Abstract
Background
Dickkopf‐3 (DKK3) is a negative regulator of the Wnt/β‐catenin signaling pathway, which is involved in inflammation. However, little is known about the relationship between DKK3 expression and the progression of atherosclerosis. The aim of the present study was to define the role of DKK3 and its potential mechanism in the development of atherosclerosis.
Methods and Results
Immunofluorescence analysis showed that DKK3 was strongly expressed in macrophages of atherosclerotic plaques from patients with coronary heart disease and in hyperlipidemic mice. The expression level was significantly increased in atherogenesis. DKK3−/−ApoE−/− mice exhibited a significant decrease in atherosclerotic lesions in the entire aorta, aortic sinus, and brachiocephalic arteries. Transplantation of bone marrow from DKK3−/−ApoE−/− mice into lethally irradiated ApoE−/− recipients resulted in a reduction of atherosclerotic lesions, compared with the lesions in recipients transplanted with ApoE−/− donor cells, suggesting that the effect of DKK3 deficiency was largely mediated by bone marrow–derived cells. A reduction in the necrotic core size, accompanied by increased collagen content and smooth muscle cells and decreased accumulation of macrophages and lipids, contributed to the stability of plaques in DKK3−/−ApoE−/− mice. Furthermore, multiple proinflammatory cytokines exhibited marked decreases in DKK3−/−ApoE−/− mice. Finally, we observed that DKK3 ablation increased β‐catenin expression in the nuclei of macrophages both in vivo and in vitro.
Conclusions
DKK3 expression in macrophages is involved in the pathogenesis of atherosclerosis through modulation of inflammation and inactivation of the Wnt/β‐catenin pathway.
Keywords: atherosclerosis, dickkopf‐3, inflammation, macrophage, β‐catenin
Subject Categories: Atherosclerosis, Vascular Disease
Introduction
Atherosclerosis is a chronic inflammatory disease1, 2 and underlies in the development of cardiovascular diseases.3 Endothelial dysfunction initiates the development of atherogenesis by secreting cytokines and chemokines, which attract peripheral monocytes. These monocytes then adhere and aggregate into the subendothelial area of blood vessels and subsequently differentiate into macrophages and then become foam cells after endocytosis of modified low‐density lipoprotein.4 Ineffective clearance results in an accumulation of apoptotic macrophages, which may then undergo post‐apoptotic necrosis. This is essential for necrotic core formation, a key determinant in the formation of vulnerable plaques.5 Chronic inflammation, plaque instability, and enlargement of the necrotic core size eventually lead to the rupture of advanced plaques, resulting in various acute cardiovascular events including myocardial infarction, sudden cardiac death, and stroke.6 The mechanisms that underlie atherogenesis and related inflammation development remain to be fully understood.
Growing evidence indicates that the Wnt/β‐catenin signaling pathway exerts an important role on the regulation of inflammation and the cell fate decision, which are important events in the development of atherogenesis.7, 8, 9 The Dickkopf (DKK) protein family, including DKK1, 2, 3, and Soggy (a unique DKK3‐related protein), is best known as a negative regulator of the Wnt signaling pathway.10, 11 All DKK proteins contain 2 conserved cysteine‐rich domains, thus facilitating the binding to cofactors and contributing to development, homeostasis, and various pathological disease processes.10 DKK3, one of the most poorly characterized members encoding 5 secreted glycoproteins, has emerged as an important regulator of cell fate determination during embryonic development.12 In addition, a large number of studies have demonstrated the important role of DKK3 in the pathological process of human tumor development. A reduction in DKK3 expression was detected in multiple cell types in human cancers including prostate,13 colon,14 and breast15 cancers, acute lymphoblastic leukemia,16 human renal clear‐cell carcinoma,17 and non‐small‐cell lung carcinomas.18 Additionally, the potential effect of DKK3 on suppressing cancer cell proliferation or accelerating the apoptotic process during cancer development suggests that DKK3 is a possible candidate tumor suppressor gene in humans.13, 14, 15, 18, 19 As a key negative modulator of Wnt/β‐catenin, DKK3 expression has been identified in human mesenchymal stem cells20 and is strongly expressed in aorta and heart during embryogenesis.21 Our recently studies have demonstrated that DKK3 plays a critical role in cardiovascular diseases22, 23 and in metabolic disease.24 However, there are no studies regarding the relationship between DKK3 expression and the development of atherosclerosis.
The present study was performed to investigate the role of DKK3 in atherogenesis and plaque destabilization. Our in vivo and in vitro experiments demonstrated that the decreased inflammation and ameliorated macrophage dysfunction associated with the development of atherosclerosis in DKK3−/−ApoE−/− mice were at least partially mediated by activation of the Wnt/β‐catenin signaling pathway.
Methods
Animals and Diets
The animal study protocols were performed in accordance with Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the Animal Care and Use Committee of the Renmin Hospital and the Animal Experiment Center of Wuhan University. DKK3 knockout mice (129S6/SvEvTac‐DKK3<tm1Tfur>), 129 background, were kindly provided by Takahisa Furukawa (Osaka Bioscience Institute, Osaka, Japan). To purify the background, the female DKK3−/− mice on a 129 background were first crossbred with male C57BL/6, and then the male F1 generation mice (DKK3 heterozygous) were mated with female C57BL/6 mice for the F2 generation mice (DKK3 heterozygous). The F2 generation mice were then repeatedly crossed with C57BL/6J mice until the F9 generation (DKK3 heterozygous). Finally, these F9 mice were then crossed to yield DKK3 KO (pure C57BL/6J background) mice as a previous study described. ApoE−/− mice and DKK3−/− mice were crossbred to obtain DKK3−/−ApoE−/− mice and ApoE−/− littermates. Eight‐week‐old mice of 2 groups were treated with a high‐fat diet (HFD; 15.8% fat and 1.25% cholesterol) or normal chow (NC) for up to 28 weeks. Serum was collected for the measurement of lipids, including triglyceride, total cholesterol, very‐low‐density lipoprotein, low‐density lipoprotein, intermediate‐density lipoprotein, and high‐density lipoprotein, and the levels of inflammatory cytokines such as tumor necrosis factor‐α (TNF‐α), monocyte chemoattractant protein‐1 (MCP‐1), interleukin (IL)‐6, and IL‐1β were tested as in a previous study.25 Euthanasia was performed by intraperitoneal injection of sodium pentobarbital (50 mg/kg).
