Abstract
Background
Most cardiovascular diseases occur in low‐ and middle‐income regions of the world, but the socioeconomic distribution within China remains unclear. Our study aims to investigate whether the prevalence of cardiovascular diseases differs among high‐, middle‐, and low‐income regions of China and to explore the reasons for the disparities.
Methods and Results
We enrolled 46 285 individuals from 115 urban and rural communities in 12 provinces across China between 2005 and 2009. We recorded their medical histories of cardiovascular diseases and calculated the INTERHEART Risk Score for the assessment of cardiovascular risk‐factor burden, with higher scores indicating greater burden. The mean INTERHEART Risk Score was higher in high‐ and middle‐income regions than in low‐income regions (9.47, 9.48, and 8.58, respectively, P<0.0001). By contrast, the prevalence of total cardiovascular disease (stroke, ischemic heart disease, and other heart diseases that led to hospitalization) was lower in high‐ and middle‐income regions than in low‐income regions (7.46%, 7.42%, and 8.36%, respectively, P trend=0.0064). In high‐ and middle‐income regions, urban communities have higher INTERHEART Risk Score and higher prevalent rate than rural communities. In low‐income regions, however, the prevalence of total cardiovascular disease was similar between urban and rural areas despite the significantly higher INTERHEART Risk Score for urban settings.
Conclusions
We detected an inverse trend between risk‐factor burden and cardiovascular disease prevalence in urban and rural communities in high‐, middle‐, and low‐income regions of China. Such asymmetry may be attributed to the interregional differences in residents’ awareness, quality of healthcare, and availability and affordability of medical services.
Keywords: cardiovascular disease, prevalence, risk‐factor burden, socioeconomic region, urban and rural
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors
Introduction
Cardiovascular disease is one of the most important public health issues in the world, including China. As estimated by the Global Burden of Disease Study, ischemic heart disease and stroke were the leading causes of death and loss of disability‐adjusted life years worldwide in 2010.1, 2 Although the incidence and mortality of cardiovascular diseases have markedly decreased in several high‐income countries in the past few decades,3, 4 the rates in middle‐ and low‐income countries are still growing rapidly, and these represent nearly 80% of the global burden.5
As one of the middle‐income countries (as classified by the World Bank), China has more than 17 million cardiovascular patients,6 with the age‐standardized cardiac‐caused years of life lost higher than the average level of the world.7 The prevalence and risk‐factor burden of cardiovascular diseases in China need more attention. Because China owns the world's fourth‐largest land area,8 the socioeconomic status of its population is highly diverse among different regions. The gross regional income per capita in some eastern areas such as Beijing and Jiangsu has reached the high‐income level, while western provinces such as Xinjiang and Qinghai remain relatively poor.9 It is necessary to investigate the prevalence of cardiovascular risk factors and cardiovascular diseases in different socioeconomic regions of China. Therefore, the aims of our study are to examine whether cardiovascular disease prevalence differs among high‐ (eastern), middle‐ (central), and low‐income (western) regions of China and to explore the reasons for the disparities.
Previous studies have shown that higher‐income countries had heavier risk‐factor burdens but lower cardiovascular disease rates than lower‐income countries because of their better health services.10 We speculate that different socioeconomic regions of China may exhibit the same patterns, and we explore the correlations between risk factors and cardiovascular diseases by regions.
Methods
Study Design
Our study came from the Prospective Urban and Rural Epidemiological (PURE) Study, which is an international, community‐based cohort study that recruited 153 996 individuals from 17 countries across 5 continents.11, 12 China is 1 of the participating countries; 46 285 Chinese aged 35 to 70 years residing in 115 urban and rural communities in 12 provinces were enrolled from January 1, 2005 to December 31, 2009. Detailed design and methods of the PURE‐China Study have been described elsewhere.13 Briefly, provinces and communities were chosen purposely to maximize economic and sociocultural diversity. For practical reasons, the PURE‐China Study did not aim for a strict proportionate sampling but instead for high‐quality data at a relatively low budget. The study protocol was approved by the ethics committees in all participating centers.
Provinces involved in the PURE‐China Study were grouped into 3 socioeconomic regions by national criteria, including 4 eastern provinces with high income levels (Beijing, Jiangsu, Shandong, and Liaoning), 3 central provinces with middle income levels (Shanxi, Jiangxi, and Inner Mongolia), and 5 western provinces with low income levels (Yunnan, Qinghai, Shaanxi, Xinjiang, and Sichuan). Communities were sampled by urban and rural stratification. All eligible households in the selected communities were recruited if they had at least 1 member aged between 35 and 70 years and intended to stay at the current address for the next 4 years. Individuals who were 35 to 70 years old and provided written informed consent were enrolled (response rate 98.3% [46 285/47 085]).
Data Collection
Data collection was performed according to standardized procedures that have been identified previously.11 From interview‐based questionnaires, family income and neighborhood walkability (as defined by the neighborhood walkability scale questionnaire)14 were recorded at the community or household level, and demographic information, cardiovascular risk factors (as described in the INTERHEART Study),15 and regular medications were recorded at the individual level. For each participant, a 10‐mL fasting blood sample was collected and measured in a centralized certified laboratory for the analysis of basic serum biochemical indexes.16
Hypertension was defined as a blood pressure higher than 140/90 mm Hg or a self‐reported history of high blood pressure. Diabetes was defined as a fasting glucose higher than 7.0 mmol/L or a self‐reported history of diabetes mellitus. Hyperlipidemia was defined as a total cholesterol higher than 5.2 mmol/L. Participants were considered to have family histories if either or both of their biological parents had heart disease. Participants were considered to have abdominal obesity if their waist‐to‐hip ratios were higher than 0.90 for males or 0.85 for females. Current smoking was defined as smoking at least 1 cigarette per day in the past 12 months. Former smoking was defined as having ceased smoking more than 1 year earlier. Current drinking was defined as drinking at least once per month in the past 12 months. Former drinking was defined as having quit drinking more than 1 year earlier. Dietary profile was described by a semiquantitative food frequency questionnaire,17 with an Alternative Healthy Eating Index Score18 less than 31 being regarded as showing an unhealthy diet. Daily exercise was evaluated by the international physical activity questionnaire,19 with metabolic equivalents per minute per week less than 600 being regarded as insufficient physical activity. Psychosocial status was assessed by self‐reported feelings of work or home life stress periodically or permanently and by feeling sad, “blue”, or depressed for 2 or more consecutive weeks in the past 12 months.
The primary outcome of our study was the medical history of cardiovascular diseases before study enrollment by self‐reporting. Major cardiovascular diseases included stroke, angina, heart attack, and coronary artery disease. Nonmajor cardiovascular diseases included all other heart diseases that led to hospitalization.
