Abstract
Background
Several models have been developed for prediction of contrast‐induced nephropathy (CIN); however, they only contain patients receiving intra‐arterial contrast media for coronary angiographic procedures, which represent a small proportion of all contrast procedures. In addition, most of them evaluate radiological interventional procedure‐related variables. So it is necessary for us to develop a model for prediction of CIN before radiological procedures among patients administered contrast media.
Methods and Results
A total of 8800 patients undergoing contrast administration were randomly assigned in a 4:1 ratio to development and validation data sets. CIN was defined as an increase of 25% and/or 0.5 mg/dL in serum creatinine within 72 hours above the baseline value. Preprocedural clinical variables were used to develop the prediction model from the training data set by the machine learning method of random forest, and 5‐fold cross‐validation was used to evaluate the prediction accuracies of the model. Finally we tested this model in the validation data set. The incidence of CIN was 13.38%. We built a prediction model with 13 preprocedural variables selected from 83 variables. The model obtained an area under the receiver‐operating characteristic (ROC) curve (AUC) of 0.907 and gave prediction accuracy of 80.8%, sensitivity of 82.7%, specificity of 78.8%, and Matthews correlation coefficient of 61.5%. For the first time, 3 new factors are included in the model: the decreased sodium concentration, the INR value, and the preprocedural glucose level.
Conclusions
The newly established model shows excellent predictive ability of CIN development and thereby provides preventative measures for CIN.
Keywords: contrast media, contrast‐induced nephropathy, percutaneous coronary intervention, risk factor, risk prediction
Subject Categories: Clinical Studies, Percutaneous Coronary Intervention, Computerized Tomography (CT), Epidemiology, Risk Factors
Introduction
Contrast‐induced nephropathy (CIN) is an important cause of acute kidney injury (AKI) in both ambulatory and hospitalized patients. With the wide use of contrast media (CM), CIN has become the third prevalent cause of all hospital‐acquired renal failure, accounting for 10%.1 Furthermore, the development of CIN has been reported to prolong hospitalization and increase mortality and morbidity.2 The precise pathophysiological mechanism of CIN remains unclear, but some studies have shown the pathogenesis of CIN to be related to the toxicity effect of CM on the tubular epithelial cells due to apoptosis, disturbances in intrarenal hemodynamics, and medullary hypoxia.3
Unfortunately, few strategies have been shown to prevent and cure CIN effectively. Therefore, it is important to comprehensively assess the risks of CIN before CM administration and to take preventative measures. Preexisting chronic kidney disease and diabetes mellitus are the most important risk factors for CIN. Age over 70, preprocedural dehydration, congestive heart failure, anemia, volume and type of CM administered, and concurrent administration of nephrotoxic drugs were found to be potential risk factors.4, 5 A number of risk prediction models with many important predisposing factors have been developed for the evaluation of an individual patient's risk of developing CIN. However, these models have exclusively focused on populations receiving intra‐arterial CM for coronary angiographic procedures, and no model developed a predictive approach for more common contrast‐enhanced computed tomography (CT) procedures.6 Indeed, the risk of CIN in a low‐risk population given intravenous contrast‐enhanced CT procedures is not small.7 What is more, most of the models evaluate the radiological interventional procedure‐related variables; thus, they complete risk assessment only after CM administration. As far as we know, there have been only 4 published models that studied the risk factors before coronary angiography.8, 9, 10, 11 Among the 4 models, only Liu et al11 developed a preprocedural model in 728 Chinese patients with chronic total occlusion undergoing percutaneous coronary intervention, but common variables such as those related to diabetes were not included in the model, so it is not particularly applicable to a diabetes population. In addition, all of the 4 models are focused on coronary angiography, and thus, they are incapable of predicting CIN before other CM procedures such as intravenous contrast‐enhanced CT.
The purpose of this study was to determine the incidence and to assess predictive factors of CIN in Chinese patients and to develop a predictive model that could provide a good prediction for CIN before patients were exposed to CM.
Materials and Methods
Ethics Statement
The study protocol was approved by the Medical Ethical Committee in the Third Xiangya Hospital of Central South University (No. 2016‐S160). All subjects were anonymized so informed consent was not required. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Patient Population
We performed a retrospective single‐center case control study in hospitalized adults from September 2007 to January 2015. According to our institute protocol, patients were included if they were treated with CM for coronary angiography or percutaneous coronary intervention or received intravenous CM such as for CT or endovascular procedures (n=69 827), identified by the electronic medical record system at the Third Xiangya Hospital of Central South University, Changsha, China. The exclusion criteria were preprocedure estimated glomerular filtration rate (eGFR) under 15 mL/(min·1.73 m2) (n=422), age ≤15 (n=635), missing variables more than 30%, which means that the number of missing variables is greater than 25 in the 83 variables (n=4914), and without serum creatinine value within 14 days before CM procedures or within 72 hours after procedures (n=55 478).
Their detailed demographic and clinical characteristics were collected from the hospital information system. The serum creatinine (Scr) concentration at the earliest within 14 days before a procedure was defined as the baseline, and the highest Scr within 72 hours after the procedure was used as the follow‐up Scr to evaluate the incidence of CIN.
Definition
In this study, CIN was defined as an increase of Scr of 0.5 mg/dl (44.2 μmol/L) or 25% relative increase in serum creatinine from the baseline value to 72 hours after exposure to CM in the absence of alternative causes for acute kidney injury according to the Contrast Media Safety Committee (CMSC). Creatinine clearance was calculated using the modification of diet in renal disease (MDRD) equation, and chronic kidney disease was defined as eGFR<60 mL/(min·1.73 m2) as estimated with the modified MDRD formula.12, 13 Anemia is defined as hemoglobin (HGB) concentration <13 g/dL for men and <12 g/dL for women.14
Statistical Analysis
Continuous variables of each group are presented as mean±standard deviation, and the categorical variables are expressed as absolute values and percentages. A t test was used to compare the normally distributed continuous variables; otherwise, the Mann‐Whiney U test was used. Categorical variables were performed by chi‐squared test. A 2‐tailed value of P<0.05 was established as the threshold of statistical significance. Data analysis was performed with the statistical package SPSS, version17.0 (SPSS Inc, Chicago, IL).
Prediction Model Development
We developed the prediction model based on machine learning. Randomization and data analysis were performed using random forests (RF), an ensemble of decision trees.15 RF is good at describing the relationship between independent and dependent variables with high flexibility and sufficient accuracy. The 2 main parameters in RF are mtry, the number of input variables randomly chosen at each split, and ntree, the number of trees in the forest. In this model, the mtry is 4 and the ntree is 1000. The training group is used to form the algorithm composed of 1000 trees, each of which is constructed using the bootstrap samples from the training data and random feature selection. Each node is best split from a random selected set. When the RF algorithm best separates all instances and this tree is able to classify all instances, this node becomes a terminal node with each unpruned tree grown to its maximum extent. After 1000 trees are achieved, the majority vote of all analogous trees in the forest was taken for the predictions for test data. RF was implemented by the RF function in the R package (ver 4.6.7).