Atherosclerotic Lesion Analysis
After treatment with a HFD for 28 weeks, the mice received intraperitoneal anesthesia with pentobarbital sodium (50 mg/kg), then were sacrificed and transcardially perfused with PBS and/or 4% paraformaldehyde. The hearts were embedded in paraffin or an optimal cutting temperature compound for histological analysis. For en face analysis, the entire aorta was removed carefully from each heart and stained with Oil Red O to quantify the size of atherosclerotic lesions by using Image Pro Plus 6.0 software as described before.26 Consecutive 5‐μm sections of the atrioventricular valve region of each heart were collected and stained with hematoxylin and eosin for morphology analysis. The plaque stability score was determined as (smooth muscle cell [SMC] area+collagen area)/(macrophage area+lipid area).27
Bone Marrow Transplantation Study
Male recipient mice at 8 weeks of age were lethally irradiated with 2 doses of 550 rads each (for a total of 11 Gy) 4 hours apart on the day of transplantation. After intraperitoneal anesthesia with pentobarbital sodium (50 mg/kg), ~5×107 nucleated bone marrow cells were obtained from donor DKK3−/−ApoE−/− or ApoE−/− mice and then injected intravenously into ApoE−/− recipient mice through the orbital plexus. Four weeks after bone marrow transplantation, peripheral blood was collected via retro‐orbital venous plexus puncture for polymerase chain reaction (PCR) analysis of bone marrow reconstitution. Then, mice were fed a HFD for an additional 16 weeks to analyze atherosclerotic lesion development.
Quantitative Real‐Time PCR and Western Blot
Total mRNA from the whole aorta was extracted using TRIzol reagent, purified with DNase, and reverse transcribed with a Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, 04897030001).28 The target genes were quantified by real‐time PCR using LightCycler 480 SYBR Green 1 Master Mix and a LightCycler 480 QPCR System (Roche Diagnostics, Indianapolis, IN). The relative transcription levels of target genes were normalized against GAPDH gene expression. Proteins were extracted from the aortas, which were homogenized in lysis buffer as described previously.29 Then, 5 μg of protein was separated by SDS‐PAGE and transferred to polyvinylidene fluoride membranes, which were blocked in Tris‐buffered saline/Tween 20 for 1 hour at room temperate and incubated with primary antibodies overnight at 4°C. Next, the membranes were incubated with secondary antibodies and treated with enhanced chemiluminescence reagents before being visualized using a FluorChem E Imager (ProteinSimple, San Jose, CA). Specific protein expression was normalized against GAPDH or Lamin B protein expression.
Immunofluorescence
Dewaxed heart slides were processed as described in our previous study30 and were then incubated overnight with the primary antibodies at 4°C. After rewarming at 37°C for 1 hour, the appropriate secondary antibodies were used, including Alexa Fluor 568 donkey anti‐goat IgG (1:200 dilution; Invitrogen, Carlsbad, CA), and Alexa Fluor 568 donkey anti‐rat IgG (1:200 dilution, Invitrogen). The nuclei were stained with 4ʹ,6‐diamidino‐2‐phenylindole.
Human Specimens
All procedures involving human samples complied with the principles outlined in the Declaration of Helsinki and were approved by the Renmin Hospital of Wuhan University Institutional Review Board in Wuhan, China. Samples of atheromatous plaques were collected from the coronary artery of coronary heart disease patients who had undergone heart transplantation, and control samples were obtained from the coronary arteries of normal heart donors who were not suitable for transplantation (Table). Written informed consent was obtained from the relevant families.
Table 1.
Relevant Information on Human Samples
| Type | Sex | Age (y) | Medications (Major) |
|---|---|---|---|
| Donor | Male | 46 | N/A |
| Donor | Male | 58 | N/A |
| Donor | Male | 25 | N/A |
| Donor | Female | 57 | N/A |
| CHD | Female | 66 | Atorvastatin, nitroglycerin, dexamethasone, |
| CHD | Male | 47 | Nicorandil, heparin sodium, spironolactone |
| CHD | Male | 58 | Atorvastatin, furosemide, trimetazidine, nicorandil |
| CHD | Male | 63 | Atorvastatin, metoprolol, heparin sodium |
CHD indicates coronary heart disease; N/A, not available.
Statistical Analysis
All statistical data were analyzed using SPSS software version 16.0 and were presented as the mean±SEM. Comparisons between 2 groups were evaluated using 2‐tailed Student t tests or Wilcoxon test according to the distribution pattern of the data, and 1‐way ANOVA for 4 groups. P<0.05 was considered to be statistically significant.
