Abstract
Background
Multiple randomized controlled trials of remote ischemic preconditioning (RIPC) prior to cardiac surgery have failed to demonstrate clinical benefit. The aim of this updated meta‐analysis was to evaluate the effect of RIPC on outcomes following cardiac surgery.
Methods and Results
Searches of PubMed, Cochrane, EMBASE, and Web of Science databases were performed for 1970 to December 13, 2015. Randomized controlled trials comparing RIPC with a sham procedure prior to cardiac surgery performed with cardiopulmonary bypass were assessed. All‐cause mortality, acute kidney injury (AKI), and myocardial infarction were the primary outcomes of interest. We identified 21 trials that randomized 5262 patients to RIPC or a sham procedure prior to undergoing cardiac surgery. The majority of patients were men (72.6%) and the mean or median age ranged from 42.3 to 76.3 years. Of the 9 trials that evaluated mortality, 188 deaths occurred out of a total of 4210 randomized patients, with 96 deaths occurring in 2098 patients (4.6%) randomized to RIPC and 92 deaths occurring in 2112 patients (4.4%) randomized to a sham control procedure, demonstrating no significant reduction in all‐cause mortality (risk ratio [RR], 0.987; 95% CI, 0.653–1.492, P=0.95). Twelve studies evaluated AKI in 4209 randomized patients. In these studies, AKI was observed in 516 of 2091 patients (24.7%) undergoing RIPC and in 577 of 2118 patients (27.2%) randomized to a sham procedure. RIPC did not result in a significant reduction in AKI (RR, 0.839; 95% CI, 0.703–1.001 [P=0.052]). In 6 studies consisting of 3799 randomized participants, myocardial infarction occurred in 237 of 1891 patients (12.5%) randomized to RIPC and in 282 of 1908 patients (14.8%) randomized to a sham procedure, resulting in no significant reduction in postoperative myocardial infarction (RR, 0.809; 95% CI, 0.615–1.064 [P=0.13]). A subgroup analysis was performed a priori based on previous studies suggesting that propofol may mitigate the protective benefits of RIPC. Three studies randomized patients undergoing cardiac surgery to RIPC or sham procedure in the absence of propofol anesthesia. Most of these patients were men (60.3%) and the mean or median age ranged from 57.0 to 70.6 years. In this propofol‐free subgroup of 434 randomized patients, 71 of 217 patients (32.7%) who underwent RIPC developed AKI compared with 103 of 217 patients (47.5%) treated with a sham procedure. In this cohort, RIPC resulted in a significant reduction in AKI (RR, 0.700; 95% CI, 0.527–0.930 [P=0.014]). In studies of patients who received propofol anesthesia, 445 of 1874 (23.7%) patients randomized to RIPC developed AKI compared with 474 of 1901 (24.9%) who underwent a sham procedure. The RR for AKI was 0.928 (95% CI, 0.781–1.102; P=0.39) for RIPC versus sham. There was no significant interaction between the two subgroups (P=0.098).
Conclusions
RIPC does not reduce morbidity or mortality in patients undergoing cardiac surgery with cardiopulmonary bypass. In the subgroup of studies in which propofol was not used, a reduction in AKI was seen, suggesting that propofol may interact with the protective effects of RIPC. Future studies should evaluate RIPC in the absence of propofol anesthesia.
Keywords: cardiac surgery, meta‐analysis
Subject Categories: Cardiovascular Surgery, Meta Analysis
Introduction
Thirty years ago, Murry et al1 first described ischemic preconditioning (IPC) after observing that anesthetized dogs subjected to prolonged circumflex coronary artery occlusion and reperfusion demonstrated a marked reduction of myocardial infarct size when exposed to 4 brief episodes of ischemia in the circumflex territory separated by 5 minutes of reperfusion prior to the prolonged occlusion. Remote IPC (RIPC) evolved from the same in vivo canine heart model where ischemia‐reperfusion injury could be attenuated in the left anterior descending coronary artery distribution after application of occlusion and reperfusion to the circumflex coronary artery.2 With this finding, Przyklenk et al2 concluded that protective mediators induced by ischemia could be transferred to distant, “regional” cardiomyocytes. Subsequent studies demonstrated that protection against ischemia‐reperfusion injury in humans could be extended to distant organs, such as the kidney and brain.3, 4 The discovery that protection could be conferred by ischemia‐reperfusion cycles in distant skeletal muscle elicited invasively by rapid stimulation of the gastrocnemius in rabbits5 and noninvasively by a tourniquet in humans6 spurred widespread clinical interest.
Given that cardiac surgery has the potential for ischemia and reperfusion injury to the heart, kidney and brain,7, 8, 9 RIPC has long been viewed as an attractive approach to mitigate the deleterious clinical consequences of these events. Prior studies have shown that RIPC before cardiac surgery results in reductions in biomarkers of renal and cardiac injury.10, 11 However, randomized controlled trials (RCTs) of RIPC evaluating clinical cardiovascular and renal outcomes as well as overall mortality have not shown benefit.12, 13 Many of these trials utilized propofol anesthesia, which has been shown to negatively impact the benefits of RIPC.14 With the recent publication of the two largest trials of RIPC to date,15, 16 we performed an updated meta‐analysis of RCTs to better evaluate the clinical merit of this intervention.
Methods
Study Selection
A systematic search of published studies in any language in the PubMed, Cochrane, EMBASE, and Web of Science databases from 1970 to December 13, 2015, was performed independently by two authors (V.P. and I.B.). Search terms included remote ischemic preconditioning, cardiac surgery, kidney injury, and renal failure, as well as combinations of these terms. A filter for RCTs was used. Bibliographies of retrieved articles and prior reviews on the subject were searched for other relevant studies.
