Abstract
Background
Soluble suppression of tumorigenicity 2 (sST2) receptor is a biomarker that is elevated in certain systemic inflammatory diseases. Comorbidity‐driven microvascular inflammation is postulated to play a key role in heart failure with preserved ejection fraction (HFpEF) pathophysiology, but data on how sST2 relates to clinical characteristics or inflammatory conditions or biomarkers in HFpEF are limited. We sought to determine circulating levels and clinical correlates of sST2 in HFpEF.
Methods and Results
At enrollment, patients (n=174) from the Phosphodiesterase‐5 Inhibition to Improve Clinical Status And Exercise Capacity in Diastolic Heart Failure (RELAX) trial of sildenafil in HFpEF had sST2 levels measured. Clinical characteristics; cardiac structure and function; exercise performance; and biomarkers of neurohumoral activation, systemic inflammation and fibrosis, and myocardial necrosis were assessed in relation to sST2 levels. Median sST2 levels in male and female HFpEF patients were 36.7 ng/mL (range 30.9–49.2 ng/mL; reference range 4–31 ng/mL) and 30.8 ng/mL (range 25.3–39.3 ng/mL; reference range 2–21 ng/mL), respectively. Among HFpEF patients, higher sST2 levels were associated with the presence of diabetes mellitus; atrial fibrillation; renal dysfunction; right ventricular pressure overload and dysfunction; systemic congestion; exercise intolerance; and biomarkers of systemic inflammation and fibrosis, neurohumoral activation, and myocardial necrosis (P<0.05 for all). sST2 was not associated with left ventricular structure or left ventricular systolic or diastolic function.
Conclusions
In HFpEF, sST2 levels were associated with proinflammatory comorbidities, right ventricular pressure overload and dysfunction, and systemic congestion but not with left ventricular geometry or function. These data suggest that ST2 may be a marker of systemic inflammation in HFpEF and potentially of extracardiac origin.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00763867.
Keywords: biomarker, diastolic heart failure
Subject Categories: Heart Failure, Biomarkers, Clinical Studies
Introduction
The suppression of tumorigenicity 2 (ST2) receptor is a member of the interleukin‐1 family of receptors and exists as transmembrane (ST2L) and soluble (sST2) isoforms differentially regulated by alternative splicing.1, 2 Interleukin‐33 (IL‐33) binds to ST2L to elicit downstream signaling, whereas sST2 acts as a nonfunctional decoy IL‐33 receptor, limiting IL‐33/ST2L signaling.3 In patients with autoimmune inflammatory conditions, sST2 levels are elevated; in related experimental models, IL‐33/ST2L signaling is proinflammatory and exacerbates clinical severity, whereas sST2 administration abrogates IL‐33/ST2L signaling and is protective.3, 4 In contrast, in cardiomyocytes and experimental heart failure (HF), IL‐33/ST2L signaling is activated with cardiomyocyte/cardiac mechanical stress and neurohumoral activation but exerts protective cardiac antiremodeling effects. In these models, sST2 administration worsens the cardiac phenotype.4, 5
Although elevated sST2 levels have been repeatedly shown to be associated with worse outcomes in HF with reduced ejection fraction (HFrEF),6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 sST2 appears to be of extracardiac origin in HFrEF.17, 18, 19, 20 To date, sST2 has not been well studied in HF with preserved ejection fraction (HFpEF). In HFpEF, comorbidity‐driven microvascular endothelial inflammation may play a key role in the genesis of myocardial, vascular, skeletal muscle, and other end‐organ damage,21, 22, 23, 24, 25 and IL‐33/ST2 signaling appears to be involved in vascular endothelial cell inflammation and remodeling.17, 26, 27 Furthermore, once established, HF may itself perpetuate inflammation in proportion to the severity of systemic congestion and hypoperfusion.28, 29 Thus, sST2 may be elevated in HFpEF caused by cardiomyocyte stress, comorbidity‐driven systemic inflammation, or inflammation related to the severity of HF. Accordingly, in the comprehensively characterized HFpEF patients enrolled in the Phosphodiesterase‐5 Inhibition to Improve Clinical Status And Exercise Capacity in Diastolic Heart Failure (RELAX) trial,30 we determined whether circulating sST2 (henceforth ST2) levels were associated with the severity of cardiac remodeling and dysfunction, proinflammatory comorbidities, or markers of clinical HF severity.
Methods
The RELAX trial was conducted by the Heart Failure Clinical Research Network (HFN) and funded by the National Heart, Lung, and Blood Institute.30 All patients provided written informed consent, and the trial was approved by the institutional review board at each participating site.
The design, entry criteria, and results of the RELAX trial were reported previously.30, 31 Briefly, RELAX enrolled 216 outpatients who had ejection fraction ≥50% and objective evidence of HF. In addition, patients were required to have elevated N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP; ≥400 pg/mL) or elevated invasively measured filling pressures and reduced exercise capacity (≤60% age, sex, and body size–specific predicted peak oxygen consumption). Patients with an estimated glomerular filtration rate (Modification of Diet in Renal Disease equation) <20 mL/min per 1.73 m2 were ineligible.