Results
Upregulation of DKK3 Expression in Atherosclerotic Vessels of Hyperlipidemic ApoE‐Deficient Mice
To explore the potential role of DKK3 in the development of atherosclerosis, we first examined the DKK3 expression level in mouse atherosclerotic plaques. Western blot revealed a strong increase of DKK3 expression in aortas from ApoE‐deficient mice fed with HFD for 28 weeks compared with NC treatment (Figure 1A). Immunofluorescence staining of the aortic root revealed a stronger immunoreactivity in the atherosclerotic lesion of HFD‐induced ApoE−/− mice than the NC group. Additionally, the upregulated expression of DKK3 was found to be primarily localized in the lesion‐infiltrated macrophages, which were identified by CD68 staining (Figure 1B). Similar results were also obtained in bone marrow–derived macrophages exposed to oxidized‐LDL stimulation (Figure 1C). However, a minimal expression and no significant changes of DKK3 were observed in vascular smooth muscle cells (VSMCs) and human umbilical vein endothelial cells after being stimulated by oxidized LDL (Figure S1A). Taken together, these data indicate that DKK3 is upregulated primarily in activated plaque macrophages during atherogenesis.
Figure 1.
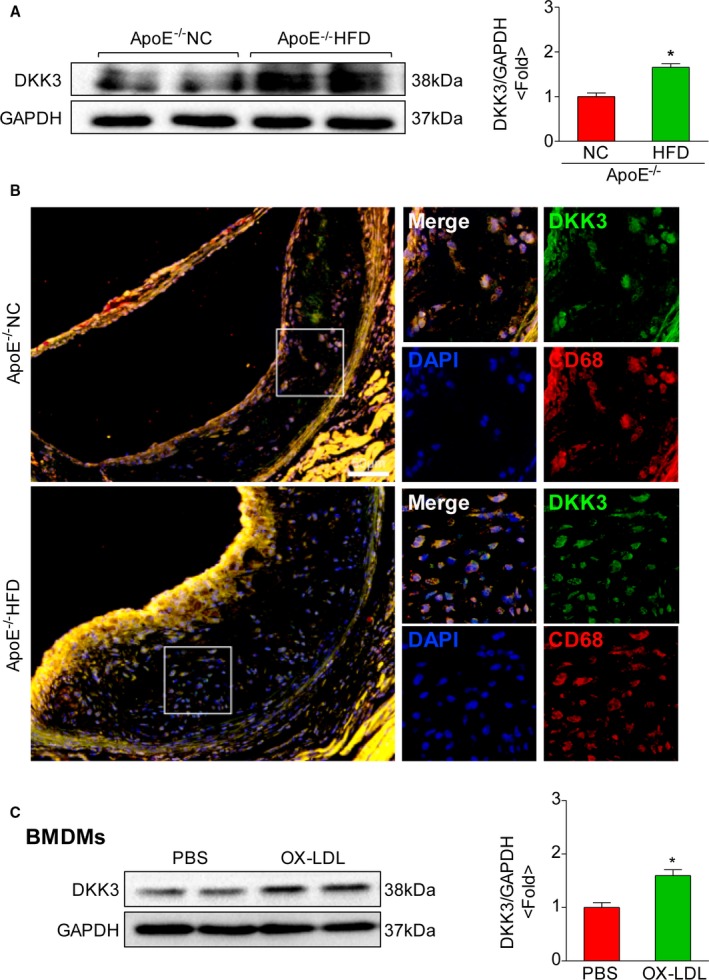
Expression of DKK3 in atheromatous lesions of mice. A, Western blotting analysis of DKK3 protein levels in aorta of mice. The expression levels were normalized to GAPDH and quantified. n=4. *P<0.05. B, Representative images showing double immunofluorescence staining of mouse aortic sinus for DKK3 (green) and macrophages (red). Scale bar=50 μm. C, Western blot analysis of DKK3 expression in BMDMs on oxidized‐LDL treatment. *P<0.05. BMDMs indicates bone marrow‐derived macrophages; DKK3, Dickkopf‐3; LDL, low‐density lipoprotein.
DKK3 Deficiency Attenuates the Development of Atherosclerosis
To further elucidate the involvement of upregulated DKK3 in the progression of atherosclerosis, male and female DKK3−/−ApoE−/− and ApoE−/− littermates were fed with NC or HFD for 28 weeks starting from 8 weeks of age. En face analysis of the whole aortas indicated that the atherosclerotic lesion area in HFD‐induced DKK3−/−ApoE−/− mice showed a significant decrease compared with ApoE−/− littermates (29.35% vs 41.57%), although no marked difference in lesions was found between the 2 groups fed with NC (Figure 2A). Additionally, we observed that the difference of lesion burden between DKK3−/−ApoE−/− and ApoE−/− mice mainly localized in the aortic arch and the thoracic aorta, whereas no significant difference was found in the abdominal aorta region (Figure 2A). The effect of DKK3 on atherogenesis was also evaluated by measuring plaque volume in the aorta at the aortic sinus level and in brachiocephalic arteries in HFD groups. Quantitative analysis revealed that the lesion areas in the aorta at valve level (Figure 2B) and brachiocephalic arteries (Figure 2C) were significantly smaller in DKK3−/−ApoE−/− mice compared to their ApoE−/− littermates. No differences were observed in body weight, plasma cholesterol levels, plasma triglycerides, and lipoprotein profiles between the 2 genotypes fed a HFD (Figure 2D and 2E).
Figure 2.
