Abstract
Background
The nonvitamin K antagonist oral anticoagulants have recently become available as an alternative to warfarin as stroke prophylaxis in atrial fibrillation, but data on real‐life patient experience, including bleeding risk, are lacking. Our objective was to compare major bleeding events and nonpersistence between the nonvitamin K antagonist oral anticoagulant apixaban and other nonvitamin K antagonist oral anticoagulants (dabigatran and rivaroxaban) and warfarin in a contemporary, nation‐wide cohort of patients with nonvalvular atrial fibrillation.
Methods and Results
Of 54 321 patients (median age, 73 years; 56% male; mean CHA 2 DS 2‐VASc score, 2.9), 7963, 6715, 15 413, and 24 230 patients initiated apixaban, rivaroxaban, dabigatran, and warfarin, respectively. Apixaban and rivaroxaban initiators were older, less often male, with higher HAS‐BLED and CHA 2 DS 2‐VASc scores compared with dabigatran and warfarin initiators. A total of 2418 patients (4.5%) experienced a major bleeding event over all available follow‐up. In this period, rivaroxaban (hazard ratio [HR] [95% CI], 1.49 [1.27–1.77]), dabigatran (HR, 1.17 [1.00–1.38]), and warfarin (HR, 1.23 [1.05–1.43]) users were significantly more likely to bleed than apixaban users. Findings were similar when restricted to the first 30 days after OAC initiation. Risk of nonpersistence was higher for dabigatran (HR, 1.45 [1.33–1.59]) and warfarin initiators (HR, 1.22 [1.12–1.33]), but not for rivaroxaban initiators (HR, 1.07 [0.96–1.20]) compared with apixaban initiators.
Conclusions
In a real‐world cohort of nonvalvular atrial fibrillation patients, apixaban had a lower adjusted major bleeding risk compared with rivaroxaban, dabigatran, and warfarin. Apixaban had a lower risk of nonpersistence compared with dabigatran and warfarin and similar risk compared with rivaroxaban.
Keywords: anticoagulant, atrial fibrillation, bleeding
Subject Categories: Epidemiology, Atrial Fibrillation, Anticoagulants, Complications
Introduction
Nonvitamin K antagonist oral anticoagulants (NOACs) may have a large impact on standard stroke prevention and management in atrial fibrillation (AF). Predictable pharmacodynamics and kinetics with fewer drug‐drug and drug‐food interactions compared with warfarin may simplify their use for health care professionals and patients. Several major trials have shown on‐par or better efficacy and safety compared with warfarin.1, 2, 3 In these trials, apixaban and low‐dose dabigatran had lower risk of major bleeding compared with warfarin, whereas the risk was similar for rivaroxaban and high‐dose dabigatran against warfarin. Despite considerable interest in the NOACs in recent years, data about their use and performance in real‐life clinical care are limited. In particular, there is a need for safety data looking at any differences in bleeding risk and persistence between NOACs in routine care. Also, whereas previous studies suggest an initially increased bleeding risk with use of warfarin, it is unknown whether this should be a concern when initiating an NOAC.4
European and American guidelines now support NOACs as an option in first‐line oral anticoagulant (OAC) therapy for patients with nonvalvular AF with 1 or more stroke risk factors.5, 6 However, transition from randomized evidence to actual clinical practice is key to optimize the management of OAC drug use in patients with AF. Data from Danish administrative registries provide comprehensive data on actual clinical practice and patient outcomes. We therefore aimed to assess and compare shorter‐term (within 30 days) and longer‐term major bleeding events and persistence comparing apixaban, rivaroxaban, dabigatran, and warfarin using Danish data sets.
Methods
Registries
From nation‐wide administrative registries, we extracted information on hospitalizations, vital status, and pharmacological treatment for each patient using a personal and unique identifier.7 The National Patient Registry contains information on hospital discharge codes with both primary and secondary diagnoses. Diagnoses are recorded by the International Classification of Diseases (ICD) 8th (until 1994) and 10th revisions (from 1995 and onward). The registry also holds information on surgical procedures according to the NOMESCO classification.7 The National Prescription Registry includes information on prescription drugs in terms of strength, number of tablets, and date of prescription according to Anatomical Therapeutical Classification codes.8 The Civil Personal Registry holds information on vital status, sex, and age.9 We use cross‐linkage through secured servers to link patients, hospitalization diagnoses, and prescription information at the individual level over time. All registries included information up to December 31, 2015. Codes used are in Table 1.
Table 1.
Codes Used
| ICD/ATC/NOMESCO Codes | Comment | |
|---|---|---|
| Inclusion criteria | ||
| AF |
Presence of: ICD8: 42793, 42794. ICD10: I48. Absence of: ICD8: 4240, 4241, 39500–39502, 39508, 39509, 39600–39604, 39608, 39609. ICD10: I05, I06, I080A, I081A, I082A, I083A, Z952, Z954. NCSP: KFKD, KFKH, KFMD, KFMH, KFGE, KFJF |
Defined from diagnosis of AF with the absence of diagnosis codes of valvular AF and mitral or aortic valve surgery |
| OAC treatment | ATC: B01AA03, B01AE07, B01AF01, B01AF02 | Includes use of warfarin, apixaban, rivaroxaban, and dabigatran |
| Exclusion criteria | ||
| Recent venous thromboembolism | ICD‐10: I801–3, I808–9, I821–3, I8289 | |
| Recent orthopedic surgery | NOMESCO: KNFB, KNFC, KNGB, KNGC | |
| Outcome | ||
| Major bleeding | ICD‐10: D62, I60, I61, I62, R31, R04, D500, H313, H356, H431, H450, I312, I850, K250, K252, K254, K256, K260, K262, K264, K266, K270, K272, K274, K276, K280, K282, K284, K286, K625, K661, K920, K921, K922, S064, S065, S066, J942, K228F, K298A, K638B, K638C, K838F, K868G, I864A, H052A, S368D, G951A | Defined from diagnosis of intracranial bleeding, major gastrointestinal bleeding, respiratory, renal/urinary tract, ocular, retroperitoneal or pericardial bleeding, and bleeding attributed to anemia. Only bleedings requiring hospitalization were used |