INTERHEART Risk Score
To quantify the risk‐factor burden of cardiovascular diseases, we used the “nonlaboratory” version of the INTERHEART Modifiable Risk Score,20 which is a validated score that considers risk factors of age, sex, medical histories, lifestyle behaviors, and psychosocial status. Table 1 presents the detailed calculation of the score. The total INTERHEART Risk Score ranges from 0 to 48, with higher scores indicating greater burden.
Table 1.
The “Nonlaboratory” Version of the INTERHEART Modifiable Risk Score
| Risk Factors | Conditions | Points | Past/Present Exposures |
|---|---|---|---|
| Age | A man ≥55 years or a woman ≥65 years | 2 | Past |
| Hypertension | Yes | 5 | Past |
| Diabetes mellitus | Yes | 6 | Past |
| Family history | Yes | 4 | Past |
| Waist‐to‐hip ratio | 0.873 to 0.963 | 2 | Past |
| ≥0.964 | 4 | ||
| Smoking status | |||
| Former smoking | Yes | 2 | Past |
| Current smoking | 1 to 5 cigarettes per day | 2 | Present |
| 6 to 10 cigarettes per day | 4 | ||
| 11 to 15 cigarettes per day | 6 | ||
| 16 to 20 cigarettes per day | 7 | ||
| >20 cigarettes per day | 11 | ||
| Secondhand smoking | ≥1 hour per week (for never or former smokers only) | 2 | Present |
| Diet | |||
| Salty foods or snacks | ≥1 time per day | 1 | Present |
| Deep fried foods or fast foods | ≥3 times per week | 1 | Present |
| Fruits | <1 time per day | 1 | Present |
| Vegetables | <1 time per day | 1 | Present |
| Meat or poultry | ≥2 times per day | 2 | Present |
| Physical activity | Mainly sedentary or perform mild exercise | 2 | Present |
| Stress | Yes | 3 | Present |
| Depression | Yes | 3 | Present |
Because the INTERHEART Risk Score is a mixture of past exposures (eg, medical histories) and present exposures (eg, current lifestyle behaviors and psychosocial factors), we further divided the score into 2 parts to better describe the situations before and after the cardiovascular events (Table 1). The past part contains variables of former smoking and specific status that are difficult or impossible to modify, such as age, sex, hypertension, diabetes, family history, and waist‐to‐hip ratio. The present part, on the other hand, contains variables of current smoking (either active or passive), eating habits, physical activity, stress, and depression, which are changeable and may be affected by the disease. The past part of the INTERHEART Risk Score ranges from 0 to 23; the present part ranges from 0 to 25.
Statistical Analysis
Continuous variables were presented as mean±standard deviation (SD). Categorical variables were presented as numbers and corresponding percentages. Comparisons between groups were made with Mann‐Whitney U tests or Kruskal‐Wallis tests for continuous variables, and with chi‐square tests for categorical variables.
Univariate and multivariate generalized‐estimating‐equation models were built to evaluate the association between risk factors and cardiovascular diseases in different regions, addressing the cluster effect of communities. To deal with the potential type I error expansion brought by multiple comparisons, the Bonferroni method was used to correct the significance level. A P value less than 0.007 was considered to be statistically significant with a 2‐sided alternative. All statistical analyses were performed with SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Among the 46 285 individuals enrolled in the PURE‐China Study, 24 807 were from high‐income (eastern) regions, 10 182 were from middle‐income (central) regions, and 11 296 were from low‐income (western) regions. Table 2 provides information on income, education, occupation, and medication in urban and rural communities in different socioeconomic regions of China. The average incomes in high‐, middle‐, and low‐income regions were 128.88, 105.22, and 90.40 dollars per month, respectively. Personal income in urban areas was much higher than that in rural areas in all regions. The rates of illiteracy (primary or no education) and unemployment (elementary or no occupation) were higher in low‐income regions and rural communities, whereas the walkability to school, work, and drugstore was higher in high‐income regions and urban communities.
Table 2.
The Allocation of Educational and Medical Resources in Urban and Rural Communities in Different Socioeconomic Regions of China
| Characteristic | High‐Income Regions | Middle‐Income Regions | Low‐Income Regions | |||
|---|---|---|---|---|---|---|
| Urban (n=12 232) | Rural (n=12 575) | Urban (n=5058) | Rural (n=5124) | Urban (n=5517) | Rural (n=5779) | |
| Personal income (dollars) | 156.62±139.05 | 102.65±141.44 | 126.96±224.09 | 77.71±104.70 | 131.13±109.77 | 42.98±57.98 |
| Primary or no education | 2113 (17.27) | 5607 (44.59) | 756 (14.95) | 2246 (43.83) | 1242 (22.51) | 3908 (67.62) |
| Elementary or no occupation | 2328 (19.03) | 10 558 (83.96) | 569 (11.25) | 4189 (81.75) | 1256 (22.77) | 5209 (90.14) |
| Time to school or work | ||||||
| ≤10 minutes | 10 611 (86.75) | 10 471 (83.27) | 3943 (77.96) | 3408 (66.51) | 4397 (79.70) | 4418 (76.45) |
| 11 to 20 minutes | 713 (5.83) | 773 (6.15) | 507 (10.02) | 492 (9.60) | 624 (11.31) | 287 (4.97) |
| 21 to 30 minutes | 478 (3.91) | 463 (3.68) | 257 (5.08) | 428 (8.35) | 279 (5.06) | 413 (7.15) |
| >30 minutes | 430 (3.52) | 868 (6.90) | 351 (6.94) | 796 (15.53) | 217 (3.93) | 661 (11.14) |
| Time to buy medicine | ||||||
| ≤10 minutes | 9049 (73.98) | 8754 (69.61) | 3423 (67.67) | 2343 (45.73) | 3511 (63.64) | 2661 (46.05) |
| 11 to 20 minutes | 2072 (16.94) | 1139 (9.06) | 1486 (29.38) | 845 (16.49) | 1489 (26.99) | 722 (12.49) |
| 21 to 30 minutes | 804 (6.57) | 1100 (8.75) | 96 (1.90) | 708 (13.82) | 325 (5.89) | 954 (16.51) |
| >30 minutes | 307 (2.51) | 1582 (12.58) | 53 (1.05) | 1228 (23.97) | 192 (3.48) | 1442 (24.95) |
Data are presented as mean±SD or n (%).