The selected 8800 patients were randomly divided into 2 separate data sets: 80% of the patients (CIN=942, non‐CIN=6098) in our database were selected to the training data set (the algorithm creation group), and the remaining 20% (CIN=231, non‐CIN=1529) were reserved as the external validation sets (validation group) to obtain unbiased estimates of correct classification rates and variable importance.
In this model RF is also used to assess the importance of variables in model quality when each variable is replaced in turn by random noise in each tree. The variable importance is measured by the resulting deterioration in model quality. The deterioration in model quality can be assessed by the change in misclassification rates for the out‐of‐bag validation. The heuristics was based on the Gini criterion. Specifically, we recorded the decrease in the Gini node impurity for the variable Xj, which was used to form the split at each split. In the forest where Xj formed the split, the average of all decreases in the Gini impurity decided the Gini variable's importance. In addition, the AUC was also used to assess the importance of selected variables: when a variable is excluded in the model, the larger the change in value of AUC is, the more important the variable is.
Five‐fold cross‐validation was primarily used as an internal validation to evaluate the prediction accuracies of the model. Briefly, we split the data set into 5 roughly equal‐sized parts, and then 4 of them were fit into the model while the other part was used to calculate the error rate. The process was repeated 5 times so that every part could be predicted as a validation set.
The prediction performance was assessed by several criteria including the overall prediction accuracy (R), sensitivity (SE), specificity (SP), and Matthews correlation coefficient (MCC). The equations are as follows:
where true positive (TP) is the number of positive samples predicted correctly, true negative (TN) is the number of negative samples predicted correctly, false positive (FP) is the number of negative samples indicated as positive, and false negative (FN) is the number of positive samples indicated as negative.
SE and SP allow computation of the percentage of correctly predicted CIN and non‐CIN, respectively, while prediction accuracy means percentage of correctly predicted CIN and non‐CIN. MCC is the statistical parameter to assess the quality of prediction and to take care of the data unbalancing. The Matthews correlation coefficient ranges from −1 to 1. MCC=1 indicates the best possible prediction, and MCC=−1 points out the worst possible prediction.
Results
Patient Characteristics
Of a total of 69 827 patients administrated CM, 8800 patients (5468 men, 3332 women; mean age 55.3±14.8 years) were included in this study. Of them, 1173 (13.3%) developed CIN. Table 1 shows the clinical characteristics of patients who developed CIN and of those who did not show this complication after CM administration.
Table 1.
Demographic, Clinical, and Angiographic Data in CIN and Non‐CIN Patients of the Training Data Set
| Variable | Non‐CIN (n=6098) | CIN (n=942) | P Value |
|---|---|---|---|
| Sex (male) | 3801 (62.33%) | 609 (64.65%) | 0.171 |
| Age (y) | 55.23±14.81 | 55.87±15.57 | 0.215 |
| eGFR (mL/[min·1.73 m2]) | 103.03±49.07 | 116.82±74.97 | 0.000* |
| CKD | 791 (12.97%) | 170 (18.05%) | 0.000* |
| Diabetes | 816 (13.38%) | 68 (7.22%) | 0.000* |
| Mechanical ventilation | 808 (13.25%) | 148 (15.71%) | 0.040* |
| Myocardial infarction | 330 (5.41%) | 42 (4.46%) | 0.224 |
| Shock | 147 (2.41%) | 44 (4.67%) | 0.000* |
| Gout | 66 (1.08%) | 5 (0.53%) | 0.115 |
| Liver cirrhosis | 322 (5.28%) | 103 (10.93%) | 0.000* |
| Kidney transplant | 42 (0.69%) | 1 (0.11%) | 0.033* |
| Atherosclerosis | 796 (13.1%) | 99 (10.51%) | 0.029* |
| ICU admission before procedure | 568 (9.31%) | 145 (12.85%) | 0.000* |
| Coronary heart disease | 371 (6.08%) | 43 (4.56%) | 0.065 |
| Anemia | 3858 (63.27%) | 667 (70.81%) | 0.000* |
| Systolic blood pressure | 126.97±27.77 | 128.70±21.32 | 0.704 |
| Diastolic blood pressure | 78.36±12.65 | 77.00±12.05 | 0.606 |
| Blood glucose | 6.47±2.92 | 7.07±4.36 | 0.000* |
| RDW | 46.05±7.23 | 46.98±7.82 | 0.000* |
| NEUT% | 75.15±12.85 | 76.59±12.92 | 0.000* |
| NEUT | 7.80±5.36 | 8.26±5.79 | 0.033* |
| Lymphocytes% | 16.35±10.33 | 15.13±10.19 | 0.000* |
| Lymphocytes | 1.30±0.93 | 1.25±1.08 | 0.000* |
| Monocytes% | 6.37±3.12 | 6.15±3.24 | 0.000* |
| Monocytes | 0.58±0.48 | 0.57±0.38 | 0.240 |
| Platelets | 198.77±112.32 | 184.44±112.83 | 0.000* |
| Mean corpuscular volume | 91.25±7.60 | 91.51±7.27 | 0.440 |
| Mean corpuscular hemoglobin | 29.76±3.06 | 29.84±2.90 | 0.740 |
| Mean corpuscular hemoglobin concentration | 326.06±19.44 | 325.83±18.90 | 0.781 |
