Abstract
Background
Cardiovascular morbidity and mortality was reduced by 25% when blood pressure (BP) was targeted to 120 mm Hg systolic compared with 140 mm Hg systolic in Systolic Blood Pressure Intervention Trial (SPRINT); however, BP was measured using a research technique. SPRINT specified 5 minutes of seated rest in a quiet room followed by 3 oscillometric measurements without an observer in the room. The relationship of this research‐grade methodology to routine BP measurements is not known.
Methods and Results
Among 275 people with chronic kidney disease who had BP <140/90 mm Hg when they came to the clinic, we measured BP as in SPRINT and recorded BP on the same day without specification of seated rest. Compared with routine measurement, the research‐grade systolic BP was 12.7 mm Hg lower with wide limits of agreement (−46.1 to 20.7 mm Hg). Research grade systolic BP was 7.9 mm Hg lower than daytime ambulatory systolic BP and had wide agreement limits (−33.2 to 17.4 mm Hg). Whereas the routine, research‐grade, and daytime ambulatory systolic BP were all related to echocardiographic left ventricular hypertrophy, the strength of the relationship between research‐grade and daytime ambulatory systolic BP to left ventricular hypertrophy was similar and stronger than the strength of the relationship between routine systolic BP and left ventricular hypertrophy.
Conclusions
Taken together, these results suggest that translation of the SPRINT results will require measurement of BP as performed in that trial. Instead of an algebraic manipulation of routine clinic measurements, the SPRINT methodology of BP measurement would be needed at minimum if implementation of the SPRINT results were to be deployed in the population at large.
Keywords: agreement, ambulatory blood pressure monitoring, blood pressure measurement, chronic kidney disease, left ventricular hypertrophy
Subject Categories: Hypertension
Introduction
There has been much debate in recent years about what the treatment targets should be for blood pressure (BP) control among hypertensive people.1 Whereas the current guidelines recommend a BP target of <140/90 mm Hg among those with hypertension, the recently released results of Systolic Blood Pressure Intervention Trial (SPRINT) challenge this notion.2 The results of SPRINT showed that when BP was targeted to <120 mm Hg, compared with a higher target of 140 mm Hg, the hazard ratio for cardiovascular morbidity and mortality was reduced by 25%.2 What has received scarce attention is that the method of measuring BP in SPRINT was, as expected, research grade. Measurement was performed after a mandatory seated rest in a quiet room for 5 minutes, after which 3 recordings were made at 1‐minute intervals.3 No observer was present in the room during the measurement. The average of these recordings was used to target BP in the participants. In the office setting, BP recordings using meticulous methods such as those recommended by the guidelines and those used in SPRINT are often not made. Accordingly, it remains unclear whether the BP targets used in SPRINT can be implemented in the general population if routine methods of BP measurement are used.
In this study, we measured BP with the methodology used in SPRINT and compared the value with a single measurement taken without specification for 5‐minute rest and using a validated device. We reported the agreement between the 2 methods and the relationship of each of these 2 methods with the reference standard of 24‐hour ambulatory BP recordings. Furthermore, we tested the strength of the association of each of these 3 methodologies with target organ damage measured using echocardiographic left ventricular hypertrophy (LVH).
Methods
Participants were recruited from a renal clinic and a general medicine clinic at the Richard L Roudebush Veterans Affairs Medical Center in Indianapolis, Indiana, as detailed previously in a report.4 To participate, patients had to have BP measured in the clinic on the day of recruitment, BP in the normotensive range (<140/90 mm Hg), and evidence of chronic kidney disease. We excluded those with kidney transplantation or dialysis and those receiving immunosuppressive drugs.
After obtaining signed informed consent, participants were invited to our research laboratory. This typically occurred in a fasting state and in the morning. BP measurement was performed using the Omron HEM 907 oscillometric monitor (Omron Healthcare) with a cuff size appropriate for the arm. This device has been validated by several investigators using standardized protocols.5, 6 The monitor was programmed such that the cuff did not start to inflate until the participant rested quietly for 5 minutes in a seated position with the arm at the level of the heart. Three consecutive recordings were made 30 seconds apart. Although 1‐minute intervals were used in SPRINT, 30‐second intervals have been widely used in the National Health and Nutrition Examination Survey.7 The average of these 3 recordings was called research‐grade clinic BP. No observer was present in the room during these measurements. This technique is similar to that reported in SPRINT.