Risk‐Factor Burden
Table 3 presents the cardiovascular risk factors of the participants in different socioeconomic regions of China. The mean INTERHEART Risk Score was higher in high‐ and middle‐income regions than in low‐income regions (9.47, 9.48, and 8.58, respectively, P<0.0001). Specifically, the past part of the score was higher in high‐income regions than in middle‐ and low‐income regions (4.85, 4.32, and 4.43, respectively, P<0.0001). Meanwhile, the present part of the score was highest in middle‐income regions, intermediate in high‐income regions, and lowest in low‐income regions (5.16, 4.62, and 4.15, respectively, P<0.0001).
Table 3.
Cardiovascular Risk Factors of Men and Women in Different Socioeconomic Regions of China
| Risk Factors | All | High‐Income Regions | Middle‐Income Regions | Low‐Income Regions | ||||
|---|---|---|---|---|---|---|---|---|
| Men (n=19 092) | Women (n=27 193) | Men (n=10 582) | Women (n=14 225) | Men (n=4063) | Women (n=6119) | Men (n=4447) | Women (n=6849) | |
| Age, y | 51.62±9.92 | 50.95±9.62 | 51.61±9.76 | 51.40±9.51 | 50.91±9.85 | 50.34±9.63 | 52.30±10.32 | 50.54±9.80 |
| Hypertension | 8606 (45.08) | 11 096 (40.80) | 5128 (48.46) | 6153 (43.25) | 1677 (41.27) | 2342 (38.27) | 1801 (40.50) | 2601 (37.98) |
| Diabetes mellitus | 1776 (9.30) | 2438 (8.97) | 997 (9.23) | 1354 (9.52) | 342 (8.42) | 509 (8.32) | 457 (10.28) | 575 (8.40) |
| Hyperlipidemia | 4135 (21.66) | 7564 (27.82) | 2217 (20.95) | 3817 (26.83) | 790 (19.44) | 1561 (25.51) | 1128 (25.37) | 2186 (31.92) |
| Family history | 2106 (11.03) | 3229 (11.87) | 1270 (12.00) | 1859 (13.07) | 441 (10.85) | 701 (11.46) | 395 (8.88) | 669 (9.77) |
| Abdominal obesity | 7719 (40.43) | 11 712 (43.07) | 4083 (38.58) | 6679 (46.95) | 1706 (41.99) | 2234 (36.51) | 1930 (43.40) | 2799 (40.87) |
| Smoking status | ||||||||
| Current smoking | 9504 (49.78) | 774 (2.85) | 5357 (50.62) | 408 (2.87) | 2317 (57.03) | 314 (5.13) | 1830 (41.15) | 52 (0.76) |
| Former smoking | 2055 (10.76) | 179 (0.66) | 892 (8.43) | 79 (0.56) | 522 (12.85) | 71 (1.16) | 641 (14.41) | 29 (0.42) |
| Never smoking | 7533 (39.46) | 26 240 (96.50) | 4333 (40.95) | 13 738 (96.58) | 1224 (30.13) | 5734 (93.71) | 1976 (44.43) | 6768 (98.82) |
| Drinking status | ||||||||
| Current drinking | 8468 (44.35) | 1243 (4.57) | 4800 (45.36) | 566 (3.98) | 2049 (50.43) | 425 (6.95) | 1619 (36.41) | 252 (3.68) |
| Former drinking | 1269 (6.65) | 246 (0.90) | 465 (4.39) | 72 (0.51) | 367 (9.03) | 84 (1.37) | 437 (9.83) | 90 (1.31) |
| Never drinking | 9355 (49.00) | 25 704 (94.52) | 5317 (50.25) | 13 587 (95.51) | 1647 (40.54) | 5610 (91.68) | 2391 (53.77) | 6507 (95.01) |
| Unhealthful diet | 4172 (21.85) | 6097 (22.42) | 2357 (22.27) | 3309 (23.26) | 717 (17.65) | 1111 (18.16) | 1098 (24.69) | 1677 (24.49) |
| Low physical activity | 2743 (14.37) | 2683 (9.87) | 1644 (15.54) | 1595 (11.21) | 420 (10.34) | 304 (4.97) | 679 (15.27) | 784 (11.45) |
| Stress | 1138 (5.96) | 1515 (5.57) | 430 (4.06) | 531 (3.73) | 286 (7.04) | 387 (6.32) | 422 (9.49) | 597 (8.72) |
| Depression | 885 (4.64) | 1619 (5.95) | 266 (2.51) | 469 (3.30) | 238 (5.86) | 511 (8.35) | 381 (8.57) | 639 (9.33) |
| INTERHEART Risk Score | ||||||||
| Past part | 5.65±4.30 | 3.90±3.96 | 5.81±4.28 | 4.12±4.03 | 5.33±4.35 | 3.64±3.86 | 5.56±4.29 | 3.70±3.89 |
| Present part | 6.05±3.92 | 3.62±2.27 | 6.05±3.90 | 3.54±2.23 | 6.81±4.05 | 4.06±2.30 | 5.34±3.71 | 3.36±2.28 |
| Total | 11.70±5.34 | 7.52±4.33 | 11.86±5.35 | 7.66±4.38 | 12.14±5.30 | 7.71±4.30 | 10.90±5.25 | 7.06±4.21 |
Data are presented as mean±SD or as n (%).
Figure 1 illustrates the distribution of the INTERHEART Risk Score by socioeconomic regions and urban or rural areas. The past score was higher in urban communities than in rural communities in all regions (high‐income regions 5.16 vs 4.54, P<0.0001; middle‐income regions 4.93 vs 3.71, P<0.0001; low‐income regions 4.95 vs 3.93, P<0.0001). The present score was higher in urban communities (4.44 vs 3.87, P<0.0001) for low‐income regions but was higher in rural communities for high‐ (4.44 vs 4.80, P<0.0001) and middle‐income regions (4.52 vs 5.79, P<0.0001).
Figure 1.