Participants underwent baseline studies that included a history and physical examination, echocardiography, cardiac magnetic resonance imaging if in sinus rhythm (n=115), cardiopulmonary exercise test, 6‐minute walk test, Minnesota Living with Heart Failure Questionnaire, and phlebotomy for biomarker measurements.31
Comprehensive Doppler echocardiography and cardiac magnetic resonance imaging were performed according to study protocols32 with measurements performed at the HFN echocardiography (Mayo Clinic, Rochester, MN) and cardiac magnetic resonance imaging (Duke University, Durham, NC) core laboratories. The cardiopulmonary exercise test was performed according to a RELAX‐specific protocol and interpreted by the HFN cardiopulmonary exercise test core laboratory (Massachusetts General Hospital, Boston, MA), as reported previously.31 Biomarker measurements were performed by the HFN biomarker core laboratory (University of Vermont, Burlington, VT), as described previously30, 31 and included NT‐proBNP, aldosterone, endothelin‐1, cystatin‐C, creatinine, uric acid, pro–collagen III N‐terminal peptide, C‐telopeptide for type I collagen, high‐sensitivity troponin I, and high‐sensitivity C‐reactive protein. ST2 levels were measured on banked samples by a high‐sensitivity sandwich monoclonal immunoassay (Presage® ST2 assay; Critical Diagnostics). The normal values for ST2 are somewhat controversial, but in a carefully screened healthy Austrian cohort with normal inflammatory markers (C‐reactive protein, procalcitonin, and interleukin‐6) and normal BNP, sex‐specific normal ranges were 4 to 31 ng/mL for male participants and 2 to 21 ng/mL for female participants using the Presage® ST2 assay.33
Statistical Analysis
Data are presented as median (25th–75th percentiles) or number (percentage) across ST2 tertiles. Differences across ST2 tertiles were tested with Kruskal–Wallis, χ2, or Fisher exact tests, as appropriate. In healthy persons, ST2 levels are higher in men than in women but are not associated with age, body mass index, or renal function.33, 34 Thus, multivariable least squares linear or logistic regression was used to assess the relationship between variables of interest and ST2 as a categorical variable (tertiles) adjusting for sex. With our sample size, we had 84% and 92% power to detect correlations of 0.22 and 0.25, respectively, between ST2 and other continuous measures. The associations between ST2 (log transformed) and other biomarkers (log transformed) are presented with Pearson correlation coefficients and were tested using linear regression models without and with sex adjustment. Analyses were performed by the HFN data coordinating center using SAS version 9.4 (SAS Institute). A P<0.05 (2‐sided) was considered statistically significant.
Results
Of the 216 participants enrolled in RELAX, stored serum from 174 patients was available to measure ST2 at enrollment. Included participants had a median age of 69 years, and 52% were men. Participants without stored serum availability were more likely to have had a HF hospitalization, worse renal function, and higher NT‐proBNP levels (Table S1).
The median ST2 levels were 34.3 ng/mL (25th–75th percentiles 26.9–46.6 ng/mL) with a range from 14.4 to 197.9 ng/mL and higher levels in male participants (median 36.7 ng/mL [25th–75th percentiles 30.9–49.2 ng/mL]) than in female participants (median 30.8 ng/mL [25th–75th percentiles 25.3–39.3 ng/mL]) (Figure 1, Table 1). Accordingly, all results described in the following sections were adjusted for sex.
Figure 1.
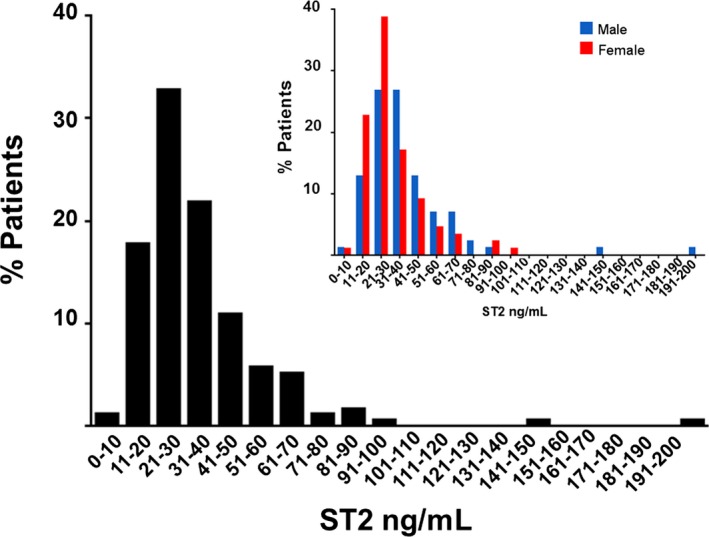
Frequency distribution of suppression of tumorigenicity 2 (ST2) levels in heart failure with preserved ejection fraction overall and by sex (insert). The distribution of baseline ST2 levels in the RELAX trial cohort (n=174). Overall, median ST2 levels were 34.3 ng/mL (25th–75th percentiles 26.9–46.6 ng/mL) and were higher in male participants (36.7 ng/mL [25th–75th percentiles 30.9–49.2 ng/mL]) than in female participants (30.8 ng/mL [25th–75th percentiles 25.3–39.3 ng/mL]).
Table 1.
