Abstract
Background
Guidelines recommend continuation or initiation of guideline‐directed medical therapy, including angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers (ACEi/ARB), in hospitalized patients with heart failure with reduced ejection fraction.
Methods and Results
Using the Get With The Guidelines‐Heart Failure Registry, we linked clinical data from 16 052 heart failure with reduced ejection fraction (ejection fraction ≤40%) patients with Medicare claims data. We divided ACEi/ARB‐eligible patients into 4 categories based on admission and discharge ACEi/ARB use: continued (reference group), started, discontinued, or not started on therapy. A multivariable Cox proportional hazard model was used to determine the association between ACEi/ARB category and outcomes. Most, 90.5%, were discharged on ACEi/ARB (59.6% continued and 30.9% newly started). Of those discharged without ACEi/ARB, 1.9% were discontinued, and 7.5% were eligible but not started. Thirty‐day mortality was 3.5% for patients continued and 4.1% for patients started on ACEi/ARB. In contrast, 30‐day mortality was 8.8% for patients discontinued (adjusted hazard ratio [HR adj] 1.92; 95% CI 1.32‐2.81; P<0.001) and 7.5% for patients not started (HR adj 1.50; 95% CI 1.12‐2.00; P=0.006). The 30‐day readmission rate was lowest among patients continued or started on therapy. One‐year mortality was 28.2% for patients continued and 29.7% for patients started on ACEi/ARB compared to 41.6% for patients discontinued (HR adj 1.35; 95% CI 1.13‐1.61; P<0.001) and 41.7% (HR adj 1.28; 95% CI 1.14‐1.43; P<0.001) for patients not started on therapy.
Conclusions
Compared with continuation, withdrawal of ACEi/ARB during heart failure hospitalization is associated with higher rates of postdischarge mortality and readmission, even after adjustment for severity of illness.
Keywords: angiotensin II receptor blockers, angiotensin‐converting enzyme inhibitors, heart failure, outcomes research, quality of care
Subject Categories: Heart Failure, Quality and Outcomes, Mortality/Survival
Introduction
Almost 6 million Americans suffer from heart failure (HF). By 2030, that figure is projected to surpass 8 million. Over the past decade, HF has accounted for over 1 million hospitalizations annually.1 Readmission rates are also high, with over 50% of discharged patients readmitted within the next 6 months.2 The age‐adjusted mortality, after an admission for acute decompensated heart failure (ADHF), is ~10% at 30 days, 30% at 1 year, and as high as 50% by 5 years.1
Angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) have been shown in multiple large clinical trials to improve symptoms, reduce hospitalizations, and improve survival in patients with HF with reduced ejection fraction (HFrEF).3, 4, 5, 6, 7 The American College of Cardiology (ACC)/American Heart Association (AHA) clinical guidelines make it a Class I, Level A recommendation to use ACEi/ARB therapy in patients with HFrEF both to “prevent symptomatic heart failure” and to “reduce morbidity and mortality.”8 In addition, the ACC/AHA HF Performance Measures recommend ACEi/ARB for outpatients with HFrEF and ACEi/ARB therapy at the time of hospital discharge for inpatients with HFrEF.9 These clinical guidelines and quality metrics, along with their associated incentives, have led to significant improvements in the rates of ACEi/ARB use over time.
Continuation of β‐blocker therapy at the time of hospital discharge has been shown to reduce both readmission and mortality rates after ADHF admissions.10, 11, 12 The impact of continuing or discontinuing ACEi/ARB after ADHF hospitalization has not been as well studied. This study aims to define the relationship between the continuation or withdrawal of ACEi/ARB therapy and the outcomes of patients with HFrEF hospitalized for ADHF.
Methods
Data Source
Data for this analysis are from the Get With The Guidelines Heart Failure (GWTG‐HF) registry linked with Medicare inpatient data. Medicare Part A (inpatient) claims and the associated denominator file from January 1, 2005, through December 31, 2013 were linked with data from the GWTG‐HF registry using previously described methods and indirect identifiers.13 Standardized data collection variables and methods for GWTG‐HF have been previously described as well.14 GWTG‐HF is a hospital‐based, voluntary data collection and quality improvement initiative that began in 2005. Participating hospitals submit clinical information regarding in‐hospital care and outcomes of consecutive patients hospitalized for ADHF using an online Patient Management Tool (Outcome Sciences, Inc, Cambridge, MA). Linkage of GWTG‐HF data with Medicare claims data allows for both short‐ and long‐term mortality and readmission assessment.
Study Population
Patients were eligible to be included if they had a diagnosis of HF based on their GWTG records and if their GWTG record could be linked with Medicare claims. To ensure high‐quality data, we excluded patients from hospitals with ≥25% missing data for past medical history. This left us with a starting population of 130 155 patients from 339 hospitals. The complete study population derivation algorithm is provided in Figure S1. We excluded patients with ejection fractions that were missing or >40% (N=79 986) and patients with contraindications to ACEi/ARB therapy (N=13 268, contraindication frequencies listed in Table S1) or patients who had missing ACEi/ARB contraindication data (N=13 379). We also excluded patients who were started on inotropes, transplanted, discharged to hospice, placed on comfort measures only, died during admission, left against medical advice, were transferred to another short‐term hospital, or who were not enrolled in Medicare fee‐for‐service. If multiple hospitalizations existed for a patient, the first hospitalization was selected as the index hospitalization. The remaining population of 16 052 was used for all analyses.