| Comorbidity/medication | ||
| Hypertension | ATC: C02A, C02B, C02C, C02L, C03A, C03B, C03D, C03E, C03X, C07B, C07C, C07D, C08G, C02DA, C09BA, C09DA, C02DB, C02DD, C02DG, C07A, C07B, C07C, C07D, C07F, C08, C09BB, C09DB, C09AA, C09BA, C09BB, C09CA, C09DA, C09DB, C09XA02, C09XA52 | Defined from combination treatment with at least 2 classes of antihypertensive drugs: adrenergic α‐antagonists, nonloop diuretics, vasodilators, beta‐blockers, calcium‐channel blockers, and renin‐angiotension system inhibitors |
| Stroke | ICD‐10: DI63, DI64, DI74, G458, G459 | Includes transient ischemic attack |
| Vascular disease | ICD‐10: I21, I22, I700, I702, I703, I704, I705, I706, I707, I708, I709 | Includes myocardial infarction and peripheral artery disease |
| Chronic kidney disease | ICD‐10: N02, N03, N04, N05, N06, N07, N08, N11, N12, N14, N18, N19, N26, N158, N159, N160, N162, N163, N164, N168, Q612, Q613, Q615, Q619, E102, E112, E132, E142, I120, M300, M313, M319, M321B | Defined from diagnosis of chronic glomerulonephritis, chronic tubulointestinal nephropathy, diabetic, and hypertensive nephropathy among others |
| Liver disease | ICD‐10: B15, B16, B17, B18, B19, C22, K70, K71, K72, K73, K74, K75, K76, K77, Z944, I982, D684C, Q618A | Defined from diagnosis of liver chronic liver disease, cirrhosis, and hepatitis |
| Alcohol abuse | ICD‐10: F10, K70, E52, T51, K860, E244, G312, I426, O354, Z714, Z721, G621, G721, K292, L278A, ATC: N07BB | Defined from alcohol‐related diagnosis codes or at least 1 dispensed prescription of an alcohol antagonist drug used to treat chronic alcoholism |
| Bleeding | ICD‐10: D62, I60, I61, I62, R31, R04, D500, H313, H356, H431, H450, I312, I850, K250, K252, K254, K256, K260, K262, K264, K266, K270, K272, K274, K276, K280, K282, K284, K286, K625, K661, K920, K921, K922, S064, S065, S066, J942, K228F, K298A, K638B, K638C, K838F, K868G, I864A, H052A, S368D, G951A | |
| Heart failure | ICD10: I42, I50, I110, J81 | |
| Diabetes mellitus | ATC: A10 | Codes for glucose‐lowering medication |
| Aspirin | ATC: B01AC06, N02BA01 | |
| Nonaspirin antiplatelets | ATC: B01AC22, B01AC24, B01AC04 | Includes clopidogrel, prasugrel, ticagrelor |
| NSAID | ATC: M01A | Excludes glucosamin (M01AX05) |
AF indicates atrial fibrillation; ICD, International Classification of Diseases; NSAID, nonsteroidal anti‐inflammatory drug; OAC, Oral anticoagulant.
Study Population and OAC Treatment
We identified all patients aged 18 years or older with a history of nonvalvular AF and grouped them into 4 cohorts according to the first OAC (apixaban, rivaroxaban, dabigatran, and warfarin) initiated in the study period (August 22, 2011 to December 31, 2015; Figure 1). We defined new initiation as no OAC prescription 6 months before inclusion; hence, the population consisted of “naïve” OAC users.10 Date of first prescription claim defined the date of inclusion. We only included dosages recommended and approved by the Danish Health and Medicines Authority for thromboprophylaxis in AF (apixaban 2.5 and 5 mg twice‐daily [BID; approved December 10, 2012], dabigatran 110 and 150 mg BID [approved August 22, 2011], and rivaroxaban 15 and 20 mg once‐daily (OD; approved February 6, 2012]). We excluded patients with recent (<6 months) venous thromboembolism or pulmonary embolism or recent (<5 weeks) hip or knee prosthetic implantation surgery to ensure patients received OAC treatment for AF.
Figure 1.
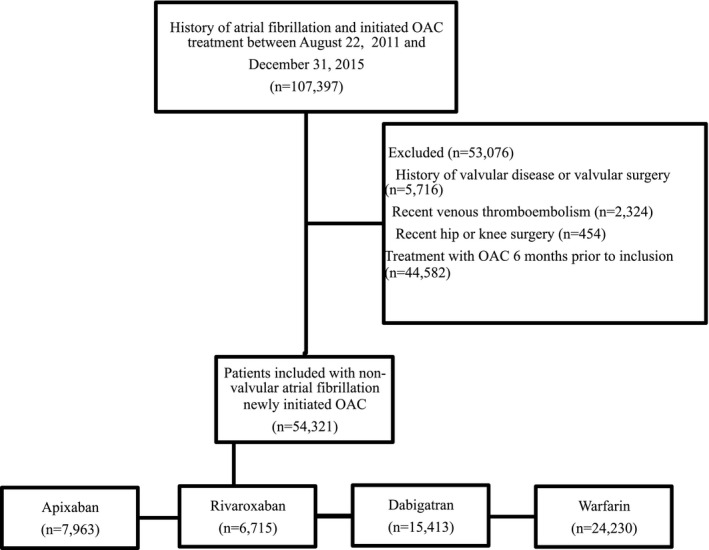
Overview of the study population. OAC indicates oral anticoagulant.
We defined exposure periods to the different OAC treatments during follow‐up using all prescription claims during the study period. Patients were considered exposed to a specific OAC when tablets were available for consumption. The algorithm to calculate this, as previously published, uses the date, number, and strength of tablets claimed for each individual from the national prescription registry.11 If hospitalization occurs, no consumption of tablets was recorded given that hospitals deliver medication during hospital stays. Exposure to more than 1 OAC was not allowed, so any subsequent prescription claim for a different OAC defined treatment switch. Patients could stop or switch OAC treatment during follow‐up according to availability of tablets (for definition of duration of exposure, please see below). The NOAC, edoxaban, was not available in Denmark in the study period.
Comorbidity and Concomitant Pharmacotherapy
Stroke risk factors comprising the CHA2DS2‐VASc (congestive heart failure, hypertension, age >75 years, diabetes mellitus, stroke/transitional cerebral ischemia, vascular disease, age 65–74 year, or female sex) score were defined from the registries as previously published.12 The score has been shown to accurately predict risk of stroke in a similar population with AF. Bleeding risk factors comprising a modified HAS‐BLED (hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [>65 years], drugs [nonsteroidal anti‐inflammatory drug {NSAID}, antiplatelets, alcohol]) score were defined in a similar manner.13 Labile INR was not included because INR values were not available. Antiplatelet treatment at baseline was defined by prescription of either aspirin and nonaspirin (ie, clopidogrel, ticagrelor, and prasugrel) antiplatelet agents in the 6 months preceding inclusion.
Major Bleeding Complications
We defined major bleeding events as first hospitalization associated with a code for bleeding and reported bleeding overall and by site, including gastrointestinal and intracranial bleedings (Table 1). This definition includes all relevant thrombolysis in myocardial infarction major bleeds14 and has been used previously.11 We examined first bleeding events during the full follow‐up time available in the registries (“total bleeding”) and also restricted to the first 30 days after OAC initiation (“30‐day bleeding”).
Persistence
We defined nonpersistence as no claim for the same as well as for any OAC for at least 30 days following the end of last OAC treatment period (ie, a treatment gap of more than 30 days).