| Mean platelet volume | 11.05±1.47 | 11.11±1.43 | 0.107 |
| Eosinophils% | 1.54±2.16 | 1.41±2.02 | 0.054 |
| Eosinophils | 0.12±0.21 | 0.11±0.17 | 0.033* |
| Basophil% | 0.32±0.41 | 0.30±0.41 | 0.009* |
| Basophil | 0.02±0.04 | 0.03±0.05 | 0.329 |
| Reticolociti% | 1.59±0.76 | 1.59±0.70 | 0.000* |
| Reticolociti | 3.80±6.16 | 5.14±8.71 | 0.000* |
| Platelet distribution width | 14.92±2.67 | 14.95±2.61 | 0.074 |
| Platelet cell ratio | 0.23±0.11 | 0.22±0.12 | 0.000* |
| Total bilirubin | 32.65±59.44 | 37.15±74.15 | 0.808 |
| Albumin | 35.12±7.25 | 33.64±7.26 | 0.000* |
| Macro‐platelet cell ratio | 36.69±7.97 | 36.95±7.69 | 0.355 |
| Albumin: globulin ratio | 1.34±0.35 | 1.31±0.37 | 0.023* |
| Creatinine | 88.26±86.04 | 122.93±137.48 | 0.000* |
| Total protein | 62.06±10.91 | 60.19±11.93 | 0.011* |
| Globulin | 27.38±6.26 | 27.02±6.92 | 0.100 |
| Alanine aminotransferase | 67.44±161.33 | 72.64±150.05 | 0.353 |
| Urea | 6.16±4.66 | 8.23±7.56 | 0.000* |
| Uric acid | 258.82±130.17 | 271.21±146.68 | 0.009* |
| Direct bilirubin | 19.03±44.45 | 22.57±54.00 | 0.715 |
| Total bile acids | 15.31±38.35 | 18.44±43.96 | 0.133 |
| Aspartate amino transferase | 71.46±191.95 | 94.95±201.63 | 0.027* |
| Chlorine | 102.97±5.36 | 102.97±5.88 | 0.984 |
| Sodium | 137.91±5.60 | 135.88±9.97 | 0.000* |
| Potassium | 4.08±0.52 | 4.16±0.60 | 0.000* |
| Calcium | 2.77±5.61 | 3.08±13.85 | 0.232 |
| Hemoglobin | 114.43±26.36 | 108.60±27.62 | 0.000* |
| Hematocrit | 35.02±7.67 | 33.25±8.27 | 0.000* |
| Total cholesterol | 4.23±1.52 | 4.18±1.78 | 0.238 |
| LDL | 2.33±1.06 | 2.23±1.13 | 0.008* |
| HDL | 1.07±0.46 | 0.99±0.45 | 0.000* |
| Triglycerides | 1.75±2.35 | 2.24±4.23 | 0.000* |
| Thrombin time | 16.16±11.03 | 17.12±15.02 | 0.018* |
| Prothrombin time | 12.89±4.01 | 13.51±4.86 | 0.000* |
| Fibrinogen | 3.67±1.25 | 3.60±1.29 | 0.304 |
| APTT | 47.88±38.01 | 33.074±13.29 | 0.409* |
| International normalized ratio | 1.11±0.35 | 1.17±0.503 | 0.000* |
| Pulse | 82.49±14.43 | 85.72±17.13 | 0.000* |
| Diuretic | 1760 (28.86%) | 317 (33.65%) | 0.003* |
| ACEI | 695 (11.38%) | 86 (9.13%) | 0.039* |
| ARB | 248 (4.07%) | 26 (2.76%) | 0.054* |
| NSAIDs | 555 (9.10%) | 76 (8.07%) | 0.301 |
| Vitamin C | 2381 (39.04%) | 363 (38.53%) | 0.765 |
| Alprostadil | 690 (11.32%) | 125 (13.27%) | 0.081 |
| Dopamine | 268 (4.39%) | 54 (5.73%) | 0.067* |
| Cephalosporin | 1173 (19.24%) | 201 (21.34%) | 0.000* |
| Glycopeptides | 162 (2.66%) | 30 (3.18%) | 0.354 |
| Quinolone | 373 (6.12%) | 65 (6.90%) | 0.354 |
| Vancomycin | 49 (0.80%) | 4 (0.42%) | 0.211 |
| Acyclovir | 47 (0.77%) | 2 (0.21%) | 0.550 |
| Aminoglycoside | 643 (10.54%) | 96 (10.19%) | 0.742 |
| Statins | 882 (14.46%) | 98 (10.40%) | 0.001* |
| Asipirin | 851 (13.96%) | 100 (10.62%) | 0.005* |
ACEI indicates angiotensin‐converting enzyme inhibitor; APTT, activated partial thromboplastin time; ARB, angiotensin receptor blocker; CIN, contrast‐induced nephropathy; CKD, chronic kidney disease (defined as eGFR <60 mL/[min·1.73 m2); eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein cholesterol; ICU, intensive care unit; LDL, low‐density lipoprotein cholesterol; NSAIDs, nonsteroidal anti‐inflammatory drugs; PT, prothrombin time; RDW, red blood cell distribution width; TG, triglyceride; TT, thrombin time.
*P<0.05.
Eighty‐three variables including demographic information, comorbidities, medications, and laboratory values were collected for each patient. Among the 8800 patients, there were 1656 patients undergoing percutaneous coronary intervention, and of them, 195 (11.8%) suffered from CIN. The incidence in the other CM procedures can be seen in Table 2. Figure 1 shows the number of patients included in analysis after exclusion criteria had been applied. The baseline characteristics of the training cohorts (7040 patients) separated by CIN and non‐CIN status are presented in Table 1. The mean baseline eGFR in 7040 patients was 104.9 mL/(min·1.73 m2) (SD 53 mL/[min·1.73 m2]). Chronic kidney disease was present in 962 patients (13.7%).
Table 2.
The Incidence of CIN in Different CM Procedures
| CIN | Non‐CIN | Incidence | |
|---|---|---|---|
| Intravenous contrast‐enhanced CT | 734 | 4600 | 13.8% |
| Percutaneous coronary intervention | 195 | 1464 | 11.8% |
| CT angiography | 77 | 408 | 15.9% |
| Noncoronary angiography | 32 | 176 | 15.4% |
| Other CM procedures | 135 | 979 | 12.1% |
| Total | 1173 | 7627 | 13.3% |
CIN indicates contrast‐induced nephropathy; CM, contrast media; CT, computed tomography.
Figure 1.
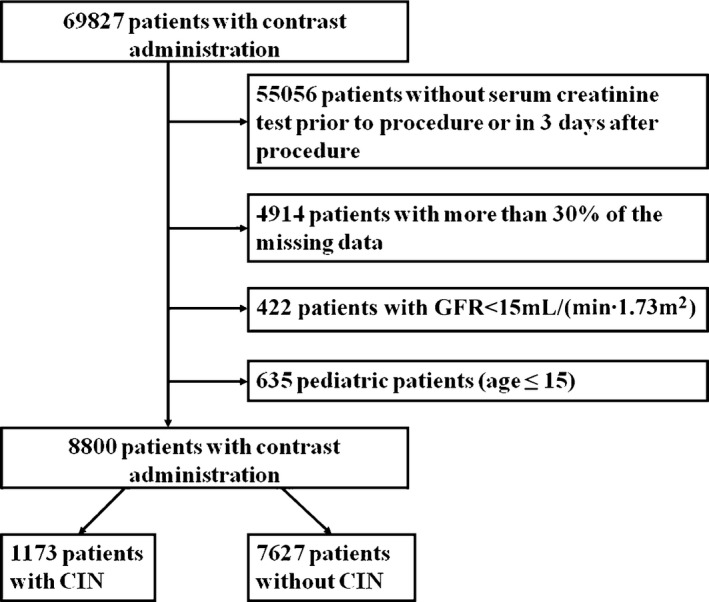
Flow chart depicting number of patients who were included in analysis after exclusion criteria. The total included encounters were divided into those with and without contrast‐induced nephrotoxicity (CIN). GFR indicates glomerular filtration rate.
