Abstract
Background
This study aimed to demonstrate the feasibility of measuring frailty in patients with cardiac implantable electrical devices while validating the physiologic significance of device‐detected physical activity by evaluating its association with frailty and mobility.
Methods and Results
Outpatients with cardiac implantable electrical devices compatible with physical activity analysis with at least 7 days of data were eligible. Office testing included frailty status (Study of Osteoporotic Fractures instrument), gait speed (m/s), mobility according to the Timed Up and Go (TUG) test (seconds), and daily physical activity (h/d) as measured by cardiac implantable electrical device. Among 219 patients, Study of Osteoporotic Fractures testing found 39.7% to be robust, 47.5% prefrail, and 12.8% frail. The mean gait speed for the cohort was 0.8±0.3 m/s, mean TUG time was 10.9±4.4 seconds, and mean activity was 2.8±1.9 h/d. Frail patients were markedly more likely to have gait speeds <0.8 m/s (OR 6.25, 95% CI 1.79‐33.3). In unadjusted analyses each 1‐hour increase in mean daily activity was associated with a 46% reduction of frail phenotype (OR 0.54, 95% CI 0.40‐0.74) versus robust and with a 27% reduction in the odds of having the prefrail phenotype (OR 0.73, 95% CI 0.62‐0.86). After adjustment this association per hour of activity persisted, with an adjusted OR for frailty of 0.71 (95% CI 0.51‐0.99) and adjusted OR for prefrailty of 0.81 (95% CI 0.67‐0.99).
Conclusions
Frailty and mobility limitation are common among cardiac implantable electrical device patients and are correlated to device‐detected physical activity.
Keywords: aging, defibrillation, pacemaker, physical exercise
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Quality and Outcomes, Aging
Introduction
Cardiac implantable electrical devices (CIEDs), including pacemakers (PM) and implantable cardioverter‐defibrillators (ICDs), are the most effective treatments for serious arrhythmias. Geriatric conditions such as frailty and decreased mobility may provide synergistic information regarding outcomes for older recipients of CIEDs.1 Clinical frailty, commonly understood as decreased biologic reserve or resiliency,2 is strongly associated with adverse outcomes for cardiovascular patients.1, 3 In a recent analysis frailty assessed by an algorithm derived from medical claims was identified in 10% of patients in a large nationwide ICD cohort,1 but to date no studies have reported more direct measurements of the frailty phenotype among individuals with CIEDs. Better tools for identifying frail patients may therefore provide opportunities for targeted testing or interventions that may improve outcomes for this challenging patient population.
Patient physical activity information is collected automatically by many CIEDs via an embedded accelerometer used to guide rate‐responsive pacing. Device‐specific algorithms use these data to generate estimates of time spent in activity, providing an accessible measure that may align with functional status and the burden of accumulated comorbidity.4, 5 Although prior studies suggest an association between these activity measures and survival,6 the relationship between device‐detected activity and clinical frailty or mobility measures has not been explored.
Accordingly, this study aimed to demonstrate the feasibility and utility of obtaining tractable measures of the frailty phenotype in patients with CIEDs as part of routine ambulatory care. In addition, we sought to validate the physiologic significance of device‐detected physical activity by evaluating its association with frailty and mobility.
Methods
Study Design and Setting
This is a cross‐sectional study of patients with CIEDs followed in the ambulatory device clinic at Beth Israel Deaconess Medical Center (BIDMC), an academic tertiary care hospital in Boston, Massachusetts. Eligible subjects were identified from the device clinic appointment schedule and were approached concurrent with their visit. Verbal informed consent was obtained, and participating subjects were provided parking vouchers as reimbursement. The BIDMC and Institute for Aging Research Institutional Review Boards both approved study conduct and procedures.
Study Population
Patients with CIEDs implanted for >2 months were eligible for inclusion. Patients who were unable to provide consent due to language barriers or cognitive dysfunction were excluded. For analysis of device data, only those patients with devices capable of recording physical activity information and those with at least 7 days of device data were eligible.