Statistical Analyses
For baseline characteristics, we described continuous variables by the mean and SD or median and interquartile range (IQR). For the persistence analyses, we calculated the duration of each OAC treatment period as the time from inclusion date to nonpersistence date as defined above (of note and as described above—switch of treatment before date of nonpersistence was allowed). For the bleeding analyses, we calculated the duration from inclusion date to first bleeding event, censoring at date of nonpersistence. For both sets of analyses, we also censored at date of claim for more than 1 OAC drug (because there is no valid indication for more than 1 concurrent OAC), death, and end of study. Figure 2 illustrates allocation of major bleeding and nonpersistence outcome and switch of treatment according to OAC exposure.
Figure 2.
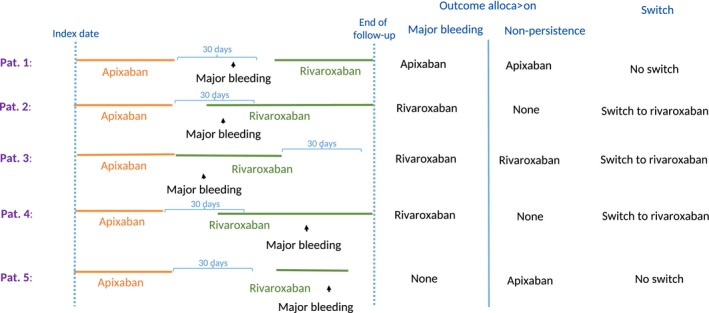
Examples of allocation of major bleeding and nonpersistence outcome and switch of treatment according to OAC exposure. OAC indicates oral anticoagulant.
We estimated risk of major bleeding and of nonpersistence by Cox regression models adjusted for age, sex, calendar year, variables in the CHA2DS2‐VASc and HAS‐BLED scores, and switch of OAC treatment. For all analyses, apixaban was used as reference. For the primary outcomes, additional analyses using warfarin as reference were conducted. We calculated cumulative incidences of major bleeding and nonpersistence (accounting for competing risks of death and switching OAC) for the first 3 years of follow‐up. Because of the time‐varying OAC exposure, we only reported cumulative incidence for patients’ first OAC.
The only clinically relevant interactions found was sex, so we undertook additional stratified analyses of major bleeding (P for interaction, 0.0016) and nonpersistence (P for interaction, <0.0001) by sex. For the nonpersistence outcome, the initial risk would differ between warfarin/rivaroxaban and apixaban/dabigatran, because typical warfarin and rivaroxaban pack sizes last for 100 days, whereas shorter for apixaban (50 days) and dabigatran (30 days), that is, the initial length of exposure differed between OAC. Hence, we performed a landmark analysis at the 90‐day mark. No other violation of model assumptions was found.
We ran the following preplanned, supplemental analyses: (1) stratified dabigatran use by 110 and 150 mg BID patients including an analysis for patients under the age of 80 years only (ie, excluding patients where 150 mg of BID is not indicated), given that different dosages were investigated separately in the RE‐LY trial; (2) major bleeding complications and nonpersistence from when all NOACs were available (January 1, 2013 [Defined data of “Debut apixaban”]) to minimize selection bias caused by change of NOAC availability during follow‐up; and (3) stratification by to patients who did and who did not switch between OAC drugs given that switchers may be a different population from nonswitchers.
All analyses used the SAS statistical package (version 9.4; SAS Institute Inc., Cary, NC) and R (version 4.1; R Foundation for Statistical Computing).
Ethics
This study was approved by the Danish Data Protection Agency (reference no. 2007‐58‐0015; internal reference: GEH‐2014‐013 I‐suite no. 02731). Information was made available to us by encrypted data access with anonymization of patients so that no individuals could be identified. By Danish law, retrospective studies do not require ethical committee approval.
Results
Population and Follow‐up
We identified 54 321 patients with nonvalvular AF who initiated OAC treatment. Median age was 73 years (IQR, 66–81), 56% were male, and mean CHA2DS2‐VASc and HAS‐BLED scores were 2.9 (SD, 1.6) and 2.2 (SD, 1.2), respectively. Mean follow‐up time per patient was 403 days and total patient‐time at‐risk was 67 764 person‐years. Patient characteristics by OAC initiated are in Table 2. Patients initiating apixaban and rivaroxaban were older and less often male, with higher CHA2DS2‐VASc scores, compared with dabigatran or warfarin users. According to year of inclusion, apixaban and rivaroxaban initiation was more frequent at the end of the study period whereas dabigatran and warfarin initiation declined. Characteristics of dabigatran 110 and 150 mg BID initiators are in Table 3. Dabigatran 110 mg BID users were considerably older (mean age, 80.1 vs 65.6 years) with higher mean HAS‐BLED score (2.5 vs 1.8) compared to dabigatran 150 mg BID users. Among warfarin initiators, 11.4% (n=2752) subsequently switched to an NOAC (564 to apixaban, 758 to rivaroxaban, and 1430 to dabigatran, comprising 20.5%, 27.5%, and 52.0%, respectively). Among initiators of an NOAC, 9.4% (n=2070) subsequently switched to warfarin. Switchers between specific OAC treatments are shown in Table 4. Mean follow‐up time ranged from 268 to 511 days between OAC groups (Table 5).
Table 2.