Primary Measures and Definitions
Among those eligible for the study and without a contradiction to ACEi/ARB therapy, we created and defined 4 patient groups for comparison: patients on ACEi/ARB at admission and discharge (continued), those not on ACEi/ARB at admission but who were discharged on ACEi/ARB (started), those on ACEi/ARB at admission but not at discharge (discontinued), and those not on ACEi/ARB at admission or discharge (not started).
The primary endpoint of this study was the difference in all‐cause mortality at 30 days between those who continued versus discontinued ACEi/ARB at the time of discharge. Secondary endpoints included differences between those who continued and those who discontinued ACEi/ARB therapy in 90‐day and 1‐year mortality rates and differences in 30‐day, 90‐day and 1‐year readmission rates. Tertiary endpoints include differences between those who continued and those who discontinued ACEi/ARB therapy in rates of the composite endpoint of all‐cause death and all‐cause readmission at 30 days, 90 days, and 1 year.
All participating institutions are required to comply with local regulatory and privacy guidelines and to submit the GWTG protocol for review and approval by their institutional review board. Because data are used mainly at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. The Duke Clinical Research Institute served as the primary analytic center for the aggregate de‐identified data. The Duke University Institutional Review Board approved the study.
Statistical Analysis
Patient characteristics and hospital characteristics are described by mean±standard deviation for normally distributed data and or median and 25th and 75th percentiles for non–normally distributed data. Categorical variables are described using percentages. Categorical variables are compared using a chi‐squared test, and continuous variables are compared using the Kruskal‐Wallis test. A multivariable Cox proportional hazard model was constructed to assess whether there were differences in clinical outcomes (30‐day, 90‐day, and 1‐year mortality, readmission, and composite endpoints) among the ACEi/ARB groups (continued, started, discontinued, not started). Patients continued on ACEi/ARB are the reference group for all hazard ratio assessments unless otherwise specified.
The covariates adjusted for in the model are specified in Table S2, and a sensitivity analysis using discharge vital signs and laboratory values is displayed in Table S3 and confirms the study's main findings. Robust standard errors are estimated in Cox models to account for the clustering of patients within hospitals. For readmission outcomes, the Fine‐Gray model15 was used to account for the competing risk of mortality. Formal tests and graphical methods (cumulative instance plots and plots of residuals vs time) were used to assess the proportional hazards assumptions in Cox and Fine‐Gray models. There was no major violation detected from the data. All P‐values are 2‐sided, with values less than 0.05 considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
The baseline patient characteristics of the 16 052 patients included in this study are displayed in Table 1. The average age was 78.3 years, 42% were women, and 78% were white. The median ejection fraction was 26% (interquartile range 20% to 33%). A total of 14 535 (90.5%) of patients were discharged on ACEi/ARB. There were 9572 (59.6%) continued on ACEi/ARB, 4963 (30.9%) newly started on ACEi/ARB, 308 (1.9%) discontinued from ACEi/ARB, and 1209 (7.5%) not started. Although there was a higher rate of hypotension (defined as systolic blood pressure <90 mm Hg) at admission among those discontinued or not started on ACEi/ARB, there was no difference in the rate of hypotension at discharge. Those discontinued or not started on ACEi/ARB did have higher rates of renal dysfunction as evidenced by higher rates of creatinine >2 mg/dL, potassium >5 mg/dL, rates of chronic kidney disease stage IV and V, and lower estimated glomerular filtration rates. There was no difference in the rate of hyperkalemia.
Table 1.
Baseline Characteristics of GWTG‐HF Subjects Meeting Eligibility Criteria
| Total | Continued ACEi/ARB | Started ACEi/ARB | Discontinued ACEi/ARB | Not Started ACEi/ARB | |
|---|---|---|---|---|---|
| N | 16 052 | 9572 | 4963 | 308 | 1209 |
| % total N | 59.63% | 30.92% | 1.92% | 7.53% | |