Variables of Importance
In general, as more variables are chosen, the error of the model will be smaller. However, increasing the number of variables does not benefit clinical practice. To identify the prominent features, we carried out variable selection using different feature subsets by the RF method. Figure 2 shows the relationship between the cross‐validation error and the number of variables. When the variables increase to 13, the error has a sharp decrease to 0.18. With the variables increasing gradually to 83, the error still remains at a similar level.
Figure 2.
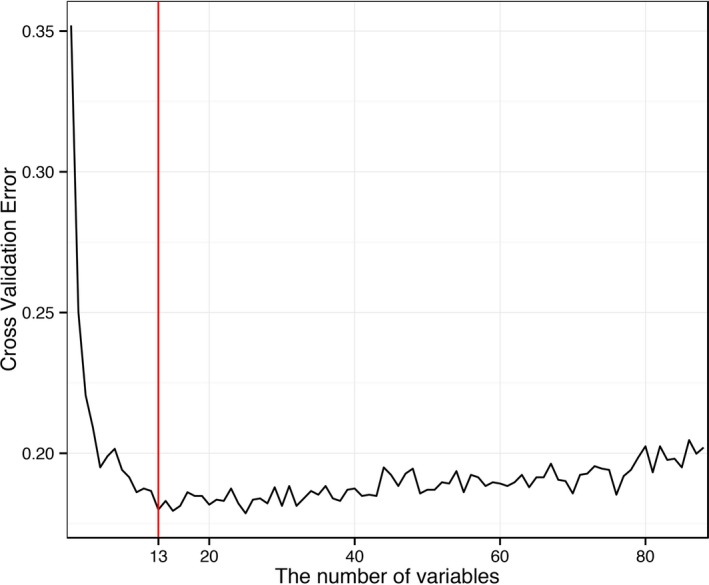
The relationship between the cross‐validation error and the number of variables.
Thus, our final model included 13 indispensable features for CIN prediction: baseline eGFR, red cell distribution width (RDW), triglycerides, the most recent serum creatinine before the procedure, high‐density lipoprotein cholesterol (HDL), total cholesterol, low‐density lipoprotein cholesterol (LDL), blood urea (BU), platelet larger cell ratio (P‐LCR), serum sodium (Na+), plateletocrit (PCT), international normalized ratio (INR), and blood glucose (BG). The importance of the 13 variables is demonstrated in Figure 3. The larger the importance number is, the more important the variable is. In addition, as shown in Table 3, the change of AUC value is also used to assess the importance of the 13 selected variables. There are some differences about the sorting on the importance of the variables as shown in Figure 3 and Table 3. In the first approach the serum creatinine is more important than serum sodium as shown in Figure 3. However, in the method based on AUC, the AUC change value of serum creatinine is bigger than that of serum sodium. Actually, the approach of the out‐of‐bag validation and Gini in Figure 3 pays more attention to the importance of the whole variables to the model, but the method of comparing the AUC change values after excluding each variable in Table 3 emphasizes the impact of individual variables on the model. Those differences do not affect the classification results.
Figure 3.
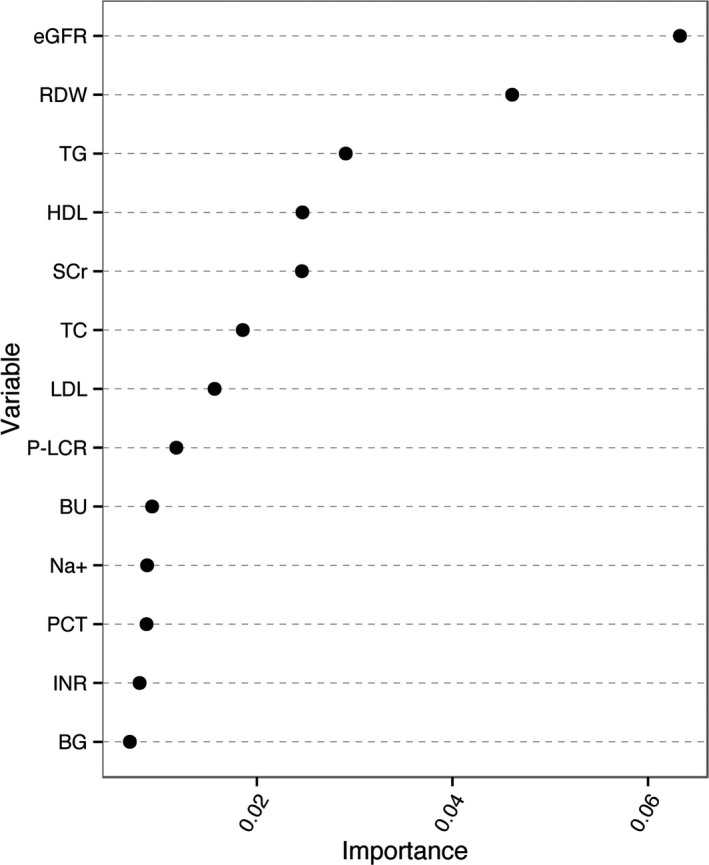
The importance of the 13 variables of the contrast‐induced nephropathy prediction model. BG indicates blood glucose; BU, blood urea; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein cholesterol; INR, International Normalized Ratio; LDL, low density lipoprotein cholesterol; Na+, serum sodium; PCT, plateletocrit; P‐LCR, platelet larger cell ratio; RDW, red cell distribution width; SCr, serum creatinine; TC, total cholesterol; TG, triglyceride.
Table 3.
The Change of AUC Value When Each Variable Is Excluded in the Model
| Variables | The Value of AUC After Excluding a Variable | The Change Value of AUC After Excluding a Variable |
|---|---|---|
| All | 0.907 | 0.000 |
| Baseline eGFR | 0.860 | 0.047 |
| Serum sodium | 0.867 | 0.040 |
| Red cell distribution width | 0.870 | 0.037 |
| Triglyceride | 0.870 | 0.037 |
| High‐density lipoprotein cholesterol | 0.871 | 0.036 |
| Low‐density lipoprotein cholesterol | 0.873 | 0.034 |
| Blood urea | 0.873 | 0.034 |
| Platelet–larger cell ratio | 0.873 | 0.034 |
| Blood glucose | 0.874 | 0.033 |
| Total cholesterol | 0.876 | 0.031 |
| International normalized ratio | 0.876 | 0.031 |
| Serum creatinine | 0.877 | 0.030 |
| Plateletocrit | 0.878 | 0.029 |
AUC indicates area under the receiver‐operating curves; eGFR, estimated glomerular filtration rate.