For inclusion, studies were required to be prospective randomized trials of preoperative RIPC or a sham procedure in patients undergoing cardiac surgery performed on cardiopulmonary bypass. In addition, studies had to report at least one clinical end point of interest as an outcome and enroll more than 50 patients. Patient characteristics, study design, and outcomes were systematically reviewed and recorded independently by 3 authors (B.P., I.B., and V.P.). Disagreements were resolved by consensus.
The methodological quality of each trial was evaluated using standard criteria: method of randomization; allocation concealment; patient, investigator, and outcome assessor blinding; selective outcome reporting; incomplete outcome ascertainment; and other potential sources of bias as recommended by the Cochrane Collaboration.17 The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach for evaluating RCTs was applied.18
The following clinical end points were analyzed: all‐cause mortality, acute kidney injury (AKI), myocardial infarction (MI), cerebrovascular accident (CVA), hospital length of stay (LOS), and intensive care unit (ICU) LOS. Discrete working definitions of AKI were reclassified as stage I, II, or III based on previous definitions described by the Acute Kidney Injury Advisory Group.19 Other end point definitions were those used in the individual trials and are summarized in Table 1.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33
Table 1.
End Point Definitions
| Trial | All‐Cause Mortality | AKI | MI | Stroke | ICU LOS | Hospital LOS |
|---|---|---|---|---|---|---|
| Rahman et al, 201020 | N/A | >0.5 mg/dL−1 creatinine rise on day 4 postoperatively | N/A | N/A | Postoperative ICU stay in days | Postoperative hospital stay in days |
| Venugopal et al, 201021 | Death from any cause within 30 d | AKIN criteria in first 72 h postoperatively | N/A | N/A | N/A | Postoperative hospital stay in days |
| Li et al, 201022 | N/A | N/A | N/A | N/A | Postoperative ICU stay in hoursa | Postoperative hospital stay in days |
| Karuppasamy et al, 201123 | N/A | N/A | N/A | N/A | Postoperative ICU stay in hoursa | Postoperative hospital stay in days |
| Wu et al, 201124 | N/A | N/A | N/A | N/A | Postoperative ICU stay in hoursa | Postoperative hospital stay in days |
| Choi et al, 201125 | N/A | AKIN criteria in first 48 h postoperatively | N/A | N/A | Postoperative ICU stay in days | Postoperative hospital stay in days |
| Zimmerman et al, 20113 | Death from any cause within the index stay | AKIN criteria in first 48 h postoperatively | N/A | N/A | N/A | Postoperative hospital stay in days |
| Lucchinetti et al, 201226 | Death from any cause within the follow‐up period (6 mo) | N/A | Perioperative increase in cTnI to 5× the 99th percentile reference range with new pathological Q waves, LBBB, or new angiographic occlusion. Postoperative increase in cTnI to 2× reference range with evidence of ischemia | N/A | N/A | N/A |
| Xie et al, 201227 | Death from any cause within the follow‐up period (3‐39 mo) | N/A | N/A | N/A | N/A | N/A |
| Young et al, 201213 | N/A | RIFLE criteria for index stay | N/A | N/A | N/A | N/A |
| Lomivorotov et al, 201241 | Death from any cause within the follow‐up period (30 d) | N/A | N/A | N/A | Postoperative ICU stay in days | N/A |
| Meybohm et al, 201328 | N/A | AKIN criteria in first 48 h postoperatively | N/A | N/A | Postoperative ICU stay in hoursa | Postoperative hospital stay in days |
| Thielmann et al, 201329 | Death from any cause within the follow‐up period (4+ y) | N/A | Perioperative increase in cTnI to 5× the 99th percentile reference range with new pathological Q waves, LBBB, or new angiographic occlusion. Postoperative increase in cTnI to 2× reference range with evidence of ischemia | Any embolic event after immediate postoperative period; a neurological event resulting in a new deficit; any neurological event lasting >24 h unless a cerebral lesion was visualized on imaging | Postoperative ICU stay in days | Postoperative hospital stay in days |
| Gallagher et al, 201530 | Death from any cause within 30 d | AKIN criteria in first 48 h postoperatively | N/A | N/A | Postoperative ICU stay in hoursa | Postoperative hospital stay in days |
| Ahmad et al, 201412 | Death from any cause within the index hospital stay | N/A | N/A | N/A | N/A | N/A |
| Slagsvold et al, 201431 | Death from any cause within 30 d | N/A | N/A | N/A | N/A | N/A |
| Candilio et al, 201532 | Death from any cause within 6 wk | RIFLE criteria in first 72 h postoperatively | Myocardial infarction (not defined) by 6 wk | Stroke (not defined) by 6 wk | Postoperative ICU stay in days | Postoperative hospital stay in days |
| Zarbock et al, 201511 | Death from any cause within 30 d | KDIGO criteria in first 72 h postoperatively | Perioperative increase in cTnI to 5× the 99th percentile of reference range with new pathological Q waves, LBBB, or new angiographic occlusion. Postoperative increase in cTnI to 2× reference range with evidence of ischemia | Any embolic event after immediate postoperative period; a neurological event resulting in a new deficit; any neurological event lasting >24 h unless a cerebral lesion was visualized on imaging | Postoperative ICU stay in days | Postoperative hospital stay in days |
| Pinaud et al, 201633 | N/A | AKIN criteria in first 72 h postoperatively | N/A | N/A | Postoperative ICU stay in hoursa | N/A |
| Meybohm et al, 201516 | Death from any cause within index hospital stay (maximum 14 d) | Increase in serum creatinine by a factor of ≥2 from baseline | Biomarker values over 5× the 99th percentile of reference range with pathological Q waves or new LBBB in first 72 h. Standard clinical criteria for MI from 72 h onward. Ischemia by echocardiography or angiography. MI diagnosed at autopsy | New, temporary, or permanent focal or global neurological deficit. Evidence of stroke at autopsy | N/A | N/A |
| Hausenloy et al, 201515 | Death from any cause within 12 mo | KDIGO criteria in first 72 h postoperatively | Biomarker values >10× the 99th percentile of reference range when associated with new pathological Q waves, new LBBB, or angiographically documented occlusion in the first 72 h postoperatively or biomarker value >100× the 99th percentile in the first 72 h postoperatively | Focal neurological deficit of cerebrovascular cause persisting beyond 24 h or interrupted by death within 24 h | Postoperative ICU stay in days | Postoperative hospital stay in days |
AKIN indicates acute kidney injury network; cTnI, cardiac troponin I; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes; LBBB, left bundle branch block; LOS, length of stay; MI, myocardial infarction; N/A, not applicable; RIFLE, risk, injury, failure, loss, end‐stage.
