Abstract
Background
Elevated high‐sensitivity C‐reactive protein (hsCRP) has been associated with increased risks of adverse outcomes of various cardiovascular diseases. The relationship between hsCRP and the prognosis of hypertrophic cardiomyopathy remains to be evaluated.
Methods and Results
The study used an observational cohort methodology. A total of 490 patients were enrolled in the Fuwai Hospital from 2001 to 2011 and were followed for 3.7±2.0 years. According to the risk category of hsCRP, subjects in the high hsCRP group (>3.0 mg/L) had a higher risk of developing adverse events than the low hsCRP group (<1.0 mg/L): cardiovascular death (adjusted hazard ratios[HR] 5.41, 95% CI 1.96–14.93, P=0.001), all‐cause mortality (adjusted HR 4.78, 95% CI 1.99–11.47, P<0.001), sudden cardiac death (adjusted HR 11.29, 95% CI 1.38–92.20, P=0.024), and heart failure–related death (adjusted HR 4.38, 95% CI 1.15–16.60, P=0.030). Similarly, the continuous variable of hsCRP was also an independent predictor for adverse outcomes: cardiovascular death (adjusted HR 1.15, 95% CI 1.06–1.25, P=0.001), all‐cause mortality (adjusted HR 1.17, 95% CI 1.09–1.26, P<0.001), sudden cardiac death (adjusted HR 1.20, 95% CI 1.06–1.36, P=0.003), and heart failure–related death (adjusted HR 1.15, 95% CI 1.02–1.30, P=0.020).
Conclusions
Our results indicate that elevated plasma hsCRP is associated with increased risk for adverse outcomes in patients with hypertrophic cardiomyopathy.
Keywords: cardiovascular death, high‐sensitivity C‐reactive protein, hypertrophic cardiomyopathy, prognosis
Subject Categories: Hypertrophy, Cardiomyopathy
Introduction
Hypertrophic cardiomyopathy (HCM) is one of the most common monogenic inherited cardiovascular diseases.1 The disease affects all age groups, with marked clinical heterogeneity, ranging from a normal lifespan without symptoms to poor outcomes such as sudden cardiac death (SCD), advanced heart failure, or stroke.2, 3 Although several factors have been proposed for risk stratification in patients with HCM, including family history of sudden death, unexplained syncope, degree of left ventricular wall thickness, resting left ventricular outflow tract obstruction, nonsustained ventricular tachycardia, and congestive symptoms, the clinical outcomes of HCM are still largely unpredictable.1, 4, 5
Inflammation is a well‐established risk factor for the development of cardiovascular disease.6 Currently, C‐reactive protein level, a nonspecific inflammatory marker,7 has been associated with adverse events in various cardiovascular diseases, including myocardial infarction,6, 8 heart failure,9, 10 ischemic stroke,6, 11 atrial fibrillation,12, 13 type 2 diabetes mellitus,14, 15 and hypertension.16 A previous study showed that the plasma level of high‐sensitivity C‐reactive protein (hsCRP) was higher in patients with HCM.17 These findings provide evidence that the plasma levels of hsCRP are closely involved in the development of HCM clinical outcomes. However, the prognostic significance of hsCRP in the clinical outcomes of HCM remains to be evaluated.
Methods
Study Population
The study used an observational cohort methodology. In total, 543 subjects were invited to participate in the study at Fuwai Hospital from 2001 to 2011. Patients with autoimmune or inflammatory disease (6 patients), active infections (1 patient), and cancer (2 patients) were excluded. Forty‐four patients (8.2%) were lost during follow‐up in the study (the baseline characteristics of the 44 patients with HCM according to risk category of hsCRP are listed in Table S1. The final study subjects consisted of 490 patients (Figure S1). HCM was ascertained by an unexplained maximal left ventricle wall thickness ≥15 mm on echocardiography and/or cardiac magnetic resonance imaging in the absence of other cardiac or systemic diseases capable of producing that magnitude of hypertrophy.1, 3 All of the patients gave informed consent to participate in this study, which was approved by the ethics committee of Fuwai Hospital. This observational cohort study was performed in keeping with the requirements of the Declaration of Helsinki.
Clinical Data Collection
The data of baseline characteristics and clinical outcomes of patients with HCM were prospectively collected with prospectively designed data forms by experienced physicians. Finally, the data were entered into the network database (Likang Times Technology Co. Ltd, Beijing, China). The twice‐entry method was used for data entry. When the values of the 2 entries were consistent, the data would enter the database. Otherwise, the system would automatically flag an error, and it could then be corrected by checking the raw data. Twice data entry of the same sample was performed by different people.
HsCRP Measurement
Baseline blood samples were obtained at enrollment. After collection, the blood samples were rapidly centrifuged at 1800 g for 10 minutes and the separated plasma samples were immediately stored at −70°C until analysis. The plasma level of hsCRP was measured using the Immunoturbidimetric Assay (Orion Diagnostica, Finland). All measurements were performed in the laboratory at Fuwai Hospital.
Clinical Outcomes
The end points of this study were cardiovascular death and all‐cause mortality. The cardiovascular death included SCD, heart failure–related death, and stroke‐related death. SCD was defined as witnessed, unexpected, and instantaneous collapse leading to death within 1 hour of the onset of symptoms. Death related to heart failure was defined as death preceded by symptoms of heart failure >1 hour.