Two‐dimensional guided M‐mode echocardiograms were performed by an accredited technician with a digital cardiac ultrasound machine (Cypress Acuson; Siemens Medical).8 The protocol specified recording of at least 12 cycles of 2‐dimensional parasternal long‐ and short‐axis left ventricular (LV) views with optimal orientation of the cursor beam to derive additional M‐mode recordings. Each patient underwent 6 M‐mode measurements of interventricular septal thickness in diastole (IVSTd), LV internal diameter in diastole (LVIDd) and systole, LV posterior wall thickness in diastole (LVPWd) and systole, and left atrial diameter using standards of the American Society of Echocardiography.9 LV mass was calculated using a previously validated formula10: LV mass (g)=0.832×[(IVSTd+LVIDd+PWTd)3−(LVIDd)3]+0.60. LVH was diagnosed if LV mass was ≥104 g/m2 among women and ≥116 g/m2 among men.
Following the echocardiogram, another BP was obtained using a validated device (Omron HEM 705 CP; Omron Healthcare).11 This measurement, however, was made only once by the echocardiographer who was present in the room at the time of the measurement. The participant was in the supine position during the measurement. We called this routine clinic measurement.
All participants then underwent 24‐hour ambulatory BP monitoring using the validated Spacelabs 90207 device,12 as reported previously.4 Participants kept diaries that were used to calculate sleep and wake times. Each participant's medical record was reviewed to ascertain underlying comorbid illnesses. The reported comorbidities are those established after review of the medical record.
Means and standard deviations are reported for continuous data, and frequency and percentages are reported for discrete data. To calculate agreement between routine and research‐grade BP measurement or between each of these 2 methods and ambulatory BP recordings, the methods proposed by Bland and Altman were used.13 The relationship of each of these 3 methods with LVH was established using logistic regression analysis. Outcome was LVH as a dichotomous variable. The sole predictor variable was the BP of interest. Models were not adjusted for multiple comparisons. Likelihood ratios in nested models were used to test the goodness of fit. Better goodness of fit indicated greater strength of association. Subgroup analyses for Bland–Altman analyses were made, and interaction effects of the variable were reported as follows: The outcome was the difference between research‐grade BP and either usual BP (Figure S1) or daytime ambulatory BP (Figure S2). In a linear regression model, the subgroup of interest was used as an indicator variable. The P value of this estimate was reported as the interaction effect. Alpha was set at 0.05, and P values were 2‐sided. All analyses were done for 275 patients. All patients had complete data on research‐grade, usual, and ambulatory BP.
The study was approved by the institutional review board, and all participants provided written informed consent.
Results
Table 1 shows the baseline characteristics of the 275 participants. Of the 333 participants recruited, 275 had echocardiograms that form the basis of this report. Ages ranged from 38 to 89 years, 65% of the participants were aged ≥65 years, and 32% were aged ≥75 years. There was diversity in race with 17% of participants being black; however, 98% of participants were men. About 65% had underlying diabetes mellitus, and there was a high prevalence of cardiovascular disease; for example, approximately a fifth each had coronary artery bypass grafting, peripheral vascular disease, or a prior hospitalization for heart failure. The distribution of chronic kidney disease stages was as follows: 5% stage 3A, 35% stage 3B, 52% stage 4, and 7% stage 5. Only 2 participants had an estimated glomerular filtration rate >60 mL/min per 1.73 m2. Albuminuria stage 2 (albumin/creatinine ratio >300 mg/g creatinine) was present in 22%, stage 1 (albumin/creatinine ratio 30–300 mg/g creatinine) was present in 27%, and 51% had no albuminuria. Of the 275 participants, 269 (98%) were being treated with antihypertensive drugs; the mean number of antihypertensive medications was 3.1.
Table 1.