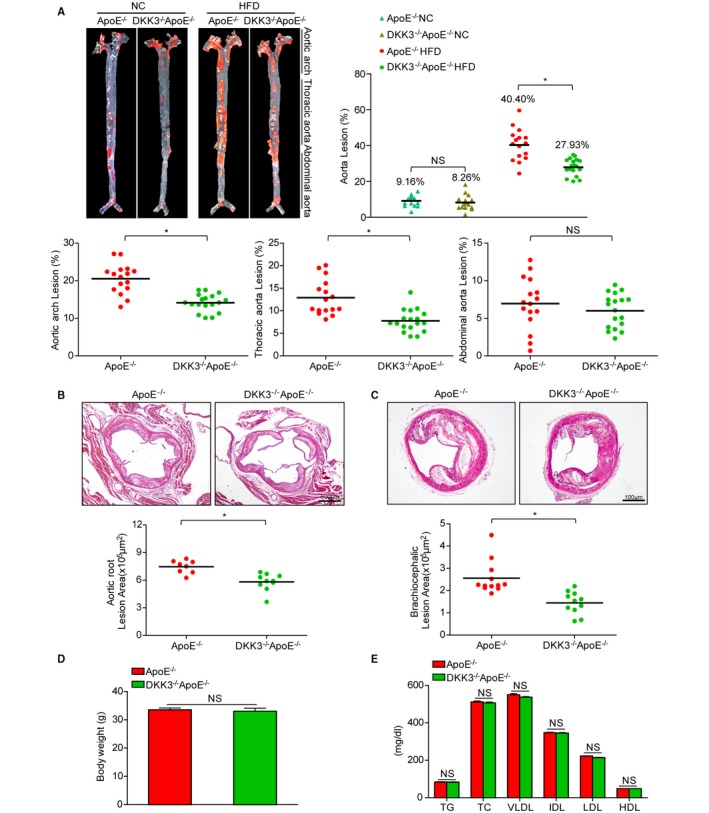
Development of atherosclerosis resulted from DKK3 deficiency. A, En face Oil Red O staining of aortas from DKK3−/−ApoE−/− mice and control mice fed NC or a HFD. Lesion occupation was quantified and shown in the right and bottom panel. n=15 to 18. B, Hematoxylin and eosin staining of the lesions in aortic root sections. Scale bar=500 μm, n=8 to 10. C, Brachiocephalic artery sections were stained with hematoxylin and eosin. Scale bar=100 μm, n=11 to 12. D and E, Body weight, triglycerides, total cholesterol, and lipoprotein profiles from DKK3−/−ApoE−/− and ApoE−/− mice fed a HFD. n=20. *P<0.05 in A through C. DKK3 indicates Dickkopf‐3; HDL, high‐density lipoprotein; HFD, high‐fat diet; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; NC, normal chow; TC, total cholesterol; TG, triglyceride; VLDL, very‐low‐density lipoprotein.
DKK3 Deficiency Decreases the Necrotic Area and Increases Features of Plaque Stability
Necrotic core formation in advanced atherosclerotic plaques promotes plaque disruption and causes acute atherothrombotic vascular events. DKK3−/−ApoE−/− mice exhibited smaller necrotic core regions in the aortic root and brachiocephalic arteries compared with ApoE−/− mice, and the fibrous caps exhibited greater thickening (Figure 3A and 3B). We also investigated the composition of lesions in the aortic root, including the collagen content, smooth muscle cells, macrophage infiltration, and lipid accumulation, which contribute to plaque stability. DKK3−/−ApoE−/− mice exhibited significantly increased collagen‐positive area, profoundly increased smooth muscle cells, decreased macrophage infiltration, and lipid accumulation (Figure 3C and 3D). Furthermore, we assessed the plaque stability score, and the results showed that DKK3 ablation improved the stability characteristics of plaque (Figure 3D).
Figure 3.
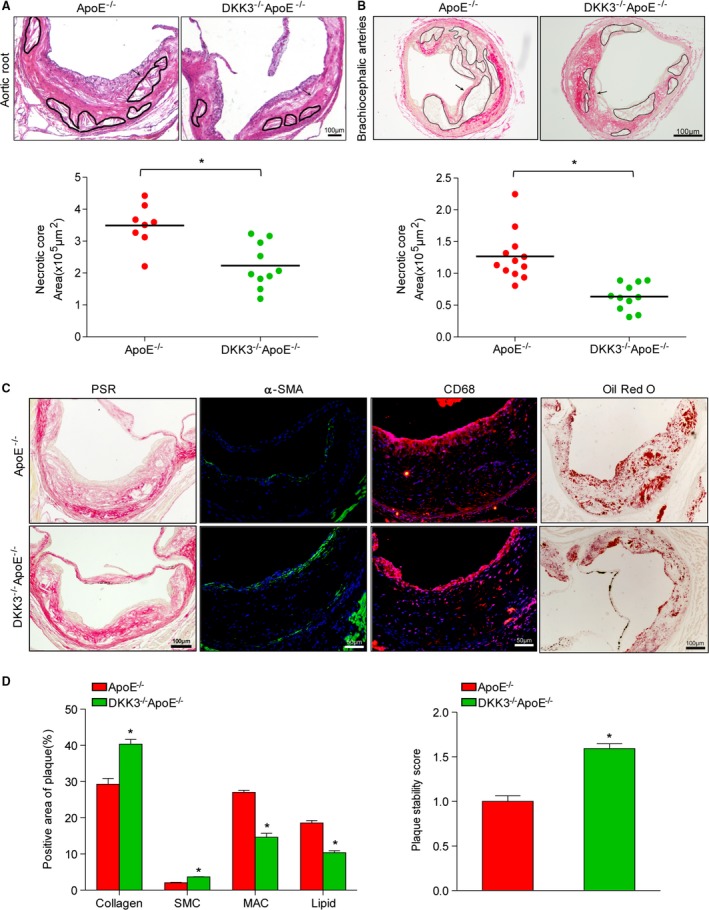
The necrotic area and plaque stability characteristics in DKK3‐deficient mice. A and B, Representative aortic sinus sections (A) and brachiocephalic arteries (B) from DKK3−/−ApoE−/− and ApoE−/− mice were stained with hematoxylin and eosin. The circular region indicates necrotic areas. Below panel: quantification of anuclear, afibrotic, and eosin‐negative necrotic areas. n=8 to 12. C and D, Cross sections of the aortic sinus plaques were stained with picrosirius red for collagen, α‐smooth muscle actin for smooth muscle cells, CD68 for macrophages, and Oil Red O for lipids. The assessment of plaque stability score in DKK3−/−ApoE−/− and ApoE−/− mice. n=5 to 10, Scale bar=100 μm. *P<0.05 in A, B, and D. DKK3 indicates Dickkopf‐3.