Statistical Analysis
Participants were divided into 3 groups according to risk category of hsCRP (low hsCRP group <1.0 mg/L, median hsCRP group 1.0–3.0 mg/L, high hsCRP group >3.0 mg/L)18 or according to tertiles of hsCRP level. The baseline characteristics among the 3 groups were analyzed by ANOVA for parametric variables, the Kruskal–Wallis test for nonparametric variables, and the χ2 test for categorical variables. Survival estimates were calculated by the Kaplan–Meier method and the log‐rank test was used for comparison. The association between hsCRP and development of adverse outcomes of HCM were estimated with univariate and multivariate Cox proportional hazards regression models. Model 1 was unadjusted. Model 2 was adjusted for age, sex, and New York Heart Association (NYHA) class III/IV. Model 3 adjusted for age, sex, NYHA class III/IV, family history of SCD, unexplained syncope, resting left ventricular outflow tract obstruction, maximal left ventricular wall thickness, and nonsustained ventricular tachycardia. Multivariable models were fitted using backward elimination of nonsignificant factors at a 10% level. Furthermore, we also used hsCRP as a continuous variable to examine the association between hsCRP and the risk of adverse outcomes. A receiver operating characteristic (ROC) curve was generated to evaluate the accuracy of hsCRP in the prediction of adverse outcomes.
All statistical testing was 2‐sided. Results were considered statistically significant at a level of P<0.05. All analyses were performed with PASW Statistics 18.0 software (SPSS Inc, Chicago, IL).
Results
Baseline Characteristics
The baseline characteristics of the 490 patients with HCM according to risk category of hsCRP are listed in Table 1. Patients were from 15 to 87 years old (mean age: 51.6±13.6 years), and consisted of 72.0% men. Plasma hsCRP ranged from 0.01 to 16.70 mg/L. Median hsCRP for the entire study population was 1.27 mg/L (interquartile range: 0.57–2.68 mg/L). Subjects in the higher hsCRP group showed higher age (low: 48.4±13.4, median: 53.3±12.4, high: 55.0±14.5, years, P<0.001), higher body mass index (low: 25.3±3.0, median: 25.8±3.1, high: 26.3±3.9, kg/m2, P=0.038), higher heart rate (low: 69.4±12.1, median: 70.8±11.3, high: 75.0±17.3, bpm, P=0.023), higher resting left ventricular outflow tract obstruction ratio (low: 38.8%, median: 40.4%, high: 53.2%, P=0.037). Subjects in the high hsCRP group showed significantly lower left ventricular ejection fraction levels (low: 67.2±9.4, median: 67.5±8.4, high: 64.1±10.0, P=0.009). In addition, the differences among the 3 groups according to tertiles of hsCRP were similar to the risk category of hsCRP (Table S2).
Table 1.
Baseline Clinical Characteristics of the Study Patients According to Risk Category of hsCRP
| hsCRP, mg/L | |||||
|---|---|---|---|---|---|
| Total (0.01–16.70) | Low (<1.0) | Median (1.0–3.0) | High (>3.0) | P Value | |
| (n=490) | (n=201) | (n=178) | (n=111) | ||
| Age, y | 51.6±13.6 | 48.4±13.4 | 53.3±12.4 | 55.0±14.5 | <0.001 |
| Male, n (%) | 353 (72.0) | 149 (74.1) | 129 (72.5) | 75 (67.6) | 0.460 |
| BMI, kg/m2 | 25.7±3.3 | 25.3±3.0 | 25.8±3.1 | 26.3±3.9 | 0.038 |
| Heart rate, bpm | 71.2±13.3 | 69.4±12.1 | 70.8±11.3 | 75.0±17.3 | 0.023 |
| CHD, n (%) | 89 (18.2) | 33 (16.4) | 29 (16.3) | 27 (24.3) | 0.160 |
| Diabetes mellitus, n (%) | 46 (9.4) | 15 (7.5) | 15 (8.4) | 16 (14.4) | 0.113 |
| NYHA III–IV, n (%) | 91 (18.6) | 36 (17.9) | 30 (16.9) | 25 (22.5) | 0.460 |
| AF, n (%) | 61 (12.4) | 22 (10.9) | 25 (14.0) | 14 (12.6) | 0.658 |
| Stroke, n (%) | 14 (2.9) | 2 (1.0) | 6 (3.4) | 6 (5.4) | 0.071 |
| LV end‐diastolic diameter, mm | 45.3±6.9 | 45.1±7.3 | 44.8±6.3 | 46.3±6.9 | 0.208 |
| LV ejection fraction, % | 66.6±9.3 | 67.2±9.4 | 67.5±8.4 | 64.1±10.0 | 0.009 |
| Left atrial diameter, mm | 40.7±7.1 | 40.7±6.9 | 40.0±6.6 | 41.7±8.2 | 0.413 |