Baseline Patient Characteristics by Tertiles of Baseline ST2 Levels
| Low ST2 Tertile (n=58) | Mid ST2 Tertile (n=58) | High ST2 Tertile (n=58) | P Value | P Valuea | |
|---|---|---|---|---|---|
| ST2 range, ng/mL | <29.5 | 29.5–38.5 | >38.5 | … | … |
| ST2, ng/mL | 23.8 (21.7–26.9) | 34.3 (31.6–36.5) | 51.1 (46.6–66.4) | … | … |
| Male | 18 (31) | 32 (55) | 36 (62) | 0.002 | … |
| Age, y | 67 (61–74) | 71 (64–79) | 69 (63–77) | 0.16 | 0.30 |
| Body mass index, kg/m2 | 32.9 (28.6–36.9) | 32.5 (28.0–38.8) | 33.8 (28.6–40.1) | 0.54 | 0.70 |
| Body surface area, m2 | 2.13 (1.95–2.23) | 2.07 (1.89–2.39) | 2.12 (1.95–2.29) | 0.80 | 0.53 |
| HF hospitalization | 18 (31) | 16 (28) | 21 (36) | 0.60 | 0.63 |
| Comorbidities | |||||
| Hypertension | 44 (76) | 56 (97) | 46 (79) | 0.005 | 0.023 |
| Ischemic heart disease | 20 (34) | 20 (34) | 27 (47) | 0.30 | 0.50 |
| Atrial fibrillation | 21 (36) | 30 (52) | 34 (59) | 0.047 | 0.049 |
| COPD | 8 (14) | 11 (19) | 13 (22) | 0.48 | 0.77 |
| Diabetes mellitus | 17 (29) | 19 (33) | 35 (60) | 0.001 | 0.005 |
| Creatinine, mg/dL (n=172) | 1.0 (0.8–1.3) | 1.0 (0.9–1.2) | 1.2 (0.9–1.7) | 0.016 | 0.018 |
| Cystatin‐C, mg/L | 1.15 (0.92–1.46) | 1.21 (1.08–1.47) | 1.58 (1.13–2.15) | 0.0003 | <0.0001 |
| Medications | |||||
| ACEI or ARB | 40 (69) | 41 (71) | 35 (60) | 0.45 | 0.36 |
| Aldosterone antagonist | 5 (9) | 6 (10) | 8 (14) | 0.66 | 0.62 |
| Beta blocker | 40 (69) | 42 (72) | 45 (78) | 0.57 | 0.80 |
| Loop diuretic | 36 (62) | 43 (74) | 51 (88) | 0.006 | 0.013 |
| Congestion and quality of life | |||||
| NT‐proBNP, pg/mL (n=173) | 382 (94–656) | 696 (356–1621) | 955 (497–1802) | <0.0001 | <0.0001 |
| Elevated JVP (n=168) | 16 (28) | 24 (44) | 33 (59) | 0.004 | 0.003 |
| Moderate or severe edema | 5 (9) | 8 (14) | 22 (38) | 0.0006 | 0.0006 |
| NYHA class II | 35 (60) | 26 (45) | 24 (41) | 0.09 | 0.029 |
| MLHFQ score (n=166) | 37 (30–63) | 49 (35–65) | 44 (25–60) | 0.16 | 0.17 |
Values are median (interquartile range) or n (%). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; HF, heart failure; JVP, jugular venous pressure; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; ST2, suppression of tumorigenicity 2.
Adjusted for sex.
Clinical Characteristics and ST2 Levels in HFpEF
There was no association between ST2 levels and age, body size, or history of HF hospitalization (Table 1). However, patients with higher ST2 levels were more likely to have diabetes mellitus (P=0.005), hypertension (P=0.023), atrial fibrillation or flutter (P=0.049), and renal dysfunction (P<0.0001) and were more likely to be treated with diuretics (P=0.013). Lung disease prevalence was similar across ST2 tertiles. Patients with higher ST2 levels also had more congestion with higher NT‐proBNP levels (P<0.0001), a higher prevalence of jugular venous pressure elevation (P=0.003), more peripheral edema (P=0.0006), and worse New York Heart Association functional class (P=0.029), although ST2 levels were not associated with the Minnesota Living with Heart Failure Questionnaire score (Table 1).
Exercise Performance and ST2 Levels in HFpEF
Six‐minute walk distance (P=0.012), peak oxygen consumption (P=0.017), percentage of predicted peak oxygen consumption (P=0.017), and peak systolic blood pressure (P=0.005) all declined with increasing ST2 levels (Table 2). There were nonsignificant trends toward lower peak heart rate (P=0.07) and chronotropic index (P=0.08) despite similar effort, as reflected in the respiratory exchange ratio. There was no association between ST2 levels and ventilatory efficiency (VE/VCO2 slope).
Table 2.
Exercise Performance by Tertile of Baseline ST2 Levels
| Low ST2 Tertile (n=58) | Mid ST2 Tertile (n=58) | High ST2 Tertile (n=58) | P Value | P Valuea | |
|---|---|---|---|---|---|
| Peak VO2, mL/kg per minute | 12.1 (10.9–14.4) | 11.9 (10.8–15.1) | 11.2 (10.0–13.3) | 0.12 | 0.017 |
| Peak VO2, % predicted | 44 (38–51) | 42 (35–48) | 39 (32–47) | 0.024 | 0.017 |
| Respiratory exchange ratio | 1.11 (1.05–1.17) | 1.09 (1.03–1.15) | 1.09 (1.02–1.15) | 0.41 | 0.41 |
| Peak systolic BP, mm Hg | 160 (140–184) | 154 (138–168) | 148 (126–168) | 0.07 | 0.005 |
| Rest HR, bpm | 67 (59–76) | 70 (60–75) | 69 (61–79) | 0.58 | 0.43 |
| Peak HR, bpm | 111 (96–136) | 107 (91–120) | 107 (91–128) | 0.12 | 0.07 |
| Chronotropic index | 0.55 (0.38–0.71) | 0.47 (0.29–0.62) | 0.49 (0.28–0.69) | 0.15 | 0.08 |
| VE/VCO2 slope | 31.7 (28.1–36.9) | 33.8 (28.6–37.5) | 34.9 (31.0–39.9) | 0.06 | 0.20 |
| 6‐minute walk distance, m | 351 (265–434) | 305 (244–372) | 311 (230–360) | 0.046 | 0.012 |
Total sample size with data (N=168–174). BP indicates blood pressure; bpm, beats per minute; HR, heart rate; ST2, suppression of tumorigenicity 2; VE/VCO2, ventilatory efficiency; VO2, oxygen consumption.
