were predominantly white women, as opposed to our predominantly black male population, the consistency of the findings suggests that the addition of hydralazine to organic nitrate therapy, a useful therapeutic approach in HFrEF,28 is not a suitable approach in HFpEF. In particular, the increased wave reflections, increased EDV, worsened exercise capacity, and poor tolerability observed in our trial argue against a potential benefit of this drug combination in HFpEF.
Interestingly, in contrast to organic nitrate, inorganic nitrate and nitrite have been shown to reduce wave reflections,53, 54 improve endothelial function,53 blunt the exercise‐induced rise in pulmonary capillary wedge pressure,55 and enhance exercise capacity in 2 separate trials of patients with HFpEF.56, 57 The effects of sustained administration of inorganic nitrate (KNO3) in HFpEF are currently being examined in a phase IIb trial sponsored by the National Heart, Lung, and Blood Institute (KNO3CK OUT HFPEF trial, ClinicalTrials.gov: NCT02840799). A trial of inhaled inorganic nitrite in HFpEF is also underway (ClinicalTrials.gov: NCT02713126).
Study Strengths and Limitations
Our study should be interpreted in the context of its strengths and limitations. The strengths of our study include its double‐blinded randomized design, the use of state‐of‐the‐art methods for central pressure and flow assessments, hemodynamic modeling, and the noninvasive characterization of myocardial structure and function. Our study also has limitations, mainly related to its small sample size. Despite flexibility in scheduling and compensation for participation,58, 59 only 27 of 44 (61%) patients who started the study medications completed the study. Unfortunately, the poor tolerability of the study interventions themselves contributed to the increased number of patients who prematurely left the study. Of the 17 patients who withdrew after starting study medications, 8 (47%) withdrew because of side effects (ISDN=4, ISDN+hydral=3, PB=1). Unlike NEAT‐HFpEF, we enrolled a predominantly black population (61%). Recruitment and retention of black patients within clinical trials represent an important yet challenging consideration.59 Future studies in this population should consider strategies to improve retention, such as involvement of community leaders, greater utilization of research nurses, and more intensive follow‐up.58, 59
The dose of study medications may also have had an impact on their tolerability. The NEAT‐HFpEF investigators noted decreased activity starting at low doses of organic nitrate (isosorbide mononitrate, 30 mg/d), suggesting that even low doses may have untoward effects.60 In addition, our population was predominantly black and male, limiting generalizability to the overall HFpEF population. However, black patients represent a unique group, with impaired endothelial function and nitric oxide bioavailability noted in health,61, 62 hypertension,63 and HFrEF.64 That the combination of organic nitrate and hydralazine did not reduce wave reflections in this population suggests that it is unlikely to do so in other HFpEF populations.
Conclusions
Despite the small sample size, the high incidence of adverse events and the within‐group changes demonstrated in the active arms in our study do not support the use of ISDN, with or without hydralazine, in patients with HFpEF.
Sources of Funding
This study was funded by a grant from the National Institute of Aging (5R21AG043802‐02, Chirinos) and a grant from the VA Health Network (VISN‐4 CPPF program, Chirinos). The project described was supported by grant number UL1RR024134 from the National Center for Research Resources and by grant number UL1TR000003 from the National Center for Advancing Translational Sciences, National Institutes of Health; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Other funding for the authors is as follows: Zamani: Institute for Translational Medicine and Therapeutics of the University of Pennsylvania (grant number: 5UL1TR000003‐09 from the National Center for Research Resources), 5‐T32‐HL007843‐17, 1‐K23‐HL‐130551‐01. Witschey: R00 HL108157, W.W. Smith Foundation, McCabe Foundation. Chirinos: 1R56HL124073‐01A1 and 1 R01 HL121510‐01A1.