DKK3 Deficiency in Marrow‐Derived Cells Decreases Atherosclerosis
Because DKK3 is primarily expressed by macrophages/foam cells in atherosclerotic plaques, it is important to know whether DKK3 deficiency in hematopoietic cells is sufficient to recapitulate the phenotype of global knockout of DKK3. For this purpose, lethally irradiated ApoE−/− recipient mice were transplanted with bone marrow from DKK3−/−ApoE−/− or ApoE−/− donors and had atherosclerosis induced with a HFD for 16 additional weeks. Success of bone marrow chimera generation was confirmed by PCR of genomic DNA isolated from circulating white blood cells (Figure 4A and 4B). The area of atherosclerotic lesions in the aorta was significantly decreased in the DKK3−/−ApoE−/− chimeras (Figure 4C). Consistent results were obtained in the aortic sinus (Figure 4D). The similar decrease in atherosclerosis between the bone marrow chimeras and global DKK3 knockouts suggests that loss of DKK3 expression in bone marrow–derived cells (presumably macrophages) contributes to the observed decrease in atherogenesis.
Figure 4.
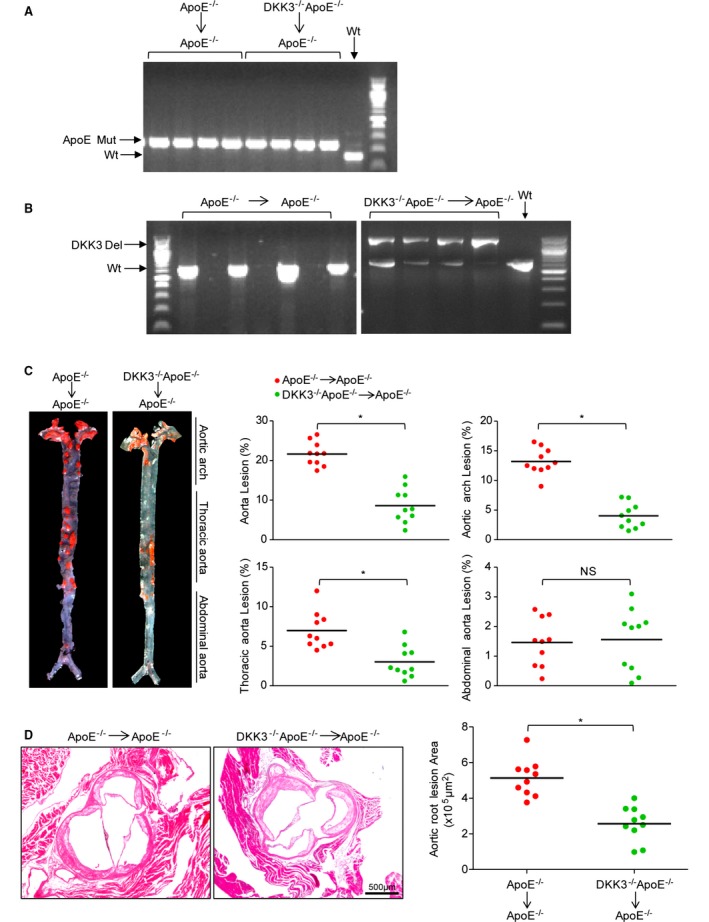
Atherogenesis of ApoE−/− mice transplanted with DKK3 deficient bone marrow cells. A and B, The genotype of ApoE (A) and DKK3 (B) in genomic DNA isolated from circulating white blood cells of recipient animals transplanted with DKK3−/−ApoE−/− or ApoE−/− bone marrow. n=4. C, Representative aorta stained with Oil Red O (n=10) (left panel) and quantification of plaque occupation in mice that received bone marrow cells from DKK3−/−ApoE−/− or ApoE−/− mice. D, Aortic root sections stained with hematoxylin and eosin (n=10) and quantification of plaque area. *P<0.05 in (C and D). DKK3 indicates Dickkopf‐3.
DKK3 Deficiency Decreases Inflammation and Inactivates the NF‐κB Signaling Pathway
The inflammatory response mediated by the secretion of cytokines, chemokines, and adhesion molecules by cells within the lesion is known to accelerate the development of atherogenesis. Thus, we used quantitative real‐time PCR to assess the expression of multiple inflammatory mediators using RNA isolated from the aortas of DKK3−/−ApoE−/− mice and ApoE−/− littermates after 28 weeks on a HFD. The mRNA levels of ICAM‐1 and VCAM‐1, 2 endothelial adhesion molecules that initiate the process of atherosclerosis, were strongly reduced in DKK3−/−ApoE−/− mice compared with ApoE−/− mice. We also observed a robust reduction of mRNA of proinflammatory markers such as IL‐6, IL‐1β, TNF‐α, MCP‐1, and inducible nitric oxide synthase in DKK3−/−ApoE−/− mice (Figure 5A). In serum, levels of TNF‐α, MCP‐1, IL‐6, and IL‐1β were significantly decreased in DKK3−/−ApoE−/− mice compared with controls (Figure 5B). In addition, by immunofluorescence staining, we observed decreased expression of ICAM‐1 and IL‐6 in the lesion area of DKK3−/−ApoE−/− mice, and reduced phosphorylation of the NF‐κB subunit p65 was observed (Figure 5C). The NF‐κB signaling pathway plays a critical role in mediating inflammation in the development of atherosclerosis. Analysis of aortas from both groups by Western blotting showed that the expression levels of phosphorylated of IKKβ, IκBα, and p65 were decreased in DKK3−/−ApoE−/− mice (Figure 5D).