Classification Results
Classification of a patient in the RF was determined by the number of votes from all classification trees in the forest. We obtained different sensitivity, specificity, and accuracy while changing the threshold of voting. The receiver‐operating curve (ROC) was developed on basis of the sensitivity and specificity of the above values. The area under the ROC curve (AUC) is often used as an additional performance index. The closer AUC was to 1, the greater was the predictive ability of the model. A model with no predictive ability would yield the diagonal line. Figure 4 shows the ROC curve for this model. It is significant that our model obtained an AUC of 0.907. Such results sufficiently indicated that a big separation for CIN and non‐CIN patients was indeed obtained from this prediction model.
Figure 4.
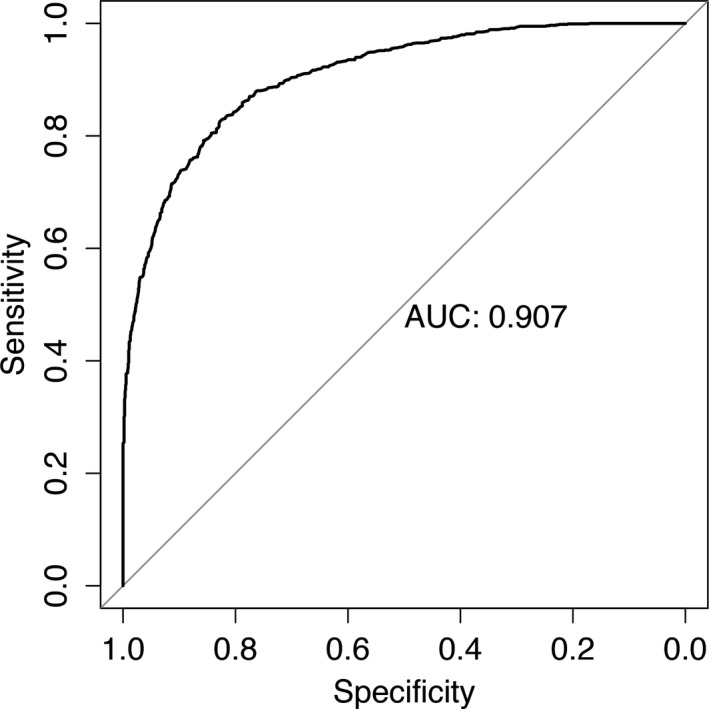
Cross‐validated receiver‐operating characteristic curves for predicting contrast‐induced nephropathy in the prediction model. AUC indicates area under the receiver‐operating curves.
The prediction accuracies of the model were internally evaluated by 5‐fold cross‐validation. On the average, the prediction model gave a prediction accuracy of 82.2%, sensitivity of 84.4%, specificity of 79.9%, and Matthews correlation coefficient of 64.4%.
In order to examine the performance of the newly developed model, we tested our training models based on a data set containing 231 patients with CIN and 1529 patients without CIN for an external validation. The basic information comparing the training and validation sets is showed in Table 4. The external validation achieved 82.4% for accuracy, 83.9% for sensitivity, 80.3% for specificity, and a Matthews correlation coefficient of 0.647, respectively. The result of high prediction accuracy and successful prediction suggested that the new model was efficiently used to predict CIN.
Table 4.
Demographic and Clinical Data in Training and Validation Cohorts
| Variable | Training Cohorts (n=7040) | Validation Cohorts (n=1760) | P Value |
|---|---|---|---|
| Sex (male) | 4410 (62.64%) | 995 (61.27%) | 0.303 |
| Age (y) | 55.31±14.91 | 55.42±14.71 | 0.800 |
| eGFR (mL/[min·1.73 m2]) | 104.86±53.47 | 105.19±60.73 | 0.222 |
| CKD | 961 (13.65%) | 233 (14.35%) | 0.463 |
| Diabetes | 884 (12.56%) | 212 (13.05%) | 0.587 |
| RDW | 46.17±7.32 | 46.08±7.31 | 0.138 |
| Triglycerides | 1.82±2.68 | 1.86±2.94 | 0.717 |
| HDL | 1.06±0.46 | 1.06±0.45 | 0.766 |
| Creatinine | 92.90±95.28 | 92.00±102.17 | 0.803 |
| LDL | 2.31±1.07 | 2.30±1.15 | 0.462 |
| Platelet‐cell ratio | 0.23±0.11 | 0.23±0.11 | 0.463 |
| Urea | 6.43±5.19 | 6.44±5.35 | 0.339 |
| Sodium | 137.64±6.40 | 137.71±6.26 | 0.811 |
| Macroplatelet‐cell ratio | 36.69±7.94 | 36.82±7.96 | 0.548 |
| Coronary heart disease | 414 (5.88%) | 81 (4.99%) | 0.162 |
| International normalized ratio | 1.12±0.38 | 1.12±0.47 | 0.736 |
| Blood glucose | 6.55±3.16 | 6.53±2.99 | 0.899 |
CKD indicates chronic kidney disease (defined as eGFR <60 mL/[min·1.73 m2); eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; RDW, red blood cell distribution width.
Discussion
With the increasing use of CM, CIN has become the third leading cause of hospital‐acquired acute renal failure, contributing to growing in‐hospital morbidity and mortality, hospitalization prolongation, and increase in costs.16, 17 Unfortunately, there are few definitively effective strategies for prophylaxis or treatment of CIN.18, 19 Therefore, it is necessary to establish a model involved in various comprehensive factors related to CIN that lets patients be protected from CM, especially for those who might be at high risk.
Several models have been developed for the prediction of CIN; however, they just focus on patients receiving intra‐arterial CM for coronary angiographic procedures, which represent only a small proportion of all contrast procedures.8, 9, 10, 11, 20, 21, 22, 23 In fact, contrast‐enhanced CT scans are much more commonly used, and the incidence of CIN resulting from contrast‐enhanced CT procedures is also high, occurring in 11% of an outpatient setting population.7 Thus, these prediction models might not be available for those who undergo these procedures, such as intravenous contrast‐enhanced CT and CT angiography. The Mehran risk score, a classic model for CIN, has widely been used for many years, but the risk factors included in the model occur only in patients receiving percutaneous coronary intervention. Furthermore, the volume of CM, a variable in this model, cannot be known before the procedure. So risk assessment cannot be completed before CM exposure. Although most of the previously established prediction models included both preprocedural and procedure‐related variables such as the volume of CM, few studies aimed to develop risk models for CIN before procedure. Liu et al11 developed a preprocedural model in a Chinese population with chronic total occlusion undergoing percutaneous coronary intervention including 3 periprocedural variables: age >75 years, LVEF <40%, and Scr >1.5 mg/dL. Some common risk factors are not included, such as diabetes, so it may not good at predicting CIN in the diabetes patients who are at higher risk of CIN. The other 3 models did not involve a Chinese population. Furthermore, all of these models also focus on coronary angiographic procedures, so they are only able to be used before coronary angiographic procedures.8, 9, 10, 11
Here, we developed a prediction model of CIN with preprocedure variables by RF, which was composed of Chinese patients administered CM. The new system was first established to provide a prediction model of contrast‐induced AKI using preprocedural variables in an unselected population. The AUC of this newly developed model was 0.907, demonstrating good discriminative power. Although the present model did not include procedure‐related variables, its predictive value was better than that of the Mehran risk score, whose AUC was 0.67. The prediction accuracies were internally evaluated by 5‐fold cross‐validation and tested by the test data set for an external validation. Thirteen of 83 variables were chosen in our risk prediction model for CIN. A strong relationship is found between decreased sodium and increased risk of CIN in patients who underwent CM administration. Additionally, INR is also observed to be a powerful factor affecting CIN prediction. In addition, we used the preprocedural glucose level in a CIN risk prediction model for the first time in the present study.