Baseline Characteristics of the Study Sample
| Variable | Result (n=275) |
|---|---|
| Age, y | 69.2±10.1 |
| Male sex, n (%) | 269 (98) |
| Race | |
| White, n (%) | 220 (80) |
| Black, n (%) | 47 (17) |
| Other, n (%) | 8 (3) |
| Body mass index, kg/m2 | 30.9±4.7 |
| Comorbid illnesses | |
| Diabetes mellitus, n (%) | 180 (65) |
| Myocardial infarction, n (%) | 77 (28) |
| Percutaneous coronary revascularization, n (%) | 73 (27) |
| Coronary artery bypass grafting, n (%) | 57 (21) |
| Congestive heart failure, n (%) | 50 (18) |
| Stroke, n (%) | 34 (12) |
| Peripheral vascular disease, n (%) | 58 (21) |
| Current smoker, n (%) | 42 (15) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 28.6±10.2 |
| Antihypertensive drugs, n | 3.1±1.4 |
| Angiotensin‐converting enzyme inhibitors, n (%) | 143 (52) |
| Angiotensin receptor blockers, n (%) | 60 (22) |
| Beta blockers, n (%) | 187 (68) |
| Dihydropyridine calcium channel blockers, n (%) | 121 (44) |
| Loop diuretics, n (%) | 104 (38) |
| Thiazide diuretics, n (%) | 67 (24) |
| Blood pressure measurements | |
| Research‐grade systolic | 121.7±17.9 |
| Research‐grade diastolic | 59.7±11.7 |
| Routine clinic systolic | 134.5±19.5 |
| Routine clinic diastolic | 71.8±12.8 |
| 24‐hour ambulatory systolic | 126.9±14.3 |
| 24‐hour ambulatory diastolic | 69.1±9.2 |
| Nighttime ambulatory systolic | 122.3±16.3 |
| Nighttime ambulatory diastolic | 65.2±10.1 |
| Daytime ambulatory systolic | 129.6±14.3 |
| Daytime ambulatory diastolic | 71.5±9.3 |
Data are shown as mean±SD except as noted.
Figure 1 shows the Bland–Altman plots. On average, compared with routine clinic BP, research‐grade systolic BP was 12.7 mm Hg lower and diastolic BP was 12.0 mm Hg lower. Notably, both measurements were recorded on the same day in each participant. The limits of agreement for systolic BP (−46.1 to 20.7 mm Hg) and diastolic BP (−34.2 to 10.1 mm Hg) were wide. Subgroup analyses are shown in Figure S1. Results indicate that bias and limits of agreement were not influenced by any one subgroup.
Figure 1.
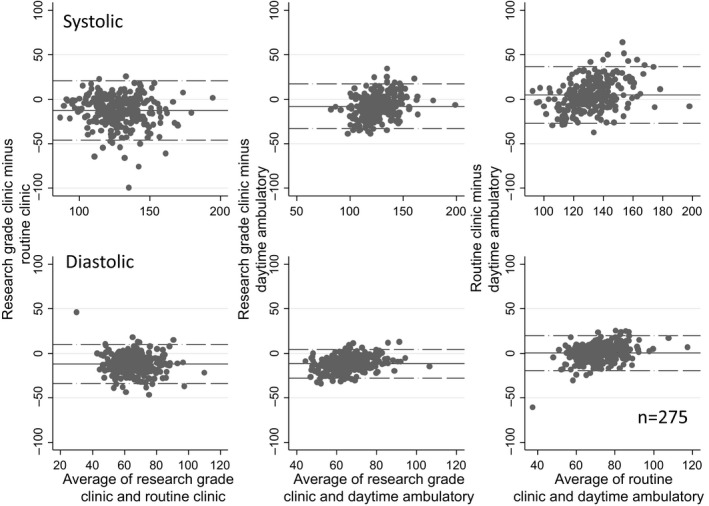
Bland–Altman plot showing the mean differences between various blood pressure (BP) recordings and their limits of agreement. The top panel shows systolic BP and the bottom panel shows diastolic BP. Research‐grade BP was, on average, 12.7/12.0 mm Hg lower (bias) than routine clinic BP and had wide limits of agreement.
Table 2 and Figure 1 show the relationship of research‐grade, routine clinic, and daytime ambulatory BP for all participants. Research‐grade systolic and diastolic BP were both lower than the corresponding daytime ambulatory BP. The systolic research‐grade clinic BP was 7.9 mm Hg lower (95% CI −9.4 to −6.4 mm Hg), and diastolic research grade clinic BP was 11.7 mm Hg lower (95% CI −12.7 to −10.8 mm Hg). In contrast, routine clinic BP was 4.8 mm Hg higher systolic and 0.3 mm Hg higher diastolic; however, for diastolic BP, the difference was not significantly different from zero.
Table 2.