Characteristics of the Study Population
| Apixaban | Rivaroxaban | Dabigatran | Warfarin | |
|---|---|---|---|---|
| No. of patients | 7963 | 6715 | 15 413 | 24 230 |
| Low dosage (%)/high dosage (%) | 3010 (37.8)/4953 (62.2) | 1840 (27.4)/4875 (72.6) | 6234 (40.4)/9179 (59.6) | N/A |
| Age, median [IQR] | 76 [68, 84] | 74 [67, 83] | 71 [65, 79] | 73 [65, 80] |
| Age, mean (SD) | 75.4 (11.10) | 74.4 (11.0) | 71.5 (11.0) | 72.1 (11.3) |
| Male sex (%) | 4049 (50.8) | 3490 (52.0) | 8740 (56.7) | 14 159 (58.4) |
| Year of inclusion (%) | ||||
| 2011 | — | — | 1465 (9.5) | 4481 (18.5) |
| 2012 | — | 447 (6.7) | 4506 (29.2) | 6658 (27.5) |
| 2013 | 632 (7.9) | 1737 (25.9) | 4596 (29.8) | 5358 (22.1) |
| 2014 | 2794 (35.1) | 1662 (24.8) | 3608 (23.4) | 4263 (17.6) |
| 2015 | 4537 (57.0) | 2869 (42.7) | 1238 (8.0) | 3470 (14.3) |
| CHA2DS2‐VASc score, mean (SD) | 3.15 (1.62) | 3.02 (1.62) | 2.73 (1.60) | 2.91 (1.66) |
| CHA2DS2‐VASc score, median [IQR] | 3 [2, 4] | 3 [2, 4] | 3 [2, 4] | 3 [2, 4] |
| CHA2DS2‐VASc score (%) | ||||
| Low (score 0) | 353 (4.4) | 294 (4.4) | 1162 (7.5) | 1675 (6.9) |
| Intermediate (score 1) | 923 (11.6) | 938 (14.0) | 2451 (15.9) | 3363 (13.9) |
| High (score >1) | 6687 (84.0) | 5483 (81.7) | 11 800 (76.6) | 19 192 (79.2) |
| HAS‐BLED score, mean (SD) | 2.25 (1.20) | 2.21 (1.16) | 2.05 (1.17) | 2.18 (1.22) |
| HAS‐BLED score, median [IQR] | 2 [1, 3] | 2 [1, 3] | 2 [1, 3] | 2 [1, 3] |
| HAS‐BLED score (%) | ||||
| Low (score 0–1) | 2273 (28.5) | 1944 (29.0) | 5115 (33.2) | 7312 (30.2) |
| Intermediate (score 2) | 2404 (30.2) | 2129 (31.7) | 4863 (31.6) | 7178 (29.6) |
| High (score >2) | 3286 (41.3) | 2642 (39.3) | 5435 (35.3) | 9740 (40.2) |
| Stroke (%) | 1692 (21.2) | 1228 (18.3) | 2451 (15.9) | 3597 (14.8) |
| Vascular disease (%) | 828 (10.4) | 619 (9.2) | 1426 (9.3) | 3343 (13.8) |
| Liver disease (%) | 115 (1.4) | 88 (1.3) | 166 (1.1) | 390 (1.6) |
| Heart failure (%) | 1362 (17.1) | 1120 (16.7) | 2442 (15.8) | 5128 (21.2) |
| Diabetes mellitus (%) | 1031 (12.9) | 830 (12.4) | 1785 (11.6) | 3354 (13.8) |
| Hypertension (%) | 3463 (43.5) | 3092 (46.0) | 6907 (44.8) | 11 699 (48.3) |
| Kidney disease (%) | 391 (4.9) | 276 (4.1) | 330 (2.1) | 1860 (7.7) |
| Bleeding (%) | 1159 (14.6) | 811 (12.1) | 1816 (11.8) | 3261 (13.5) |
| Alcohol misuse (%) | 287 (3.6) | 227 (3.4) | 522 (3.4) | 753 (3.1) |
| Nonaspirin antiplatelets (%) | 917 (11.5) | 690 (10.3) | 1274 (8.3) | 2173 (9.0) |
| Aspirin antiplatelet (%) | 2982 (37.4) | 2697 (40.2) | 6212 (40.3) | 10 267 (42.4) |
| NSAIDs (%) | 1119 (14.1) | 951 (14.2) | 2279 (14.8) | 3379 (13.9) |
IQR indicates interquartile range; N/A, not applicable; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Table 3.
Patient Characteristics and Risk of Major Bleeding According to Initial Dabigatran Dosage
| Characteristics | Dabigatran 110 mg BID (n=6234) | Dabigatran 150 mg BID (n=9179) |
|---|---|---|
| Males, n (%) | 2793 (44.8) | 5947 (64.8) |
| Age, y | ||
| Mean (SD) | 80.1 (8.1) | 65.6 (8.6) |
| Median (IQR) | 81 [76, 85] | 67 [61, 72] |
| CHA2DS2‐VASc score, mean (SD) | 3.7 (1.4) | 2.1 (1.4) |
| CHA2DS2‐VASc score, median [IQR] | 4 [3, 5] | 2 [1, 3] |
| CHA2DS2‐VASc score (%) | ||
| Low (score 0) | 47 (0.8) | 1115 (12.1) |
| Intermediate (score 1) | 237 (3.8) | 2214 (24.1) |
| High (score >1) | 5950 (95.4) | 5850 (63.7) |
| HAS‐BLED score, mean (SD) | 2.48 (1.06) | 1.76 (1.15) |
| HAS‐BLED score, median [IQR] | 2 [2, 3] | 2 [1, 3] |
| HAS‐BLED score (%) | ||
| Low (score 0–1) | 1181 (18.9) | 3934 (42.9) |
| Intermediate (score 2) | 1982 (31.8) | 2881 (31.4) |
| High (score >2) | 3071 (49.3) | 2364 (25.8) |
| Major bleedinga | ||
| 30‐day major bleeding | 1.62 [1.11–2.38] | 1.53 [0.99–2.36] |
| Total follow‐up | 1.29 [1.09–1.52] | 0.98 [0.81–1.20] |
| 30‐day major bleeding (age <80 years) | 0.89 [0.46–1.70] | 1.26 [0.75–2.10] |
| Total follow‐up (age <80 years) | 1.07 [0.82–1.38] | 0.92 [0.73–1.17] |
| Nonpersistencea | ||
| Nonpersistence | 1.23 [1.12–1.36] | 1.65 [1.50–1.80] |
BID indicates twice‐daily; IQR, interquartile range.
Presented as adjusted hazard ratio (apixaban is reference).
Table 4.
Number and Percentage of First‐Time Switchers
| Initiation OAC Group | Switch to (Row %) | ||||
|---|---|---|---|---|---|
| Apixaban | Rivaroxaban | Dabigatran | Warfarin | Total No. of Switchers (% of Total Initiated) | |
| Apixaban (n=7963) | — | 93 (22) | 63 (15) | 270 (63) | 426 (5) |
| Rivaroxaban (n=6715) | 182 (27) | — | 198 (29) | 305 (45) | 685 (10) |
| Dabigatran (n=15 413) | 550 (20) | 703 (26) | — | 1495 (54) | 2748 (19) |
| Warfarin (n=24 230) | 564 (20) | 758 (28) | 1430 (52) | — | 2752 (11) |
OAC indicates oral anticoagulant.
Table 5.
Risk of Major Bleeding and Nonpersistence
| Apixaban | Rivaroxaban | Dabigatran | Warfarin | |
|---|---|---|---|---|
| Availability of follow‐up, days | ||||
| Mean (SD) | 268.4 (220.1) | 348.5 (324.1) | 511.4 (438.0) | 398.0 (374.6) |
| Median (IQR) | 214 (85–397) | 230 (89–549) | 392 (119–821) | 251 (129–564) |
| Population (Total No. of Events) | No. Events | HR [95% CI] | No. Events | HR [95% CI] | No. Events | HR [95% CI] | No. Events | HR [95% CI] | |
|---|---|---|---|---|---|---|---|---|---|
| Major bleeding | |||||||||
| Total major bleeding | 54 321 (2418) | 252 | Reference | 343 | 1.49 [1.27–1.77] | 695 | 1.17 [1.00–1.38] | 1128 | 1.23 [1.05–1.43] |
| 30‐day major bleeding | 54 321 (448) | 52 | Reference | 71 | 1.69 [1.18–2.43] | 123 | 1.64 [1.15–2.34] | 202 | 1.41 [1.01–1.96] |
| Nonpersistence | |||||||||
| Risk of 30‐day gap in treatment | 54 321 (14 800) | 688 | Reference | 605 | 1.07 [0.96–1.20] | 3418 | 1.45 [1.33–1.59] | 10 089 | 1.22 [1.12–1.33] |
HR indicates hazard ratio; IQR, interquartile range.