| Symptoms, n (%) | 395 (80.6) | 162 (80.6) | 142 (79.8) | 91 (82.0) | 0.899 |
| Chest pain, n (%) | 196 (40.0) | 78 (38.8) | 68 (38.2) | 50 (45.0) | 0.464 |
| Palpitations, n (%) | 205 (41.8) | 89 (44.3) | 70 (39.3) | 46 (41.4) | 0.619 |
| Syncope or presyncope, n (%) | 118 (24.1) | 43 (21.4) | 49 (27.5) | 26 (23.4) | 0.372 |
| NYHA III–IV, n (%) | 91 (18.6) | 36 (17.9) | 30 (16.9) | 25 (22.5) | 0.460 |
| Unexplained syncope, n (%) | 77 (15.7) | 30 (14.9) | 30 (16.9) | 17 (15.3) | 0.868 |
| Family history of SCD, n (%) | 63 (13.3) | 28 (13.9) | 21 (11.8) | 14 (12.6) | 0.822 |
| Resting LVOT obstruction, n (%) | 209 (42.7) | 78 (38.8) | 72 (40.4) | 59 (53.2) | 0.037 |
| Maximal LV wall thickness, mm | 21.0±5.0 | 20.9±5.3 | 20.9±4.9 | 21.3±5.0 | 0.724 |
| NSVT, n (%) | 15 (3.1) | 6 (3.0) | 4 (2.2) | 5 (4.5) | 0.554 |
| Family history of HCM, n (%) | 94 (19.2) | 34 (16.9) | 34 (19.1) | 26 (23.4) | 0.376 |
| ICD implantation | 3 (0.6) | 1 (0.5) | 0 (0) | 2 (1.8) | 0.156 |
| Septal reduction therapy, n (%) | 84 (17.1) | 37 (18.4) | 30 (16.9) | 17 (15.3) | 0.780 |
| Surgical septal myectomy, n (%) | 15 (3.1) | 6 (3.0) | 3 (1.7) | 6 (5.4) | 0.202 |
| Alcohol septal ablation, n (%) | 69 (14.1) | 31 (15.4) | 27 (15.2) | 11 (9.9) | 0.355 |
| β‐Blocker, n (%) | 378 (77.1) | 157 (78.1) | 135 (75.8) | 86 (77.5) | 0.868 |
| Verapamil or diltiazem, n (%) | 92 (18.8) | 41 (20.4) | 35 (19.7) | 16 (14.4) | 0.402 |
Continuous variables are presented as mean±SD; categorical variables are presented as numbers or percentages. AF indicates atrial fibrillation; BMI, body mass index; CHD, coronary heart disease; HCM, hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein; ICD, implantable cardioverter‐defibrillator; LV, left ventricular; LVOT, left ventricular outflow tract; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; SCD, sudden cardiac death.
Survival Analysis
During the follow‐up of 3.7±2.0 years, there were 30 (6.1%) cardiovascular deaths, including SCD in 11 patients, heart failure–related deaths in 14 patients, and stroke‐related deaths in 5 patients. Baseline clinical characteristics of patients with cardiovascular death are listed in Table S3. During follow‐up, the rate of cardiovascular death in the high hsCRP group, median hsCRP group, and low hsCRP group were 13.5% (15/111), 5.6% (10/178), and 2.5% (5/201) (P<0.001), respectively. According to the risk category of hsCRP, survival free of cardiovascular death was lower in the high hsCRP group (log‐rank P<0.001, Figure 1A). Similarly, 38 (7.8%) of the 490 patients had all‐cause mortality. Subjects in the high hsCRP group had lower all‐cause mortality‐free survival (log‐rank P<0.001, Figure 1B). Furthermore, survival free of SCD and heart failure–related death were lower in the high hsCRP group (log‐rank P=0.005, Figure 2A; log‐rank P=0.008, Figure 2B, respectively). There were 5 stroke‐related deaths during the follow‐up. The incidence of stroke‐related deaths was low and did not allow for further analysis.
Figure 1.
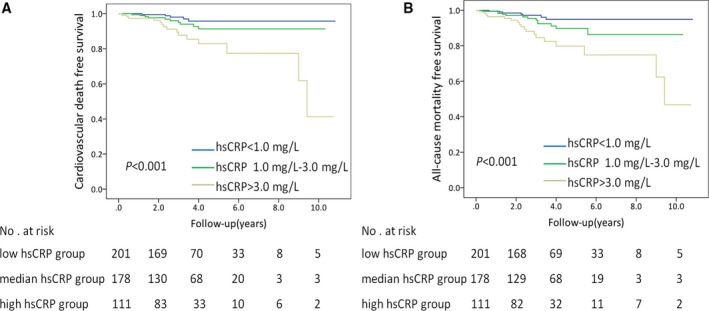
Survival free of cardiovascular death (A) and all‐cause mortality (B) in patients with HCM. According to risk category of hsCRP, subjects in the high hsCRP group had lower cardiovascular death and all‐cause mortality‐free survival in the patients with HCM. P‐values were calculated using the log‐rank test. HCM indicates hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein.
Figure 2.
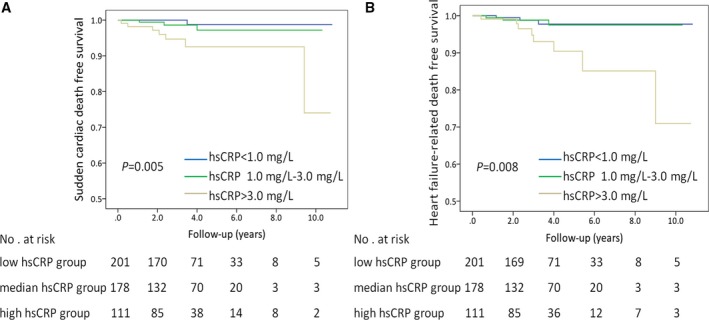
Survival free of sudden cardiac death (A) and heart failure–related death (B) in patients with HCM. According to the risk category of hsCRP, subjects in the high hsCRP group had lower sudden cardiac death and heart failure–related death‐free survival than in the patients with HCM. P‐values were calculated using the log‐rank test. HCM indicates hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein.