Disclosures
Dr Chirinos reports consulting fees from Bristol‐Myers Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft Research, Vital Labs, and Merck. Chirinos received research grants from the National Institutes of Health, American College of Radiology Network, Fukuda‐Denshi, Bristol‐Myers Squibb, Microsoft Research, and CVRx Inc, and device loans from Atcor Medical. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of heart failure and preserved ejection fraction. Dr Townsend is a consultant for Medtronic, Fukuda Denshi, and Relypsa.
Acknowledgments
We would like to thank Priyanka Bhattacharya, MD, and Uzma Kewan, MD, for their assistance with data collection. We would also like to thank Dr Kenneth B. Margulies for his critical review of the manuscript.
(J Am Heart Assoc. 2017;6:e004262. DOI: 10.1161/JAHA.116.004262.)
References
- 1. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; ADHERE Scientific Advisory Committee and Investigators . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 2. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB; OPTIMIZE‐HF Investigators and Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF Registry. J Am Coll Cardiol. 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 3. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC; Get With the Guidelines Scientific Advisory Committee and Investigators . Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 4. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J. 2013;34:1424–1431. [DOI] [PubMed] [Google Scholar]
- 5. Safar ME, Levy BI, Struijker‐Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. [DOI] [PubMed] [Google Scholar]
- 6. Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. 2009;15:658–664. [DOI] [PubMed] [Google Scholar]
- 7. Weber T, Wassertheurer S, O'Rourke MF, Haiden A, Zweiker R, Rammer M, Hametner B, Eber B. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61:1874–1883. [DOI] [PubMed] [Google Scholar]
- 8. Weber T, Auer J, O'Rourke MF, Punzengruber C, Kvas E, Eber B. Prolonged mechanical systole and increased arterial wave reflections in diastolic dysfunction. Heart. 2006;92:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle‐dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. [DOI] [PubMed] [Google Scholar]
- 11. Chirinos JA, Segers P, Gillebert TC, Gupta AK, De Buyzere ML, De Bacquer D, St John‐Sutton M, Rietzschel ER; Asklepios Investigators . Arterial properties as determinants of time‐varying myocardial stress in humans. Hypertension. 2012;60:64–70. [DOI] [PubMed] [Google Scholar]
- 12. Chirinos JA, Segers P, Gupta AK, Swillens A, Rietzschel ER, De Buyzere ML, Kirkpatrick JN, Gillebert TC, Wang Y, Keane MG, Townsend R, Ferrari VA, Wiegers SE, St John Sutton M. Time‐varying myocardial stress and systolic pressure‐stress relationship: role in myocardial‐arterial coupling in hypertension. Circulation. 2009;119:2798–2807. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure‐overload left ventricular hypertrophy in rats. Circulation. 1996;94:3362–3368. [DOI] [PubMed] [Google Scholar]
- 14. Gillebert TC, Lew WY. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol. 1991;261:H805–H813. [DOI] [PubMed] [Google Scholar]
- 15. Zamani P, Bluemke DA, Jacobs DR Jr, Duprez DA, Kronmal R, Lilly SM, Ferrari VA, Townsend RR, Lima JA, Budoff M, Segers P, Hannan P, Chirinos JA. Resistive and pulsatile arterial load as predictors of left ventricular mass and geometry: the Multi‐Ethnic Study of Atherosclerosis. Hypertension. 2015;65:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC; Asklepios Investigators . Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle‐aged adults: the Asklepios study. Hypertension. 2013;61:296–303. [DOI] [PubMed] [Google Scholar]
- 17. Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. [DOI] [PubMed] [Google Scholar]
- 18. Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–1202. [DOI] [PubMed] [Google Scholar]
- 19. Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. [DOI] [PubMed] [Google Scholar]
- 20. Stokes GS, Barin ES, Gilfillan KL. Effects of isosorbide mononitrate and AII inhibition on pulse wave reflection in hypertension. Hypertension. 2003;41:297–301. [DOI] [PubMed] [Google Scholar]