Major Bleeding Complications
Of all included patients, 4.5% (2418) experienced a major bleeding event during total follow‐up. Most common bleeding sites were gastrointestinal, renal, and intracranial (Table 6). Crude incidence rates during follow‐up were 3.6 (252 events), 4.3 (343 events), 2.9 (695 events), and 3.9 (1128 events) per 100 person‐years for apixaban, rivaroxaban, dabigatran, and warfarin, respectively. Figure 3 shows cumulative incidences of major bleeding event by OAC initiated. Table 5 shows adjusted risk of total and 30‐day major bleeding for the different OAC groups. Compared with apixaban, rivaroxaban, dabigatran, and warfarin were all associated with increased total and 30‐day major bleeding risk. Compared with warfarin, rivaroxaban was associated with a higher and apixaban with a lower total major bleeding risk (hazard ratio [HR], 0.82 [0.70–0.95]); dabigatran had a similar total major bleeding risk to warfarin. The 30‐day major bleeding risk was lower for apixaban (HR, 0.71 [0.51–0.99]) compared to warfarin and comparable for rivaroxaban (HR, 1.20 [0.90–1.60]) and dabigatran (HR, 1.17 [0.93–1.47]). In the sex‐stratified analyses (Table 7), there was an increased total major bleeding risk versus apixaban for rivaroxaban, but not for dabigatran or warfarin, among men. There was an increased total major bleeding risk for all OACs versus apixaban among women.
Table 6.
Distribution and Type of Major Bleeding
| Type of Major Bleeding | No. (%) |
|---|---|
| Intracranial | 399 (17) |
| Gastrointestinal | 1022 (42) |
| Respiratory | 276 (11) |
| Pericardial | 16 (1) |
| Ocular | 19 (1) |
| Renal/urinary | 454 (19) |
| Anemia | 232 (10) |
| Total | 2418 (100) |
Figure 3.
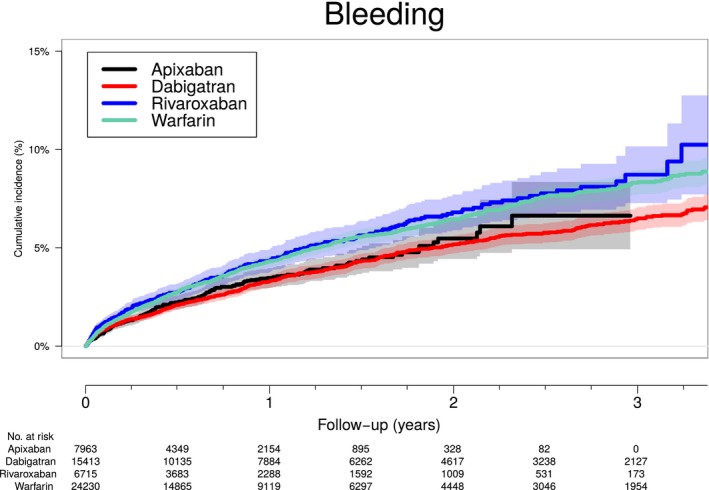
Cumulative incidence of major bleeding according to OAC treatment. Cumulative incidence of major bleeding according to OAC treatment (nonswitch patients only) taking into account competing of death. OAC indicates oral anticoagulant.
Table 7.
Sex‐Stratified Analyses of Risk of Major Bleeding and Nonpersistence
| Population (Total No. of Events) | Apixaban | Rivaroxaban | Dabigatran | Warfarin | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. Events | HR [95% CI] | No. Events | HR [95% CI] | No. Events | HR [95% CI] | No. Events | HR [95% CI] | ||
| Major bleeding | |||||||||
| Total major bleeding (Female) | 23 883 (966) | 102 | Reference | 133 | 1.47 [1.12–1.91] | 308 | 1.45 [1.13–1.85] | 423 | 1.46 [1.14–1.86] |
| Total major bleeding (Male) | 30 438 (1452) | 150 | Reference | 210 | 1.51 [1.22–1.88] | 387 | 1.01 [0.82–1.24] | 705 | 1.08 [0.89–1.32] |
| 30‐day major bleeding (Female) | 23 883 (182) | 18 | Reference | 32 | 2.31 [1.29–4.15] | 49 | 2.15 [1.20–3.86] | 83 | 2.06 [1.19–3.57] |
| 30‐day major bleeding (Male) | 30 438 (266) | 34 | Reference | 39 | 1.35 [0.85–2.16] | 74 | 1.36 [0.87–2.14] | 119 | 1.08 [0.71–1.64] |
| Nonpersistence | |||||||||
| Risk of 30 day gap in treatment (female) | 23 883 (6226) | 260 | Reference | 235 | 1.10 [0.92–1.32] | 1307 | 1.64 [1.43–1.89] | 4424 | 1.66 [1.45–1.90] |
| Risk of 30‐day gap in treatment (male) | 30 438 (8574) | 428 | Reference | 370 | 1.05 [0.91–1.20] | 2111 | 1.32 [1.18–1.47] | 5665 | 0.97 [0.88–1.08] |
HR indicates hazard ratio.
Nonpersistence
Of all included patients, 14 800 (27.2%) experienced a break of at least 30 days during follow‐up. Figure 4 shows the cumulative incidence of nonpersistence by OAC initiated for patients who did not switch OAC. After ≈3 years, 15% of apixaban and rivaroxaban, 30% of dabigatran, and 60% of warfarin patients were nonpersistent. In the adjusted analyses with apixaban as reference, dabigatran or warfarin initiators were more likely to be nonpersistent whereas rivaroxaban initiators had similar risk (Table 5). In the adjusted analyses with warfarin as reference, apixaban (HR, 0.82 [0.75–0.89]) and rivaroxaban (HR, 0.88 [0.81–0.95]) had lower risk of nonpersistence whereas dabigatran had a higher risk (HR, 1.19 [1.14–1.24]). Sex‐stratified analyses are in Table 7. In the landmark analyses of nonpersistence, dabigatran (HR, 1.57 [1.34–1.85]) had an increased risk, warfarin (HR, 0.31 [0.26–0.37]) a decreased risk, and rivaroxaban a similar risk (HR, 1.01 [0.81–1.26]) compared with apixaban during the first 90 days of follow‐up. After the first 90 days of follow‐up, the risk of nonpersistence was increased for all OACs compared with apixaban, that is, dabigatran (HR, 1.56 [1.41–1.74]), rivaroxaban (HR, 1.20 [1.05–1.36]), and warfarin (HR, 1.76 [1.59–1.95]).