| Demographics | |||||
| Age, y | 78.34±7.92 | 77.99±7.82 | 78.65±8.1 | 78.97±7.77 | 79.58±7.85 |
| Sex (% female) | 41.85 | 41.19 | 43.2 | 44.48 | 40.78 |
| Race (% white) | 77.97 | 78.02 | 77.31 | 76.3 | 80.73 |
| Body mass index | 27.65±6.83 | 27.91±6.82 | 27.34±6.84 | 27.38±7.32 | 26.8±6.62 |
| Past medical history | |||||
| Atrial fibrillation/flutter, % | 36.19 | 37.33 | 32.77 | 33.66 | 41.4 |
| Diabetes mellitus—insulin‐treated, % | 16.8 | 17.52 | 15.21 | 15.18 | 17.78 |
| Diabetes mellitus—non–insulin‐treated, % | 24.85 | 26.24 | 21.99 | 29.04 | 24.12 |
| Hyperlipidemia, % | 51.18 | 53.65 | 46.81 | 46.2 | 50.17 |
| Hypertension, % | 76.51 | 79.59 | 71.6 | 75.58 | 71.79 |
| ICD, % | 14.9 | 16.55 | 11.35 | 13.2 | 16.44 |
| Ischemic heart disease, %* | 67.09 | 70.23 | 59.71 | 68.32 | 71.2 |
| PVD, % | 13.51 | 13.86 | 12.56 | 11.22 | 15.11 |
| Renal insufficiency, % | 14.63 | 13.77 | 13.86 | 19.47 | 23.37 |
| Smoking, % | 12.05 | 11.35 | 13.82 | 10.71 | 10.75 |
| Stroke, % | 15.72 | 16.16 | 14.5 | 16.83 | 16.78 |
| HF characteristics | |||||
| History of HF, % | 67.91 | 72.79 | 58.8 | 63.31 | 67.91 |
| Ejection fraction, %a | 26.36±7.33 | 26.34±7.28 | 26.27±7.41 | 26.35±7.29 | 26.9±7.37 |
| Number of hospital admissions in prior 1 year for HF (n) | |||||
| Unknown (%) | 21.99 | 23.03 | 18.56 | 27.6 | 26.47 |
| >2 (%) | 2.21 | 2.44 | 1.51 | 2.92 | 3.06 |
| 2 (%) | 3.11 | 3.45 | 2.16 | 2.6 | 4.55 |
| 1 (%) | 12.46 | 13.59 | 10.03 | 12.99 | 13.32 |
| 0 (%) | 37.07 | 39.77 | 32.66 | 37.66 | 33.66 |
| Vital signs (at admission) | |||||
| Heart rate, bpm | 85.3±19.91 | 83.66±19.29 | 88.67±20.69 | 84.85±20.39 | 84.99±19.81 |
| Systolic blood pressure, mm Hg | 137.67±26.86 | 137.58±26.8 | 139.29±27.12 | 134.27±26.47 | 132.72±25.6 |
| % with systolic blood pressure <90 mm Hg | 1.44% | 1.57% | 1.03% | 0.97% | 2.23% |
| Diastolic blood pressure, mm Hg | 76.94±17.18 | 76.25±17.07 | 79.06±17.44 | 74.9±17.65 | 74.39±15.95 |
| Vital signs (at discharge) | |||||
| Heart rate, bmp | 74.77±12.81 | 74.14±12.55 | 75.61±13.01 | 76.1±13.32 | 76.26±13.65 |
| Systolic blood pressure, mm Hg | 119.82±19.29 | 120.22±19.55 | 119.13±18.95 | 119.23±20.34 | 119.64±18.17 |
| % with systolic blood pressure <90 mm Hg | 2.63% | 2.80% | 2.42% | 3.90% | 1.82% |
| Diastolic blood pressure, mm Hg | 65.46±11.63 | 65.42±11.73 | 65.51±11.63 | 65.31±11.56 | 65.61±10.72 |
| Labs (at admission)b | |||||
| BNP, pg/mL | 1078 (552, 2010) | 1032 (521, 1928) | 1136 (590, 2150) | 1170 (577, 2061) | 1190 (632, 2142) |
| NT‐proBNP, pg/mL | 7315 (3284, 15 310) | 7205 (3107, 14 115) | 7155 (3594, 16 017) | 7306 (4297, 19 602) | 9693 (4065, 20 896) |
| Creatinine, mg/dL | 1.3 (1.0, 1.6) | 1.3 (1.0, 1.6) | 1.2 (1.0, 1.6) | 1.5 (1.1, 2.1) | 1.5 (1.1, 2.0) |
| % with creatinine >2 mg/dL | 11.15% | 10.31% | 9.39% | 23.38% | 21.92% |
| eGFR, mL/[min·1.73 m2] | 52.7 (38.7, 69.4) | 52.7 (39.1, 69.2) | 55.9 (41.3, 72.3) | 41.7 (29.8, 61.3) | 45.0 (30.4, 61.4) |
| % with CKD Stage IV or Vc | 10.09% | 9.31% | 8.36% | 22.40% | 20.26% |
| Labs (at discharge)b | |||||
| Potassium, mEq/L | 4.1 (3.8, 4.4) | 4.1 (3.8, 4.4) | 4.1 (3.8, 4.4) | 4.1 (3.7, 4.4) | 4.1 (3.7, 4.4) |
| % with potassium >5 mEq/L | 2.16% | 2.37% | 1.59% | 3.90% | 2.40% |
| BNP, pg/mL | 706 (348, 1417) | 671 (334, 1350) | 717 (344, 1480) | 906 (414, 1582) | 974 (501, 1700) |
| NT‐proBNP, pg/mL | 5419 (2787, 12 019) | 5470 (2839, 10 556) | 5049 (2629, 12 611) | 4656 (4142, 14 231) | 8471 (3172, 15 356) |
| Creatinine, mg/dL | 1.3 (1.0, 1.7) | 1.3 (1.0, 1.6) | 1.2 (1.0, 1.6) | 1.5 (1.2, 2.0) | 1.5 (1.1, 2.1) |
| % with creatinine >2 mg/dL | 9.89% | 9.02% | 8.54% | 19.16% | 19.93% |
| eGFR, mL/[min·1.73 m2] | 51.6 (37.9, 68.3) | 51.6 (37.9, 68.0) | 54.9 (40.5, 70.8) | 43.7 (30.5, 61.3) | 44.2 (30.7, 61.5) |
| % with CKD stage IV or Vc | 9.11% | 8.33% | 8.04% | 17.86% | 17.54% |
| % with worsening renal functiond | 11.87% | 11.84% | 11.38% | 15.58% | 13.23% |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B‐type natriuretic peptide; bpm, beats per minute; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GWTG, Get With the Guidelines; HF, heart failure; ICD, implantable cardiac defibrillator; NT‐proBNP, N‐terminal prohormone BNP; PVD, peripheral vascular disease.
Ischemic heart disease: a history of myocardial infarction, coronary artery disease, percutaneous coronary intervention or coronary artery bypass grafting.