Subjects in the higher hsCRP group showed significantly lower 3‐year survival rates of cardiovascular death and all‐cause mortality (Table S4) and showed significantly higher event rates (per 100‐patient years) of adverse outcomes in patients with HCM (Table S5).
Relation of hsCRP to Adverse Outcomes
To further evaluate the effect of hsCRP on development of adverse outcomes of HCM, we used univariate and multivariate Cox proportional hazards regression models to reveal whether the plasma hsCRP level could predict the adverse outcomes of patients with HCM.
In univariable Cox regression models (Model 1), compared with patients in the low hsCRP group, the hazard ratios (HR) for cardiovascular deaths in the median hsCRP group and high hsCRP group were 2.32 (95% CI 0.79–6.80, P=0.124) and 5.88 (95% CI 2.13–16.20, P=0.001), respectively. Similarly, the high hsCRP group had a higher risk of developing all‐cause mortality than the low hsCRP group (HR 5.04, 95% CI 2.10–12.09, P<0.001). There was no significant difference in relative risk of all‐cause mortality between the low hsCRP group and the median hsCRP group (HR 2.16, 95% CI 0.86–5.42, P=0.101). Furthermore, compared with patients in the low hsCRP group, the HR for SCD and heart failure–related death in the high hsCRP group were 12.57 (HR 12.57, 95% CI 1.54–102.45, P=0.018) and 4.98 (HR 4.98, 95% CI 1.31–18.86, P=0.018), respectively (Table 2).
Table 2.
Univariate and Multivariate Cox Analysis According to Risk Category of hsCRP
| Cardiovascular Death | All‐Cause Mortality | Sudden Cardiac Death | Heart Failure–Related Death | |||||
|---|---|---|---|---|---|---|---|---|
| HRa (95% CI) | P a Value | HRa (95% CI) | P a Value | HRa (95% CI) | P a Value | HRa (95% CI) | P a Value | |
| Model 1b | ||||||||
| Low hsCRP (n=201) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Median hsCRP (n=178) | 2.32 (0.79–6.80) | 0.124 | 2.16 (0.86–5.42) | 0.101 | 3.45 (0.36–33.21) | 0.258 | 1.15 (0.23–5.70) | 0.866 |
| High hsCRP (n=111) | 5.88 (2.13–16.20) | 0.001 | 5.04 (2.10–12.09) | <0.001 | 12.57 (1.54–102.45) | 0.018 | 4.98 (1.31–18.86) | 0.018 |
| Model 2c | ||||||||
| Low hsCRP (n=201) | 1.0 | 1.00 | 1.00 | 1.00 | ||||
| Median hsCRP (n=178) | 2.57 (0.87–7.55) | 0.087 | 2.35 (0.93–5.91) | 0.070 | 3.70 (0.38–35.73) | 0.258 | 1.31 (0.26–6.52) | 0.745 |
| High hsCRP (n=111) | 5.41 (1.96–14.93) | 0.001 | 4.78 (1.99–11.47) | <0.001 | 11.16 (1.37–91.17) | 0.024 | 4.38 (1.15–16.60) | 0.030 |
| Model 3d | ||||||||
| Low hsCRP (n=201) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Median hsCRP (n=178) | 2.57 (0.87–7.55) | 0.087 | 2.35 (0.93–5.91) | 0.070 | 3.53 (0.36–34.16) | 0.276 | 1.31 (0.26–6.52) | 0.745 |
| High hsCRP (n=111) | 5.41 (1.96–14.93) | 0.001 | 4.78 (1.99–11.47) | <0.001 | 11.29 (1.38–92.20) | 0.024 | 4.38 (1.15–16.60) | 0.030 |
HR indicates hazard ratio; hsCRP, high‐sensitivity C‐reactive protein.
Compared with the low hsCRP group.
Model 1: unadjusted.
Model 2: multivariate adjustment was made for age, sex, New York Heart Association class III/IV.
Model 3: multivariate adjustment was made for age, sex, New York Heart Association class III/IV, a family history of sudden cardiac death events, unexplained syncope, resting left ventricular outflow tract obstruction, maximal left ventricular wall thickness, and nonsustained ventricular tachycardia.
Multivariate analysis showed that plasma hsCRP was independently and positively related to cardiovascular death (adjusted HR in the high hsCRP group: 5.41, 95% CI 1.96–14.93, P=0.001, compared with the low hsCRP group) after adjusting for age, sex, and NYHA class III/IV (Model 2). With further adjustment for age, sex, NYHA class III/IV, family history of SCD, unexplained syncope, resting left ventricular outflow tract obstruction, maximal left ventricular wall thickness, and nonsustained ventricular tachycardia (Model 3), the HR for cardiovascular deaths in the high hsCRP group showed no change (HR 5.41, 95% CI 1.96–14.93, P=0.001). Similarly, the high hsCRP group had a higher risk of developing all‐cause mortality than the low hsCRP group (Model 2: HR 4.78, 95% CI 1.99–11.47, P<0.001; Model 3: HR 4.78, 95% CI 1.99–11.47, P<0.001). Furthermore, subjects in the high hsCRP group were associated with an increased risk of SCD (Model 2: HR 11.16, 95% CI 1.37–91.17, P=0.024; Model 3: HR 11.29, 95% CI 1.38–92.20, P=0.024; compared with the low hsCRP group) and heart failure–related death (Model 2: HR 4.38, 95% CI 1.15–16.60, P=0.030; Model 3: HR 4.38, 95% CI 1.15–16.60, P=0.030; compared with the low hsCRP group) (Table 2).