Figure 4.
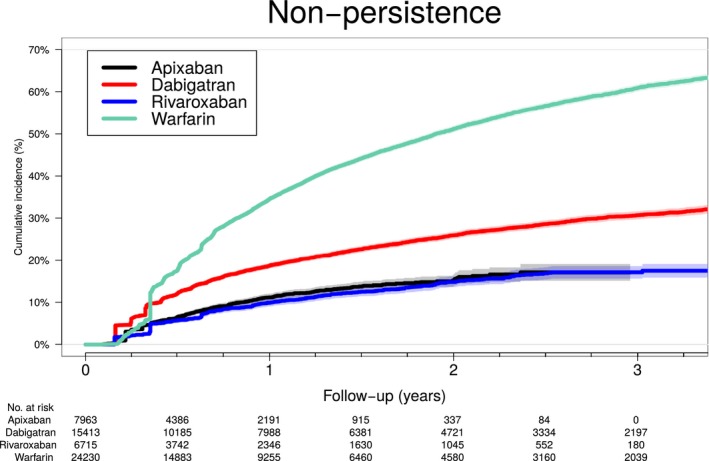
Cumulative incidence of nonpersistence according to OAC treatment. Cumulative incidence of nonpersistence (defined as more than a 30‐day gap in treatment) according to OAC treatment (nonswitch patients only) taking into account competing of death. OAC indicates oral anticoagulant.
Supplementary Analyses
Compared with apixaban, we found similar 30‐day and total major bleeding risk for dabigatran 150 mg BID, but higher major bleeding risk for dabigatran 110 mg BID (Table 3). There were no differences in major bleeding risk with dabigatran 150 mg or dabigatran 110 mg BID compared to apixaban in patients aged <80 years. Findings (major bleeding and nonpersistence) were similar to those in the main analyses when restricted to the period with availability of all NOACs and for OAC switch and nonswitch patients (Table 8).
Table 8.
Risk of Major Bleeding and Nonpersistence Following Full NOAC Availability and According to Switch Status
| Population (Total No. of Events) | Apixaban | Rivaroxaban | Dabigatran | Warfarin | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. Events | HR [95% CI] | No. Events | HR [95% CI] | No. Events | HR [95% CI] | No. Events | HR [95% CI] | ||
| Major bleedinga | |||||||||
| Total major bleeding (debut apixaban) | 36 762 (1427) | 244 | Reference | 283 | 1.48 [1.24–1.76] | 337 | 1.15 [0.96–1.37] | 563 | 1.23 [1.04–1.45] |
| 30‐day major bleeding (debut apixaban) | 36 762 (312) | 52 | Reference | 65 | 1.66 [1.15–2.40] | 70 | 1.53 [1.04–2.24] | 125 | 1.45 [1.03–2.04] |
| Total major bleeding (OAC nonswitch) | 54 321 (2180) | 221 | Reference | 280 | 1.49 [1.25–1.79] | 612 | 1.13 [0.95–1.34] | 1067 | 1.21 [1.02–1.42] |
| 30‐day major bleeding (OAC nonswitch) | 54 321 (440) | 51 | Reference | 70 | 1.73 [1.20–2.49] | 118 | 1.62 [1.13–2.32] | 201 | 1.42 [1.02–1.99] |
| Total major bleeding (OAC switch) | 6611 (238) | 31 | Reference | 63 | 1.51 [0.98–2.34] | 83 | 1.47 [0.95–2.29] | 61 | 1.25 [0.80–1.97] |
| Nonpersistence | |||||||||
| Risk of 30‐day gap in treatment (debut apixaban) | 36 762 (6921) | 683 | Reference | 505 | 1.02 [0.91–1.15] | 1756 | 1.50 [1.37–1.65] | 3977 | 1.20 [1.10–1.30] |
| Risk of 30‐day gap in treatment (OAC nonswitch) | 54 321 (14 597) | 664 | Reference | 579 | 1.07 [0.96–1.20] | 3371 | 1.45 [1.32–1.58] | 9983 | 1.22 [1.12–1.33] |
| Risk of 30‐day gap in treatment (OAC switch) | 1001 (203) | 24 | Reference | 26 | 1.01 [0.58–1.77] | 47 | 1.97 [1.16–3.33] | 106 | 1.13 [0.71–1.80] |
HR indicates hazard ratio; NOAC, nonvitamin K antagonist oral anticoagulant; OAC, oral anticoagulant.
Too few events to assess 30‐day major bleeding risk in OAC switch patients.
Discussion
This study reports contemporary data on OAC use and safety in real‐life nonvalvular AF patients following the marketing of NOACs apixaban, dabigatran, and rivaroxaban. We had 3 main findings. First, apixaban and rivaroxaban initiators had a different clinical profile from dabigatran and warfarin initiators, because they were older and less often male, with higher CHA2DS2‐VASc and HAS‐BLED scores. Second, apixaban had lower 30‐day and total major bleeding risk compared with rivaroxaban, dabigatran, and warfarin. Third, dabigatran and warfarin users were at higher risk of nonpersistence compared to apixaban users, whereas rivaroxaban users had similar risk.
The 3 major trials, comparing NOACs with warfarin—ARISTOTLE (apixaban), RE‐LY (dabigatran), and ROCKET‐AF (rivaroxaban)—have resulted in grade 1 recommendations by American and European cardiac societies.5, 6 American guidelines recommend NOAC in patients with AF with at least 2 stroke risk factors whereas European Society of Cardiology guidelines support NOAC use with 1 or more risk factors (excluding female sex without other stroke risk factors). Indirect comparisons of trial data showed no noteworthy difference in efficacy between NOACs, but a potentially lower risk of major bleeding for apixaban.15, 16
Overall, the rate of major bleeding events ranged from 2.9 to 4.3 per 100 person‐years in our study. These are higher than those reported in trial data (2.1–3.6%/year) and are readily explained by inclusion of older and unselected patients in this real‐world setting. In addition, definitions of bleeding were not identical, given that our study included only serious bleeding requiring hospitalization whereas trial events were adjudicated from clinical workup. Given that new agents are approved beyond a controlled setting, the addition of real‐world complications should be appreciated because the degree of clinically meaningful events from trials have been recognized as a major limitation.17 Dabigatran had the lowest major bleeding rate, whereas apixaban had a lower adjusted risk of major bleeding (both total and within 30 days of initiation) than rivaroxaban, dabigatran, and warfarin. A recent post‐hoc analysis of ROCKET‐AF showed that rivaroxaban users had significantly higher risk of major and nonmajor gastrointestinal bleeding compared with warfarin users (HR, 1.42), which support potential differences found between apixaban and rivaroxaban in the present study.18 A study of elderly Medicare beneficiaries found that dabigatran users had increased gastrointestinal bleeding compared to warfarin users, although overall major bleeding risk was similar.19 In contrast to the clinical trials, we found the increased major bleeding risk most pronounced for females taking dabigatran and warfarin compared to apixaban. There was no difference by sex in bleeding risk in the ARISTOTLE (apixaban) and ROCKET‐AF (rivaroxaban) trials; RE‐LY did not report these data. Although not supported by the pivotal trials, a meta‐analysis including 7 trials of NOACs concluded that women have a higher bleeding risk than men in acute venous thromboembolism treated by NOAC.20 Possible mechanisms include differences in creatinine clearance, volume distribution, and drug uptake between men and women.21 Our finding suggests a need for future investigations (preferably randomized) of sex‐related differences in bleeding between OAC drugs.