Hours were divided by 24 for unit of measurement uniformity.
Statistical Analysis
Because patient‐level data from each trial were not available, a meta‐analysis of summary statistics from individual trials was performed. Data from each trial were analyzed on an intention‐to‐treat basis according to the recommendations of the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement.34 Trial results for each end point were summarized with risk ratios (RRs) and standardized mean differences as the measures of effect. RRs were employed because accurate time‐to‐event data were not available in all trials. Summary RRs or standardized mean differences and 95% CIs were calculated using a random‐effects model for combining results across studies, which incorporates between‐ and within‐study variance and provides a more conservative summary. A random‐effects model was preferred because heterogeneity across patient characteristics and clinical trial design would be unlikely to result in a consistent treatment effect across trials.35 When no events were observed within a treatment group, a 0.5 correction factor was added to all values of that end point for calculation of the RR and its variance.36, 37 To determine whether there was heterogeneity between individual trials, we assessed the Q statistic (a weighted index of effect estimate differences across studies assuming a χ2 distribution) and I 2 statistic ([Q−df]/Q×100). Because the I 2 value quantifies heterogeneity on a scale of 0% to 100% and represents the extent of inconsistency among trial results rather than a sampling error independent of the number of studies, an I 2 of ≥75% was considered representative of high heterogeneity.38 To assess for publication bias, funnel plots were evaluated by visual inspection and confirmed by Egger's test.39 If analysis yielded plot asymmetry, Duval and Tweedie's trim and fill method, a quantitative assessment of publication bias, was performed.40
Heterogeneity was explored in subgroup analyses by study quality (high versus low), intraoperative propofol use, additive European System for Cardiac Operative Risk Evaluation (EuroSCORE), and preoperative potassium‐ATP (K‐ATP) antagonist use. Unless an anesthetic regimen without propofol was detailed, it was assumed that propofol was administered. In the event of protocol ambiguity, primary authors were contacted for clarification. In trials that did not exclude diabetic patients, it was assumed that K‐ATP antagonists were used unless specifically prohibited preoperatively. Sensitivity analyses were performed for each outcome to determine whether any single study disproportionately influenced the pooled estimate by excluding individual trials one at a time and recalculating the combined RR or standardized mean difference for the remaining studies. P<0.05 was considered statistically significant and all tests were 2‐sided. Statistical analyses were performed with Comprehensive Meta‐Analysis (version 2) software (Biostat, Englewood, NJ).
Results
Literature Search
The electronic search yielded 833 citations that were screened by reviewing the title or abstract with subsequent removal of duplicates. Of these, 45 articles were reviewed in full and 21 studies were included for analysis (Figure 1). Characteristics of the studies are listed in Table 2. Eight studies tested RIPC in patients undergoing isolated coronary artery bypass grafting (CABG),12, 20, 21, 23, 26, 29, 31, 41 four studies in patients undergoing isolated valve surgery,22, 24, 27, 33 and the remaining 9 in patients undergoing any cardiac surgery.1 One study included additional perconditioning, the application of short periods of ischemia and reperfusion at a distant site delivered during target organ ischemia; only data from the preconditioning intervention and the sham procedure were included.22
Figure 1.

Study selection. Flow diagram depicts study selection for inclusion in the meta‐analysis according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses.34 RIPC indicates remote ischemic preconditioning.
Table 2.