Adjusted for sex.
Cardiac Structure and Function and ST2 Levels in HFpEF
There were no associations between ST2 levels and left ventricular (LV) diastolic or systolic function parameters or LV geometry as assessed by echocardiography (Table 3) or in patients in sinus rhythm, by cardiac magnetic resonance (Table S2). Participants with higher ST2 levels had higher right ventricular (RV) systolic pressure (P=0.016) and worse RV function (lower tricuspid annular plane systolic excursion, P=0.015) by echocardiography (Table 3). In contrast, NT‐proBNP levels were strongly associated with all diastolic function parameters, LV geometry, and RV load and systolic function but not with LV ejection fraction (Table S3).
Table 3.
Baseline Cardiac Structure and Function by Tertiles of Baseline ST2 Levels
| Na | Low ST2 Tertile (n=58) | Mid ST2 Tertile (n=58) | High ST2 Tertile (n=58) | P Value | P Valueb | |
|---|---|---|---|---|---|---|
| Diastolic function parameters | ||||||
| E/A ratio | 118 | 1.4 (1.0–1.9) | 1.3 (0.9–3.0) | 1.6 (1.0–3.0) | 0.70 | 0.68 |
| Medial e′, m/s | 160 | 0.06 (0.04–0.07) | 0.07 (0.05–0.08) | 0.06 (0.05–0.08) | 0.56 | 0.77 |
| Medial E/e′ | 155 | 14.9 (11.3–22.0) | 15.0 (10.0–20.0) | 17.9 (13.1–24.5) | 0.17 | 0.14 |
| Deceleration time, ms | 159 | 192 (159–215) | 180 (158–219) | 181 (151–219) | 0.86 | 0.97 |
| LA volume/BSA, mL/m2 | 123 | 41 (33–50) | 47 (34–62) | 51 (35–62) | 0.08 | 0.16 |
| Left ventricular systolic function and geometry | ||||||
| Ejection fraction, % | 173 | 61 (57–66) | 61 (56–66) | 60 (55–63) | 0.29 | 0.47 |
| LVEDd/BSA, cm/m2 | 133 | 2.3 (2.1–2.5) | 2.2 (2.0–2.4) | 2.2 (2.0–2.5) | 0.58 | 0.91 |
| Relative wall thickness | 128 | 0.38 (0.34–0.44) | 0.42 (0.36–0.52) | 0.42 (0.37–0.46) | 0.06 | 0.10 |
| LV mass/BSA, g/m2 | 128 | 76 (64–85) | 72 (61–89) | 80 (60–100) | 0.98 | 0.60 |
| Right ventricular load and function | ||||||
| PASP, mm Hg | 113 | 39 (32–48) | 46 (34–58) | 43 (32–51) | 0.045 | 0.016 |
| TAPSE, mm | 172 | 19.0 (16.0–23.0) | 17.5 (14.0–24.0) | 16.0 (13.0–20.0) | 0.013 | 0.015 |
| Vascular function | ||||||
| Systolic BP, mm Hg | 174 | 128 (114–140) | 123 (113–137) | 124 (112–131) | 0.44 | 0.25 |
| Diastolic BP, mm Hg | 174 | 70 (64–80) | 70 (62–78) | 69 (62–78) | 0.66 | 0.50 |
| Ao distensibility, 10−3 mm Hg−1 | 68 | 1.21 (0.67–1.46) | 1.08 (0.58–2.25) | 1.09 (0.67–1.76) | 0.77 | 0.50 |
Data are median (interquartile range). Ao indicates aortic; BP, blood pressure; BSA, body surface area; LA, left atrial; LV, left ventricular; LVEDd, left ventricular end‐diastolic dimension; PASP, pulmonary artery systolic pressure; ST2, suppression of tumorigenicity 2; TAPSE, tricuspid annular plane systolic excursion.
Total sample with data.
Adjusted for sex.
ST2 and Other Biomarkers in HFpEF
ST2 concentrations were correlated with endothelin‐1 (r=0.33, P<0.0001) and with biomarkers of systemic inflammation (high‐sensitivity C‐reactive protein, r=0.22, P=0.002), fibrosis (C‐telopeptide for type I collagen, r=0.30, P=0.0004), and myocardial necrosis (high‐sensitivity troponin‐I, r=0.33, P<0.0001) but not with aldosterone or pro–collagen III N‐terminal peptide (Figure 2).
Figure 2.