Figure 5.
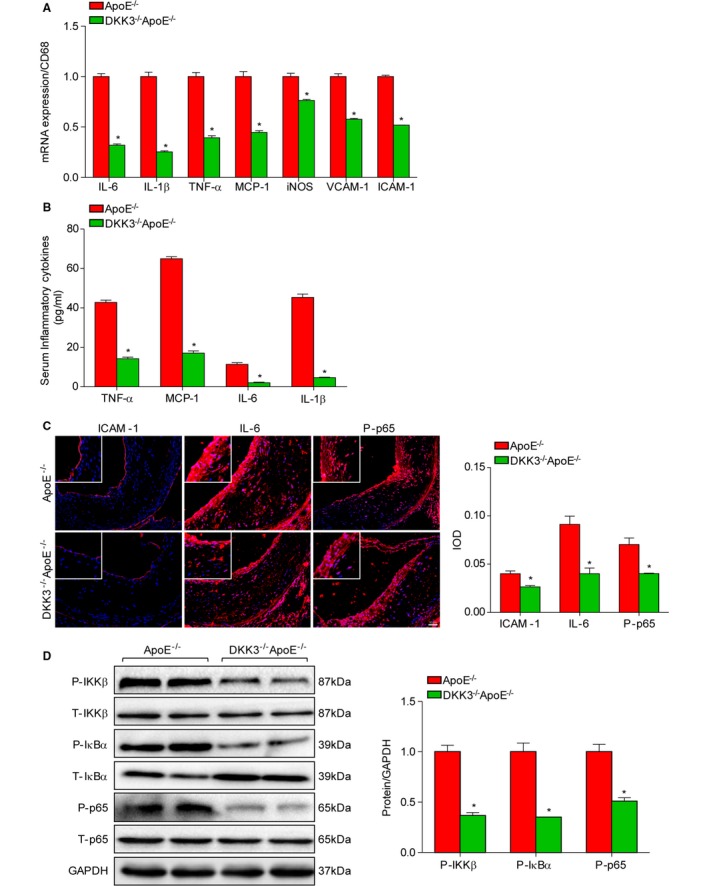
Inflammatory cytokine production and activity of the NF‐κB signaling pathway in DKK3 knockout mice. A, mRNA levels of proinflammatory markers in the aortas of ApoE−/− and DKK3−/−ApoE−/− mice were measured by real‐time PCR. n=6. B, Serum TNF‐α, MCP‐1, IL‐6 and IL‐1β levels were measured by ELISA in ApoE−/− and DKK3−/−ApoE−/− mice. n=10. C, Immunofluorescence staining for ICAM‐1, IL‐6, and p65 phosphorylation in the aortic sinuses of ApoE−/− and DKK3−/−ApoE−/− mice. n=5. D, Western blotting analysis of the NF‐κB signaling pathway in the aortas of ApoE−/− and IRF3−/−ApoE−/− mice, as assessed by the levels of IKKβ, IκBα, and p65 phosphorylation. Protein expression levels were normalized to GAPDH. *P<0.05 in A, B, and D. DKK3 indicates Dickkopf‐3; IL‐6, interleukin‐6; IL‐1β, interleukin‐1β; MCP‐1, monocyte chemoattractant protein‐1; TNF‐α, tumor necrosis factor‐α;
DKK3 Deficiency Activates the Wnt/β‐Catenin Pathway
Sustained activation of Wnt proteins causes the disassembly of the β‐catenin destruction complex, which can no longer phosphorylate β‐catenin for proteasome‐mediated degradation. The accumulation of dephosphorylated β‐catenin eventually translocates from the cytoplasm to the nucleus to induce expression of target genes. To further investigate the molecular mechanism by which DKK3−/−ApoE−/− mice showed attenuated development of atherosclerosis, we investigated β‐catenin activity in the aortas of mice. Western blotting analysis revealed a significant decrease of phosphorylated β‐catenin but increased dephosphorylated β‐catenin in the cytoplasm of DKK3−/−ApoE−/− mice compared with ApoE−/− littermates. Meanwhile, dephosphorylated β‐catenin in the nuclear fraction was strongly increased in DKK3‐deficient mice compared with the control group (Figure 6A). Immunofluorescence staining indicated the translocation of β‐catenin in the nuclei of macrophages in DKK3−/−ApoE−/− mice (Figure 6B). The similar results in accordance with experiment in vivo were also obtained in peritoneal macrophages isolated from ApoE−/− and DKK3−/− ApoE−/− mice treated with oxidized LDL (Figure 6C). The PCR analysis showed DKK3 deficiency significantly reduced the expression of several proinflammatory cytokines in macrophages, including IL‐6, IL‐1β, TNF‐α, inducible nitric oxide synthase, and Cox2 on stimulation with oxidized LDL (Figure 6D).
Figure 6.