Clinical Variables
Demographics (age and sex) and medical history were ascertained from electronic health records. Coronary artery disease was defined as prior percutaneous coronary intervention, coronary artery bypass surgery, or myocardial infarction. Congestive heart failure (CHF) was classified according to the New York Heart Association (NYHA) system (I‐IV). Presence or absence of chronic lung disease, lower extremity peripheral arterial disease (defined as clinical claudication or prior revascularization intervention), and prior stroke or transient ischemic attack were recorded, as well as device type (pacemaker, ICD, or implantable loop recorder).
Additionally, participants' self‐rated health was obtained by asking participants to classify their overall health status as excellent, very good, good, fair, or poor.
Clinical Frailty Assessment
Clinical frailty was assessed using the Study of Osteoporotic Fractures (SOF) frailty measure, which has been validated in men and women and is predictive of falls, mobility disability, and mortality.7, 8, 9, 10 In contrast to some other measures of the frailty phenotype commonly used in the research literature,2, 11 the SOF index is intended to maximize tractability of measurement in a clinical setting, making it ideal for our design. This measure has shown strong agreement with the construct by Fried and colleagues obtained from the Cardiovascular Health Study, widely considered the research standard.7, 8, 9 We previously showed that the prevalence of prefrailty and frailty by the SOF criteria were equal to those of the more complex Cardiovascular Health Study measure in a large sample of randomly selected, community‐dwelling individuals.12
Components of the SOF construct include involuntary weight loss (positive response to the query “In the last year, have you lost more than 10 pounds unintentionally, that is, not due to dieting or exercise?”), lethargy (negative response to the query “Do you feel full of energy?”), and inability to perform a repeated chair stands task (moving from a seated to a standing position without use of one's arms 5 times in succession). Individuals meeting none of these 3 criteria are considered “robust,” those meeting 1 of the 3 are consider “prefrail,” and those meeting 2 or 3 are considered “frail.”
Mobility and Gait Assessments
Mobility was assessed with the Timed Up and Go (TUG) test, which has been shown to be a reliable and valid measure in several studies.13, 14 After instructed on the test, patients were timed as they rose from a chair, walked 3 m at their usual pace, turned around, walked back, and sat down. Each subject performed the TUG test twice, and the average of these 2 measurements was used in analyses.
Gait speed was measured via the 4‐m walk test, which has been previously shown to predict clinical outcomes in select cardiovascular patients.15, 16 Starting from a standing position, subjects were instructed to walk at their usual speed. The faster of the 2 speeds was selected for analyses as this has been previously considered reflective of usual speed after the subject has become accustomed to the experimental setting.17 Prevalent “slow gait speed” was defined as a 4‐m gait speed less than 0.8 m/s, consistent with other investigations.18, 19
Patient Activity
Patient activity in CIEDs is measured through an integrated circuit accelerometer embedded in the pulse generator, which in applicable patients can also be used for rate‐responsive pacing. The accelerometer detects both the frequency and amplitude of patient motion and translates this into a proportional electrical signal updated each minute. The specific algorithm for translating these signals into an adjudicated minute of “activity” is proprietary and may vary by manufacturer. For example, in Boston Scientific devices, force of motion of at least 25 milligravities—corresponding to an approximate walking speed of 2 mph—denotes an “active” minute.6 Device platforms for storing activity information vary, but they generally track minutes per day of activity according to calendar days. Some specific models that are common in clinical practice (such as the Medtronic Adapta and Sensia pacemaker systems) do not store activity in an analyzable format, so these and similar devices were excluded from our analysis.
All activity information was directly downloaded from the applicable programmer in device clinic at the time of clinical device interrogations. For all manufacturers, the raw data files are encrypted and require translation into an analyzable format using proprietary software specific to each company. These data were then used to calculate activity in hours per day for each patient in the 30 days prior to their in‐office interviews. For analyses reported here we restricted attention to participants with at least 7 days' evaluable data.
Statistical Analysis
Summary statistics were generated for baseline demographics and clinical variables. Characteristics of patients with and without physical activity data available from their devices were compared with Student t tests and Fisher exact tests.