Characteristics of Trials
| Study | Country, Enrollment Years | No. of Patients Enrolled (RIPC/Sham) | Mean or Median Age (RIPC/Sham) | Male (RIPC/Sham), % | Inclusion Criteria | Primary End Point | CABG, Valve Surgery, or Mixed | Propofol Use | K‐ATP Antagonist Use | Preconditioning Site and Duration (Cycles×min) |
|---|---|---|---|---|---|---|---|---|---|---|
| Rahman et al, 201020 | UK, 2007–2009 | 162 (80/82) | 63/65 | 89.0/88.0 | Isolated first‐time multivessel CABG | Troponin AUC at 48 h | CABG | Yes | No | UL, 3×5 |
| Venugopal et al, 201021 | UK, 2006–2008 | 78 (38/40) | 64.0/66.0 | 79.0/85.0 | Elective CABG | Perioperative AKI in first 72 h | CABG | Unknown | No | UL, 3×5 |
| Li et al, 201022 | China, 2009 | 53 (26/27) | 45.8/42.3 | 30.0/50.0 | Age 18 to 65 y, rheumatic heart valve disease | Troponin release at 30 min and 4, 12, and 72 h after declamping | Valve replacement | Yes | No | LL, 4×4 |
| Karuppasamy et al, 201123 | UK, 2008–2009 | 54 (27/27) | 66.9/67.3 | 81.0/85.0 | Elective CABG | Troponin release at 6, 12, 24, and 48 h postoperatively | CABG | Yes | No | UL, 3×5 |
| Wu et al, 201124 | Japan, 2009–2010 | 75 (50/25) | 46.2/43.6 | 40.0/28.0 | Age 18 to 60 y, mitral valve replacement | Postoperative inotrope requirement, ICU LOS, hospital LOS | Valve replacement | Unknown | No | UL, 3×5 |
| Choi et al, 201125 | South Korea, 2008–2009 | 76 (38/38) | 57.0/60.0 | 39.5/39.5 | Elective, complex valvular heart surgery | Postoperative biomarkers of renal injury and incidence of AKI | Mixed | No | No | LL, 3×10 |
| Zimmerman et al, 20113 | US, 2008–2009 | 118 (59/59) | 62.0/65.0 | 69.0/68.0 | Elective cardiac surgery on CPB | Postoperative AKI | Mixed | No | Yes | LL, 3×5 |
| Lucchinetti et al, 201226 | Canada, 2008–2010 | 55 (27/28) | 59.0/62.0 | 96.0/86.0 | Elective CABG, age 50 to 85 y | Postoperative high‐sensitivity troponin release | CABG | Yes | No | LL, 4×5 |
| Xie et al, 201227 | China, 2007–2011 | 73 (38/35) | 51.1/50.4 | 50.0/47.1 | Elective valve surgery | Troponin I level at 6, 12, 24, 48 and 72 h | Valve | Yes | Yes | UL, 3×5 |
| Young et al, 201213 | New Zealand, 2010–2011 | 96 (48/48) | 65.5/64.4 | 60.4/64.6 | Double‐valve or triple‐valve surgery, mitral valve surgery, CABG | Postoperative high‐sensitivity troponin T at 6 and 12 h and AKI | Mixed | Yes | Yes | UL, 3×5 |
| Lomivorotov et al, 201241 | Russia 2010–2011 | 80 (40/40) | 56.6/58.1 | 90.0/92.5 | Adults undergoing CABG on CPB | Postoperative hemodynamic markers and troponin I and CK‐MB at 6, 24, and 48 h | CABG | Yes | No | UL, 3×5 |
| Meybohm et al, 201328 | Germany 2009–2010 | 180 (90/90) | 70.0/68.0 | 76.7/85.6 | Age >18 y undergoing cardiac surgery on CPB | Postoperative neurocognitive dysfunction at days 5 to 7 | Mixed | Yes | No | UL, 4×5 |
| Thielmann et al, 201329 | Germany 2008–2012 | 329 (162/167) | 68.2/69.1 | 83.0/80.0 | Adults with triple‐vessel disease undergoing primary, isolated, elective CABG on CPB | Perioperative myocardial injury reflected by AUC for troponin I | CABG | Yes | Unknown | UL, 3×5 |
| Gallagher et al, 201530 | UK, 2011–2012 | 86 (43/43) | 68.7/72.8 | 76.7/83.7 | Patients with chronic kidney disease undergoing CABG | Postoperative AKI within 48 h | Mixed | Unknown | Yes | UL, 3×5 |
| Ahmad et al, 201412 | Pakistan, 2012–2013 | 67 (35/32) | 54.5/55.2 | 77.1/78.1 | Patients with class III angina and triple‐vessel disease | Postoperative CK‐MB levels at 1, 6, 12, and 24 h | CABG | Yes | Unknown | UL, 3×5 |
| Slagsvold et al, 201431 | Norway, 2011 | 60 (30/30) | 64.0/68.0 | 90.0/76.7 | Urgent or elective first‐time CABG surgery | Mitochondrial respiration in situ as assessed by left ventricular biopsy | CABG | Yes | Yes | UL, 3×5 |
| Candilio et al, 201532 | UK, 2010–2012 | 178 (89/89) | 65.0/66.0 | 81.0/75.0 | Adult patients undergoing CABG and/or valve surgery | Perioperative myocardial infarction, measured by 72‐h AUC hsTnT | Mixed | Yes | No | UL, 3×5 |
| Zarbock et al, 201511 | Germany, 2013–2014 | 240 (120/120) | 70.1/70.6 | 63.3/62.5 | Adults at high risk for acute kidney injury undergoing cardiac surgery with CPB | Postoperative acute kidney injury at 72 h | Mixed | No | No | UL, 3×5 |
| Pinaud et al, 201633 | France, 2011–2012 | 99 (50/49) | 75.8/72.9 | 54.0/48.9 | Age >18 y undergoing elective aortic valve replacement | Postoperative AUC troponin I at 72 h | Valve | Yes | No | UL, 3×5 |
| Meybohm et al, 201516 | Germany, 2011–2014 | 1385 (692/693) | 65.8/66.0 | 73.4/75.0 | Age >18 y undergoing elective cardiac surgery requiring CPB | Composite of death, MI, stroke or acute renal failure | Mixed | Yes | No | UL, 4×5 |
| Hausenloy et al, 201515 | UK, 2010–2015 | 1612 (801/811) | 76.1/76.3 | 70.4/72.7 | Age >18 y; additive EuroSCORE ≥5 undergoing on‐pump CABG (with or without valve surgery) | Composite of death from cardiovascular causes, nonfatal MI, coronary revascularization, or stroke | Mixed | Yes | No | UL, 4×5 |
AKI indicates acute kidney injury; AUC, area under the curve; CABG, coronary artery bypass grafting; CK‐MB, creatinine kinase, myocardial B fraction; CPB, cardiopulmonary bypass; EuroSCORE, European System for Cardiac Operative Risk Evaluation; hsTnT, high‐sensitivity troponin T; ICU, intensive care unit; K‐ATP, potassium‐ATP; LL, lower limb; LOS, length of stay; MI, myocardial infarction; RIPC, remote ischemic preconditioning; UK, United Kingdom; UL, upper limb; US, United States.