- 21. Stokes GS, Barin ES, Gilfillan KL, Kaesemeyer WH. Interactions of L‐arginine, isosorbide mononitrate, and angiotensin II inhibitors on arterial pulse wave. Am J Hypertens. 2003;16:719–724. [DOI] [PubMed] [Google Scholar]
- 22. Stokes GS, Ryan M, Brnabic A, Nyberg G. A controlled study of the effects of isosorbide mononitrate on arterial blood pressure and pulse wave form in systolic hypertension. J Hypertens. 1999;17:1767–1773. [DOI] [PubMed] [Google Scholar]
- 23. Kelly RP, Gibbs HH, O'Rourke MF, Daley JE, Mang K, Morgan JJ, Avolio AP. Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery. Eur Heart J. 1990;11:138–144. [DOI] [PubMed] [Google Scholar]
- 24. Munzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, Freeman BA, Harrison DG. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane‐bound NADH oxidase. A new action for an old drug. J Clin Invest. 1996;98:1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gogia H, Mehra A, Parikh S, Raman M, Ajit‐Uppal J, Johnson JV, Elkayam U. Prevention of tolerance to hemodynamic effects of nitrates with concomitant use of hydralazine in patients with chronic heart failure. J Am Coll Cardiol. 1995;26:1575–1580. [DOI] [PubMed] [Google Scholar]
- 26. Bauer JA, Fung HL. Concurrent hydralazine administration prevents nitroglycerin‐induced hemodynamic tolerance in experimental heart failure. Circulation. 1991;84:35–39. [DOI] [PubMed] [Google Scholar]
- 27. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 28. Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN; African‐American Heart Failure Trial Investigators . Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. [DOI] [PubMed] [Google Scholar]
- 29. Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, Claessens T, Gillebert TC, St John‐Sutton M, Rietzschel ER. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz‐Menger J. Optimization and validation of a fully‐integrated pulse sequence for modified look‐locker inversion‐recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26:1081–1086. [DOI] [PubMed] [Google Scholar]
- 31. Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JA. Evaluation of age‐related interstitial myocardial fibrosis with cardiac magnetic resonance contrast‐enhanced T1 mapping: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;62:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 33. Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure‐flow and pressure‐volume relations in humans. Hypertension. 2010;56:563–570. [DOI] [PubMed] [Google Scholar]
- 34. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 35. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 36. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gori T, Mak SS, Kelly S, Parker JD. Evidence supporting abnormalities in nitric oxide synthase function induced by nitroglycerin in humans. J Am Coll Cardiol. 2001;38:1096–1101. [DOI] [PubMed] [Google Scholar]
- 38. Munzel T. Does nitroglycerin therapy hit the endothelium? J Am Coll Cardiol. 2001;38:1102–1105. [DOI] [PubMed] [Google Scholar]
- 39. van Heerebeek L, Hamdani N, Falcao‐Pires I, Leite‐Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. [DOI] [PubMed] [Google Scholar]
- 40. Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite‐Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ, Hamdani N. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:312–324. [DOI] [PubMed] [Google Scholar]
- 41. Yaginuma T, Avolio A, O'Rourke M, Nichols W, Morgan JJ, Roy P, Baron D, Branson J, Feneley M. Effect of glyceryl trinitrate on peripheral arteries alters left ventricular hydraulic load in man. Cardiovasc Res. 1986;20:153–160. [DOI] [PubMed] [Google Scholar]
- 42. Simon AC, Levenson JA, Levy BY, Bouthier JE, Peronneau PP, Safar ME. Effect of nitroglycerin on peripheral large arteries in hypertension. Br J Clin Pharmacol. 1982;14:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pierpont GL, Brown DC, Franciosa JA, Cohn JN. Effect of hydralazine on renal failure in patients with congestive heart failure. Circulation. 1980;61:323–327. [DOI] [PubMed] [Google Scholar]