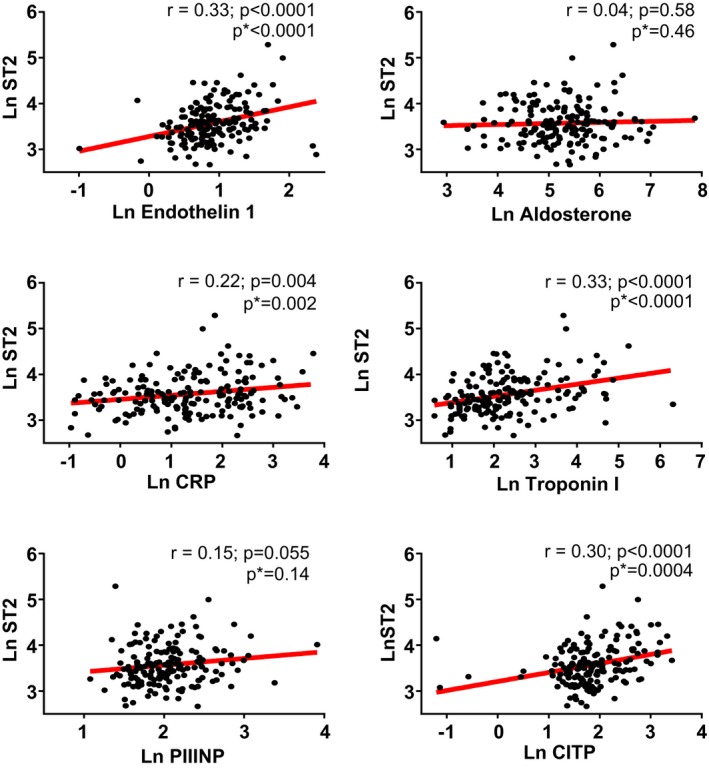
The relationship between suppression of tumorigenicity 2 (ST2) and biomarkers in heart failure with preserved ejection fraction. ST2 was associated with endothelin 1, high‐sensitivity C‐reactive protein (CRP), C‐telopeptide for type I collagen (CITP), and troponin I but not aldosterone or pro–collagen III N‐terminal peptide (PIIINP) levels. *Adjusted for sex. Ln indicates log transformed.
Discussion
In this comprehensively phenotyped cohort of patients with HFpEF, ST2 levels were elevated compared with published normative sex‐specific values. ST2 was higher in the presence of several proinflammatory comorbidities (diabetes mellitus, atrial fibrillation, renal dysfunction) and associated with greater RV pressure overload and dysfunction; central venous congestion; exercise intolerance; and biomarkers reflective of systemic inflammation and fibrosis, neurohumoral activation, and myocardial necrosis. ST2 levels were not associated with LV geometry or LV systolic or diastolic function. In contrast, NT‐proBNP—a biomarker of myocardial origin, the production of which is stimulated by wall stress—correlated with the severity of LV remodeling and diastolic dysfunction. These data add to our understanding of ST2 in HFpEF and suggest that in HFpEF, ST2 is predominately a marker of systemic inflammation activated by interplay between proinflammatory comorbidities and HF severity–related systemic congestion.
Association of ST2 Levels With Sex
Consistent with findings in other cohorts,33, 34, 35 male RELAX participants had higher ST2 levels than their female counterparts. Although the mechanism of sex differences in ST2 levels remains unclear, the considerable differences of ST2 levels between sexes warrant adjustment for sex when analyzing the association between ST2 levels and disease severity.36
ST2 as a Biomarker in HFpEF
Despite the lower percentage of male participants in RELAX compared with studies of ambulatory HFrEF patients, the median level of ST2 in this HFpEF cohort was higher than that observed in studies of ambulatory HFrEF patients8, 10 and approached the 33–35 ng/mL cut point associated with a higher risk of cardiovascular outcomes in chronic HFrEF.8, 10, 11 Median ST2 levels in RELAX were also higher than those reported in patients with ischemic heart disease (19–24 ng/mL)37, 38 and in the general population (22 ng/mL)34 but lower than those reported in acute HF or pulmonary disease (42–76 ng/mL)15, 33, 39 and much lower than observed in critically ill intensive care unit patients (555–745 ng/mL).33, 40 Importantly, all of these studies used the same Presage® ST2 assay. Although relatively few studies have evaluated ST2 levels in HFpEF,6, 18, 35 the only one to assess ST2 using this assay was a post hoc analysis of the PARAMOUNT (Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction) trial that described elevated median ST2 levels (33 ng/mL), similar to the HFpEF patients in RELAX.35
Association of ST2 Levels With Clinical Characteristics
The associations we observed between ST2 levels and comorbidities including diabetes mellitus, renal dysfunction, and atrial fibrillation as well as congestion, diuretic use, and New York Heart Association functional status have also been reported in studies of ambulatory patients with HFrEF8, 10, 11 and the PARAMOUNT HFpEF cohort.35 Diabetes mellitus, renal dysfunction, atrial fibrillation, and chronic venous congestion are all linked to inflammation.41 It is perhaps surprising that obesity was not associated with ST2 levels in this cohort or in the PARAMOUNT HFpEF cohort,35 given a previous study showing higher ST2 levels in severe obesity and the known proinflammatory effect of obesity.42 However, the intensity and types of inflammation linked to activation of ST2 are not well defined.33 It is important to note that ST2 levels are not associated with renal function in the general population34 or elevated in a small sample of chronic kidney disease patients without HF,33 suggesting a unique interaction among HF, renal dysfunction, and ST2 levels.
Association of ST2 Levels With Functional Capacity
In HFpEF, as in HFrEF, ST2 levels were associated with worse exercise capacity. Because skeletal muscle function is a potent determinant of peak oxygen consumption in HFpEF,25 this association may reflect effects of systemic inflammation on skeletal muscle or peripheral vascular function, given the lack of association of ST2 and resting LV structure and function.