When separating dabigatran into 110 and 150 mg BID, we confirmed previous registry‐based findings that lower‐dose dabigatran was associated with higher major bleeding risk when compared to warfarin.22, 23 This is in contrast to RE‐LY and partly in contrast to results from a Medicare claims analysis.19 The latter study consisted of an elderly AF population (aged >65 years) using the nontrial dosage of dabigatran 75 mg BID and reported only gastrointestinal (nonsignificant increase in bleeding with high‐dose dabigatran) and intracranial bleeding (nonsignificant increase in bleeding with low‐dose dabigatran). Our finding may be influenced by unmeasured confounders or selection bias: For example, dabigatran 110 mg BID users were, on average, >14 years older and had higher HAS‐BLED (2.5 vs 1.8) than dabigatran 150 mg BID users. When restricting the analyses to patients aged <80 years (ie, excluding patients where only low dosage is recommended), we found no differences in major bleeding risk using apixaban as reference. Thus, prescribing physicians may systematically select patient for lower dosages because of perceived (and possibly correctly) increased bleeding risk, which is reflected in the observed higher bleeding rates in the dabigatran 110 mg group. Studies investigating potential physician preferences and prescription patterns according to dosages are most welcome. In the clinical trials, discontinuation risk was lower for apixaban (2310 of 9088 [25.4%] vs 2493 of 9052 [27.5%] patients), but higher for rivaroxaban (23.7% vs 22.2%) and dabigatran (14.5–15.5% vs 10.2%) compared with warfarin. In our study, the cumulative incidence curve of nonpersistence (Figure 4) showed better persistence to the NOACs than to warfarin. Although definitions of persistence between the trials and the present study are not readily comparable, it is reassuring that these new agents seem to yield persistence in 70% to 85% of patients after 3 years; this is considerably higher than the 40% observed for warfarin. However, it should be recognized that the method used is based on prescription claims. Following the initiation of treatment, some treatment episodes last longer (warfarin and rivaroxaban), attributed to differences in pack sizes, and this likely affect the results. Notably, findings from our landmark analyses suggest that warfarin users initially had very good persistence, but when looking beyond the first 90 days, we found, from the cumulative incidence and the adjusted Cox model, higher persistence for apixaban and rivaroxaban and lower for dabigatran and warfarin. The fact that gastrointestinal side effects have frequently been reported in dabigatran users could potentially be a mechanism behind our finding that dabigatran users were more likely to be nonpersistent compared with apixaban users.19, 24 Several compliance studies have shown that persistence to treatment is strongly affected by the number of tablets taken daily.25 Perhaps surprisingly, our data suggested that rivaroxaban (given OD) users did not have lower risk of nonpersistence compared with apixaban users (given BID). Periods off treatment are very worrisome given short half‐life elimination (range, 10–17 hours) and may place patients at markedly increased thromboembolic risk.26 The degree of suboptimal protection needs to be investigated further as well as any markers of interruption of treatment.
Do these data aid in the selection of OAC for the practicing clinician and patients? We found highest persistence to apixaban and rivaroxaban, fewer major bleeding complications with apixaban overall, and some evidence that women taking dabigatran and warfarin may be at further increased risk of major bleeding compared to men. Even though causality is a challenge in observational data, these findings warrant attention. Our findings suggest that, in a real‐life clinical setting, uptake and safety issues differ between NOACs. Clarification for health care providers and patients will require future observational studies with information on prescription patterns (including sex differences) and assessment of effectiveness, given that randomization between NOACs seems unimaginable at the moment.
Limitations
Despite providing updated and real‐life data on experience from OAC use, our study is limited by its observational nature. The Danish population consists of a racially homogeneous population of mainly whites, which makes extrapolation of the results to other populations limited. Although indications of use should be similar across the different OAC cohorts in our study, the potential for selection bias should be recognized. Concerning unmeasured confounders, we did not have information on left ventricle ejection fraction, on tobacco use, degree of potential renal dysfunction, body mass index, or patient and physician preferences. Our definition of major bleeding includes all episodes requiring hospitalization; and hospital databases have shown high precision for registration of bleeding events.27 The diagnostic coding for AF has previously been validated with high positive predictive value (97%).28 Also, our analyses do not take local or regional recommendations or policies of first‐line OAC use into account. We confirm data from a previous study that the proportions initiating OAC treatment change over time (dabigatran and warfarin initiation declined, whereas apixaban and rivaroxaban increased).29 The decline of warfarin use is most likely explained by increased NOAC use, whereas the decline in dabigatran partly can be explained that elderly is less likely to be initiated.29, 30 The observation period of especially apixaban was shorter compared to the other cohorts attributed to later entrance to market. The possibility exists that treatment courses that span multiple prescriptions claims might be subject to more episodes off treatment. However, we do not believe this would strongly affect our findings given that we took the switch to another OAC into account (and also performed analyses with censoring at time of switch), performed sensitivity analyses in the period when all NOACs were available, and used methods that allowed for continuous exposure assessment. All prescriptions of OAC are captured by the databases, but our method used to define OAC exposure is an approximation. Given that actual intake of tablets is unknown (data based on prescription claims) and likely to be closely related to a recent prescription claim, this could have affected our findings. However, because there is only partial reimbursement of drug expenses in Denmark, patient copayment would indicate an intention to take the claimed prescription. Furthermore, given that analyses of shorter‐term major bleeding risk (within 30 days) are consistent with results from the total follow‐up period, we believe our method to be valid in assessing an association between OAC exposure and bleeding.