Agreement Assessed With the Bland–Altman Method Using the 3 Blood Pressure Measurement Techniques
| Variable | Bias (95% CI) | Limits of Agreement |
|---|---|---|
| Research grade, routine SBP | −12.7 (−14.7 to −10.7) | −46.1 to 20.7 |
| Research grade, routine DBP | −12.0 (−13.4 to −10.7) | −34.2 to 10.1 |
| Research grade, day ABPM SBP | −7.9 (−9.4 to −6.4) | −33.2 to 17.4 |
| Research grade, day ABPM DBP | −11.7 (−12.7 to −10.8) | −27.8 to 4.3 |
| Routine clinic, day ABPM SBP | 4.8 (2.9–6.7) | −26.9 to 36.5 |
| Routine clinic, day ABPM DBP | 0.3 (−0.9 to 1.5) | −19.5 to 20.1 |
ABPM indicates ambulatory blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Analyses in 10 subgroups indicated that bias (difference between research‐grade systolic BP and daytime ambulatory systolic BP) was not modified by 8 of 10 subgroups (Figure S2). Nevertheless, both diabetes mellitus (P=0.01) and marginally peripheral vascular disease (P=0.06) influenced bias. Compared with daytime ambulatory systolic BP, the research‐grade systolic BP was 4.8 mm Hg lower in those without diabetes mellitus and 9.6 mm Hg lower in those with diabetes mellitus. Compared with daytime ambulatory systolic BP, the research‐grade systolic BP was 8.7 mm Hg lower in those without peripheral vascular disease and 5 mm Hg lower in those with peripheral vascular disease; however, the limits of agreement remained similarly wide.
To further explore the association of routine clinic and research‐grade clinic BP measurements, we calculated the odds of echocardiographic LVH with each type of BP measurement. Figure 2 shows the odds ratios for each type of systolic BP measured in this analysis. The strength of the association of LVH and systolic BP was greater for ambulatory and research‐grade measurements compared with routine clinic measurements.
Figure 2.

Relationship of odds ratio for echocardiographic left ventricular hypertrophy (LVH) and systolic blood pressure (SBP) measured using 3 different methods. Odds ratios and their 95% CIs are plotted together with the chi‐square and P values. As measured by the likelihood ratio test, the strength of the relationship between SBP and LVH was stronger for daytime ambulatory BP (ABPM) than routine SBP and LVH (P=0.032). The strength of the relationship of LVH and research‐grade SBP was stronger than that with routine SBP (P=0.005) The strength of the relationship of LVH and daytime ambulatory SBP was similar to that with research‐grade SBP (P=0.052).
Discussion
The major findings of this study are that research‐grade BP recordings are substantially lower than routinely measured single recordings in the clinic. The limits of agreement between these 2 recordings are wide and cannot be predicted by any one factor. Finally, the relationship of routine clinic recordings to target organ damage measured by echocardiographic recordings is weaker compared with research‐grade or ambulatory BP recordings.
In the intensive treatment group, the mean systolic BP over a 3.26‐year average follow‐up in SPRINT was 121.5 mm Hg; this was achieved with the use of 2.8 medications. The mean systolic research‐grade BP in our study was 121.7 mm Hg, and this was achieved with the use of 3.1 medications. Consequently, the 2 populations are comparable, at least with respect to achieved BP and antihypertensive medications. Research‐grade BP was 12.7/12.0 mm Hg lower compared with routine clinic BP. Adding 12.7 mm Hg to the target BP of 120 mm Hg systolic measured in SPRINT yields 132.7 mm Hg systolic. This may be considered the “routine BP goal”: however, this would be a naïve approach, given that the limits of agreement between measurements were wide. As an example, for a target BP of 120 mm Hg systolic measured using research‐grade methodology, patients may have differences from routine clinic BP that may be 46.1 mm Hg lower or 20.7 mm Hg higher, making such recommendations worthless.
Contrary to the popular belief that clinic BP is always higher, on average, the research‐‐grade systolic and diastolic BP was considerably lower than daytime ambulatory BP. Systolic BP was 7.9 mm Hg lower and diastolic BP was 11.7 mm Hg lower. In comparison, the routine clinic BP was, on average, 4.8 mm Hg higher systolic and no different from diastolic BP. Neither the research‐grade BP nor the routine clinic BP was able to estimate daytime ambulatory BP accurately; the agreement limits for each measure were wide. Even among participants without diabetes mellitus or with peripheral vascular disease, for which the bias was lower, the limits of agreement remained wide. Consequently, neither of the 2 measurements is an adequate replacement for ambulatory BP measurement.