- 44. Francis GS, Cohn JN, Johnson G, Rector TS, Goldman S, Simon A. Plasma norepinephrine, plasma renin activity, and congestive heart failure. Relations to survival and the effects of therapy in V‐HeFT II. The V‐HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI40–VI48. [PubMed] [Google Scholar]
- 45. Parker LM, Damanhuri HA, Fletcher SP, Goodchild AK. Hydralazine administration activates sympathetic preganglionic neurons whose activity mobilizes glucose and increases cardiovascular function. Brain Res. 2015;1604:25–34. [DOI] [PubMed] [Google Scholar]
- 46. Levick SP, Murray DB, Janicki JS, Brower GL. Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension. 2010;55:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Perlini S, Palladini G, Ferrero I, Tozzi R, Fallarini S, Facoetti A, Nano R, Clari F, Busca G, Fogari R, Ferrari AU. Sympathectomy or doxazosin, but not propranolol, blunt myocardial interstitial fibrosis in pressure‐overload hypertrophy. Hypertension. 2005;46:1213–1218. [DOI] [PubMed] [Google Scholar]
- 48. Maekawa K, Liang CS, Tsui A, Chen BT, Kawashima S. Vasodilative effect of hydralazine in awake dogs: the roles of prostaglandins and the sympathetic nervous system. Circulation. 1984;70:908–916. [DOI] [PubMed] [Google Scholar]
- 49. Everett RJ, Stirrat CG, Semple SI, Newby DE, Dweck MR, Mirsadraee S. Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol. 2016;71:768–778. [DOI] [PubMed] [Google Scholar]
- 50. Schelbert EB, Moon JC. Exploiting differences in myocardial compartments with native T1 and extracellular volume fraction for the diagnosis of hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2015;8:e004232 [DOI] [PubMed] [Google Scholar]
- 51. Taylor AJ, Salerno M, Dharmakumar R, Jerosch‐Herold M. T1 mapping: basic techniques and clinical applications. JACC Cardiovasc Imaging. 2016;9:67–81. [DOI] [PubMed] [Google Scholar]
- 52. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E; NHLBI Heart Failure Clinical Research Network . Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double‐blind, placebo‐controlled study. Hypertension. 2015;65:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Omar SA, Fok H, Tilgner KD, Nair A, Hunt J, Jiang B, Taylor P, Chowienczyk P, Webb AJ. Paradoxical normoxia‐dependent selective actions of inorganic nitrite in human muscular conduit arteries and related selective actions on central blood pressures. Circulation. 2015;131:381–389; discussion 389 [DOI] [PubMed] [Google Scholar]
- 55. Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eggebeen J, Kim‐Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2016;4:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zamani P, Rawat D, Shiva‐Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380; discussion 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gul RB, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. J Clin Nurs. 2010;19:227–233. [DOI] [PubMed] [Google Scholar]
- 59. Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. [DOI] [PubMed] [Google Scholar]
- 60. Redfield MM, Velazquez EJ, Braunwald E. Nitrates in heart failure with preserved ejection fraction. N Engl J Med. 2016;374:1589. [DOI] [PubMed] [Google Scholar]
- 61. Kalinowski L, Dobrucki IT, Malinski T. Race‐specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–2517. [DOI] [PubMed] [Google Scholar]
- 62. Cardillo C, Kilcoyne CM, Cannon RO III, Panza JA. Attenuation of cyclic nucleotide‐mediated smooth muscle relaxation in blacks as a cause of racial differences in vasodilator function. Circulation. 1999;99:90–95. [DOI] [PubMed] [Google Scholar]
- 63. Kahn DF, Duffy SJ, Tomasian D, Holbrook M, Rescorl L, Russell J, Gokce N, Loscalzo J, Vita JA. Effects of black race on forearm resistance vessel function. Hypertension. 2002;40:195–201. [DOI] [PubMed] [Google Scholar]
- 64. Androne AS, Hryniewicz K, Hudaihed A, Dimayuga C, Yasskiy A, Qureshi G, Katz SD. Comparison of metabolic vasodilation in response to exercise and ischemia and endothelium‐dependent flow‐mediated dilation in African‐American versus non‐African‐American patients with chronic heart failure. Am J Cardiol. 2006;97:685–689. [DOI] [PubMed] [Google Scholar]