Of the 5262 patients included in the analysis, 2624 were randomized to RIPC and 2638 were randomized to a sham procedure. Baseline characteristics of the study populations showed that most patients were men (72.6%). The mean or median ages of patients ranged from 42.3 to 76.3 years. The majority of studies were double‐blinded, randomized, and had adequate descriptions of patient attrition. Study quality is summarized in Table 3.
Table 3.
Study Quality and Risk of Bias Using the GRADE Criteria18
| Quality Assessment | No. of Patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Remote Ischemic Preconditioning | Sham Preconditioning | Relative (95% CI) | Absolute (95% CI) | ||
| All‐cause mortality | ||||||||||||
| 12 | Randomized trials | Seriousa , b | Seriousc , d | Not serious | Not serious | None | 96/2098 (4.6%) | 92/2112 (4.4%) | RR 0.98 (0.63–1.53) | 1 fewer per 1000 (from 16 fewer to 23 more) | ⨁⨁◯◯ Low | CRITICAL |
| Acute kidney injury | ||||||||||||
| 12 | Randomized trials | Seriousa , b | Not serious | Seriouse | Not serious | None | 516/2091 (24.7%) | 577/2118 (27.2%) | RR 0.85 (0.69–1.03) | 41 fewer per 1000 (from 8 more to 84 fewer) | ⨁⨁◯◯ Low | CRITICAL |
| Myocardial infarction | ||||||||||||
| 6 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 237/1891 (12.5%) | 282/1908 (14.8%) | RR 0.80 (0.61–1.04) | 30 fewer per 1000 (from 6 more to 58 fewer) | ⨁⨁⨁⨁ High | IMPORTANT |
| Stroke | ||||||||||||
| 6 | Randomized trials | Not serious | Seriousc | Not serious | Not serious | None | 34/1864 (1.8%) | 37/1880 (2.0%) | RR 0.94 (0.59–1.49) | 1 fewer per 1000 (from 8 fewer to 10 more) | ⨁⨁⨁◯ Moderate | IMPORTANT |
| ICU length of stay | ||||||||||||
| 12 | Randomized trials | Very seriousa , b , f | Very seriousc , d , g | Not serious | Not serious | None | 1381 | 1396 | — | SMD 0.004 SD more (0.11 fewer to 0.12 more) | ⨁◯◯◯ very low | IMPORTANT |
| Hospital LOS | ||||||||||||
| 13 | Randomized trials | Very seriousa , b , f | Very seriousc , d , g | Not serious | Not serious | None | 559 | 567 | — | SMD 0.005 SD fewer (0.12 fewer to 0.11 more) | ⨁◯◯◯ very low | IMPORTANT |
GRADE indicates Grades of Recommendation, Assessment, Development, and Evaluation; ICU, intensive care unit; LOS, length of stay; RR, risk ratio; SMD, standardized mean difference. GRADE score quality is reflected by: high quality (at least 4 ⨁ overall), moderate quality (3 ⨁ ), low quality (2 ⨁ ), and very low quality (one ? or less)
Randomization methods not consistently described.
Multiple trials were not double‐blinded.
Direction of effect not consistent.
Point estimates varied widely.
Definition of outcome varied.
Patient attrition not described.
Significant statistical heterogeneity.
Quantitative Outcomes
Of the 188 deaths in the 4210 randomized patients undergoing cardiac surgery, 96 deaths occurred in the 2098 patients (4.6%) randomized to RIPC, whereas 92 deaths occurred in the 2112 patients (4.4%) randomized to a sham control procedure. The RR for mortality for RIPC versus sham was 0.987 (95% CI, 0.653–1.492; P=0.95 [I 2=16%]) (Figure 2A). AKI occurred in 516 of 2091 patients (24.7%) undergoing RIPC and in 577 of 2118 patients (27.2%) who underwent a sham procedure. The RR for AKI for RIPC versus sham procedure was 0.839 (95% CI, 0.703–1.001; P=0.052 [I 2=41%]) (Figure 2B). Postoperative MI occurred in 237 of 1891 patients (12.5%) randomized to RIPC and in 282 of 1908 patients (14.8%) randomized to a sham procedure. The RR for MI for RIPC versus sham was 0.809 (95% CI, 0.615–1.064; P=0.13 [I 2=27%]) (Figure 2C). Postoperative CVA was diagnosed in 34 of 1864 patients (1.82%) who underwent RIPC and in 37 of 1880 patients (1.97%) who underwent a sham procedure. The RR for CVA for RIPC versus sham was 0.939 (95% CI, 0.592–1.489; P=0.79 [I 2=0%]) (Figure 2D). The standardized difference in mean ICU LOS was 0.010 days (95% CI, −0.116 to 0.137; P=0.87 [I 2=41%]) between the 1381 patients in the RIPC group and the 1396 patients in the sham control group (Figure 2E). Similarly, the standardized difference in mean hospital LOS was 0.026 days (95% CI, −0.091 to 0.143; P=0.67 [I 2=0%]) for the 559 patients undergoing RIPC versus 567 patients having a sham procedure (Figure 2F). Summarized quantitative data for the entire sample can be seen in Table 3.
Figure 2.

Comparison of outcomes between remote ischemic preconditioning (RIPC) and sham procedure. A, All‐cause mortality. B, Acute kidney injury. C, Myocardial infarction. D, Stroke. E, Intensive care unit length of stay. F, Hospital length of stay. The sizes of the squares representing the point estimates for each study are proportional to the weight of the study. Diamonds indicate the overall risk ratio (RR) or standardized mean difference and 95% CIs for the outcome of interest.