Our study is the first to show a relationship between decreased serum sodium and increased risk of CIN among patients who underwent CM administration. For 1 thing, hospital‐acquired lower serum sodium is found to coincide with various inflammatory conditions.24, 25 Inflammatory cytokines such as IL‐1β and IL‐6 have been reported as mediators in the development of hyponatremia related to ADH secretion.25, 26, 27 Inflammation is associated with impaired renal function. In addition, activation of the signaling pathway for inflammation by CM in human renal proximal tubular cells has been reported.28 Furthermore, some articles have shown that hyponatremia might be a surrogate marker for the severity of certain pathologies such as heart failure, pneumonia, and liver disease,29, 30 which may promote the development of CIN, so patients with lower plasma sodium are susceptible to CIN after CM exposure.
Our research indicates that baseline eGFR is an important risk factor, as has been found in previous studies.16, 31, 32 The eGFR of CIN patients was lower than that of non‐CIN patients in the previous studies because they got different eGFR values from using different preprocedural time points, such as the first admission time or 7 or 14 days before the procedure. However, in the present research, the eGFR from 14 days preprocedure of CIN is greater than that of non‐CIN patients. In addition, the most recent SCr before procedure is 1 of the strongest prediction factors for CIN development. The SCr of CIN is greater than that of non‐CIN patients, which is consistent with the previous studies. In addition, we also compared the increased value of Scr, defined as the most recent Scr value before CM procedures minus that at admission, between the CIN group and the non‐CIN group. This result showed that Scr increased 5.3% on average in the CIN group and decreased 5.2% in the non‐CIN group. In view of the above factors, the renal function of some patients is prone to be affected by medical interventions such as nephrotoxic agents and operations. Although these patients have a normal renal function at admission, Scr will increase rapidly under admission conditions and leave these patients more prone to develop CIN from CM.
Blood urea, a parameter for evaluation renal function, is also in our model, although the renal function is mainly evaluated by eGFR. Furthermore, blood urea plays a fundamental and direct role in fluid and sodium homeostasis regulated by neurohormonal systems.33, 34, 35, 36 The decreased intravascular effective volume and decompensated heart failure reduce the rate of urea excretion and increase blood urea levels.37, 38 The decreased intravascular effective volume would cause disturbances in intrarenal hemodynamics that potentially could result in CIN. Thus, blood urea levels provide an effective way to assess circulatory volume and play an important role in the prediction of CIN.
The RDW, reported routinely as part of an automated full blood count and used to evaluate the size of circulating red blood cells and the possible causes of anemia, is a main risk factor in the development of CIN. It has been found that RDW correlates with kidney function. Moreover, recent studies have reported an independent association between increased RDW and CIN in patients who underwent PCI.39, 40, 41 Mizuno et al added the RDW to the Mehran risk score for predicting CIN in patients with ST‐elevation acute myocardial infarction.42 Elevated RDW has been shown to be an effective biomarker for chronic inflammation and oxidative stress.43 Therefore, patients with an increased RDW may have a high level of oxidative stress and chronic inflammation, which may lead to renal dysfunction after CM administration.
Although several studies have indicated that elevated glucose level is a risk factor of CIN,44, 45, 46 our study found that the blood glucose level preprocedure enters the CIN risk prediction model in both diabetic and nondiabetic patients. Additionally, glycemic control using insulin in critically ill patients has been shown to reduce the rates of AKI.47, 48 The mechanism of the underlying relationship between acute hyperglycemia and the risk of CIN is still unknown. Studies demonstrate that elevated glucose levels are associated such factors as endothelial dysfunction,49 increased activation of prothrombotic factors,50, 51 markers of vascular inflammation,52, 53 and generation of reactive oxygen species.54, 55 An animal study has demonstrated that hyperglycemia exacerbates kidney damage through mitochondrial dysfunction.51 Such factors may lead to kidney impairment if patients are exposed to CM.
By examining the relationships among HDL cholesterol, LDL cholesterol, triglycerides, serum total cholesterol, and CIN, our study found that hypercholesterolemia, hypertriglyceridemia, or low HDL would raise the risk for CIN. Such blood lipid factors result in reducing the production of nitric oxide and increasing oxidative stress and inflammation in the kidney.56, 57, 58
For the first time, the elevated INR has been reported in a CIN prediction model. INR monitoring is essential during oral anticoagulation therapy to minimize bleeding complications and thrombotic events. INR elevation indicates that the glomerulus may hemorrhage, and red blood cell casts obstruct renal tubules.59 Thus, INR is an important risk factor for CIN.
We also find that platelet activity biomarkers may correlate with the development of CIN. Those reflecting the platelet reactivity, including platelet count (PC), platelet–larger cell ratio (P‐LCR), mean platelet volume (MPV), and platelet distribution width (PDW), were evaluated in this study. PC and P‐LCR, the index of the platelet reactivity, are significant variables in this model. Thrombocytopenia has often been cited as an indicator of critical illness severity,60, 61 and a novel association between thrombocytopenia and postoperative AKI has been established.61 Activated platelets have been found as a source of vasoactive inflammatory mediators related to the endothelial integrity,62 which is a key player in the development of CIN.
Conclusion
A risk prediction model with excellent predictive ability for CIN in Chinese patients has been successfully established. This model can be applied to patients administered CM for coronary procedures and other contrast procedures such as intravenous contrast‐enhanced CT, CT angiography, and noncoronary angiography. For the first time, there are 3 new factors included in the model: the decreased sodium concentration, the INR value, and the preprocedural glucose level.
Limitations
The potential limitations of our study should be mentioned. First, this study is limited by its retrospective design, whose inherent weakness cannot be avoided. Second, our prediction model is derived and validated by a single center. For the wide application of the prediction model, it still needs to be validated in a multicenter trial. Third, any variable that was missing for more than 30% of the population was not assessed in the present study. Finally, we ignored unstructured clinical notes. Future studies addressing these limitations are necessary.