For the analytic cohort with device data, multiple linear regression was used to determine associations between gait speed and TUG time on frailty. Logistic regression was used to assess association of slow gait speed with frailty. We then fit a multinomial logistic regression model to estimate the association between clinical frailty and device‐detected activity. Age, body mass index, NYHA Class (collapsed into a binary variable comprising Class I versus Classes II through IV), and SF‐12 were included in the multivariate model based on the independent strength of association with frailty or activity, theoretical plausibility, and evidence from literature review. Odds ratios (ORs) and 95% confidence intervals (CIs) were generated to quantify associations. Similarly, the association between gait speed, TUG time and device‐detected activity was assessed with linear regression, also adjusted for age, body mass index, NYHA class, and SF‐12 status.
Prespecified hypothesis testing of the relationships between (1) gait speed, TUG time and frailty, (2) slow gait speed and frailty, and (3) TUG time, gait speed, and frailty and activity were evaluated at the 0.05 level. Analyses were performed in R 3.3.020 using the MASS and nnet packages.21
Results
Derivation of Analytic Sample
Of 448 eligible individuals screened, 418 (93%) consented to enrollment (Figure 1). Of these, device data could be extracted for 219 patients who constituted our analytic sample. Descriptive statistics comparing patients with and without available device data are presented in Table S1. We found no significant difference in distribution of frailty phenotype, gait speed, NYHA Class, or SF‐12 status among patients with device activity data compared to those lacking device activity data. There were slight differences in prevalence of coronary artery disease, congestive heart failure, and stroke/transient ischemic attack (TIA) among patients with device activity data. All enrolled patients completed the study protocol, and no adverse events were recorded.
Figure 1.

Study flow and derivation of analytic sample.
Baseline Characteristics
The table provides summary characteristics for the analytic cohort (N=219) according to frailty status. A total of 87 (39.7%) participants were robust, 104 (47.5%) were prefrail, and 28 (12.8%) were frail. Frail individuals had a greater prevalence of cardiovascular comorbidities including CHF and preexisting stroke or TIA. Frail patients also had a greater likelihood of reporting fair or poor health (45%) than their prefrail (27%) or robust (3%) counterparts. The mean gait speed for the cohort was 0.8±0.3 m/s, and the mean TUG time was 10.9±4.4 seconds. Slow gait speed (<0.8 m/s) was more prevalent among frail patients (89%) than in prefrail (54%) or robust individuals (38%). Physical activity as adjudicated by patients' device averaged 2.8±1.0 hours overall and 3.6±2.0 hours among robust patients, with expected trends toward lower activity in prefrail (2.5±1.8 hours) and frail (1.8±1.2 hours) subjects.
Table 1.
Characteristics of Study Cohort at Time of Office Testing
| Overall 219 (100%) | Frail 28 (12.8%) | Prefrail 104 (47.5%) | Robust 87 (39.7%) | |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, y (mean±SD) | 68±13 | 73±12 | 69±12 | 65±14 |
| Male | 153 (70) | 19 (68) | 70 (67) | 64 (74) |
| Body mass index (mean±SD) | 28±6 | 27±5 | 30±6 | 27±5 |
| Coronary artery disease | 101 (46) | 14 (50) | 51 (49) | 36 (41) |
| Congestive heart failure | 116 (53) | 21 (75) | 56 (54) | 39 (45) |
| New York Heart Association Class | ||||
| Class I | 146 (67) | 9 (32) | 60 (58) | 146 (67) |
| Class II | 57 (26) | 11 (39) | 37 (36) | 57 (26) |
| Class III | 13 (6) | 7 (25) | 5 (5) | 13 (6) |
| Class IV | 3 (1) | 1 (4) | 2 (2) | 3 (1) |
| Chronic lung disease | 35 (16) | 5 (18) | 11 (13) | 35 (16) |
| Peripheral arterial disease | 12 (5) | 3 (11) | 4 (4) | 5 (6) |
| Stroke or transient ischemic attack | 40 (18) | 9 (32) | 19 (18) | 12 (14) |
| Device type | ||||
| Implantable defibrillator | 132 (60) | 15 (54) | 63 (61) | 132 (60) |
| Pacemaker | 74 (34) | 12 (43) | 37 (36) | 74 (34) |
| Implantable loop recorder | 13 (6) | 1 (4) | 4 (4) | 13 (6) |
| Office testing results | ||||
| Timed Up and Go Test, seconds (mean±SD) | 10.9±4.4 | 15.4±5.8 | 11.0±4.1 | 9.5±3.1 |
| Gait speed, m/s (mean±SD) | 0.8±0.3 | 0.6±0.2 | 0.8±0.3 | 0.9±0.3 |
| Mobility limitation* | 114 (52) | 25 (89) | 56 (54) | 33 (38) |
| Physical activity, hours (mean±SD) | 2.8±1.9 | 1.8±1.2 | 2.5±1.8 | 3.6±2.0 |
Values are N (%) unless otherwise stated. *Mobility limitation was defined as a gait speed of less than 0.8 m/s.