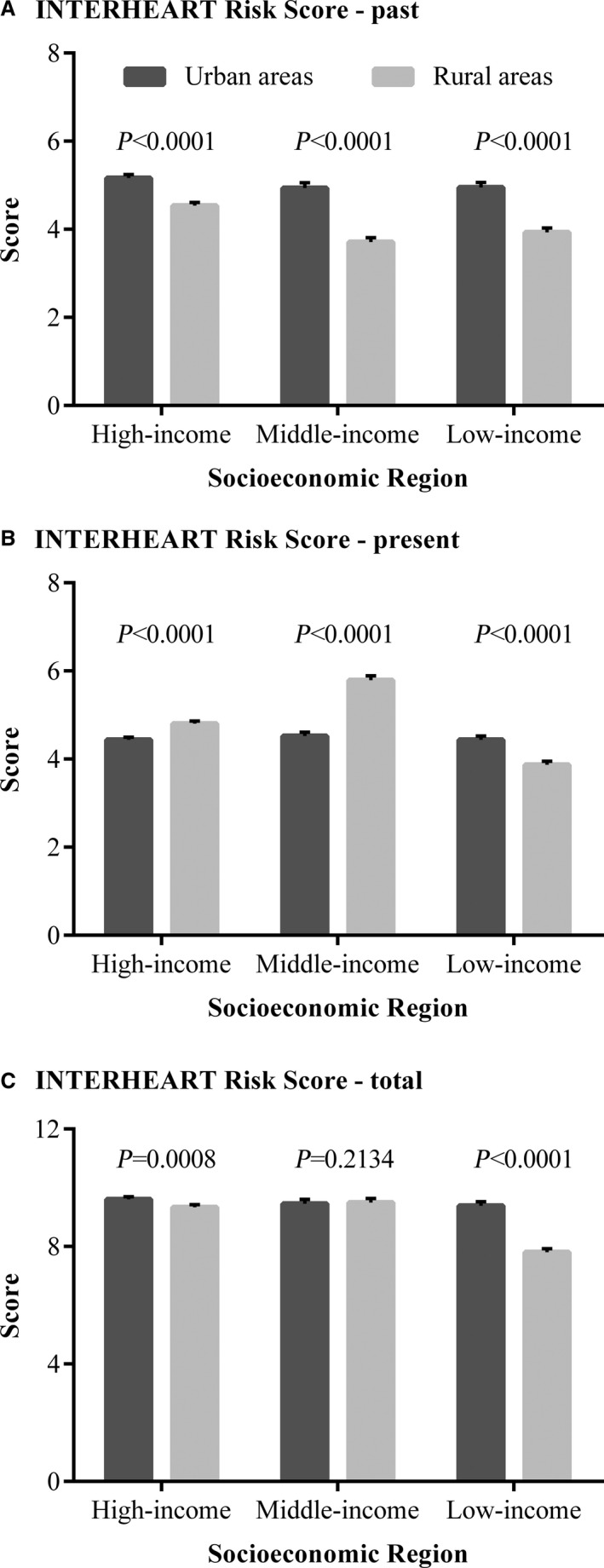
The mean INTERHEART Risk Score in urban and rural communities in different socioeconomic regions of China. A, The distribution of the past INTERHEART Risk Score. B, The distribution of the present INTERHEART Risk Score. C, The distribution of the total INTERHEART Risk Score.
Cardiovascular Diseases
Of the 46 285 participants with baseline medical history records, 872 (1.88%) had stroke, 2407 (5.20%) had ischemic heart disease (angina/heart attack/coronary artery disease), and 3070 (6.63%) had at least one major cardiovascular disease. Additionally, there were 479 (1.03%) participants suffering from nonmajor cardiovascular disease, constituting 3549 (7.67%) cardiovascular patients in total. The median time since diagnosis was 5 years (interquartile range 2‐10).
Figure 2 presents the prevalence of cardiovascular diseases in different socioeconomic regions. Stroke prevalence was highest in high‐income regions, intermediate in middle‐income regions, and lowest in low‐income regions (2.13%, 1.92%, and 1.31%, respectively, P trend<0.0001). Ischemic heart disease showed an opposite tendency, with lower prevalence in high‐ and middle‐income regions, and higher prevalence in low‐income regions (5.05%, 4.72%, and 5.97%, respectively, P trend=0.0017). Major cardiovascular disease had no consistent trend (6.70%, 6.24%, and 6.83%, respectively, P trend=0.9079). Nonmajor cardiovascular disease (0.75%, 1.18%, and 1.52%, respectively, P trend<0.0001) as well as total cardiovascular disease (7.46%, 7.42%, and 8.36%, respectively, P trend=0.0064) followed a similar pattern of ischemic heart disease in high‐, middle‐, and low‐income regions.
Figure 2.
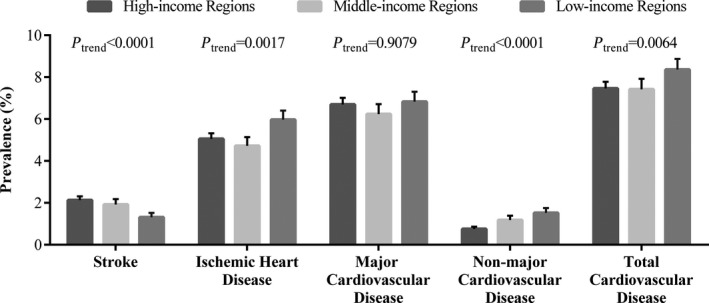
Prevalence of cardiovascular diseases in different socioeconomic regions of China.
Figure 3 and Figure S1 display the prevalence of cardiovascular diseases in urban and rural communities. The prevalence of total cardiovascular disease was higher in urban areas than in rural areas in high‐ (9.22% vs 5.74%, P<0.0001) and middle‐income regions (10.48% vs 4.39%, P<0.0001), the same as for major cardiovascular disease (high‐income regions 8.27% vs 5.18%, P<0.0001; middle‐income regions 9.31% vs 3.20%, P<0.0001). In low‐income regions, however, both total and major cardiovascular diseases showed similar prevalence between urban and rural areas (total cardiovascular disease 8.68% vs 8.05%, P=0.2222; major cardiovascular disease 6.49% vs 7.16%, P=0.1554). The prevalences of stroke and ischemic heart disease are described in Data S1.
Figure 3.
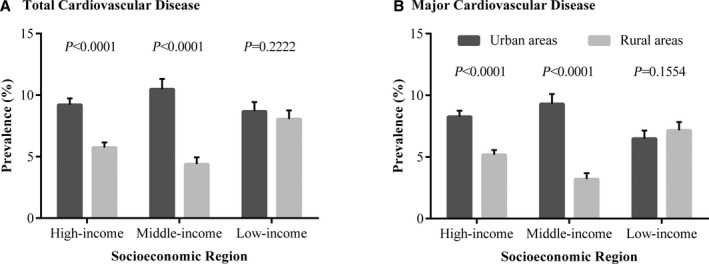
Prevalence of total and major cardiovascular diseases in urban and rural communities in different socioeconomic regions of China. A, The distribution of total cardiovascular disease prevalence. B, The distribution of major cardiovascular disease prevalence.
Associations Between Risk Factors and Cardiovascular Diseases
The INTERHEART Risk Score was positively correlated to total cardiovascular disease (odds ratio [OR] 1.08; 95% CI 1.07‐1.09) and major cardiovascular disease (OR, 1.08; 95% CI, 1.07‐1.09), adjusted for age, sex, socioeconomic region, urban or rural location, and region by location interaction. After excluding present exposures that might lead to reverse causality, the past part of the score showed higher correlations, with odds ratio 1.12 (1.11‐1.14) for total cardiovascular disease and 1.13 (1.12‐1.14) for major cardiovascular disease.