Author Contributions
Yin and Zuo conceived and designed the study. Yin, Zuo, and Yi performed data acquisition and statistical analyses. Yin and Guan managed the patient database. All authors were involved in the data interpretation and discussion of the results. Yin, Wang, and Li prepared the figures. Yin and Zuo drafted the manuscript. All authors approved the final version of the manuscript.
Sources of Funding
This study was supported by the Program for New Century Excellent Talents in University (NCET‐13‐0605), the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (No. 20150218), National Natural Science Foundation of China (81102512), and the National Clinical Pharmacy Key Specialty Construction Project.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e004498. DOI: 10.1161/JAHA.116.004498.)
References
- 1. Nash K, Hafeez A, Hou S. Hospital‐acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. [DOI] [PubMed] [Google Scholar]
- 2. Tepel M, Aspelin P, Lameire N. Contrast‐induced nephropathy: a clinical and evidence‐based approach. Circulation. 2006;113:1799–1806. [DOI] [PubMed] [Google Scholar]
- 3. Sadat U. Radiographic contrast‐media‐induced acute kidney injury: pathophysiology and prophylactic strategies. ISRN Radiol. 2013;2013:496438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neyra JA, Shah S, Mooney R, Jacobsen G, Yee J, Novak JE. Contrast‐induced acute kidney injury following coronary angiography: a cohort study of hospitalized patients with or without chronic kidney disease. Nephrol Dial Transplant. 2013;28:1463–1471. [DOI] [PubMed] [Google Scholar]
- 5. Section 4: contrast‐induced AKI. Kidney Int Suppl. 2012;2:69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351:h4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell AM, Jones AE, Tumlin JA, Kline JA. Incidence of contrast‐induced nephropathy after contrast‐enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010;5:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inohara T, Kohsaka S, Abe T, Miyata H, Numasawa Y, Ueda I, Nishi Y, Naito K, Shibata M, Hayashida K, Maekawa Y, Kawamura A, Sato Y, Fukuda K. Development and validation of a pre‐percutaneous coronary intervention risk model of contrast‐induced acute kidney injury with an integer scoring system. Am J Cardiol. 2015;115:1636–1642. [DOI] [PubMed] [Google Scholar]
- 9. Maioli M, Toso A, Gallopin M, Leoncini M, Tedeschi D, Micheletti C, Bellandi F. Preprocedural score for risk of contrast‐induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med (Hagerstown). 2010;11:444–449. [DOI] [PubMed] [Google Scholar]
- 10. Gurm HS, Seth M, Kooiman J, Share D. A novel tool for reliable and accurate prediction of renal complications in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2013;61:2242–2248. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Liu YH, Chen JY, Tan N, Zhou YL, Li HL, Guo W, Duan CY, Chen PY. A simple pre‐procedural risk score for contrast‐induced nephropathy among patients with chronic total occlusion undergoing percutaneous coronary intervention. Int J Cardiol. 2015;180:69–71. [DOI] [PubMed] [Google Scholar]
- 12. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 13. Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. [DOI] [PubMed] [Google Scholar]
- 14. Studies on trachoma. World Health Organization. Report of the Fourth Scientific Group, WHO. Rev Int Trach. 1967;44:48–58. [PubMed] [Google Scholar]
- 15. Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods. 2009;14:323–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital‐acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. [DOI] [PubMed] [Google Scholar]
- 17. Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast‐induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. [DOI] [PubMed] [Google Scholar]
- 18. Weisbord SD, Palevsky M. Strategies for the prevention of contrast‐induced acute kidney injury. Curr Opin Nephrol Hypertens. 2010;19:539–549. [DOI] [PubMed] [Google Scholar]
- 19. Kwok CS, Pang CL, Yeong JK, Loke YK. Measures used to treat contrast‐induced nephropathy: overview of reviews. Br J Radiol. 2013;86:20120272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 21. Tziakas D, Chalikias G, Stakos D, Apostolakis S, Adina T, Kikas P, Alexoudis A, Passadakis P, Thodis E, Vargemezis V, Konstantinides S. Development of an easily applicable risk score model for contrast‐induced nephropathy prediction after percutaneous coronary intervention: a novel approach tailored to current practice. Int J Cardiol. 2013;163:46–55. [DOI] [PubMed] [Google Scholar]
- 22. Ozturk D, Celik O, Erturk M, Kalkan AK, Uzun F, Akturk IF, Akin F, Yildirim A. Utility of the logistic clinical syntax score in the prediction of contrast‐induced nephropathy after primary percutaneous coronary intervention. Can J Cardiol. 2016;32:240–246. [DOI] [PubMed] [Google Scholar]
- 23. Fu N, Li X, Yang S, Chen Y, Li Q, Jin D, Cong H. Risk score for the prediction of contrast‐induced nephropathy in elderly patients undergoing percutaneous coronary intervention. Angiology. 2013;64:188–194. [DOI] [PubMed] [Google Scholar]
- 24. Ortega O, Cobo G, Rodriguez I, Camacho R, Gallar P, Mon C, Herrero JC, Ortiz M, Oliet A, Di Gioia C, Vigil A. Lower plasma sodium is associated with a microinflammatory state among patients with advanced chronic kidney disease. Nephron Clin Pract. 2014;128:312–318. [DOI] [PubMed] [Google Scholar]
- 25. Ohta M, Ito S. Hyponatremia and inflammation. Rinsho Byori. 1999;47:408–416. [PubMed] [Google Scholar]
- 26. Swart RM, Hoorn EJ, Betjes MG, Zietse R. Hyponatremia and inflammation: the emerging role of interleukin‐6 in osmoregulation. Nephron Physiol. 2011;118:45–51. [DOI] [PubMed] [Google Scholar]
- 27. Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin‐6 in human disease. Ann Intern Med. 1998;128:127–137. [DOI] [PubMed] [Google Scholar]
- 28. Andreucci M, Lucisano G, Faga T, Bertucci B, Tamburrini O, Pisani A, Sabbatini M, Salzano S, Vitale M, Fuiano G, Michael A. Differential activation of signaling pathways involved in cell death, survival and inflammation by radiocontrast media in human renal proximal tubular cells. Toxicol Sci. 2011;119:408–416. [DOI] [PubMed] [Google Scholar]
- 29. Arroyo V, Rodés J, Gutiérrez‐Lizárraga MA, Revert L. Prognostic value of spontaneous hyponatremia in cirrhosis with ascites. Am J Dig Dis. 1976;21:249–256. [DOI] [PubMed] [Google Scholar]