The recently released Canadian Hypertension and Education Program guidelines recommend the use of automated office BP recordings without an observer in the room.14 These guidelines, however, do not mandate the 5‐minute period of rest that is specified in SPRINT14; therefore, the automated office BP recordings are not synonymous with measurements made in SPRINT. Nonetheless, recordings made using an automated device are often much lower than routine measurements,15, 16 and the agreement limits are wide.15 These findings are similar to what we reported in this study; however, in contrast to the observation that the automated office BP recording is similar to daytime ambulatory BP,17 we noted that the research‐grade BP was substantially lower than daytime ambulatory BP with wide agreement limits.
SPRINT excluded people with diabetes mellitus. The ACCORD (Action to Control Cardiovascular Risk in Diabetes) study randomized patients with diabetes mellitus who were hypertensive to the same systolic BP targets as those used in SPRINT.18 The sample size of ACCORD was less than half that of SPRINT, and a factorial design included interventions to test lipid lowering with fenofibrate and intensive or standard glucose lowering. The hazard ratio for the primary end point was 0.89, favoring intensive BP lowering. Furthermore, there was 48% reduction in relative risk for stroke. Although the ACCORD study used the same automated device as SPRINT (Omron HEM 907) and measured BP in triplicate at each visit, it is less clear whether ACCORD required and enforced a mandatory period of 5 minutes of rest and whether the readings were observed or unobserved (supplementary appendix 2 of Cushman et al18). The overall results of the ACCORD trial for intensive BP lowering, providing little additional benefit for cardiovascular end points, could be partly related to the cointerventions of lipid and glucose lowering, which may have diluted the results of aggressive BP lowering. It is possible, however, that the BP measurement methodology could be operative.
SPRINT excluded people with stroke. Patients with recent lacunar strokes were randomized to intensive (target systolic BP <130 mm Hg) or standard (target systolic BP <140 mm Hg) groups and followed for an average of 3.7 years in the SPS3 (Secondary Prevention of Small Subcortical Strokes) study.19 The more intensive treatment reduced the rate of recurrent stroke by 19% (P=0.08) and the rate of intracerebral hemorrhage by 63% (P=0.03). In this study, the Colin 8800C electronic device was used (Colin Medical Instruments) to measure BP in triplicate after 15 minutes of sitting quietly in a seated position; BP was measured at 2‐minute intervals in the right arm (unless the left arm had 10‐mm Hg higher systolic BP).20 These BP recordings were obtained with an observer in the room. Whether the study mandated and enforced 15 minutes of quiet rest prior to cuff inflation is not clear. Aside from the smaller difference in BP targets in SPS3 compared with SPRINT, the measurement technique may have made a difference in outcomes.
The results of our study suggest that there is great variability in research‐grade BP and routinely obtained BP, even when done on the same day in the same patient; therefore, algebraic correction of clinic BP would be insufficient for target BP in individual patients. Research‐grade BP obtained after a mandated 5 minutes of seated rest is, on average, lower than daytime ambulatory systolic BP by 7.9 mm Hg. In contrast, routine systolic BP is 4.8 mm Hg higher. Given the wide limits of agreement, neither is sufficient to predict ambulatory BP. Research‐grade BP is a stronger determinant of echocardiographic LVH compared with routine clinic BP. A limitation of this work is that it was performed predominantly in men; however, we have little reason to believe these data would not be applicable to women. We did not randomize the order in which the routine and research‐grade BP measurements was made. Because routine BP recordings were always made after the research‐grade recordings, it is expected that routine recordings would be lower; the fact that they were higher may suggest that we may have underestimated the height of overestimation. Furthermore, our study was limited to patients with chronic kidney disease.
Taken together, these findings suggest that if the SPRINT findings were to be translated to clinic practice, the first step would be to measure the clinic BP as was done in the trial. Without these research‐grade measurements, the likelihood of harm (or lack of benefit) may be real.
Sources of Funding
This work was supported by a grant from the U.S. Veterans Administration Merit Review.
Disclosures
Agarwal has consulted for several pharmaceutical companies that make antihypertensive drugs including Merck, Takeda, Novartis, Daiichi Sankyo, Abbvie, Bayer, and Johnson and Johnson.