Table 4 and Table S1 provide the overall odds ratio estimations for cardiovascular risk factors. All past exposures were associated with both total and major cardiovascular diseases in univariate analysis. By adjusting for age, sex, socioeconomic region, urban or rural location, location by region interaction, and other risk factors in a multivariate generalized‐estimating‐equation model, family history and former drinking showed strongest correlations to total (or major) cardiovascular disease, with odds ratios exceeding to 2.00.
Table 4.
Association of Risk Factors With Total and Major Cardiovascular Diseases
| Past Risk Factors | Total Cardiovascular Disease | Major Cardiovascular Disease | ||
|---|---|---|---|---|
| Univariate Analysisa | Multivariate Analysisb | Univariate Analysisa | Multivariate Analysisb | |
| Hypertension | 2.88 (2.56‐3.23) | 1.92 (1.75‐2.11) | 3.16 (2.79‐3.58) | 2.04 (1.85‐2.24) |
| Diabetes mellitus | 2.37 (2.07‐2.72) | 1.43 (1.27‐1.61) | 2.49 (2.18‐2.85) | 1.47 (1.31‐1.66) |
| Hyperlipidemia | 1.51 (1.35‐1.68) | 1.04 (0.93‐1.15) | 1.50 (1.34‐1.69) | 1.02 (0.93‐1.13) |
| Family history | 2.08 (1.80‐2.39) | 2.31 (2.00‐2.67) | 2.16 (1.85‐2.51) | 2.43 (2.08‐2.84) |
| Abdominal obesity | 1.67 (1.53‐1.82) | 1.24 (1.14‐1.36) | 1.73 (1.58‐1.91) | 1.27 (1.16‐1.40) |
| Former smoking | 2.38 (2.04‐3.78) | 1.72 (1.49‐1.98) | 2.45 (2.07‐2.89) | 1.72 (1.48‐2.01) |
| Former drinking | 2.81 (2.31‐3.41) | 2.22 (1.86‐2.66) | 2.71 (2.18‐3.37) | 2.07 (1.72‐2.48) |
Data are presented as odds ratio (95% confidence interval).
Univariate analysis is performed with the use of a generalized‐estimating‐equation model to address clustering of data.
Multivariate analysis includes all past and present risk factors, adjusted for age, sex, socioeconomic region, urban or rural location, and region×location interaction.
The past INTERHEART Risk Score was risk‐related to total cardiovascular disease in high‐ (OR 1.13; 95% CI 1.11‐1.15), middle‐ (OR 1.12; 95% CI 1.11‐1.14), and low‐income regions (OR 1.11; 95% CI 1.08‐1.15). Figure 4 and Figure S2 show the different association patterns of risk factors and cardiovascular diseases among regions. Former lifestyle behaviors were most strongly correlated to total cardiovascular disease in high‐income regions, intermediate in middle‐income regions, and weakest in low‐income regions (ORs were 2.81, 2.31, and 1.63, respectively, for former drinking; 2.01, 1.76, and 1.19, respectively, for former smoking). Medical histories, especially for family history and hypertension, were remarkable risk factors because the odds ratios in all regions were fairly high (ORs were 2.29, 2.19, and 2.54, respectively, for family history; 1.99, 2.16, and 1.70, respectively, for hypertension). Similar association patterns were also observed in major cardiovascular disease. The associations of risk factors with stroke and ischemic heart disease are presented in Data S2.
Figure 4.
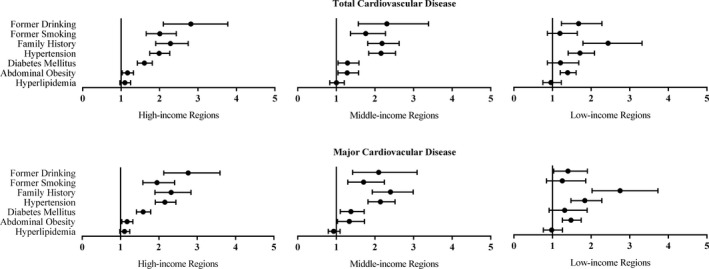
Association of risk factors with total and major cardiovascular diseases in different socioeconomic regions of China, adjusted for age, sex, and urban or rural location.
Medications
Among participants with total cardiovascular disease, the use of antiplatelet drugs was higher in high‐ and middle‐income regions than in low‐income regions (22.59%, 21.99%, and 5.51%, respectively, P trend<0.0001). Similar tendencies were also observed in the use of angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers (9.95%, 7.68%, and 4.77%, respectively, P trend<0.0001), diuretics (17.57%, 12.85%, 5.93%, respectively, P trend<0.0001), calcium‐channel blockers (15.03%, 23.71%, and 5.08%, respectively, P trend<0.0001), and any of the blood‐pressure‐lowering drugs (37.68%, 40.26%, and 16.00%, respectively, P trend<0.0001).
Table 5 displays the rates of drug use in urban and rural communities in different socioeconomic regions of China. The use of secondary prevention drugs was generally higher in urban than in rural communities, and the relative differences in drug use between urban and rural areas were least pronounced in high‐income regions, intermediate in middle‐income regions, and most pronounced in low‐income regions.
Table 5.
Regular Medications for Participants With Total Cardiovascular Disease in Urban and Rural Communities in Different Socioeconomic Regions of China
| Medications | High‐Income Regions | Middle‐Income Regions | Low‐Income Regions | |||
|---|---|---|---|---|---|---|
| Urban (n=1128) | Rural (n=722) | Urban (n=530) | Rural (n=225) | Urban (n=479) | Rural (n=465) | |
| Antiplatelet drugs | 205 (18.17) | 213 (29.50) | 136 (25.66) | 30 (13.33) | 38 (7.93) | 14 (3.01) |
| β‐Blockers | 83 (7.36) | 23 (3.19) | 58 (10.94) | 11 (4.89) | 32 (6.68) | 5 (1.08) |
| ACE inhibitors or ARBs | 114 (10.11) | 70 (9.70) | 44 (8.30) | 14 (6.22) | 30 (6.26) | 15 (3.23) |
| Diuretics | 182 (16.13) | 143 (19.81) | 71 (13.40) | 26 (11.56) | 40 (8.35) | 16 (3.44) |
| Calcium‐channel blockers | 178 (15.78) | 100 (13.85) | 157 (29.62) | 22 (9.78) | 45 (9.39) | 3 (0.65) |
| Blood‐pressure‐lowering drugsa | 436 (38.65) | 261 (36.15) | 252 (47.55) | 52 (23.11) | 117 (24.43) | 34 (7.31) |
| Statins | 25 (2.22) | 2 (0.28) | 20 (3.77) | 5 (2.22) | 4 (0.84) | 0 |
Data are presented as n (%). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker.