In addition, when divided into 3 groups according to tertiles of hsCRP, the survival analysis and multivariate Cox regression analysis were similar to the risk category of hsCRP, which shows that elevated levels of hsCRP predict high risk of adverse outcomes in patients with HCM (Figures S2 and S3; Table S6).
When analyzed as a continuous variable, elevated hsCRP predicted increased risk for cardiovascular death (Model 1: HR 1.16, 95% CI 1.08–1.26, P<0.001; Model 2: HR 1.15, 95% CI 1.06–1.25, P=0.001; Model 3: HR 1.15, 95% CI 1.06–1.25, P=0.001) and all‐cause mortality (Model 1: HR 1.18, 95% CI 1.10–1.26, P<0.001; Model 2: HR 1.17, 95% CI 1.09–1.26, P<0.001; Model 3: HR 1.17, 95% CI 1.09–1.26, P<0.001). The continuous variable of hsCRP was also independently and positively related to SCD (Model 1: HR 1.21, 95% CI 1.07–1.36, P=0.002; Model 2: HR 1.21, 95% CI 1.06–1.37, P=0.004; Model 3: HR 1.20, 95% CI 1.06–1.36, P=0.003) and heart failure–related death (Model 1: HR 1.16, 95% CI 1.04–1.31, P=0.009; Model 2: HR 1.15, 95% CI 1.02–1.30, P=0.020; Model 3: HR 1.15, 95% CI 1.02–1.30, P=0.020) (Table 3).
Table 3.
Univariate and Multivariate Cox Analysis as a Continuous Variable of hsCRP
| Events | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Cardiovascular deaths | 1.16 (1.08–1.26) | <0.001 | 1.15 (1.06–1.25) | 0.001 | 1.15 (1.06–1.25) | 0.001 |
| All‐cause mortality | 1.18 (1.10–1.26) | <0.001 | 1.17 (1.09–1.26) | <0.001 | 1.17 (1.09–1.26) | <0.001 |
| Sudden cardiac death | 1.21 (1.07–1.36) | 0.002 | 1.21 (1.06–1.37) | 0.004 | 1.20 (1.06–1.36) | 0.003 |
| Heart failure–related death | 1.16 (1.04–1.31) | 0.009 | 1.15 (1.02–1.30) | 0.020 | 1.15 (1.02–1.30) | 0.020 |
HR indicates hazard ratio; hsCRP, high‐sensitivity C‐reactive protein.
Model 1: unadjusted.
Model 2: multivariate adjustment was made for age, sex, New York Heart Association class III/IV.
Model 3: multivariate adjustment was made for age, sex, New York Heart Association class III/IV, a family history of sudden cardiac death events, unexplained syncope, resting left ventricular outflow tract obstruction, maximal left ventricular wall thickness, and nonsustained ventricular tachycardia.
Prognostic Value of hsCRP for Adverse Outcomes
The ROC analysis indicated that hsCRP had reasonable accuracy for prediction of adverse outcomes. The area under the ROC curve was 0.71 (95% CI 0.62–0.80, P<0.001) for cardiovascular death (Figure 3). A cutoff of hsCRP >3.0 mg/L had a 96.0% negative predictive value for predicting cardiovascular death (Table S7). The area under the ROC curve was 0.70 for all‐cause mortality (95% CI 0.62–0.79, P<0.001), 0.77 for SCD (95% CI 0.64–0.89, P=0.002), and 0.69 for heart failure–related death (95% CI 0.54–0.84, P=0.017) (Figure S4).
Figure 3.
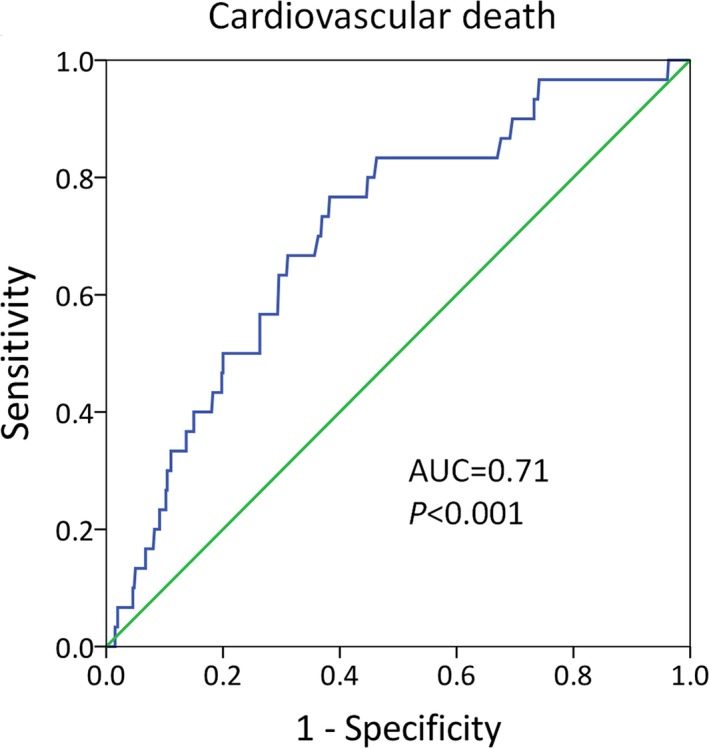
A ROC curve of hsCRP to predict cardiovascular death in patients with HCM. The AUC was 0.71 (95% CI 0.62–0.80, P<0.001). AUC indicates area under curves; HCM, hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein; ROC, receiver operating characteristic.