Subgroup Analyses, Sensitivity Analyses, and Publication Bias
Subgroup analysis showed no differences in outcomes when compared by the use of K‐ATP antagonists (results not shown). In the subgroup of studies of patients who did not receive propofol, we observed that most of these patients were men (60.3%) and the mean or median age ranged from 57.0 to 70.6 years. In this propofol‐free subgroup, 71 of 217 patients (32.7%) who underwent RIPC developed AKI compared with 103 of 217 patients (47.5%) treated with a sham procedure. The RR for AKI was 0.700 (95% CI, 0.527–0.930; P=0.014) for RIPC versus sham. In studies of patients who received propofol, 445 of 1874 (23.7%) who received RIPC developed AKI compared with 474 of 1901 (24.9%) who underwent a sham procedure. The RR for AKI was 0.928 (95% CI, 0.781–1.102; P=0.39) for RIPC versus sham (Figure 3). Summarized quantitative data for these subgroups can be seen in Table 4. There was no significant interaction between the two subgroups (P=0.098). Additionally, there were no differences in the effect of RIPC on development of stage I, II, or III AKI, and there was no difference in the effect of RIPC on the development of severe AKI, defined as stage II or III AKI (results not shown).
Figure 3.
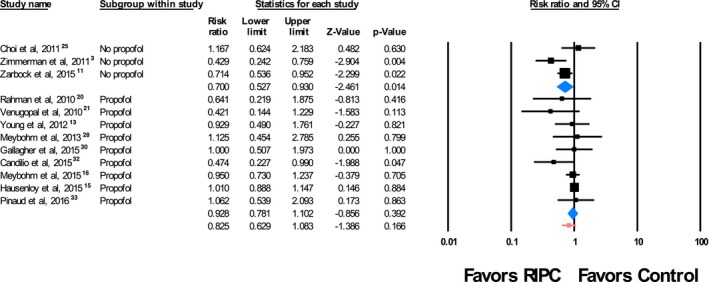
Subgroup analysis of cardiac surgeries performed with and without propofol anesthesia. The sizes of the squares representing the point estimates for each study are proportional to the weight of the study. Diamonds indicate the overall risk ratio (RR) and 95% CIs. RIPC indicates remote ischemic preconditioning.
Table 4.
Subgroup Analysis of Perioperative Propofol Use
| No. (RIPC) | AKI (RIPC) | No. (Sham) | AKI (Sham) | RR (95% CI) | P Value |
|---|---|---|---|---|---|
| Propofol used (N=9 trials)13, 15, 16, 20, 21, 28, 30, 32, 33 | |||||
| 1874 | 445 (23.7%) | 1901 | 474 (24.9%) | 0.928 (0.781–1.102) | 0.39 |
| Propofol not used (N=3 trials)3, 11, 25 | |||||
| 217 | 71 (32.7%) | 217 | 103 (47.5%) | 0.700 (0.527–0.930) | 0.014 |
AKI indicates acute kidney injury; RIPC, remote ischemic preconditioning; RR, risk ratio.
Sensitivity analyses showed no significant differences in outcomes when results were compared by study quality (high versus low), type of surgery performed (CABG, valve, or mixed), severity of illness (based on additive EuroSCORE), or duration or site of RIPC (results not shown). Visual inspection of the funnel plots suggested possible publication bias (Figure 4). This was further analyzed using the trim and fill method. The RR of AKI of 0.839 (95% CI, 0.702–1.001) was unchanged by the trim and fill method, suggesting no publication bias. This was confirmed by the Egger's test, which indicated lack of publication bias (P=0.055).
Figure 4.

Assessment of publication bias. This funnel plot is a plot of a measure of study size on the vertical axis as a function of effect size on the horizontal axis for acute kidney injury. Large studies appear toward the top of the graph and tend to cluster near the mean effect size. Smaller studies appear toward the bottom of the graph and (since there is more sampling variation in effect size estimates in the smaller studies) will be dispersed across a range of values. In the absence of publication bias, the studies, represented by circles, are distributed symmetrically about the combined effect size. The dashed diamond appearing below the x axis represents the summary effect.
In addition, sensitivity analyses to assess potential effects of qualitative differences on study design and patient selection showed that exclusion of any one trial from analysis of mortality, AKI, MI, CVA, ICU LOS, and hospital LOS did not change the overall findings (data not shown).
Discussion
In this meta‐analysis of 5262 patients undergoing RIPC for cardiac surgery with cardiopulmonary bypass, we found that RIPC conferred no clinical benefit. The intervention failed to reduce the incidence of all‐cause mortality, MI, CVA, and ICU or hospital LOS. There was a strong trend towards reduction of AKI in patients who underwent RIPC.
Previous meta‐analyses have also failed to demonstrate clinical benefit of RIPC.42, 43 However, two aspects of this meta‐analysis differentiate it from prior studies. First, this analysis includes two recent, large, high‐quality RCTs of RIPC in patients undergoing cardiac surgery not included in previous meta‐analyses.15, 16 The inclusion of these trials increased the study population 2‐fold. Second, as far as we are aware, this is the first meta‐analysis to evaluate outcomes as a function of propofol and K‐ATP antagonist use.
Although the mechanisms of RIPC have not been fully elucidated, many believe there are components of both humoral and sensory‐neuronal pathways that confer organ protection.44 The neuronal pathway was first described by Jones et al,45 who demonstrated that myocardial protection could be produced through activation of sensory C fibers by an abdominal incision in mice. Furthermore, transection of the spinal cord and blockade of sensory C fibers by lidocaine abrogated the benefit of preconditioning, suggesting neuronal signal transmission. Similarly, propofol may disrupt mediators of the neuronal pathway and diminish the clinical benefits of RIPC when compared with isoflurane anesthesia.14, 46 Other investigators have suggested propofol itself may be protective and any incremental benefits of RIPC are too small to be detected.47 Our analysis is congruent with these theories, as we observed a highly significant reduction in AKI in the subgroup of patients who did not receive propofol, despite no benefit in the overall cohort.