Blood‐pressure‐lowering drugs include β‐blockers, ACE inhibitors, ARBs, diuretics, and calcium‐channel blockers.
Discussion
Our study found opposite trends of risk‐factor burden and total cardiovascular disease in urban and rural communities in different socioeconomic regions of China. Despite the lowest risk‐factor burden in low‐income regions, prevalence there was the highest. Compared with urban communities, rural communities in low‐income regions had lower risk‐factor burdens but similar prevalence of total cardiovascular disease.
The main finding of our study is generally consistent with the conclusion from the previous global research,10 that is, although the risk‐factor burden (evaluated by the INTERHEART Risk Score) was positively correlated to the socioeconomic levels of the countries, the incidence and case fatality rate of major cardiovascular events (defined as deaths from cardiovascular causes, nonfatal stroke, myocardial infarction, and heart failure) showed a reverse pattern. Investigators attributed this phenomenon to some country‐level factors including the quality of health services, the frequency of proven therapies used, and the educational background of the population.10 Our study differs from the previous study in the following ways. First, we did not consider follow‐up data but only cross‐sectional data; thus we used prevalence rather than incidence or mortality to assess the disease occurrences. Second, the definitions of cardiovascular diseases were varied. Despite these differences, we observed similar trends with the global results among eastern, central, and western provinces in China, which represented high‐, middle‐, and low‐income regions of China, respectively.
The total INTERHEART Risk Score was higher in high‐ and middle‐income regions than in low‐income regions, indicating the lightest risk‐factor burden in low‐income regions. Specifically, the past INTERHEART Risk Score, which represents the burden of lifestyle behaviors and medical histories before cardiovascular events, was higher in high‐income regions than in middle‐ and low‐income regions. The present INTERHEART Risk Score, which represents the burden of lifestyle behaviors and psychosocial factors after cardiovascular events, was highest in middle‐income regions, intermediate in high‐income regions, and lowest in low‐income regions. Situations in different socioeconomic regions of China well paralleled the development process of risk factors in high‐, middle‐, and low‐income countries in the world in the last century. At the very beginning, risk factors increased in high‐income countries but remained less prevalent in middle‐ and low‐income countries.21 After several decades of development, risk factors in high‐income countries declined as a result of the enhancement of resident awareness and improvement of disease management.4 In contrast, risk factors in middle‐income countries increased markedly because of the working manner and lifestyle transition to sedentary, crapulent, and gluttonous.22 For low‐income countries, people were too poor to smoke, drink, and entertain; therefore the prevalence of risk factors was still relatively low.23
The prevalence of total cardiovascular disease was lower in high‐ and middle‐income regions than in low‐income regions, demonstrating the most severe heart problems in low‐income regions. The prevalence of major cardiovascular disease was comparable among regions because of the opposed trends of stroke and ischemic heart disease with income levels. Similar tendencies were also reported in other studies,24, 25 and the decreased prevalence of stroke and the increased prevalence of ischemic heart disease in low‐income settings were explained by worse health surveillance and less adequate disease management, respectively. Socioeconomic status other than risk factors played a decisive role in determining the spectrum of the disease. The inferior availability and affordability of health services, the lacked consciousness of prevention, the poorer control of risk factors, and the poorer quality of diagnoses and treatments in low‐income regions of China13 may jointly lead to the asymmetry of risk‐factor burden and disease rates.
Within each socioeconomic region, the past risk‐factor burden and the disease prevalence differed between urban and rural communities. For high‐ and middle‐income regions, the greater past risk‐factor burden in urban communities (compared with rural) well explained the higher prevalence of total cardiovascular disease in urban communities. For low‐income regions, large imbalances in the allocation of educational and medical resources might contribute to different past risk‐factor burdens but to similar prevalences between urban and rural areas. Although the past risk‐factor burden was greater in urban than rural communities in low‐income regions, the theoretical higher prevalence of total cardiovascular disease in urban settings was neutralized by the better socioeconomic status and neighborhood walkability. A parallel but more significant phenomenon was observed from urban versus rural settings of middle‐ and low‐income countries in the global study.10
After diagnosis with total cardiovascular disease, the present risk‐factor burden also varied from urban to rural in different socioeconomic regions. Residents in urban communities in high‐ and middle‐income regions were more likely to develop healthier living habits to reduce their present risk‐factor burdens because of their higher awareness. In contrast, people in urban communities in low‐income regions were unaware of risk factors. But they were richer and cozier, enough to suffer more from unhealthy lifestyle behaviors and psychosocial problems, than people in rural communities. Therefore, citizens in low‐income regions undertook greater present risk‐factor burden than their countrymen.
All the past risk factors were associated with total cardiovascular disease. Among them, family history and former drinking showed the strongest associations. Family history, which represented the genetic background, was positively correlated to total cardiovascular disease in all regions. Former drinking, which represented for the frequency of lifestyle intervention, showed a decreased strength of correlations to total cardiovascular disease with the decline of socioeconomic status. As a result of the different levels of awareness, treatment, and control rates of cardiovascular diseases in different regions,13 residents in higher‐income regions might be more educated and more active in quitting drinking due to illness, and vice versa.
Healthcare accessibility was another possible influential factor for total cardiovascular disease. We used regular medications in our study to evaluate the healthcare accessibility in different socioeconomic regions of China. The drug use for total cardiovascular patients was higher in high‐ and middle‐income regions than in the low‐income regions, indicating the worst healthcare accessibility in low‐income regions. Meanwhile, the drug use was higher in urban than rural settings in all regions, especially in low‐income regions. These giant differences in healthcare accessibility between urban and rural areas further explained the reverse trend of risk‐factor burden and disease prevalence in urban and rural communities in low‐income regions.
Our study provides the first regional estimations of risk‐factor burden and prevalence of cardiovascular diseases in urban and rural communities in different socioeconomic regions of China. Results showed that poorer areas had lower risks but higher prevalence, especially for rural settings in low‐income regions. This finding implies important public health inequalities across provinces and between urban and rural communities, which have been discussed in the previous literature.13, 26 The imbalance in economic development directly affected the different healthcare provision among regions. Because the association pattern of risk factors and cardiovascular diseases varied among regions, giving different regions different intervention and prevention approaches would be a more efficient approach. Our study provides scientific evidence that may lead to better allocation of medical resources and more rational public health decision making.