Ejection fraction: defined as original variable (%) when present or, if categorized only, imputed to 30% if described as moderate/severe or 50% if described as mild/moderate.
Labs at admission and discharge are displayed as median with interquartile ranges. There is a high rate of missing data among some variables: admission BNP 37.2% missing, admission NT‐proBNP 88.1% missing, and admission creatinine 11.6% missing; discharge BNP 67.8% missing, discharge NT‐proBNP 97.1% missing, and discharge creatinine 24.6% missing.
Chronic kidney disease (CKD) stage IV or V: defined as eGFR <30 mL/(min·1.73 m2).
Worsening renal function: defined as a rise of ≥0.3 mg/dL in serum creatinine between admission and discharge.
Hospital characteristics are displayed in Table 2. The rates of in‐hospital procedures were low and are presented in Table S4. The rates of conformity with hospital performance measures were lower among patients discontinued or not started on ACEi/ARB. In addition to lower rates of β‐blocker and ACEi/ARB prescription at discharge, those discontinued or not started on ACEi/ARB also had lower rates of being provided discharge instructions, smoking cessation counseling, timely follow‐up appointments, and ICD counseling.
Table 2.
Baseline Hospital Characteristics
| Hospital characteristics | |||||
| Number of beds, n | 443.77±280.13 | 447.53±285.13 | 440.03±270.97 | 442.33±289.78 | 429.71±274.31 |
| Academic hospital, % | 64.38 | 63.37 | 66.63 | 68.51 | 62.03 |
| Rural hospital, % | 6.77 | 6.48 | 6.27 | 8.77 | 10.67 |
| Region | |||||
| West, % | 5.86 | 5.87 | 6.04 | 4.87 | 5.21 |
| South, % | 37.78 | 38.65 | 35.16 | 46.43 | 39.37 |
| Midwest, % | 27.96 | 28.4 | 27.34 | 20.78 | 28.87 |
| Northeast, % | 28.41 | 27.08 | 31.45 | 27.92 | 26.55 |
| Primary PCI performed for MI, % | 82.09 | 81.84 | 82.01 | 82.47 | 84.28 |
| Interventional capabilities, % | 73.6 | 73.57 | 74.57 | 70.45 | 70.72 |
| Cardiac surgery on site, % | 73.95 | 74.03 | 74.87 | 72.08 | 70.06 |
| Cardiac transplant center | 10.37 | 11.31 | 9.17 | 6.17 | 8.85 |
| Performance measures | |||||
| Discharge instructions provided, % | 89.81 | 90.7 | 91.02 | 77.18 | 79.98 |
| HF patients with LVSD discharged on β‐blocker, % | 93.59 | 94.96 | 94.79 | 75.95 | 82.38 |
| HF patients with smokers with smoking cessation, % | 94.63 | 95.03 | 95.34 | 87.88 | 89.23 |
| HF patients with follow‐up appointment at discharge, % | 61.99 | 64.46 | 59.77 | 50 | 46.27 |
| HF patients with LVSD with ICD placed/prescribed at discharge, % | 61.06 | 65.25 | 52.56 | 64.29 | 48.89 |
HF signifies heart failure; ICD, implantable cardiac defibrillator; LVSD, left ventricular systolic dysfunction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Mortality
The unadjusted results for 30‐day, 90‐day, and 1‐year mortality, readmission, and composite mortality or readmission rates are displayed in Table 3. The adjusted hazard ratios (HRadj) and corresponding 95% CI from the multivariate Cox proportional hazard model are displayed in Table 4.
Table 3.
30‐Day, 90‐Day, and 1‐Year Mortality, Readmission, and Composite Mortality and Readmission Rates by Group, Observed
| Continued | Started | Discontinued | Not Started | P Value | |
|---|---|---|---|---|---|
| N | 9572 | 4963 | 308 | 1209 | |
| 30‐day mortality | 338 (3.5%) | 202 (4.1%) | 27 (8.8%) | 90 (7.5%) | <0.0001 |
| 90‐day mortality | 950 (10.0%) | 550 (11.2%) | 64 (20.8%) | 215 (18.0%) | <0.0001 |
| 1‐year mortality | 2590 (28.2%) | 1413 (29.7%) | 126 (41.6%) | 492 (41.7%) | <0.0001 |
| 30‐day readmission | 1738 (18.3%) | 941 (19.1%) | 88 (28.6%) | 281 (23.3%) | <0.0001 |
| 90‐day readmission | 3301 (35.0%) | 1687 (34.5%) | 132 (43.0%) | 488 (40.9%) | <0.0001 |
| 1‐year readmission | 5639 (61.6%) | 2827 (59.6%) | 198 (66.0%) | 753 (64.3%) | <0.0001 |
| 30‐day mortality/readmission | 1910 (20.1%) | 1042 (21.1%) | 102 (33.1%) | 331 (27.5%) | <0.0001 |
| 90‐day mortality/readmission | 3652 (38.7%) | 1888 (38.6%) | 153 (49.9%) | 579 (48.5%) | <0.0001 |
| 1‐year mortality/readmission | 6202 (67.7%) | 3144 (66.2%) | 225 (74.9%) | 886 (75.6%) | <0.0001 |
Table 4.