Association of ST2 Levels With Cardiac Structure and Function
Although an association between ST2 and ventricular dysfunction was demonstrated in animal studies,4 a relationship between circulating ST2 levels and the severity of cardiac remodeling or dysfunction in clinical HF is less clear.8, 35, 43, 44, 45
In general population cohorts with a spectrum of LV geometry and function, ST2 levels were not associated with LV mass46 or ejection fraction47, 48 but were associated with a greater likelihood of asymptomatic diastolic dysfunction in one study.47 In a diverse cohort of patients evaluated in an emergency department for dyspnea and subsequently found to have HFrEF, HFpEF, or noncardiac dyspnea, ST2 levels were higher in HF than noncardiac dyspnea and, across the combined HF groups, were associated with LV ejection fraction, RV systolic dysfunction, higher pulmonary artery pressures, and more severe tricuspid regurgitation but not with LV mass or diastolic function.43 In another study of patients with acute dyspnea and normal ejection fraction (most with noncardiac dyspnea), ST2 was not associated with LV mass or most Doppler measures of diastolic function.44 ST2 levels were not meaningfully associated with LV remodeling in HFrEF.8 The PARAMOUNT analysis in HFpEF found a modest association of ST2 levels with left atrial volumes but no other parameter of cardiac structure or function, although RV indices were not assessed.35
In the RELAX HFpEF cohort, we found no association between LV structure or function and ST2 levels, whereas NT‐proBNP (of myocardial origin20) was strongly associated with cardiac structure and function. The association of ST2 levels with RV function observed in this study may be related to the impact of RV dysfunction on systemic venous congestion and its known proinflammatory effects.29
Mechanism of ST2 Activation in HFpEF
We did not measure the transcardiac ST2 gradient, and thus the systemic versus cardiac source of elevated levels of ST2 in HFpEF cannot be established in our study. The lack of a relationship between ST2 and LV structure and function and the associations between ST2 and proinflammatory comorbidities, severity of congestion, and biomarkers reflective of systemic inflammation and fibrosis (high‐sensitivity C‐reactive protein and C‐telopeptide for type I collagen) and myocardial injury or inflammation (high‐sensitivity troponin I) may implicate an extracardiac source of ST2 in HFpEF, as has been established in HFrEF.17, 18, 19, 20 Indeed, human saphenous venous and arterial endothelial cells exposed to inflammatory stimuli have been shown to secrete ST2,17, 18, 49 and we speculate that that activation of ST2 reflects systemic inflammation in HFpEF. Whether the inflammatory stimulus putatively responsible for ST2 activation is mediated by comorbidity‐driven microvascular endothelial inflammation or the systemic congestion and end‐organ hypoperfusion common to advanced HFpEF or HFrEF is deserving of further study.
Limitations
Given the post hoc nature of this study and its modest sample size, this analysis was deemed to be exploratory with the aim of generating hypotheses that could be tested in larger mechanistic HFpEF studies. The RELAX cohort had relatively advanced HFpEF,30, 50 and this may limit correlations, although the range of ST2 levels was fairly broad. Those patients missing stored serum appeared to have more severe HF; some associations may have been stronger if the entire cohort had available serum. The association of ST2 levels and outcomes was not assessed in RELAX, given the short duration of follow‐up.
Conclusion
In HFpEF, ST2 levels were elevated and associated with proinflammatory comorbidities; RV pressure overload and dysfunction; systemic congestion; exercise intolerance; and biomarkers reflective of systemic inflammation and fibrosis, neurohumoral activation, and myocardial injury but not with LV structure and function. These data add to our understanding of ST2 as a biomarker in HFpEF and suggest that systemic inflammation may mediate or contribute to activation of ST2 in HFpEF.
Sources of Funding
This study was supported by grants from the National Heart, Lung, and Blood Institute: U01HL084904 (for the data coordinating center), and U01HL084861, U01HL084875, U01HL084877, U01HL084889, U01HL084890, U01HL084891, U01HL084899, U01HL084907, and U01HL084931 (for the clinical centers). This study was not supported by industry.
Disclosures
Dr Felker consults for Singulex, Roche Diagnostics, and receives grant support from the National Institutes of Health and Roche Diagnostics. Dr Chen reports that he and Mayo Clinic have patented and licensed designer natriuretic peptides to Anexon Inc and Capricor Therapeutics. Dr Chen is the cofounder of Zumbro Discovery. Dr Braunwald receives grant support to his institution from Duke University for his role as chair of the National Heart, Lung, and Blood Institute–sponsored Heart Failure Network. All others report no disclosures relevant to this manuscript.
Supporting information
Table S1. Characteristics of Patients With or Without Stored Serum for Suppression of Tumorigenicity 2 Levels
Table S2. Association of Suppression of Tumorigenicity 2 With Left Ventricular Systolic Function and Geometry by Cardiac Magnetic Resonance Imaging
Table S3. Association of N‐Terminal Pro‐B‐Type Natriuretic Peptide Levels With Cardiac Structure and Function
(J Am Heart Assoc. 2017;6:e004382. DOI: 10.1161/JAHA.116.004382.)