- 30. Dargie HJ, Cleland JG, Leckie BJ, Inglis CG, East BW, Ford I. Relation of arrhythmias and electrolyte abnormalities to survival in patients with severe chronic heart failure. Circulation. 1987;75:IV98–IV107. [PubMed] [Google Scholar]
- 31. Gao YM, Li D, Cheng H, Chen YP. Derivation and validation of a risk score for contrast‐induced nephropathy after cardiac catheterization in Chinese patients. Clin Exp Nephrol. 2014;18:892–898. [DOI] [PubMed] [Google Scholar]
- 32. Chen YL, Fu NK, Xu J, Yang SC, Li S, Liu YY, Cong HL. A simple preprocedural score for risk of contrast‐induced acute kidney injury after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2014;83:E8–E16. [DOI] [PubMed] [Google Scholar]
- 33. Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol. 2005;288:F881–F896. [DOI] [PubMed] [Google Scholar]
- 34. Fenton RA. Essential role of vasopressin‐regulated urea transport processes in the mammalian kidney. Pflugers Arch. 2009;458:169–177. [DOI] [PubMed] [Google Scholar]
- 35. Cowley AJ. Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol. 1997;273:R1–R15. [DOI] [PubMed] [Google Scholar]
- 36. Sands JM, Layton HE. The physiology of urinary concentration: an update. Semin Nephrol. 2009;29:178–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lindenfeld J, Schrier RW. Blood urea nitrogen a marker for adverse effects of loop diuretics? J Am Coll Cardiol. 2011;58:383–385. [DOI] [PubMed] [Google Scholar]
- 38. Schrier RW. Blood urea nitrogen and serum creatinine: not married in heart failure. Circ Heart Fail. 2008;1:2–5. [DOI] [PubMed] [Google Scholar]
- 39. Akin F, Celik O, Altun I, Ayca B, Ozturk D, Satilmis S, Ayaz A, Tasbulak O. Relation of red cell distribution width to contrast‐induced acute kidney injury in patients undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2015;26:289–295. [DOI] [PubMed] [Google Scholar]
- 40. Zhao K, Li Y, Gao Q. Role of red blood cell distribution width in predicting contrast induced nephropathy in patients with stable angina pectoris undergoing percutaneous coronary intervention. Int J Cardiol. 2015;197:276–278. [DOI] [PubMed] [Google Scholar]
- 41. Akkoyun DC, Akyuz A, Kurt O, Bilir B, Alpsoy S, Güler N. Relationship between red cell distribution width and contrast‐induced nephropathy in patients who underwent primary percutaneous coronary intervention. Turk Kardiyol Dern Ars. 2015;43:613–620. [DOI] [PubMed] [Google Scholar]
- 42. Mizuno A, Ohde S, Nishizaki Y, Komatsu Y, Niwa K. Additional value of the red blood cell distribution width to the Mehran risk score for predicting contrast‐induced acute kidney injury in patients with ST‐elevation acute myocardial infarction. J Cardiol. 2015;66:41–45. [DOI] [PubMed] [Google Scholar]
- 43. Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, Fried LP. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women's Health and Aging Study I. Clin Nutr. 2010;29:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alpert MA, Carlino C. Pre‐procedural blood glucose levels: a new risk marker for contrast‐induced acute kidney injury in patients without diabetes with acute myocardial infarction. J Am Coll Cardiol. 2010;55:1441–1443. [DOI] [PubMed] [Google Scholar]
- 45. Stolker JM, McCullough PA, Rao S, Inzucchi SE, Spertus JA, Maddox TM, Masoudi FA, Xiao L, Kosiborod M. Pre‐procedural glucose levels and the risk for contrast‐induced acute kidney injury in patients undergoing coronary angiography. J Am Coll Cardiol. 2010;55:1433–1440. [DOI] [PubMed] [Google Scholar]
- 46. Naruse H, Ishii J, Hashimoto T, Kawai T, Hattori K, Okumura M, Motoyama S, Matsui S, Tanaka I, Izawa H, Nomura M, Ozaki Y. Pre‐procedural glucose levels and the risk for contrast‐induced acute kidney injury in patients undergoing emergency coronary intervention. Circ J. 2012;76:1848–1855. [DOI] [PubMed] [Google Scholar]
- 47. van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. [DOI] [PubMed] [Google Scholar]
- 48. Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. [DOI] [PubMed] [Google Scholar]
- 49. Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow‐mediated endothelium‐dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999;34:146–154. [DOI] [PubMed] [Google Scholar]
- 50. Gresele P, Guglielmini G, De Angelis M, Ciferri S, Ciofetta M, Falcinelli E, Lalli C, Ciabattoni G, Davì G, Bolli GB. Acute, short‐term hyperglycemia enhances shear stress‐induced platelet activation in patients with type II diabetes mellitus. J Am Coll Cardiol. 2003;41:1013–1020. [DOI] [PubMed] [Google Scholar]
- 51. Melin J, Hellberg O, Fellstrom B. Hyperglycaemia and renal ischaemia‐reperfusion injury. Nephrol Dial Transplant. 2003;18:460–462. [DOI] [PubMed] [Google Scholar]
- 52. Morohoshi M, Fujisawa K, Uchimura I, Numano F. Glucose‐dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes. 1996;45:954–959. [DOI] [PubMed] [Google Scholar]
- 53. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. [DOI] [PubMed] [Google Scholar]
- 54. Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor α gene expression in monocytic cells via hyperglycemia‐induced oxidant stress‐dependent and ‐independent pathways. J Biol Chem. 2000;275:17728–17739. [DOI] [PubMed] [Google Scholar]
- 55. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–2973. [DOI] [PubMed] [Google Scholar]
- 56. Prasad K. Vitamin E and regression of hypercholesterolemia‐induced oxidative stress in kidney. Mol Cell Biochem. 2014;385:17–21. [DOI] [PubMed] [Google Scholar]
- 57. Yang D, Lin S, Yang D, Shang W. Effects of short‐ and long‐term hypercholesterolemia on contrast‐induced acute kidney injury. Am J Nephrol. 2012;35:80–89. [DOI] [PubMed] [Google Scholar]
- 58. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. [DOI] [PubMed] [Google Scholar]
- 59. Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, Hebert L, Calomeni E, Nadasdy T. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54:1121–1126. [DOI] [PubMed] [Google Scholar]
- 60. Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30:1765–1771. [DOI] [PubMed] [Google Scholar]
- 61. Kertai MD, Zhou S, Karhausen JA, Cooter M, Jooste E, Li YJ, White WD, Aronson S, Podgoreanu MV, Gaca J, Welsby IJ, Levy JH, Stafford‐Smith M, Mathew JP, Fontes ML. Platelet counts, acute kidney injury, and mortality after coronary artery bypass grafting surgery. Anesthesiology. 2016;124:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. [DOI] [PubMed] [Google Scholar]