Conclusions
In a contemporary real‐life cohort of nonvalvular AF patients, differences in major bleeding complications and persistence between OAC therapies exist. Apixaban had lower 30‐day and total major bleeding risk compared to rivaroxaban, dabigatran, and warfarin. Nonpersistence was highest for dabigatran and warfarin, compared to apixaban and rivaroxaban. Given that the landscape of stroke prevention in AF is rapidly evolving, results from nonrandomized studies of potential safety issues need to come to the attention to patients and health care providers. As more real‐world data on NOACs accumulate, future studies should also focus on effectiveness to help inform optimal treatment decisions.
Author Contributions
Lamberts and Gislason had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Lamberts, Staerk, Olesen, Fosbøl, Hansen, and Gislason are responsible for the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, and approval of the manuscript. The sponsors of the study, Bristol Meyers‐Squibb/Pfizer (Harboe, Lefevre, Evans), contributed with the design and conduct of the study and preparation, review, and approval of the manuscript.
Sources of Funding
The study was supported by Bristol Meyers‐Squibb/Pfizer.
Disclosures
Lamberts reports receiving speaker fees from Bristol Meyers‐Squibb and Bayer. Staerk has no disclosures to report. Olesen reports receiving speaker fees from Bristol‐Myers Squibb and Boehringer Ingelheim, and funding for research from the Lundbeck Foundation, Bristol‐Myers Squibb, and The Capital Region of Denmark, Foundation for Health Research. Fosbøl reports receiving an independent research grant from Janssen Pharmaceutical. Hansen reports receiving speaker fees from Bristol Meyers‐Squibb. Harboe is currently employed at Bristol Meyers‐Squibb (Denmark). Lefevre is currently employed at Bristol Meyers‐Squibb (France). Evans has formerly been employed at Bristol Meyers‐Squibb (France). Gislason reports receiving speaker fees from AstraZeneca, Pfizer, and Bristol‐Myers Squibb. Gislason reports research grants from Bristol‐Myers Squibb and a scientific advisor position for AstraZeneca.
(J Am Heart Assoc. 2017;6:e004517. DOI: 10.1161/JAHA.116.004517.)
References
- 1. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; Investigators RA . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 2. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; Committees A, Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; Committee R‐LS, Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 4. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest P . Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e44S–e88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; Guidelines ESCCfP . 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice G . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kildemoes HW, Sorensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. [DOI] [PubMed] [Google Scholar]
- 10. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 11. Lamberts M, Lip GY, Hansen ML, Lindhardsen J, Olesen JB, Raunso J, Olsen AM, Andersen PK, Gerds TA, Fosbol EL, Torp‐Pedersen C, Gislason GH. Relation of nonsteroidal anti‐inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161:690–698. [DOI] [PubMed] [Google Scholar]
- 12. Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp‐Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olesen JB, Lip GY, Hansen PR, Lindhardsen J, Ahlehoff O, Andersson C, Weeke P, Hansen ML, Gislason GH, Torp‐Pedersen C. Bleeding risk in ‘real world’ patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost. 2011;9:1460–1467. [DOI] [PubMed] [Google Scholar]
- 14. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 15. Schneeweiss S, Gagne JJ, Patrick AR, Choudhry NK, Avorn J. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lip GY, Mitchell SA, Liu X, Liu LZ, Phatak H, Kachroo S, Batson S. Relative efficacy and safety of non‐vitamin K oral anticoagulants for non‐valvular atrial fibrillation: network meta‐analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol. 2016;204:88–94. [DOI] [PubMed] [Google Scholar]
- 17. Steg PG, Huber K, Andreotti F, Arnesen H, Atar D, Badimon L, Bassand JP, De Caterina R, Eikelboom JA, Gulba D, Hamon M, Helft G, Fox KA, Kristensen SD, Rao SV, Verheugt FW, Widimsky P, Zeymer U, Collet JP. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2011;32:1854–1864. [DOI] [PubMed] [Google Scholar]
- 18. Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh EY, Becker RC, Singer DE, Halperin JL, Hankey GJ, Berkowitz SD, Fox KA, Patel MR. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol. 2015;66:2271–2281. [DOI] [PubMed] [Google Scholar]
- 19. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. [DOI] [PubMed] [Google Scholar]
- 20. Loffredo L, Violi F, Perri L. Sex related differences in patients with acute venous thromboembolism treated with new oral anticoagulants. A meta‐analysis of the interventional trials. Int J Cardiol. 2016;212:255–258. [DOI] [PubMed] [Google Scholar]
- 21. Group EUCCS , Regitz‐Zagrosek V, Oertelt‐Prigione S, Prescott E, Franconi F, Gerdts E, Foryst‐Ludwig A, Maas AH, Kautzky‐Willer A, Knappe‐Wegner D, Kintscher U, Ladwig KH, Schenck‐Gustafsson K, Stangl V. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016;37:24–34. [DOI] [PubMed] [Google Scholar]
- 22. Sorensen R, Gislason G, Torp‐Pedersen C, Olesen JB, Fosbol EL, Hvidtfeldt MW, Karasoy D, Lamberts M, Charlot M, Kober L, Weeke P, Lip GY, Hansen ML. Dabigatran use in Danish atrial fibrillation patients in 2011: a nationwide study. BMJ Open. 2013;3:e002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsen TB, Rasmussen LH, Skjoth F, Due KM, Callreus T, Rosenzweig M, Lip GY. Efficacy and safety of dabigatran etexilate and warfarin in “real‐world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–2273. [DOI] [PubMed] [Google Scholar]
- 24. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hernandez Madrid A, Potpara TS, Dagres N, Chen J, Larsen TB, Estner H, Todd D, Bongiorni MG, Sciaraffia E, Proclemer A, Cheggour S, Amara W, Blomstrom‐Lundqvist C. Differences in attitude, education, and knowledge about oral anticoagulation therapy among patients with atrial fibrillation in Europe: result of a self‐assessment patient survey conducted by the European Heart Rhythm Association. Europace. 2016;18:463–467. [DOI] [PubMed] [Google Scholar]
- 26. Heidbuchel H, Vrijens B. Non‐vitamin K antagonist oral anticoagulants (NOAC): considerations on once‐ vs. twice‐daily regimens and their potential impact on medication adherence. Europace. 2015;17:1317–1318. [DOI] [PubMed] [Google Scholar]
- 27. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112:1736–1742. [DOI] [PubMed] [Google Scholar]
- 29. Staerk L, Fosbol EL, Gadsboll K, Sindet‐Pedersen C, Pallisgaard JL, Lamberts M, Lip GY, Torp‐Pedersen C, Gislason GH, Olesen JB. Non‐vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: temporal trends 2011–2015 in Denmark. Sci Rep. 2016;6:31477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. AbuDagga A, Stephenson JJ, Fu AC, Kwong WJ, Tan H, Weintraub WS. Characteristics affecting oral anticoagulant therapy choice among patients with non‐valvular atrial fibrillation: a retrospective claims analysis. BMC Health Serv Res. 2014;14:310. [DOI] [PMC free article] [PubMed] [Google Scholar]