References
- 1. Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos‐responsive gene Fit‐1 generates mRNA isoforms coding for either secreted or membrane‐bound proteins related to the IL‐1 receptor. EMBO J. 1994;13:1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pascual‐Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. 2015;115:3B–7B. [DOI] [PubMed] [Google Scholar]
- 3. Kakkar R, Lee RT. The IL‐33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL‐33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin‐1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manzano‐Fernandez S, Mueller T, Pascual‐Figal D, Truong QA, Januzzi JL. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol. 2011;107:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Broch K, Ueland T, Nymo SH, Kjekshus J, Hulthe J, Muntendam P, McMurray JJ, Wikstrand J, Cleland JG, Aukrust P, Gullestad L. Soluble ST2 is associated with adverse outcome in patients with heart failure of ischaemic aetiology. Eur J Heart Fail. 2012;14:268–277. [DOI] [PubMed] [Google Scholar]
- 8. Anand IS, Rector TS, Kuskowski M, Snider J, Cohn JN. Prognostic value of soluble ST2 in the Valsartan Heart Failure Trial. Circ Heart Fail. 2014;7:418–426. [DOI] [PubMed] [Google Scholar]
- 9. Rehman SU, Mueller T, Januzzi JL Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–1465. [DOI] [PubMed] [Google Scholar]
- 10. Felker GM, Fiuzat M, Thompson V, Shaw LK, Neely ML, Adams KF, Whellan DJ, Donahue MP, Ahmad T, Kitzman DW, Piña IL, Zannad F, Kraus WE, O'Connor CM. Soluble ST2 in ambulatory patients with heart failure: association with functional capacity and long‐term outcomes. Circ Heart Fail. 2013;6:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries DL, Tang WH, Wu AH, Fang JC, Boxer R, Sweitzer NK, Levy WC, Goldberg LR, Jessup M, Cappola TP. High‐sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bayes‐Genis A, de Antonio M, Galan A, Sanz H, Urrutia A, Cabanes R, Cano L, González B, Díez C, Pascual T, Elosúa R, Lupón J. Combined use of high‐sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Eur J Heart Fail. 2012;14:32–38. [DOI] [PubMed] [Google Scholar]
- 13. Boisot S, Beede J, Isakson S, Chiu A, Clopton P, Januzzi J, Maisel AS, Fitzgerald RL. Serial sampling of ST2 predicts 90‐day mortality following destabilized heart failure. J Card Fail. 2008;14:732–738. [DOI] [PubMed] [Google Scholar]
- 14. Maisel A, Xue Y, van Veldhuisen DJ, Voors AA, Jaarsma T, Pang PS, Butler J, Pitt B, Clopton P, de Boer RA. Effect of spironolactone on 30‐day death and heart failure rehospitalization (from the COACH Study). Am J Cardiol. 2014;114:737–742. [DOI] [PubMed] [Google Scholar]
- 15. Januzzi JL Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, O'Donoghue M, Sakhuja R, Chen AA, van Kimmenade RR, Lewandrowski KB, Lloyd‐Jones DM, Wu AH. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro‐Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–613. [DOI] [PubMed] [Google Scholar]
- 16. Pascual‐Figal DA, Manzano‐Fernandez S, Boronat M, Casas T, Garrido IP, Bonaque JC, Pastor‐Perez F, Valdés M, Januzzi JL. Soluble ST2, high‐sensitivity troponin T‐ and N‐terminal pro‐B‐type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13:718–725. [DOI] [PubMed] [Google Scholar]
- 17. Chen WY, Hong J, Gannon J, Kakkar R, Lee RT. Myocardial pressure overload induces systemic inflammation through endothelial cell IL‐33. Proc Natl Acad Sci USA. 2015;112:7249–7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartunek J, Delrue L, Van Durme F, Muller O, Casselman F, De Wiest B, Croes R, Verstreken S, Goethals M, de Raedt H, Sarma J, Joseph L, Vanderheyden M, Weinberg EO. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol. 2008;52:2166–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Truong QA, Januzzi JL, Szymonifka J, Thai WE, Wai B, Lavender Z, Sharma U, Sandoval RM, Grunau ZS, Basnet S, Babatunde A, Ajijola OA, Min JK, Singh JP. Coronary sinus biomarker sampling compared to peripheral venous blood for predicting outcomes in patients with severe heart failure undergoing cardiac resynchronization therapy: the BIOCRT study. Heart Rhythm. 2014;11:2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaye DM, Mariani JA, van Empel V, Maeder MT. Determinants and implications of elevated soluble ST2 levels in heart failure. Int J Cardiol. 2014;176:1242–1243. [DOI] [PubMed] [Google Scholar]
- 21. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 22. Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–H1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985). 2015;119:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yndestad A, Marshall AK, Hodgkinson JD, Tham el L, Sugden PH, Clerk A. Modulation of interleukin signalling and gene expression in cardiac myocytes by endothelin‐1. Int J Biochem Cell Biol. 2010;42:263–272. [DOI] [PubMed] [Google Scholar]
- 27. Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL‐33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paulus WJ. Cytokines and heart failure. Heart Fail Monit. 2000;1:50–56. [PubMed] [Google Scholar]
- 29. Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, Elsner S, Sliziuk V, Scherbakov N, Murín J, Anker SD, Sandek A. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J. 2016;37:1684–1691. [DOI] [PubMed] [Google Scholar]