Multivariate Cox Proportional Hazard Model Comparing Mortality, Readmission, and Composite Mortality and Readmissions Between Groups
| Outcome | Groups Compared | Adjusted Hazard Ratio and 95% CI | Adjusted P Value |
|---|---|---|---|
| Mortality rates | |||
| 30‐day mortality | Started vs continued | 1.15 (0.96, 1.38) | 0.134 |
| Discontinued vs continued | 1.92 (1.32, 2.81) | <0.001 | |
| Not started vs continued | 1.50 (1.12, 2.00) | 0.006 | |
| 90‐day mortality | Started vs continued | 1.13 (1.01, 1.25) | 0.026 |
| Discontinued vs continued | 1.68 (1.31, 2.15) | <0.001 | |
| Not started vs continued | 1.37 (1.17, 1.60) | 0.000 | |
| 1‐year mortality | Started vs continued | 1.09 (1.01, 1.17) | 0.019 |
| Discontinued vs continued | 1.35 (1.13, 1.61) | 0.001 | |
| Not started vs continued | 1.28 (1.14, 1.43) | <0.001 | |
| Readmission rates | |||
| 30‐day readmission | Started vs continued | 1.07 (0.98, 1.17) | 0.148 |
| Discontinued vs continued | 1.40 (1.16, 1.71) | <0.001 | |
| Not started vs continued | 1.14 (1.01, 1.29) | 0.038 | |
| 90‐day readmission | Started vs continued | 1.01 (0.94, 1.08) | 0.769 |
| Discontinued vs continued | 1.18 (0.98, 1.41) | 0.074 | |
| Not started vs continued | 1.09 (1.00, 1.20) | 0.061 | |
| 1‐year readmission | Started vs continued | 0.98 (0.93, 1.03) | 0.434 |
| Discontinued vs continued | 1.07 (0.92, 1.25) | 0.353 | |
| Not started vs continued | 1.01 (0.93, 1.09) | 0.836 | |
| Composite mortality or readmission rates | |||
| 30‐day mortality/readmission | Started vs continued | 1.08 (0.99, 1.17) | 0.091 |
| Discontinued vs continued | 1.47 (1.21, 1.79) | <0.001 | |
| Not started vs continued | 1.20 (1.07, 1.36) | 0.003 | |
| 90‐day mortality/readmission | Started vs continued | 1.02 (0.96, 1.09) | 0.509 |
| Discontinued vs continued | 1.24 (1.04, 1.49) | 0.020 | |
| Not started vs continued | 1.18 (1.08, 1.29) | <0.001 | |
| 1‐year mortality/readmission | Started vs continued | 0.99 (0.95, 1.04) | 0.803 |
| Discontinued vs continued | 1.14 (0.96, 1.34) | 0.130 | |
| Not started vs continued | 1.12 (1.03, 1.22) | 0.008 | |
The unadjusted 30‐ and 90‐day mortality rates were higher among those who discontinued or did not start ACEi/ARB therapy, compared to those who continued or started therapy. After adjustment, the HRadj for 30‐day mortality among patients who discontinued ACEi/ARB was 1.92 (95% CI 1.32, 2.81; P<0.001) compared to patients continued on therapy. Among those not started on therapy, it was 1.50 (95% CI 1.12, 2.06; P=0.006). By 1 year, patients discontinued from ACEi/ARB had an HRadj of 1.35 (95% CI 1.13, 1.61; P=0.001), and those not started on ACEi/ARB had an HRadj of 1.28 (95% CI 1.14, 1.43; P<0.001). Estimates for 1‐year mortality are displayed in Figure — panel A.
Figure 1.
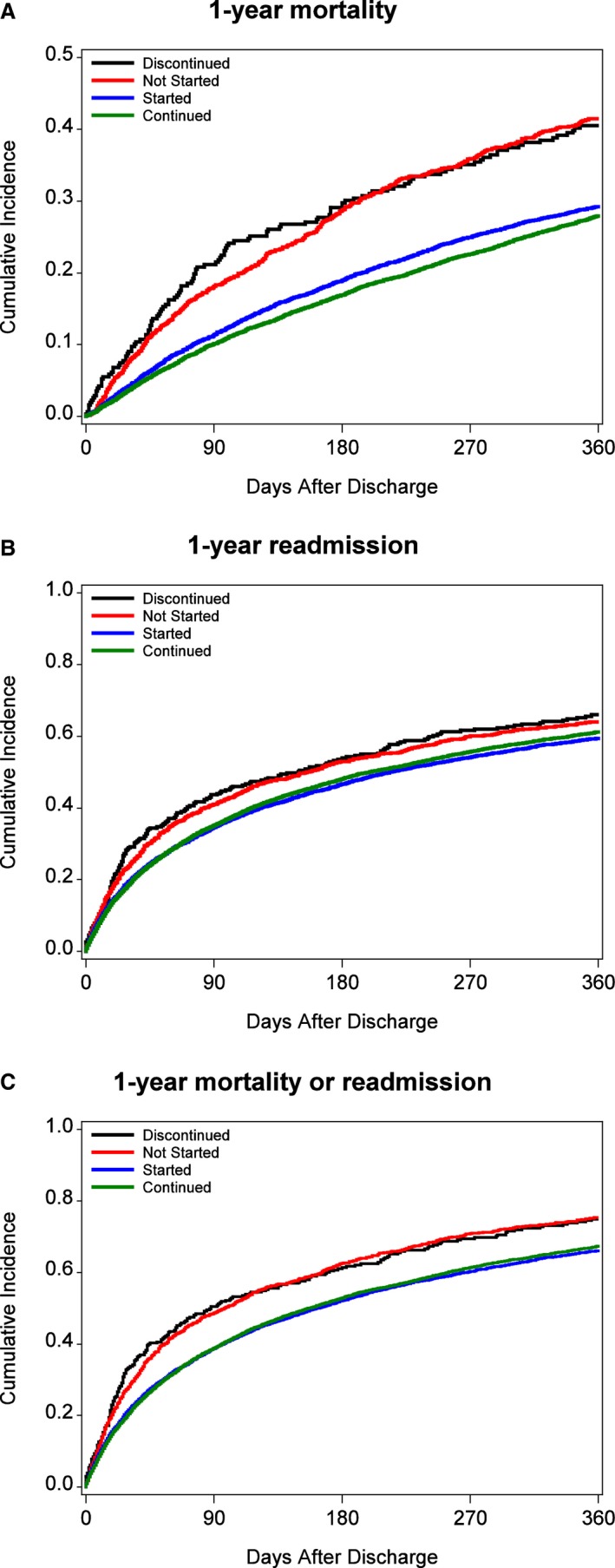
A, One‐year mortality. B, One‐year readmission rates. C, One‐year composite endpoint (mortality or readmission) rates.