Association Between Mobility and Frailty
Average gait speed in the frail group was significantly lower than that in the robust group (mean difference −0.02 m/s, 95% CI −0.33 to −0.07), but there was no significant difference in gait speed when prefrail and robust groups were compared (mean difference −0.004, 95% CI −0.09 to 0.082). TUG testing for the frail group took over 3 seconds longer on average compared to the robust group (mean difference 3.341, 95% CI 1.53‐5.152), but there was no significant difference in TUG time between the prefrail and robust groups (mean difference 0.092 seconds, 95% CI −1.103 to 1.286). There was a strong association between frailty status and mobility. Frail patients were markedly more likely to have gait speeds <0.8 m/s (OR 6.25, 95% CI 1.79‐33.3), but this association was not statistically significant for prefrail patients (OR 1.18, 95% CI 0.59‐2.38).
Association Between Activity and Frailty
Unadjusted multinomial regression demonstrated a robust association between activity and frailty status (Figure 2), indicating that, on average, a 1‐hour increase in mean daily activity was associated with a 46% reduction of frail phenotype (OR 0.54, 95% CI 0.40‐0.74) versus robust, and a 27% reduction in the odds of having the prefrail phenotype (OR 0.73, 95% CI 0.62‐0.86). This association persisted after adjustment for covariates: each additional hour of activity was associated with a 29% reduction in the odds of frailty (adjusted OR 0.71, 95% CI 0.51‐0.99) and a 19% reduction in the odds of prefrailty phenotype (adjusted OR 0.81, 95% CI 0.67‐0.99) versus being robust.
Figure 2.
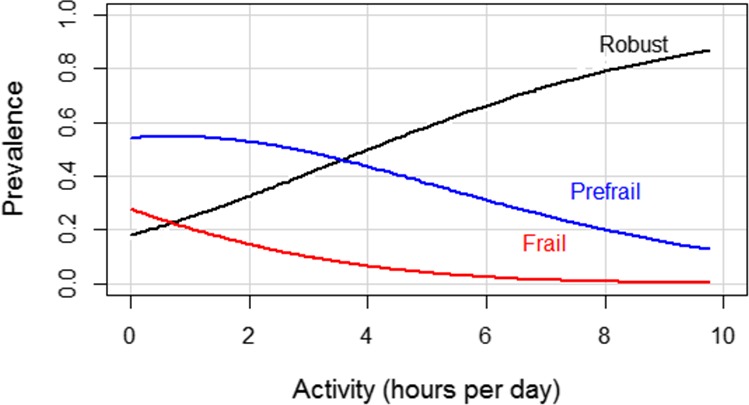
Unadjusted association between activity and frailty.
Association Between Mobility and Activity
Unadjusted linear regression evaluating mobility endpoints and device‐detected activity demonstrated that each hour increase in activity was associated with a decrease in TUG time of 0.83 seconds (95% CI 0.54‐1.11, P<0.001) and an increase in gait speed of 0.04 m/s (95% CI 0.02‐0.06, P<0.001). After adjustment for covariates, the relationship with TUG remained statistically significant, with a decrease in TUG time of 0.36 seconds, (95% CI 0.08‐0.65, P=0.013). The adjusted relationship with gait speed, however, was no longer statistically significant (increase of 0.02 m/s, 95% CI 0‐0.04, P=0.09).