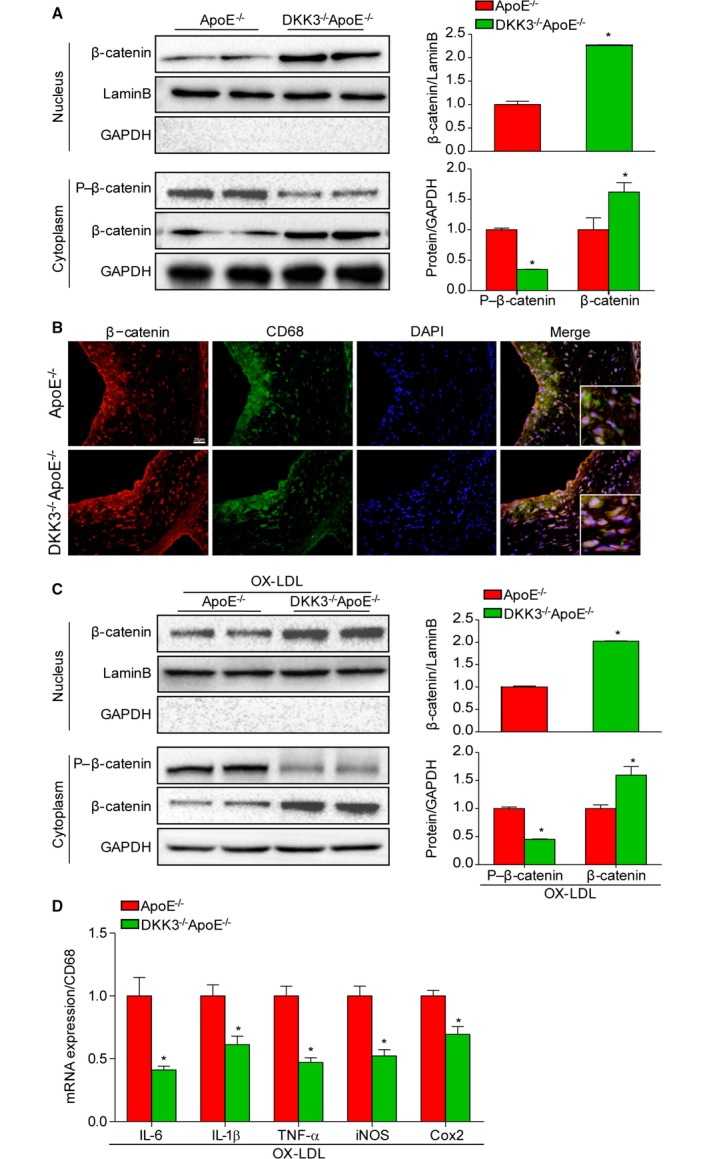
Activation of the Wnt/β‐catenin pathway in DKK3 deficiency. A, The total protein levels of β‐catenin in the nucleus and the phosphorylated and total protein levels of β‐catenin in the cytoplasm of DKK3−/−ApoE−/− and ApoE−/− mice. *P<0.05. B, Immunofluorescence costaining of atherosclerotic plaques with β‐catenin (red) and CD68 (green). n=3. Scale bar=20 μm. C, Western blot analysis of β‐catenin in the nucleus and the phosphorylated and total protein levels of β‐catenin in the cytoplasm of peritoneal macrophages isolated from ApoE−/− and DKK3−/− ApoE−/− upon oxidized‐LDL stimulation. *P<0.05. D, mRNA levels of proinflammatory markers in peritoneal macrophages isolated from ApoE−/− and DKK3−/− ApoE−/− treatment with oxidized LDL. *P<0.05. DKK3 indicates Dickkopf‐3; LDL, low‐density lipoprotein.
Enhanced DKK3 Expression in Human Coronary Artery Plaques
To evaluate the response of DKK3 in human atherosclerotic lesion, we analyzed the DKK3 expression level in the right coronary artery of patients with coronary heart disease, which is an atherosclerosis‐prone region. The western blot analysis showed a remarkable increased DKK3 expression in atherosclerotic plaque of patients (Figure 7A). More importantly, immunofluorescence staining of human plaque also revealed significantly increased DKK3 expression in the macrophages of atherosclerotic arteries compared with normal arteries (Figure 7B). In addition, VSMCs and endothelial cells also exhibited modest DKK3 expression in human plaques (Figure S1B).
Figure 7.
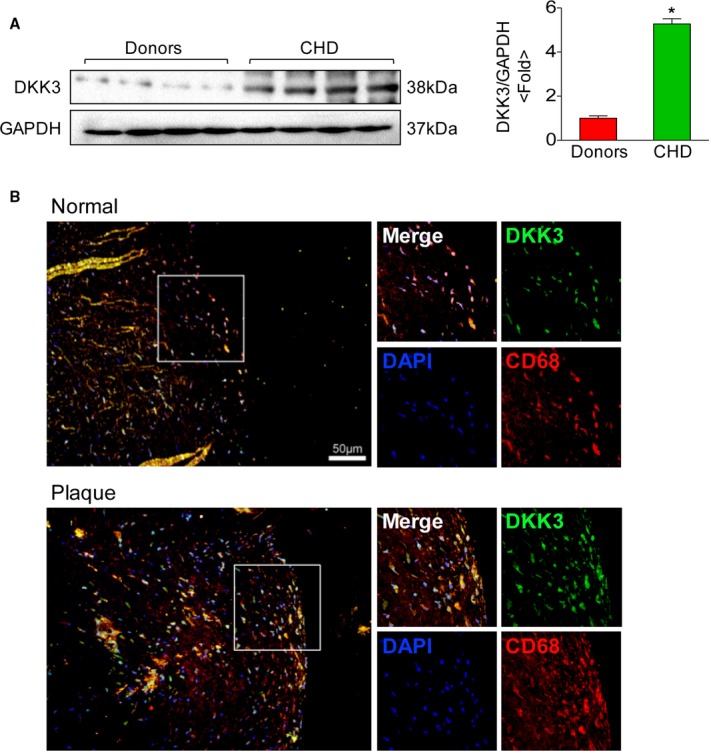
Expression of DKK3 in human atheromatous lesions. A, Western blotting analysis of DKK3 protein levels in the right coronary artery in humans. The expression levels were normalized to GAPDH and quantified. n=4. *P<0.05. B, Representative images showing double‐immunofluorescence staining of human coronary arteries for DKK3 (green) and macrophages (red). Scale bar=50 μm. DKK3 indicates Dickkopf‐3.