There were several limitations of our study. First of all, the sampling framework of the PURE‐China Study was not nationally representative; thus caution is needed in extrapolating our findings. To minimize the selection biases brought by nonrandom sampling, we enrolled residents from high‐ (eastern), middle‐ (central), and low‐income (western) regions as well as from urban and rural communities, and we followed standardized protocols to approach households and individuals. In addition, our overall prevalence of total cardiovascular disease is generally consistent with that reported by the China Health Statistical Yearbook,27 suggesting our sampling methods may not be a major concern.
Second, we used the INTERHEART Modifiable Risk Score, which was established for the prediction of myocardial infarction, to evaluate the risk‐factor burden of all cardiovascular diseases. Because the risk factors involved in the score were applicable for many noncommunicable chronic diseases, and the score was highly correlated to cardiovascular diseases other than myocardial infarction, it was widely employed in cardiovascular risk assessments.10, 28, 29, 30
Third, the medical history of cardiovascular diseases was self‐reported, so its prevalence may be underestimated. However, previous studies showed that self‐reports and hospital records were highly consistent for stroke and ischemic heart disease,31, 32, 33 and the adjudication committee of the PURE‐China Study had verified most of the disease cases; therefore, we assumed that all individuals who reported such events were cardiovascular patients.
Last but not least, the association between risk factors and cardiovascular diseases can not define any causality. Given the cross‐sectional feature of our study, we can not decide the temporal relationship; hence, our findings were just implications but not confirmations. The correlations between risk factors and cardiovascular diseases to some extent reflected the impact of cardiovascular diseases on risk factors. Nevertheless, we distinguished the cardiovascular risk factors into past and present parts, to represent the situations before and after cardiovascular events, and just used past risk factors to do the association; then the potential reverse causality can be minimized. Because the follow‐up data from the Chinese cohort were not yet available, prospective results were left for future analysis.
In conclusion, our study found an inverse tendency between cardiovascular risks and cardiovascular diseases among socioeconomic regions, with lowest risk‐factor burden but highest prevalence in low‐income regions and rural communities in China. These findings may be interpreted in terms of the different education levels, healthcare quality, and medical availability in different areas; therefore, appropriate treatment and prevention strategies are needed for specific regions.
Appendix
PURE‐China Investigators
China Coordination Center Beijing Office: Lisheng Liu**, Wei Li**, Bing Liu, Bo Hu, Chunming Chen, Jin Guo, Hongye Zhang, Hui Chen, Jian Bo, Jian Li, Juan Li, Jun Yang, Kean Wang, Li Zhang, Qing Deng, Bing Ren, Tao Chen, Tao Xu, Wei Wang, Wenhua Zhao, Xiaohong Chang, Xiaoru Cheng, Xinye He, Xixin Hou, Xingyu Wang, Xiulin Bai, Xiuwen Zhao, Xu Liu, Xuan Jia, Yang Wang, Yi Sun, Yi Zhai. Beijing: Dong Li*, Di Chen*, Hui Jin, Jiwen Tian, Yumin Ma; Yindong Li*, Chao He, Kai You, Songjian Zhang; Xiuzhen Tian*, Xu Xu*, Jinling Di, Jianquan Wu, Mei Wang, Qiang Zhou. Inner Mongolia Autonomous Region: Shiying Zhang*, Aiying Han, Minzhi Cao. Jiangsu Province: Jianfang Wu*, Weiping Jiang*, Deren Qiang, Jing Qin, Shan Qian, Suyi Shi, Yihong Zhou; Zhenzhen Qian*, Zhengrong Liu; Changlin Dong*, Ming Wan; Jun Li*, Jinhua Tang; Jun Li*, Yongzhen Mo*, Rongwen Bian, Qinglin Lou. Jiangxi Province: Rensheng Lei*, Lihua Hu, Shuwei Xiong, Yan Zhong; Ning Li*, Xincheng Tang*, Shuli Ye. Liaoning Province: Yu Liu*, Chunyi Li, Yujin Li; Minfan Fu*, Qiuyuan Wang, Xiaoli Fu; Xiaojie Xing*, Baoxia Guo*, Huilian Feng, Lihui Xu. Qinghai Province: Yuqing Yang*, Haibin Ma, Ruiqi Wu, Yali Wang; Xiaolan Ma*, Hongze Liu, Yurong Ma. Sichuan Province: Xiaoyang Liao*, Bo Yuan, Qian Zhao; Guofan Xu*, Hui He, Jiankang Liu, Xin Wang; Ming Chen*, Wenqing Deng*. Shandong Province: Fanghong Lu*, Zhendong Liu*, Hua Zhang, Shangwen Sun, Shujian Wang, Yingxin Zhao, Yutao Diao; Mei Wang*, Xuezheng Shi; Debin Ren*, Chuanrui Wei. Shanxi Province: Liangqing Zhang*, Jufang Wang; Lianghou Fan*, Guoqin Liu; Yan Hou*, Cuiying Wu, Guilan Ma, Hua Wei, Junying Wang, Xiongfei Bao, Yue Tang; Tianlu Liu*, Yahong Zhi. Shaanxi Province: Peng Zhang*, Ailing Wang, Huijuan Wang, Jianna Liu, Qinzhou Liu, Rong Wang. Xinjiang Uygur Autonomous Region: Jianguo Wu*, Aideer Aili*, Ayoufumiti Wula, Aibi Bula, Dongmei Yang, Qian Wen, Resha Laiti. Yunnan Province: Yize Xiao*, Qingping Shi, Ying Shao; Jing He*, Kehua Li, Wuba Bai, Jinkui Yang; Yunchun Jiang*, Huaxing Liu*, Shunyun Yang.
*Regional Coordinator.
**National Coordinator.
Sources of Funding
The PURE‐China Study is supported by the Population Health Research Institute, Hamilton, ON, Canada, which administered the funds received from a variety of sources, including the Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, unrestricted grants from several pharmaceutical companies, and grants from the National Center for Cardiovascular Diseases, China.
Disclosures
None.
Supporting information
Data S1. Prevalence of stroke and ischemic heart disease.
Data S2. Associations of risk factors with stroke and ischemic heart disease.
Table S1. Association of Risk Factors With Stroke, Ischemic Heart Disease, and Nonmajor Cardiovascular Disease
Figure S1. Prevalence of stroke and ischemic heart disease in urban and rural communities in different socioeconomic regions of China.
Figure S2. Association of risk factors with stroke in different socioeconomic regions of China, adjusted for age, sex, and urban or rural location.
Acknowledgments
The authors are grateful for the contributions of all the participants of the PURE‐China Study as well as the local medical personnel for data collection.
(J Am Heart Assoc. 2017;6:e004445. DOI: 10.1161/JAHA.116.004445.)