Supporting information
Figure S1. Systolic blood pressure bias and limits of agreement for 10 subgroups.
Figure S2. Systolic blood pressure (BP) bias between research systolic and daytime ambulatory BP and limits of agreement for 10 subgroups.
(J Am Heart Assoc. 2017;6:e004536. DOI: 10.1161/JAHA.116.004536.)
References
- 1. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 2. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel‐Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA. 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agarwal R, Pappas MK, Sinha AD. Masked uncontrolled hypertension in CKD. J Am Soc Nephrol. 2016;27:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El Assaad MA, Topouchian JA, Darne BM, Asmar RG. Validation of the Omron HEM‐907 device for blood pressure measurement. Blood Press Monit. 2002;7:237–241. [DOI] [PubMed] [Google Scholar]
- 6. White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM‐907 monitor in adults. Blood Press Monit. 2001;6:107–110. [DOI] [PubMed] [Google Scholar]
- 7. Ostchega Y, Prineas RJ, Paulose‐Ram R, Grim CM, Willard G, Collins D. National Health and Nutrition Examination Survey 1999–2000: effect of observer training and protocol standardization on reducing blood pressure measurement error. J Clin Epidemiol. 2003;56:768–774. [DOI] [PubMed] [Google Scholar]
- 8. Agarwal R. Longitudinal study of left ventricular mass growth: comparative study of clinic and ambulatory systolic blood pressure in chronic kidney disease. Hypertension. 2016;67:710–716. [DOI] [PubMed] [Google Scholar]
- 9. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 10. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 11. O'Brien E, Mee F, Atkins N, Thomas M. Evaluation of three devices for self‐measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM‐705CP, Philips HP5332, and Nissei DS‐175. Blood Press Monit. 1996;1:55–61. [PubMed] [Google Scholar]
- 12. O'Brien E, Mee F, Atkins N, O'Malley K. Accuracy of the SpaceLabs 90207 determined by the British Hypertension Society protocol. J Hypertens. 1991;9:573–574. [DOI] [PubMed] [Google Scholar]
- 13. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 14. Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, Cloutier L, Gelfer M, Lamarre‐Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Tobe SW, Ruzicka M, Burns KD, Vallee M, Prasad GV, Lebel M, Feldman RD, Selby P, Pipe A, Schiffrin EL, McFarlane PA, Oh P, Hegele RA, Khara M, Wilson TW, Penner SB, Burgess E, Herman RJ, Bacon SL, Rabkin SW, Gilbert RE, Campbell TS, Grover S, Honos G, Lindsay P, Hill MD, Coutts SB, Gubitz G, Campbell NR, Moe GW, Howlett JG, Boulanger JM, Prebtani A, Larochelle P, Leiter LA, Jones C, Ogilvie RI, Woo V, Kaczorowski J, Trudeau L, Petrella RJ, Hiremath S, Drouin D, Lavoie KL, Hamet P, Fodor G, Gregoire JC, Lewanczuk R, Dresser GK, Sharma M, Reid D, Lear SA, Moullec G, Gupta M, Magee LA, Logan AG, Harris KC, Dionne J, Fournier A, Benoit G, Feber J, Poirier L, Padwal RS, Rabi DM. Hypertension Canada's 2016 Canadian Hypertension Education Program Guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569–588. [DOI] [PubMed] [Google Scholar]
- 15. Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Grant FC, Kaczorowski J. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ. 2011;342:d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burgess SE, MacLaughlin EJ, Smith PA, Salcido A, Benton TJ. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484–488. [DOI] [PubMed] [Google Scholar]
- 17. Myers MG. The great myth of office blood pressure measurement. J Hypertens. 2012;30:1894–1898. [DOI] [PubMed] [Google Scholar]
- 18. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons‐Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail‐Beigi F. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood‐pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pergola PE, White CL, Graves JW, Coffey CS, Tonarelli SB, Hart RG, Benavente OR. Reliability and validity of blood pressure measurement in the Secondary Prevention of Small Subcortical Strokes study. Blood Press Monit. 2007;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Systolic blood pressure bias and limits of agreement for 10 subgroups.
Figure S2. Systolic blood pressure (BP) bias between research systolic and daytime ambulatory BP and limits of agreement for 10 subgroups.