In addition to the neuronal pathway, humoral‐mediated pathways have also been described. After Huffman et al48 demonstrated that transfer of serum from preconditioned to recipient rats prior to an induced MI conferred cardiac protection, the mediators of this pathway were explored. Adenosine and bradykinin, among other mediators, have been shown to induce preconditioning of myocytes, thought to be via the activation of the K‐ATP channel pathway.44, 49, 50 Loukogeorgakis et al51 implicated the K‐ATP pathway in IPC‐mediated endothelial protection by demonstrating abolition of the protective effect after administration of glibenclamide, a K‐ATP antagonist. In our subgroup analysis, after removal of studies that included patients treated with K‐ATP antagonists, clinical benefit of RIPC was still not observed.
Study Limitations
The results of this meta‐analysis should be interpreted with consideration of its limitations. First, the majority of trials included in the review were single‐center studies with varying inclusion and exclusion criteria. Second, definitions for outcomes and duration of follow‐up differed between included trials. Third, because we assumed that all patients in a given trial either did or did not receive propofol, a portion of patients within individual trials may have been miscategorized. Additionally, variability in RIPC protocols may have led to heterogeneity in the analysis. Finally, data were extracted only from RCTs and may not be representative of patients treated in usual practice.
Conclusions
RIPC does not prevent morbidity or mortality in patients undergoing cardiac surgery with cardiopulmonary bypass. In the subgroup of studies in which propofol was not used, a reduction in AKI was seen, suggesting that propofol may interact with the protective effects of RIPC. To evaluate the independent effect of RIPC on outcomes, future studies on RIPC should be performed in the absence of propofol anesthesia.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e004666. DOI: 10.1161/JAHA.116.004666.)
Note
References
- 1. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 2. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. [DOI] [PubMed] [Google Scholar]
- 3. Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, Kramer RS. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int. 2011;80:861–867. [DOI] [PubMed] [Google Scholar]
- 4. Hu S, Dong HL, Li YZ, Luo ZJ, Sun L, Yang QZ, Yang LF, Xiong L. Effects of remote ischemic preconditioning on biochemical markers and neurologic outcomes in patients undergoing elective cervical decompression surgery: a prospective randomized controlled trial. J Neurosurg Anesthesiol. 2010;22:46–52. [DOI] [PubMed] [Google Scholar]
- 5. Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–1646. [DOI] [PubMed] [Google Scholar]
- 6. Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. [DOI] [PubMed] [Google Scholar]
- 7. Costa MA, Carere RG, Lichtenstein SV, Foley DP, de Valk V, Lindenboom W, Roose PC, van Geldorp TR, Macaya C, Castanon JL, Fernandez‐Avilez F, Gonzales JH, Heyer G, Unger F, Serruys PW. Incidence, predictors, and significance of abnormal cardiac enzyme rise in patients treated with bypass surgery in the arterial revascularization therapies study (ARTS). Circulation. 2001;104:2689–2693. [DOI] [PubMed] [Google Scholar]
- 8. Selnes OA, Goldsborough MA, Borowicz LM Jr, Enger C, Quaskey SA, McKhann GM. Determinants of cognitive change after coronary artery bypass surgery: a multifactorial problem. Ann Thorac Surg. 1999;67:1669–1676. [DOI] [PubMed] [Google Scholar]
- 9. Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. [DOI] [PubMed] [Google Scholar]
- 10. Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–664. [DOI] [PubMed] [Google Scholar]
- 11. Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Gorlich D, Kellum JA, Meersch M, Renal RI. Effect of remote ischemic preconditioning on kidney injury among high‐risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. [DOI] [PubMed] [Google Scholar]
- 12. Ahmad AM, Ali GS, Tariq W. Remote ischemic preconditioning is a safe adjuvant technique to myocardial protection but adds no clinical benefit after on‐pump coronary artery bypass grafting. Heart Surg Forum. 2014;17:E220–E223. [DOI] [PubMed] [Google Scholar]
- 13. Young PJ, Dalley P, Garden A, Horrocks C, La Flamme A, Mahon B, Miller J, Pilcher J, Weatherall M, Williams J, Young W, Beasley R. A pilot study investigating the effects of remote ischemic preconditioning in high‐risk cardiac surgery using a randomised controlled double‐blind protocol. Basic Res Cardiol. 2012;107:256. [DOI] [PubMed] [Google Scholar]
- 14. Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–38. [DOI] [PubMed] [Google Scholar]
- 15. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM; ERICCA Trial Investigators. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. [DOI] [PubMed] [Google Scholar]
- 16. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg‐Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer‐Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K; RIPHeart Study Collaborators . A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S; GRADE Working Group . Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology . World incidence of AKI: a meta‐analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, Townsend P, Townend JN, Green D, Bonser RS. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation. 2010;122:S53–S59. [DOI] [PubMed] [Google Scholar]
- 21. Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis. 2010;56:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li L, Luo W, Huang L, Zhang W, Gao Y, Jiang H, Zhang C, Long L, Chen S. Remote perconditioning reduces myocardial injury in adult valve replacement: a randomized controlled trial. J Surg Res. 2010;164:e21–e26. [DOI] [PubMed] [Google Scholar]