Discussion
This cross‐sectional study demonstrates the ease with which clinical frailty and mobility testing can be integrated into ambulatory device assessment, with high rates of enrollment and no adverse events. We found that a relatively high proportion of ambulatory CIED patients are frail, and over half manifest slow gait speed. Our analysis also provides further validation of device‐detected activity as a clinically meaningful covariate with clear associations with tractable measures of function and frailty status. Device‐detected activity may therefore be clinically useful for identifying patients at risk for frailty or prefrailty.
Formal measurement of geriatric conditions such as frailty, as well as physiologic measures such as gait speed, have been increasingly embedded in the care of cardiovascular patients.3 These measures are associated with outcomes among patients undergoing cardiac surgery23, 24 and transcatheter aortic valve replacement,16 and in the setting of acute coronary syndromes,25 and may be superior to traditional covariates included routinely in risk models.26 However, frailty and mobility testing have not been routinely integrated into studies of electrophysiology patients. Larger observational cohorts such as the National Cardiovascular Data Registry—ICD Registry have been used to focus on outcomes of importance to older adults, such as hospice enrollment,27 but currently do not include frailty or mobility testing. Our study demonstrates the ease and safety with which such measures can be included alongside routine CIED management and provide estimates of the prevalence of frailty and average gait speed and TUG times applicable to future studies.
The results of our analysis of CIED physical activity data and frailty demonstrate that device‐detected physical activity is predictive of frailty status and bolsters the potential for the use of activity data as an indicator of risk of frailty and mobility disability. This work thus builds on prior assessments of physical activity among CIED recipients, which have predominantly focused on patients with clinical heart failure and ICDs. For example, Conraads et al evaluated pooled data for 781 heart failure patients from separate clinical trials and found activity shortly after ICD implantation to be predictive of survival (hazard ratio 0.93 per 10 min/day of activity) after adjustment for clinical covariates.4 Baseline and time‐varying activity predicted survival in a large nationwide ICD sample,6 and patterns of longitudinal activity have been evaluated as a marker of cardiac resynchronization therapy response. Activity has also been included alongside markers of autonomic function and arrhythmia burden in risk models evaluating heart failure events,28, 29 although correlations with 6‐minute walk tests have been only modestly successful.5, 30 Our study thus broadens the device‐detected activity population to include patients without heart failure or ICDs and strengthens the evidence supporting the physiologic importance of these measures.
The ubiquity of well‐validated device‐detected activity measures provides a tempting target not just for identifying frail patients but to improve clinical outcomes. Identifying patients whose functional trajectories may be trending toward frailty, for example, may trigger additional diagnostic testing or interventions designed to improve or preserve patients' mobility, quality of life, and/or independence.24 Formal frailty testing of patients found to have new or very depressed activity levels may identify conditions such as incompletely treated heart failure or peripheral arterial disease. Gait speed and TUG testing (or components such as chair stands) contribute to formal assessment of frailty in some measures (such as the Fried criteria and short physical performance battery) while they also independently contribute to a fuller picture of patients' functional status.2, 31, 32, 33 Thus, for patients in whom frailty, prefrailty, or mobility limitations are identified, comanagement shared between cardiology and geriatrics may streamline care and rehabilitative efforts focused on preservation of independence.
In addition, remote monitoring, now routine for most CIED patients,34 may support tracking of individual patients' activity trajectories without the need for in‐office assessments. Importantly, frailty itself is not a fixed state but may be dynamic and potentially modifiable, with physical activity itself consistently demonstrated to be the most powerful intervention.3, 26, 35 The strong and significant independent association between SOF frailty assessment and mobility or mobility limitation we identified appeared to be more consequential and clinically meaningful in magnitude for the complex TUG task than for gait speed itself, which exhibited a statistically significant but modest mean difference of only 0.02 m/s between robust and frail individuals. This finding suggests that frailty status may manifest more noticeably in complex functional activities rather than in gait speed itself. Thus, linking activity measures to both frailty and mobility may not be straightforward at the individual level. However, pairing activity patterns or thresholds aligned with treatment pathways in a prospective way may be an opportunity to leverage the extraordinary amount of data already being collected by patients' devices.