References
- 1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker‐Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez‐Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez‐Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui‐Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker‐Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan‐Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere‐Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz‐Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez‐Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina‐Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi‐Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez‐Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez‐Riera L, Sanman E, Schwebel DC, Scott JG, Segui‐Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability‐adjusted life years (DALYS) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 3. Feigin VL, Lawes CM, Bennett DA, Barker‐Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population‐based studies: a systematic review. Lancet Neurol. 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 4. O'Flaherty M, Buchan I, Capewell S. Contributions of treatment and lifestyle to declining CVD mortality: why have CVD mortality rates declined so much since the 1960s? Heart. 2013;99:159–162. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Global Status Report on Noncommunicable Diseases 2010. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 6. National Center for Cardiovascular Diseases, China . Report on Cardiovascular Diseases in China (2014). Beijing: Encyclopedia of China Publishing House; 2015. [Google Scholar]
- 7. Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, Vos T, Wang H, Lopez AD, Murray CJL. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Bureau of Statistics of China . International Statistical Yearbook 2014. Beijing: China Statistic Press; 2015. [Google Scholar]
- 9. National Bureau of Statistics of China . China Statistical Yearbook 2014. Beijing: China Statistic Press; 2015. [Google Scholar]
- 10. Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez‐Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G; Investigators P . Cardiovascular risk and events in 17 low‐, middle‐, and high‐income countries. N Engl J Med. 2014;371:818–827. [DOI] [PubMed] [Google Scholar]
- 11. Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S. The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low‐, middle‐, and high‐income countries. Am Heart J. 2009;158:1–7.e1. [DOI] [PubMed] [Google Scholar]
- 12. Corsi DJ, Subramanian SV, Chow CK, McKee M, Chifamba J, Dagenais G, Diaz R, Iqbal R, Kelishadi R, Kruger A, Lanas F, Lopez‐Jaramilo P, Mony P, Mohan V, Avezum A, Oguz A, Rahman MO, Rosengren A, Szuba A, Li W, Yusoff K, Yusufali A, Rangarajan S, Teo K, Yusuf S. Prospective Urban Rural Epidemiology (PURE) study: baseline characteristics of the household sample and comparative analyses with national data in 17 countries. Am Heart J. 2013;166:636–646.e634. [DOI] [PubMed] [Google Scholar]
- 13. Li W, Gu H, Teo KK, Bo J, Wang Y, Yang J, Wang X, Zhang H, Sun Y, Jia X, He X, Zhao X, Cheng X, Li J, Rangarajan S, Chen C, Yusuf S, Liu L; PURE China Investigators . Hypertension prevalence, awareness, treatment, and control in 115 rural and urban communities involving 47 000 people from China. J Hypertens. 2016;34:39–46. [DOI] [PubMed] [Google Scholar]
- 14. Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood‐based differences in physical activity: an environment scale evaluation. Am J Public Health. 2003;93:1552–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 16. Peng Y, Li W, Wang Y, Bo J, Chen H. The cut‐off point and boundary values of waist‐to‐height ratio as an indicator for cardiovascular risk factors in Chinese adults from the PURE study. PLoS One. 2015;10:e0144539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food‐frequency questionnaires—a review. Public Health Nutr. 2002;5:567–587. [DOI] [PubMed] [Google Scholar]
- 18. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–1271. [DOI] [PubMed] [Google Scholar]
- 19. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71:S114–S120. [PubMed] [Google Scholar]
- 20. McGorrian C, Yusuf S, Islam S, Jung H, Rangarajan S, Avezum A, Prabhakaran D, Almahmeed W, Rumboldt Z, Budaj A, Dans AL, Gerstein HC, Teo K, Anand SS; INTERHEART Investigators . Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J. 2011;32:581–589. [DOI] [PubMed] [Google Scholar]
- 21. Walker AR, Walker BF, Segal I. Some puzzling situations in the onset, occurrence and future of coronary heart disease in developed and developing populations, particularly such in sub‐Saharan Africa. J R Soc Promot Health. 2004;124:40–46. [DOI] [PubMed] [Google Scholar]
- 22. Stringhini S, Viswanathan B, Gedeon J, Paccaud F, Bovet P. The social transition of risk factors for cardiovascular disease in the African region: evidence from three cross‐sectional surveys in the Seychelles. Int J Cardiol. 2013;168:1201–1206. [DOI] [PubMed] [Google Scholar]
- 23. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker‐Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan‐Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez‐Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez‐Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker‐Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C; Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010), the GBD Stroke Experts Group . Global and regional burden of stroke during 1990‐2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 Study. Circulation. 2014;129:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ling RE, Liu F, Lu XQ, Wang W. Emerging issues in public health: a perspective on China's healthcare system. Public Health. 2011;125:9–14. [DOI] [PubMed] [Google Scholar]
- 27. Ministry of Health of the People's Republic of China . China Health Statistical Yearbook 2010. Beijing: Peking Union Medical College Press; 2011. [Google Scholar]
- 28. Cordero A, Lopez‐Palop R, Carrillo P, Miralles B, Masia MD, Bertomeu‐Martinez V. Prognostic value of the INTERHEART‐cholesterol risk score in patients hospitalized for chest pain. Rev Esp Cardiol. 2014;67:578–580. [DOI] [PubMed] [Google Scholar]
- 29. Fathima FN, Joshi R, Agrawal T, Hegde S, Xavier D, Misquith D, Chidambaram N, Kalantri SP, Chow C, Islam S, Devereaux PJ, Gupta R, Pais P, Yusuf S. Rationale and design of the primary prevention strategies at the community level to promote adherence of treatments to prevent cardiovascular diseases trial number (CTRI/2012/09/002981). Am Heart J. 2013;166:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahanta TG, Joshi R, Mahanta BN, Xavier D. Prevalence of modifiable cardiovascular risk factors among tea garden and general population in Dibrugarh, Assam, India. J Epidemiol Glob Health. 2013;3:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH, Curb JD. Comparison of self‐report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;160:1152–1158. [DOI] [PubMed] [Google Scholar]
- 32. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self‐report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. [DOI] [PubMed] [Google Scholar]
- 33. Yamagishi K, Ikeda A, Iso H, Inoue M, Tsugane S; JPHC Study Group . Self‐reported stroke and myocardial infarction had adequate sensitivity in a population‐based prospective study JPHC (Japan Public Health Center)‐based prospective study. J Clin Epidemiol. 2009;62:667–673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Prevalence of stroke and ischemic heart disease.
Data S2. Associations of risk factors with stroke and ischemic heart disease.
Table S1. Association of Risk Factors With Stroke, Ischemic Heart Disease, and Nonmajor Cardiovascular Disease
Figure S1. Prevalence of stroke and ischemic heart disease in urban and rural communities in different socioeconomic regions of China.
Figure S2. Association of risk factors with stroke in different socioeconomic regions of China, adjusted for age, sex, and urban or rural location.