- 30. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E; RELAX Trial . Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E; Heart Failure Clinical Research Network . PhosphdiesteRasE‐5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale and design. Circ Heart Fail. 2012;5:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 33. Dieplinger B, Januzzi JL Jr, Steinmair M, Gabriel C, Poelz W, Haltmayer M, Mueller T. Analytical and clinical evaluation of a novel high‐sensitivity assay for measurement of soluble ST2 in human plasma—the Presage ST2 assay. Clin Chim Acta. 2009;409:33–40. [DOI] [PubMed] [Google Scholar]
- 34. Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, Cheng S, Fradley MG, Kretschman D, Gao W, O'Connor G, Wang TJ, Januzzi JL. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem. 2012;58:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, Prescott MF, Shi V, Lefkowitz M, McMurray JJ, Solomon SD; Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) Investigators . Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: data from the prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction study. Circ Heart Fail. 2016;9:e002551. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mueller T, Jaffe AS. Soluble ST2—analytical considerations. Am J Cardiol. 2015;115:8B–21B. [DOI] [PubMed] [Google Scholar]
- 37. Dieplinger B, Egger M, Haltmayer M, Kleber ME, Scharnagl H, Silbernagel G, de Boer RA, Maerz W, Mueller T. Increased soluble ST2 predicts long‐term mortality in patients with stable coronary artery disease: results from the Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem. 2014;60:530–540. [DOI] [PubMed] [Google Scholar]
- 38. Kohli P, Bonaca MP, Kakkar R, Kudinova AY, Scirica BM, Sabatine MS, Murphy SA, Braunwald E, Lee RT, Morrow DA. Role of ST2 in non‐ST‐elevation acute coronary syndrome in the MERLIN‐TIMI 36 trial. Clin Chem. 2012;58:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lassus J, Gayat E, Mueller C, Peacock WF, Spinar J, Harjola VP, van Kimmenade R, Pathak A, Mueller T, Disomma S, Metra M, Pascual‐Figal D, Laribi S, Logeart D, Nouira S, Sato N, Potocki M, Parenica J, Collet C, Cohen‐Solal A, Januzzi JL Jr, Mebazaa A; GREAT‐Network . Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168:2186–2194. [DOI] [PubMed] [Google Scholar]
- 40. Bajwa EK, Volk JA, Christiani DC, Harris RS, Matthay MA, Thompson BT, Januzzi JL; National Heart, Lung and Blood Institute Acute Respiratory Distress Syndrome Network . Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity‐2 concentrations in acute respiratory distress syndrome. Crit Care Med. 2013;41:2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luscher TF. Heart failure and comorbidities: renal failure, diabetes, atrial fibrillation, and inflammation. Eur Heart J. 2015;36:1415–1417. [DOI] [PubMed] [Google Scholar]
- 42. Zeyda M, Wernly B, Demyanets S, Kaun C, Hämmerle M, Hantusch B, Schranz M, Neuhofer A, Itariu BK, Keck M, Prager G, Wojta J, Stulnig TM. Severe obesity increases adipose tissue expression of interleukin‐33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes (Lond). 2013;37:658–665. [DOI] [PubMed] [Google Scholar]
- 43. Shah RV, Chen‐Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Serum levels of the interleukin‐1 receptor family member ST2, cardiac structure and function, and long‐term mortality in patients with acute dyspnea. Circ Heart Fail. 2009;2:311–319. [DOI] [PubMed] [Google Scholar]
- 44. Shah KB, Kop WJ, Christenson RH, Diercks DB, Henderson S, Hanson K, Li SY, deFilippi CR. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem. 2011;57:874–882. [DOI] [PubMed] [Google Scholar]
- 45. Daniels LB, Clopton P, Iqbal N, Tran K, Maisel AS. Association of ST2 levels with cardiac structure and function and mortality in outpatients. Am Heart J. 2010;160:721–728. [DOI] [PubMed] [Google Scholar]
- 46. Xanthakis V, Larson MG, Wollert KC, Aragam J, Cheng S, Ho J, Coglianese E, Levy D, Colucci WS, Michael Felker G, Benjamin EJ, Januzzi JL, Wang TJ, Vasan RS. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: implications for screening. J Am Heart Assoc. 2013;2:e000399 DOI: 10.1161/JAHA.113.000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seliger SL, Ginsberg E, Gottdiener J, Christenson R, DeFilippi C. Soluble ST2 and galectin‐3 are associated with subclinical diastolic dysfunction in older adults. Paper presented at: American College of Cardiology (ACC) Scientific Sessions; March 29, 2014; Washington, DC. [Google Scholar]
- 48. Chen LQ, de Lemos JA, Das SR, Ayers CR, Rohatgi A. Soluble ST2 is associated with all‐cause and cardiovascular mortality in a population‐based cohort: the Dallas Heart Study. Clin Chem. 2013;59:536–546. [DOI] [PubMed] [Google Scholar]
- 49. Demyanets S, Kaun C, Pentz R, Krychtiuk KA, Rauscher S, Pfaffenberger S, Zuckermann A, Aliabadi A, Gröger M, Maurer G, Huber K, Wojta J. Components of the interleukin‐33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J Mol Cell Cardiol. 2013;60:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, Fiuzat M, Zannad F , Pitt B, O'Connor CM, Lam CS. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol. 2015;65:1668–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Patients With or Without Stored Serum for Suppression of Tumorigenicity 2 Levels
Table S2. Association of Suppression of Tumorigenicity 2 With Left Ventricular Systolic Function and Geometry by Cardiac Magnetic Resonance Imaging
Table S3. Association of N‐Terminal Pro‐B‐Type Natriuretic Peptide Levels With Cardiac Structure and Function