Our study includes potential limitations, including the cross‐sectional nature of the data collection, the convenience sampling frame, and restriction of enrollment to individuals at a single ambulatory clinic. Future longitudinal assessments of randomly selected cohorts over a more diverse geographic range will be critical in confirming generalizability of our results to the broader population of men and women with implantable devices. The activity measurements from the devices used in our study have not to our knowledge been validated against other accepted measures of activity such as omnidirectional accelerometry, and the question of whether different manufacturers' algorithms vary from each other remains proprietary and unknown. Although evaluation of activity in a clinical setting is straightforward, because of data encryption the export of more granular data for statistical analysis requires significant cooperation from manufacturers, which may limit further studies in this area.
In summary, more than half of ambulatory CIED patients are prefrail or frail, and mobility limitations are similarly common. Device‐detected physical activity is correlated with these measures and may be clinically useful for identifying patients at high risk for adverse events.
Sources of Funding
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. Dr Kramer is supported by a Paul B. Beeson Career Development Award in Aging Research (K23AG045963). Dr Mitchell is supported by NIH‐NIA K24AG033640.
Disclosures
None.
Supporting information
Table S1. Characteristics at Time of Office Testing for Final Cohort of Patients With Device Data Available (N=219) Compared With Patients Who Completed the In‐Office Testing but Whose Cardiac Devices Did Not Support Analysis of Activity Data (N=199). For covariates with categories (such as NYHA Class or device type), test statistics were generated by comparing the largest groups to each other.
Acknowledgments
Drs Kramer and Travison had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2017;6:e004659. DOI: 10.1161/JAHA.116.004659.)
References
- 1. Green AR, Leff B, Wang Y, Spatz ES, Masoudi FA, Peterson PN, Daugherty SL, Matlock DD. Geriatric conditions in patients undergoing defibrillator implantation for prevention of sudden cardiac death: prevalence and impact on mortality. Circ Cardiovasc Qual Outcomes. 2016;9:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 3. Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conraads VM, Spruit MA, Braunschweig F, Cowie MR, Tavazzi L, Borggrefe M, Hill MR, Jacobs S, Gerritse B, van Veldhuisen DJ. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ Heart Fail. 2014;7:279–287. [DOI] [PubMed] [Google Scholar]
- 5. Kadhiresan VA, Pastore J, Auricchio A, Sack S, Doelger A, Girouard S, Spinelli JC; PATH‐CHF Study Group: Pacing therapies in congestive heart failure. . A novel method—the activity log index—for monitoring physical activity of patients with heart failure. Am J Cardiol. 2002;89:1435–1437. [DOI] [PubMed] [Google Scholar]
- 6. Kramer DB, Mitchell SL, Monteiro J, Jones PW, Normand SL, Hayes DL, Reynolds MR. Patient activity and survival following implantable cardioverter‐defibrillator implantation: the ALTITUDE Activity Study. J Am Heart Assoc. 2015;4:e001775 doi: 10.1161/JAHA.115.001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. [DOI] [PubMed] [Google Scholar]
- 8. Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, Dam TT, Marshall LM, Orwoll ES, Cummings SR; Osteoporotic Fractures in Men Research Group . A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiely DK, Cupples LA, Lipsitz LA. Validation and comparison of two frailty indexes: the MOBILIZE Boston Study. J Am Geriatr Soc. 2009;57:1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bilotta C, Nicolini P, Case A, Pina G, Rossi S, Vergani C. Frailty syndrome diagnosed according to the study of osteoporotic fractures (SOF) criteria and adverse health outcomes among community‐dwelling older outpatients in Italy. A one‐year prospective cohort study. Arch Gerontol Geriatr. 2012;54:e23–e28. [DOI] [PubMed] [Google Scholar]
- 11. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Travison TG, Nguyen AH, Naganathan V, Stanaway FF, Blyth FM, Cumming RG, Le Couteur DG, Sambrook PN, Handelsman DJ. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: the Concord Health and Ageing in Men Project. J Clin Endocrinol Metab. 2011;96:2464–2474. [DOI] [PubMed] [Google Scholar]