In addition, although it did not reach statistical significance, we observed a trend toward increased mortality at 30 and 90 days among those who discontinued compared with those not started on ACEi/ARB (Table S5). In addition, there was a slight mortality advantage for those who continued on ACEi/ARB compared with those started on therapy at the time of discharge.
Readmission
The unadjusted 30‐day, 90‐day, and 1‐year readmission rates were higher in patients discontinued or not started on ACEi/ARB therapy compared with patients who continued or started on ACEi/ARB. After adjustment, the HRadj for 30‐day readmission was 1.40 (95% CI 1.16, 1.71, P<0.001) among those who discontinued and 1.14 (95% CI 1.01, 1.29, P=0.038) among those who did not start ACEi/ARB. There was no difference in 30‐day readmission rates between those who continued and those who started ACEi/ARB therapy. By 90 days, after adjustment, there was no difference in readmission rates between the groups. Estimates for 1‐year readmission are displayed in Figure — panel B.
Composite Endpoint (Mortality or Readmission)
Similar to mortality and readmission rates, the unadjusted 30‐day, 90‐day, and 1‐year composite endpoint of mortality and readmission was higher among those discontinued or not started on ACEi/ARB, compared with patients who continued or started on therapy. As observed with mortality, patients who were continued or started on ACEi/ARB had lower 30‐ and 90‐day composite endpoint rates. By 1 year, those who were not started on ACEi/ARB had higher rates of the composite endpoint compared to patients continued on ACEi/ARB, and there was a trend toward higher rates among those discontinued from ACEi/ARB. Estimates for 1‐year composite endpoint rates are displayed in Figure — panel C.
Discussion
In this study of 16 052 patients with HFrEF admitted for ADHF, we found that discontinuation of ACEi/ARB at the time of hospital discharge was associated with higher 30‐day, 90‐day, and 1‐year mortality compared with continuation of ACEi/ARB among eligible patients. We also found that continuation or initiation of ACEi/ARB was associated with lower 30‐ and 90‐day readmission and composite endpoint rates. Patients who had ACEi/ARB discontinued during hospitalization had the highest rates of mortality and readmission.
These findings are consistent with prior cardiovascular work demonstrating improved outcomes with greater adherence to clinical guidelines. The REACH investigators examined 37 154 outpatients diagnosed with atherothrombotic disease and found that nonadherence was associated with an increased risk of cardiovascular death, myocardial infarction, or stroke.16 In this study we similarly found improved outcomes for patients when clinicians adhered closely to guidelines and continued or initiated ACEi/ARB therapy.
Compared to some prior studies, we found higher rates of ACEi/ARB prescription at discharge, 90.6%.17, 18, 19 This rate, however, is consistent with a prior GWTG‐HF study.20 The high rates of ACEi/ARB prescription in this study may reflect the impact of including ACEi/ARB as a performance measure9, 21 and the results of quality improvement efforts made during this time (2005‐2013). The percentage may also be high due to selection bias, and hospitals that choose to participate in GWTG may be different from those that do not.
This study confirms and extends prior work regarding patterns of guideline‐directed medical therapy use and outcomes in HF patients. Data from Tran et al using the Atherosclerosis Risk in Communities Study found that the initiation or cessation of guideline‐directed medical therapy for HF occurs in roughly 12% of hospitalized patients and that cessation of guideline‐directed medical therapy was associated with higher mortality rates.22 Our work is also consistent with prior work analyzing continuation versus withdrawal of β‐blocker therapy. Data from the OPTIMIZE‐HF study found a significant benefit (HR 0.60, 95% CI 0.37, 0.99) for all‐cause mortality at 60 to 90 days associated with β‐blocker continuation at the time of discharge among HFrEF patients.12
This study also confirms and extends prior work regarding ACEi/ARB use patterns. Data from over 17 000 patients enrolled in the National Health Care Project found that a discharge prescription for ACEi/ARB was associated with a 17% relative reduction in mortality (RR 0.83, 95% CI 0.79, 0.88).19 Work by Sanam et al analyzing 1384 Medicare beneficiaries in Alabama found lower 30‐day and 1‐year readmission and mortality rates among patients with HF discharged on ACEi/ARB.23
In contrast to prior work this study separated patients not discharged on ACEi/ARB into discontinued and not started categories and separated patients discharged on ACEi/ARB into continued and newly started categories. By doing this we were able to observe a trend toward increased mortality at 30 and 90 days among those discontinued from ACEi/ARB, compared with those not started on ACEi/ARB. This trend may merit further investigation. We also observed a mortality advantage for those who continued on ACEi/ARB compared with those started on therapy at discharge.