- 23. Karuppasamy P, Chaubey S, Dew T, Musto R, Sherwood R, Desai J, John L, Shah AM, Marber MS, Kunst G. Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury and inflammation? Basic Res Cardiol. 2011;106:511–519. [DOI] [PubMed] [Google Scholar]
- 24. Wu Q, Gui P, Wu J, Ding D, Purusram G, Dong N, Yao S. Effect of limb ischemic preconditioning on myocardial injury in patients undergoing mitral valve replacement surgery. A randomized controlled trial. Circ J. 2011;75:1885–1889. [DOI] [PubMed] [Google Scholar]
- 25. Choi YS, Shim JK, Kim JC, Kang KS, Seo YH, Ahn KR, Kwak YL. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2011;142:148–154. [DOI] [PubMed] [Google Scholar]
- 26. Lucchinetti E, Bestmann L, Feng J, Freidank H, Clanachan AS, Finegan BA, Zaugg M. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on‐pump coronary artery bypass graft surgery: lack of synergy or evidence of antagonism in cardioprotection? Anesthesiology. 2012;116:296–310. [DOI] [PubMed] [Google Scholar]
- 27. Xie JJ, Liao XL, Chen WG, Huang DD, Chang FJ, Chen W, Luo ZL, Wang ZP, Ou JS. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing heart valve surgery: randomised controlled trial. Heart. 2012;98:384–388. [DOI] [PubMed] [Google Scholar]
- 28. Meybohm P, Renner J, Broch O, Caliebe D, Albrecht M, Cremer J, Haake N, Scholz J, Zacharowski K, Bein B. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double‐blind randomized controlled pilot study. PLoS One. 2013;8:e64743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhauser M, Peters J, Jakob H, Heusch G. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single‐centre randomised, double‐blind, controlled trial. Lancet. 2013;382:597–604. [DOI] [PubMed] [Google Scholar]
- 30. Gallagher SM, Jones DA, Kapur A, Wragg A, Harwood SM, Mathur R, Archbold RA, Uppal R, Yaqoob MM. Remote ischemic preconditioning has a neutral effect on the incidence of kidney injury after coronary artery bypass graft surgery. Kidney Int. 2015;87:473–481. [DOI] [PubMed] [Google Scholar]
- 31. Slagsvold KH, Moreira JB, Rognmo O, Hoydal M, Bye A, Wisloff U, Wahba A. Remote ischemic preconditioning preserves mitochondrial function and activates pro‐survival protein kinase Akt in the left ventricle during cardiac surgery: a randomized trial. Int J Cardiol. 2014;177:409–417. [DOI] [PubMed] [Google Scholar]
- 32. Candilio L, Malik A, Ariti C, Barnard M, Di Salvo C, Lawrence D, Hayward M, Yap J, Roberts N, Sheikh A, Kolvekar S, Hausenloy DJ, Yellon DM. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. 2015;101:185–192. [DOI] [PubMed] [Google Scholar]
- 33. Pinaud F, Corbeau JJ, Baufreton C, Binuani JP, De Brux JL, Fouquet O, Angoulvant D, Furber A, Prunier F. Remote ischemic preconditioning in aortic valve surgery: results of a randomized controlled study. J Cardiol. 2016;67:36–41. [DOI] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 35. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 36. Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta‐analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cox DR. The continuity correction. Biometrika. 1970;57:217–219. [Google Scholar]
- 38. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 39. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 41. Lomivorotov VV, Shmyrev VA, Nepomnyaschih VA, Ponomarev DN, Knyazkova LG, Lomivorotov VN, Karaskov AM. Remote ischaemic preconditioning does not protect the heart in patients undergoing coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. 2012;15:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haji Mohd Yasin NA, Herbison P, Saxena P, Praporski S, Konstantinov IE. The role of remote ischemic preconditioning in organ protection after cardiac surgery: a meta‐analysis. J Surg Res. 2014;186:207–216. [DOI] [PubMed] [Google Scholar]
- 43. Remote Preconditioning Trialists' Group , Healy DA, Khan WA, Wong CS, Moloney MC, Grace PA, Coffey JC, Dunne C, Walsh SR, Sadat U, Gaunt ME, Chen S, Tehrani S, Hausenloy DJ, Yellon DM, Kramer RS, Zimmerman RF, Lomivorotov VV, Shmyrev VA, Ponomarev DN, Rahman IA, Mascaro JG, Bonser RS, Jeon Y, Hong DM, Wagner R, Thielmann M, Heusch G, Zacharowski K, Meybohm P, Bein B, Tang TY. Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta‐analysis. Int J Cardiol. 2014;176:20–31. [DOI] [PubMed] [Google Scholar]
- 44. Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120:S1–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kottenberg E, Musiolik J, Thielmann M, Jakob H, Peters J, Heusch G. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;147:376–382. [DOI] [PubMed] [Google Scholar]
- 47. Shirakawa M, Imura H, Nitta T. Propofol protects the immature rabbit heart against ischemia and reperfusion injury: impact on functional recovery and histopathological changes. Biomed Res Int. 2014;2014:601250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huffman LC, Koch SE, Butler KL. Coronary effluent from a preconditioned heart activates the JAK‐STAT pathway and induces cardioprotection in a donor heart. Am J Physiol Heart Circ Physiol. 2008;294:H257–H262. [DOI] [PubMed] [Google Scholar]
- 49. Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther. 1994;270:681–689. [PubMed] [Google Scholar]
- 50. Grover GJ, Sleph PG, Dzwonczyk S. Role of myocardial ATP‐sensitive potassium channels in mediating preconditioning in the dog heart and their possible interaction with adenosine A1‐receptors. Circulation. 1992;86:1310–1316. [DOI] [PubMed] [Google Scholar]
- 51. Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM, Deanfield JE, MacAllister RJ. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)‐channel dependent mechanism. Circulation. 2007;116:1386–1395. [DOI] [PubMed] [Google Scholar]