- 13. Hafsteinsdottir TB, Rensink M, Schuurmans M. Clinimetric properties of the Timed Up and Go Test for patients with stroke: a systematic review. Top Stroke Rehabil. 2014;21:197–210. [DOI] [PubMed] [Google Scholar]
- 14. Botolfsen P, Helbostad JL, Moe‐Nilssen R, Wall JC. Reliability and concurrent validity of the Expanded Timed Up‐and‐Go test in older people with impaired mobility. Physiother Res Int. 2008;13:94–106. [DOI] [PubMed] [Google Scholar]
- 15. Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. [DOI] [PubMed] [Google Scholar]
- 16. Alfredsson J, Stebbins A, Brennan JM, Matsouaka R, Afilalo J, Peterson ED, Vemulapalli S, Rumsfeld JS, Shahian D, Mack MJ, Alexander KP. Gait speed predicts 30‐day mortality after transcatheter aortic valve replacement: results from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2016;133:1351–1359. [DOI] [PubMed] [Google Scholar]
- 17. Quach L, Galica AM, Jones RN, Procter‐Gray E, Manor B, Hannan MT, Lipsitz LA. The nonlinear relationship between gait speed and falls: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. J Am Geriatr Soc. 2011;59:1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community‐dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. [DOI] [PubMed] [Google Scholar]
- 19. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. Available at: https://www.R-project.Org/. Accessed October 1, 2016. [Google Scholar]
- 21. Venables WN, Ripley BD. Modern Applied Statistics With S. 4th ed New York, NY: Springer; 2002. ISBNsbn 0‐387‐95457‐0. [Google Scholar]
- 22. Venables WN, Ripley BD. Modern Applied Statistics With S. 4th ed New York, NY: Springer; 2002. ISBN 0‐387‐95457‐0. [Google Scholar]
- 23. Afilalo J, Kim S, O'Brien S, Brennan JM, Edwards FH, Mack MJ, McClurken JB, Cleveland JC Jr, Smith PK, Shahian DM, Alexander KP. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol. 2016;1:314–321. [DOI] [PubMed] [Google Scholar]
- 24. Kim DH, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative frailty assessment and outcomes at 6 months or later in older adults undergoing cardiac surgical procedures: a systematic review. Ann Intern Med. 2016;165:650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ekerstad N, Swahn E, Janzon M, Alfredsson J, Lofmark R, Lindenberger M, Carlsson P. Frailty is independently associated with short‐term outcomes for elderly patients with non‐ST‐segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. [DOI] [PubMed] [Google Scholar]
- 26. Afilalo J. The road to frailty is paved with good intentions. Circ Cardiovasc Qual Outcomes. 2016;9:194–196. [DOI] [PubMed] [Google Scholar]
- 27. Kramer DB, Reynolds MR, Normand SL, Parzynski CS, Spertus JA, Mor V, Mitchell SL. Hospice use following implantable cardioverter‐defibrillator implantation in older patients: results from the National Cardiovascular Data Registry. Circulation. 2016;133:2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whellan DJ, Ousdigian KT, Al‐Khatib SM, Pu W, Sarkar S, Porter CB, Pavri BB, O'Connor CM; Partners Study Investigators . Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) study. J Am Coll Cardiol. 2010;55:1803–1810. [DOI] [PubMed] [Google Scholar]
- 29. Cowie MR, Sarkar S, Koehler J, Whellan DJ, Crossley GH, Tang WH, Abraham WT, Sharma V, Santini M. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur Heart J. 2013;34:2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vegh EM, Kandala J, Orencole M, Upadhyay GA, Sharma A, Miller A, Merkely B, Parks KA, Singh JP. Device‐measured physical activity versus six‐minute walk test as a predictor of reverse remodeling and outcome after cardiac resynchronization therapy for heart failure. Am J Cardiol. 2014;113:1523–1528. [DOI] [PubMed] [Google Scholar]
- 31. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 33. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PubMed] [Google Scholar]
- 34. Hindricks G, Varma N. Remote monitoring and heart failure: monitoring parameters, technology, and workflow. Eur Heart J. 2016;37:3141–3146. [DOI] [PubMed] [Google Scholar]
- 35. Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics at Time of Office Testing for Final Cohort of Patients With Device Data Available (N=219) Compared With Patients Who Completed the In‐Office Testing but Whose Cardiac Devices Did Not Support Analysis of Activity Data (N=199). For covariates with categories (such as NYHA Class or device type), test statistics were generated by comparing the largest groups to each other.