Based on the results of this study, additional work adequately powered to determine if there is indeed a true disadvantage to discontinuing versus not starting therapy or if there is a true advantage to continuing versus starting therapy, should be considered. In addition, using a different data set, exploring the clinical reasons underlying the decision not to follow clinical guidelines and whether these reasons affect clinical outcomes would be very helpful.
Limitations
Certain limitations should be considered when interpreting the results of this study. First, because of the retrospective design of this study, we are unable to make any comments on causation. This study reports associations only. Because the data come from a registry, many patients were excluded because of missing data. These results should therefore only be applied to patients meeting the study's inclusion/exclusion criteria. However, to address concern regarding excluded patients, we compared baseline characteristics of the study population and those excluded due to missing data. The results are displayed in Table S6. Although their interpretation is limited by a high rate of missing data in the excluded group, the only notable differences are a higher rate of prior HF diagnosis, higher number of prior HF hospitalizations, and higher B‐type natriuretic peptide and N‐terminal prohormone B‐type natriuretic peptide in the excluded group.
Although we have adjusted for many relevant clinical characteristics, it is possible that some of the associations we observe are due to unmeasured or residual confounding. We are also unable to track patient adherence, a powerful predictor of cardiovascular outcomes in a registry such as this.24 Prior studies of HF patients have demonstrated that patients discharged on ACEi/ARB therapy have relatively high persistence rates, whereas those discharged not on therapy have low rates of outpatient initiation.25 Finally, these findings are from hospitals participating in GWTG and may not necessarily generalize to other settings and populations.
Conclusions
In this large, multicenter cohort of 16 052 patients with HFrEF, we found that continuation or initiation of ACEi/ARB at the time of discharge after admission for ADHF was associated with lower 30‐day, 90‐day, and 1‐year mortality rates. In addition, patients who continued or initiated ACEi/ARB therapy had lower readmission rates at 30 and 90 days compared with those who discontinued or did not start therapy. Additional research to better understand the determinants of guideline adherence and ACEi/ARB initiation and discontinuation may be warranted.
Sources of Funding
This research was funded by a Young Investigator Grant awarded to Dr Gilstrap by the American Heart Association.
Disclosures
Dr Gilstrap discloses the following relationships: research funding from the American Heart Association, The Commonwealth Fund, Laura and John Arnold Foundation, Novartis, and the Pharmaceutical Research and Manufactures of America. Dr Fonarow discloses the following relationships: Amgen (modest), Janssen (modest), and Novartis (significant). Dr Desai discloses the following relationships: honoraria for consulting from Novartis (modest), St. Jude Medical (modest), Relypsa (modest), Merck (modest), Janssen (modest), Sanofi (modest); research support from Novartis (significant). Dr DeVore discloses the following relationships: research funding from American Heart Association, Amgen, and Novartis. Dr Hernandez discloses the following relationships: honoraria for consulting from Amgen (modest), Bayer (modest), Boston Scientific (modest), Luitpold (modest), Pfizer (modest), and Novartis (significant); and research funding from Amgen (modest), AstraZeneca (modest), Bayer (modest), GSK (modest), Merck (modest), Novartis (modest), and Portola Pharmaceuticals (modest). Dr Bhatt discloses the following relationships: advisory board of Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors of Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair, American Heart Association Quality Oversight Committee; Data‐Monitoring Committees of Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; honoraria from American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor‐in‐Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor‐in‐Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); research funding from Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); site coinvestigator for Biotronik, Boston Scientific, St. Jude Medical; Trustee, American College of Cardiology; unfunded research for FlowCo, PLx Pharma, Takeda. The remaining authors have no disclosures to report.
Supporting information
Table S1. Documented Reasons for ACEi/ARB Contraindication
Table S2. Covariates Included in Multivariable Cox Regression
Table S3. Comparison of Primary Analysis and Sensitivity Analysis Using Complete Discharge Data
Table S4. Rates of In‐Hospital Procedures
Table S5. Multivariate Cox Proportional Hazard Model Comparing Mortality, Readmission, and Composite Mortality and Readmissions Between Patients Discontinued From and Not Started on ACEi/ARB
Table S6. Comparison of Baseline Characteristics Between Patients Excluded Because of Missing Data and Those Included in the Study Population
Figure S1. Study population derivation.
(J Am Heart Assoc. 2017;6:e004675. DOI: 10.1161/JAHA.116.004675.)