Discussion
In this study, we found that elevated hsCRP was associated with adverse outcomes in patients with HCM. Patients in the high hsCRP group had higher risk for cardiovascular death, as well as all‐cause mortality, SCD, and heart failure–related death. Furthermore, when analyzed as a continuous variable, elevated hsCRP predicted increased risk for adverse outcomes in patients with HCM. The ROC analysis indicated that hsCRP had reasonable predictive accuracy for the development of adverse outcomes.
CRP measured by a highly sensitive assay (hsCRP) has considerable chemical stability, requires no special precautions for sampling, and has a relatively long half‐life.19 Therefore, it has emerged as a leading biomarker of inflammation for clinical application. HsCRP has been regarded as a traditional risk factor in cardiovascular diseases.20 A previous study found that obstructive HCM was associated with increased CRP levels, compared with nonobstructive HCM.21 Similarly, the plasma level of hsCRP was significantly higher in patients with obstructive HCM than nonobstructive HCM in this study. However, the prognostic value of hsCRP in HCM has not yet been reported. To our knowledge, this is the first study to demonstrate that hsCRP was an independent predictor of adverse prognosis in patients with HCM.
Histological studies support a possible association between inflammation and HCM, and infiltration of chronic inflammatory cells is found in the myocardium of patients with HCM.22, 23 Recently, the study has demonstrated that a low‐grade inflammatory response may play a significant role in the phenotypic expression of myocardial fibrosis in HCM.17 Previous studies found that CRP may have a pathogenic role in the development of cardiac hypertrophy and fibrosis, possibly through the nuclear factor kappa B signaling pathways.24 Zhang et al also found that activation of the nuclear factor kappa B signaling pathways may be the mechanisms by which CRP promotes cardiac fibrosis.25 Myocardial fibrosis is a hallmark of hypertrophic cardiomyopathy. It is an early manifestation of HCM even before hypertrophic remodeling.26 Meanwhile, myocardial fibrosis is a major determinant of SCD, ventricular tachyarrhythmia, left ventricular dysfunction, and heart failure.26, 27, 28 Our results showed that elevated hsCRP predicted an increased risk for adverse outcomes in patients with HCM. These studies suggest that prolonged and low‐grade myocardial inflammation induces nuclear factor kappa B upregulation and activates inflammatory cell invasion and fibroblasts, and finally leads to myocardial fibrosis.17, 25, 29, 30 Myocardial fibrosis is thought to play a significant role in clinical progression.
In the present study, the event rates were 7.8% (2.12 per 100 person‐year) for all‐cause mortality and 6.1% (1.67 per 100 person‐year) for cardiovascular deaths. Recently, Maron et al have shown that the event rate was 8.2% (1.16 per 100 person‐year) for all‐cause mortality and 4.0% (0.53 per 100 person‐year) for HCM‐related death (7.2±5.2 years of follow‐up).31 Another study shows that the event rate of all‐cause mortality was 13.1% and HCM‐related death was 3.7% (6.6±5.3 years of follow‐up).32 Compared with these studies, the event rates of cardiovascular deaths in our study were somewhat higher. In other studies, Coats et al showed an event rate of all‐cause mortality of 8% (68/847) (a median duration of 3.5 years of follow‐up),33 and Geske et al showed that the incidence per 100 person‐year of all‐cause mortality was 2.29.34 The event rates of our study were comparable to these reports. The discrepancy of event rates among different studies may be due to the collection bias of patients, or ethnic differences. In our study, all included patients were of the Chinese Han ethnic group, and their disease may be more severe because the Fuwai Hospital is the National Center for Cardiovascular Diseases. In spite of the above, our results are still able to clearly indicate that hsCRP is a risk factor of adverse outcomes in patients with HCM.
In this study, we found that patients with higher hsCRP levels had a higher risk for adverse outcomes of HCM. Although a causal relationship between hsCRP and the prognosis of HCM had not yet been established, our findings suggest a possible association of an inflammatory state and the clinical progression of HCM.
The patients enrolled in this study come from a single center and were limited to the native Chinese population. There were 8.2% of patients lost during follow‐up in the present study, which might introduce biases. In addition, the values of hsCRP were measured at enrollment. We did not have serial measurements of hsCRP in this study. These facts limit the generalizability of our findings.
Conclusions
Our results indicate that elevated plasma hsCRP is associated with increased risk for adverse outcomes in the patients with HCM. Although a causal relationship between hsCRP and the prognosis of HCM had not yet been established, our findings suggest a possible association of an inflammatory state and the clinical progression of HCM.
Sources of Funding
This study was supported by CAMS Innovation Fund for Medical Sciences (CIFMS, 2016‐I2M‐1‐015), the National Natural Science Foundation of China (grant No. 81470380) and the Ministry of Science and Technology of China (grant No. 2015AA020407, 2011BAI11B04 and 2011CB503901).
Disclosures
None.
Supporting information
Table S1. Baseline Clinical Characteristics of the Patients Lost to Follow‐Up
Table S2. Baseline Clinical Characteristics of the Study Patients According to Tertiles of hsCRP
Table S3. Baseline Clinical Characteristics of Patients With Cardiovascular Death
Table S4. Three‐Year Survival Rate of Cardiovascular Death and All‐Cause Mortality According to Risk Category of hsCRP
Table S5. Event Rates of Adverse Outcomes According to Risk Category of hsCRP
Table S6. Univariate and Multivariate Cox Analysis According to Tertiles of hsCRP*
Table S7. Operating Characteristics of hsCRP >3.0 mg/L Thresholds to Predict Adverse Outcomes of HCM
Figure S1. Patient flowchart.
Figure S2. Survival free of cardiovascular death (A) and all‐cause mortality (B) in patients with HCM. According to tertiles of hsCRP, subjects in the highest tertile of hsCRP had lower cardiovascular death and all‐cause mortality‐free survival in the patients with HCM. P‐values were calculated using the log‐rank test. HCM indicates hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein.
Figure S3. Survival free of sudden cardiac death (A) and heart failure–related death (B) in patients with HCM. According to relative risk category of hsCRP, subjects in the highest tertile of hsCRP had lower sudden cardiac death and heart failure–related death‐free survival in the patients with HCM. P‐values were calculated using the log‐rank test. HCM indicates hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein.
Figure S4. A ROC curve of hsCRP to predict all‐cause mortality (A), sudden cardiac death (B), and heart failure–related death (C) in patients with HCM. The AUC was 0.70 for all‐cause mortality (95% CI 0.62–0.79, P<0.001), 0.77 for sudden cardiac death (95% CI 0.64–0.89, P=0.002), and 0.69 for heart failure–related death (95% CI 0.54–0.84, P=0.017). AUC indicates area under curves; HCM, hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein; ROC, receiver operating characteristic.
(J Am Heart Assoc. 2017;6:e004529. DOI: 10.1161/JAHA.116.004529.)
Contributor Information
Lei Song, Email: lsongqd@yahoo.com.
Jizheng Wang, Email: jzwang@hotmail.com.
References
- 1. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212–e260. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–255. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH III, Spirito P, Ten Cate FJ, Wigle ED. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J. 2003;24:1965–1991. [DOI] [PubMed] [Google Scholar]
- 4. Kofflard MJ, Ten Cate FJ, van der Lee C, van Domburg RT. Hypertrophic cardiomyopathy in a large community‐based population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J Am Coll Cardiol. 2003;41:987–993. [DOI] [PubMed] [Google Scholar]
- 5. Maron BJ, Olivotto I, Bellone P, Conte MR, Cecchi F, Flygenring BP, Casey SA, Gohman TE, Bongioanni S, Spirito P. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;39:301–307. [DOI] [PubMed] [Google Scholar]
- 6. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 7. Kraus VB, Stabler TV, Luta G, Renner JB, Dragomir AD, Jordan JM. Interpretation of serum C‐reactive protein (CRP) levels for cardiovascular disease risk is complicated by race, pulmonary disease, body mass index, gender, and osteoarthritis. Osteoarthritis Cartilage. 2007;15:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ridker PM, Glynn RJ, Hennekens CH. C‐reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–2011. [DOI] [PubMed] [Google Scholar]
- 9. Alonso‐Martinez JL, Llorente‐Diez B, Echegaray‐Agara M, Olaz‐Preciado F, Urbieta‐Echezarreta M, Gonzalez‐Arencibia C. C‐reactive protein as a predictor of improvement and readmission in heart failure. Eur J Heart Fail. 2002;4:331–336. [DOI] [PubMed] [Google Scholar]
- 10. Kardys I, Knetsch AM, Bleumink GS, Deckers JW, Hofman A, Stricker BH, Witteman JC. C‐reactive protein and risk of heart failure. The Rotterdam Study. Am Heart J. 2006;152:514–520. [DOI] [PubMed] [Google Scholar]
- 11. Rost NS, Wolf PA, Kase CS, Kelly‐Hayes M, Silbershatz H, Massaro JM, D'Agostino RB, Franzblau C, Wilson PW. Plasma concentration of C‐reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32:2575–2579. [DOI] [PubMed] [Google Scholar]
- 12. Aviles RJ, Martin DO, Apperson‐Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 13. Liu T, Li G, Li L, Korantzopoulos P. Association between C‐reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta‐analysis. J Am Coll Cardiol. 2007;49:1642–1648. [DOI] [PubMed] [Google Scholar]
- 14. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C‐reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. [DOI] [PubMed] [Google Scholar]
- 15. Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, O'Reilly DS, Packard CJ, Sattar N. C‐reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–1600. [DOI] [PubMed] [Google Scholar]
- 16. Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C‐reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. [DOI] [PubMed] [Google Scholar]