Accompanying Tables S1 through S6 and Figure S1 are available at http://jaha.ahajournals.org/content/6/2/e004675/DC1/embed/inline-supplementary-material-1.pdf
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–506. [DOI] [PubMed] [Google Scholar]
- 3. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 4. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 5. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med. 1992;327:685–691. [DOI] [PubMed] [Google Scholar]
- 6. Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. [DOI] [PubMed] [Google Scholar]
- 7. Cleland JG, Dargie HJ, Ball SG, Gillen G, Hodsman GP, Morton JJ, East BW, Robertson I, Ford I, Robertson JI. Effects of enalapril in heart failure: a double blind study of effects on exercise performance, renal function, hormones, and metabolic state. Br Heart J. 1985;54:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 9. Bonow RO, Ganiats TG, Beam CT, Blake K, Casey DE Jr, Goodlin SJ, Grady KL, Hundley RF, Jessup M, Lynn TE, Masoudi FA, Nilasena D, Pina IL, Rockswold PD, Sadwin LB, Sikkema JD, Sincak CA, Spertus J, Torcson PJ, Torres E, Williams MV, Wong JB; American College of Cardiology Foundation, American Heart Association Task Force on Performance Measures and American Medical Association‐Physician Consortium for Performance Improvement . ACCF/AHA/AMA‐PCPI 2011 performance measures for adults with heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association‐Physician Consortium for Performance Improvement. Circulation. 2012;125:2382–2401. [DOI] [PubMed] [Google Scholar]
- 10. Butler J, Young JB, Abraham WT, Bourge RC, Adams KF Jr, Clare R, O'Connor C; ESCAPE Investigators . Beta‐blocker use and outcomes among hospitalized heart failure patients. J Am Coll Cardiol. 2006;47:2462–2469. [DOI] [PubMed] [Google Scholar]
- 11. Metra M, Torp‐Pedersen C, Cleland JG, Di Lenarda A, Komajda M, Remme WJ, Dei Cas L, Spark P, Swedberg K, Poole‐Wilson PA; COMET Investigators . Should beta‐blocker therapy be reduced or withdrawn after an episode of decompensated heart failure? Results from COMET. Eur J Heart Fail. 2007;9:901–909. [DOI] [PubMed] [Google Scholar]
- 12. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB; OPTIMIZE‐HF Investigators and Coordinators . Influence of beta‐blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF program. J Am Coll Cardiol. 2008;52:190–199. [DOI] [PubMed] [Google Scholar]
- 13. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterson PN, Chan PS, Spertus JA, Tang F, Jones PG, Ezekowitz JA, Allen LA, Masoudi FA, Maddox TM. Practice‐level variation in use of recommended medications among outpatients with heart failure: insights from the NCDR PINNACLE program. Circ Heart Fail. 2013;6:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16. Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Hoffman E, Goto S, Ohman EM, Bhatt DL. Adherence to secondary prevention medications and four‐year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126:693–700.e1. [DOI] [PubMed] [Google Scholar]
- 17. Fonarow GC, Yancy CW, Heywood JT; Adhere Scientific Advisory Committee, Study Group, and Investigators . Adherence to heart failure quality‐of‐care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–1477. [DOI] [PubMed] [Google Scholar]
- 18. Komajda M, Lapuerta P, Hermans N, Gonzalez‐Juanatey JR, van Veldhuisen DJ, Erdmann E, Tavazzi L, Poole‐Wilson P, Le Pen C. Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J. 2005;26:1653–1659. [DOI] [PubMed] [Google Scholar]
- 19. Masoudi FA, Rathore SS, Wang Y, Havranek EP, Curtis JP, Foody JM, Krumholz HM. National patterns of use and effectiveness of angiotensin‐converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation. 2004;110:724–731. [DOI] [PubMed] [Google Scholar]
- 20. Krantz MJ, Ambardekar AV, Kaltenbach L, Hernandez AF, Heidenreich PA, Fonarow GC. Patterns and predictors of evidence‐based medication continuation among hospitalized heart failure patients (from Get With the Guidelines‐Heart Failure). Am J Cardiol. 2011;107:1818–1823. [DOI] [PubMed] [Google Scholar]
- 21. Bonow RO, Bennett S, Casey DE Jr, Ganiats TG, Hlatky MA, Konstam MA, Lambrew CT, Normand SL, Pina IL, Radford MJ, Smith AL, Stevenson LW, Burke G, Eagle KA, Krumholz HM, Linderbaum J, Masoudi FA, Ritchie JL, Rumsfeld JS, Spertus JA. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures): endorsed by the Heart Failure Society of America. Circulation. 2005;112:1853–1887. [DOI] [PubMed] [Google Scholar]
- 22. Tran R. Mortality following initiation or discontinuation of guideline directed medical therapies in hospitalized heart failure patients in the Atherosclerosis Risk in Communities Study. J Am Coll Cardiol. 2016;67:1549. [Google Scholar]
- 23. Sanam K, Bhatia V, Bajaj NS, Gaba S, Morgan CJ, Fonarow GC, Butler J, Deedwania P, Prabhu SD, Wu WC, White M, Love TE, Aronow WS, Fletcher RD, Allman RM, Ahmed A. Renin‐angiotensin system inhibition and lower 30‐day all‐cause readmission in Medicare beneficiaries with heart failure. Am J Med. 2016;129:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kolandaivelu K, Leiden BB, O'Gara PT, Bhatt DL. Non‐adherence to cardiovascular medications. Eur Heart J. 2014;35:3267–3276. [DOI] [PubMed] [Google Scholar]
- 25. Butler J, Arbogast PG, Daugherty J, Jain MK, Ray WA, Griffin MR. Outpatient utilization of angiotensin‐converting enzyme inhibitors among heart failure patients after hospital discharge. J Am Coll Cardiol. 2004;43:2036–2043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Documented Reasons for ACEi/ARB Contraindication
Table S2. Covariates Included in Multivariable Cox Regression
Table S3. Comparison of Primary Analysis and Sensitivity Analysis Using Complete Discharge Data
Table S4. Rates of In‐Hospital Procedures
Table S5. Multivariate Cox Proportional Hazard Model Comparing Mortality, Readmission, and Composite Mortality and Readmissions Between Patients Discontinued From and Not Started on ACEi/ARB
Table S6. Comparison of Baseline Characteristics Between Patients Excluded Because of Missing Data and Those Included in the Study Population
Figure S1. Study population derivation.