- 17. Kuusisto J, Karja V, Sipola P, Kholova I, Peuhkurinen K, Jaaskelainen P, Naukkarinen A, Yla‐Herttuala S, Punnonen K, Laakso M. Low‐grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart. 2012;98:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 19. Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein‐associated phospholipase A2, high‐sensitivity C‐reactive protein, and risk for incident coronary heart disease in middle‐aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–842. [DOI] [PubMed] [Google Scholar]
- 21. Dimitrow PP, Undas A, Bober M, Tracz W, Dubiel JS. Obstructive hypertrophic cardiomyopathy is associated with enhanced thrombin generation and platelet activation. Heart. 2008;94:e21. [DOI] [PubMed] [Google Scholar]
- 22. Tazelaar HD, Billingham ME. The surgical pathology of hypertrophic cardiomyopathy. Arch Pathol Lab Med. 1987;111:257–260. [PubMed] [Google Scholar]
- 23. Lamke GT, Allen RD, Edwards WD, Tazelaar HD, Danielson GK. Surgical pathology of subaortic septal myectomy associated with hypertrophic cardiomyopathy. A study of 204 cases (1996–2000). Cardiovasc Pathol. 2003;12:149–158. [DOI] [PubMed] [Google Scholar]
- 24. Nagai T, Anzai T, Kaneko H, Mano Y, Anzai A, Maekawa Y, Takahashi T, Meguro T, Yoshikawa T, Fukuda K. C‐reactive protein overexpression exacerbates pressure overload‐induced cardiac remodeling through enhanced inflammatory response. Hypertension. 2011;57:208–215. [DOI] [PubMed] [Google Scholar]
- 25. Zhang R, Zhang YY, Huang XR, Wu Y, Chung AC, Wu EX, Szalai AJ, Wong BC, Lau CP, Lan HY. C‐reactive protein promotes cardiac fibrosis and inflammation in angiotensin II‐induced hypertensive cardiac disease. Hypertension. 2010;55:953–960. [DOI] [PubMed] [Google Scholar]
- 26. Ho CY, Lopez B, Coelho‐Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, Gonzalez A, Colan SD, Seidman JG, Diez J, Seidman CE. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ, Maron MS. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1374. [DOI] [PubMed] [Google Scholar]
- 28. Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase‐2 and activation of nuclear factor‐kappaB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–103. [DOI] [PubMed] [Google Scholar]
- 30. Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. [DOI] [PubMed] [Google Scholar]
- 31. Maron BJ, Rowin EJ, Casey SA, Link MS, Lesser JR, Chan RH, Garberich RF, Udelson JE, Maron MS. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J Am Coll Cardiol. 2015;65:1915–1928. [DOI] [PubMed] [Google Scholar]
- 32. Maron BJ, Rowin EJ, Casey SA, Garberich RF, Maron MS. What do patients with hypertrophic cardiomyopathy die from? Am J Cardiol. 2016;117:434–435. [DOI] [PubMed] [Google Scholar]
- 33. Coats CJ, Gallagher MJ, Foley M, O'Mahony C, Critoph C, Gimeno J, Dawnay A, McKenna WJ, Elliott PM. Relation between serum N‐terminal pro‐brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur Heart J. 2013;34:2529–2537. [DOI] [PubMed] [Google Scholar]
- 34. Geske JB, McKie PM, Ommen SR, Sorajja P. B‐type natriuretic peptide and survival in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;61:2456–2460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Clinical Characteristics of the Patients Lost to Follow‐Up
Table S2. Baseline Clinical Characteristics of the Study Patients According to Tertiles of hsCRP
Table S3. Baseline Clinical Characteristics of Patients With Cardiovascular Death
Table S4. Three‐Year Survival Rate of Cardiovascular Death and All‐Cause Mortality According to Risk Category of hsCRP
Table S5. Event Rates of Adverse Outcomes According to Risk Category of hsCRP
Table S6. Univariate and Multivariate Cox Analysis According to Tertiles of hsCRP*
Table S7. Operating Characteristics of hsCRP >3.0 mg/L Thresholds to Predict Adverse Outcomes of HCM
Figure S1. Patient flowchart.
Figure S2. Survival free of cardiovascular death (A) and all‐cause mortality (B) in patients with HCM. According to tertiles of hsCRP, subjects in the highest tertile of hsCRP had lower cardiovascular death and all‐cause mortality‐free survival in the patients with HCM. P‐values were calculated using the log‐rank test. HCM indicates hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein.
Figure S3. Survival free of sudden cardiac death (A) and heart failure–related death (B) in patients with HCM. According to relative risk category of hsCRP, subjects in the highest tertile of hsCRP had lower sudden cardiac death and heart failure–related death‐free survival in the patients with HCM. P‐values were calculated using the log‐rank test. HCM indicates hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein.
Figure S4. A ROC curve of hsCRP to predict all‐cause mortality (A), sudden cardiac death (B), and heart failure–related death (C) in patients with HCM. The AUC was 0.70 for all‐cause mortality (95% CI 0.62–0.79, P<0.001), 0.77 for sudden cardiac death (95% CI 0.64–0.89, P=0.002), and 0.69 for heart failure–related death (95% CI 0.54–0.84, P=0.017). AUC indicates area under curves; HCM, hypertrophic cardiomyopathy; hsCRP, high‐sensitivity C‐reactive protein; ROC, receiver operating characteristic.