Discussion
Numerous published papers have demonstrated that the Wnt/β‐catenin signaling pathway is involved in many human diseases. However, the direct assessment of Wnt signaling in atherosclerosis is incomplete. In the current study we provide the first report that DKK3, an inhibitor of the Wnt pathway, is a positive regulator of the deleterious outcomes of atherosclerosis. Within the atherosclerotic plaques of coronary heart disease patients or hyperlipidemic mice, increased DKK3 expression was predominantly located in macrophages. Using a loss‐of‐function strategy and bone marrow transplantation experiments, we demonstrated that the antiatherogenesis effect of DKK3 deficiency largely originated from its role in macrophages. The absence of DKK3 led to a reduction in the inflammatory response and inactivation of the NF‐κB signaling pathway. Collectively, multiple pathological processes contribute to the decreased size of the necrotic core and the increased stability of plaques in DKK3 deficiency. Mechanistically, DKK3 ablation activates the Wnt/β‐catenin pathway and promotes accumulation of β‐catenin in macrophages.
During the initiation and development of atherosclerosis, accumulative inflammatory response has been involved in all stages of atherogenesis, which ultimately destabilizes plaques and leads to the occurrence of severe cardiovascular events.1, 2 In the current work, DKK3 deficiency significantly attenuated the atherosclerotic lesion burden in the whole aorta, aortic root, and brachiocephalic arteries, and the hallmark of DKK3 ablation on atherogenesis is characterized as decreased secretion of inflammatory mediators and inactivation of proinflammatory NF‐κB signaling. Notably, the prediction of plaque disruption and acute clinical events in humans is associated with plaque morphology rather than plaque size.31 In parallel with the decreased size of the necrotic core, the collagen fiber content and contractility of vascular smooth muscle cells were increased, whereas the accumulation of macrophages and lipids was decreased in DKK3−/−ApoE−/− mice, thus collectively contributing to plaque stability. Further investigation using bone marrow transplantation demonstrated that ablation of DKK3 in macrophages accounted for the decrease in atherosclerotic plaques observed in DKK3‐deficienct mice. Meanwhile, we observed a moderate DKK3 expression in the VSMCs and endothelium in human atherosclerotic plaque, suggesting a potential role of non‐macrophage‐derived DKK3 on atherogenesis. Although it has been reported that DKK3 has an effect on smooth muscle differentiation,32, 33 we found that the expression of DKK3 in VSMCs and human umbilical vein endothelial cells was at a minimal level and did not show response to oxidized‐LDL stimulation. Additionally, the DKK3−/−ApoE−/− mice that received transplanted ApoE−/− bone marrow developed similar amounts of atherosclerotic plaques as the ApoE−/− mice transplanted with ApoE−/− bone marrow. Collectively, these data indicate that the role of DKK3 deficiency in hematopoietic cells on the development of atherosclerosis is more critical than it is in other vascular cells. Recently, our research has revealed a versatile role for DKK3 in diverse cell types under different pathological stimulations. In cardiomyocytes the pressure overload‐induced decrease in DKK3 expression represses the development of cardiac remodeling,22 and DKK3 protects against ventricular remodeling after myocardial infarction.23 In hepatocytes DKK3 has been identified as a negative modulator of insulin resistance, hepatic steatosis, and inflammatory responses.24
The Wnt signaling pathway was originally found to play a key role in embryogenesis and cellular development.34, 35, 36 However, growing evidence has demonstrated that the Wnt/β‐catenin signaling pathway exerts an important role on the regulation of inflammation and cell fate decision determination, proliferation, differentiation, cell polarity, and migration.7, 8, 9 In multiple experimental systems and diseases it has been verified that the activation of different intricate intracellular signaling pathways by Wnt proteins is highly dependent on their interaction with transmembrane‐spanning Frizzled receptors and the association of endogenous coreceptors, of which the Wnt3a and LDL‐related proteins 5 and 6 (LRP5/6) are required for activation of canonical Wnt/β‐catenin signaling.37 When Wnt3a proteins bind to the Frizzled–LRP5/6‐receptor complex, disassembly of the β‐catenin destruction complex results in the rapid accumulation of dephosphorylated β‐catenin in the cytoplasm; β‐catenin subsequently translocates to the nucleus and triggers the expression of a variety of target genes.38 In both in vivo and in vitro experiments we observed a significant reduction in phosphorylated β‐catenin and an increased presence of dephosphorylated β‐catenin in the cytoplasm, with increased accumulation of β‐catenin expression in the nuclear fraction of DKK3−/−ApoE−/− mice, which was accompanied by decreased proinflammatory cytokines. Recently, compelling evidence has demonstrated that the Wnt‐LRP5/6–β‐catenin axis plays an important role in the development of atherosclerosis. First, a reduction in LRP6 receptor expression has been identified in human carotid atherosclerotic lesions,39 and a familial missense mutation in the human LRP6 gene has been found to be associated with an increased incidence of early coronary artery disease and high levels of circulating LDL cholesterol.39, 40 Furthermore, an augmentation of atherosclerotic lesion formation has been observed in LRP5‐null mice,41 whereas mice with LRP6 mutation crossed to the LDLR−/− background develop fulminant proliferative and obstructive coronary artery disease.42 Second, enhanced expression of Wnt3a was observed in murine bone marrow–derived macrophages, and Wnt/β‐catenin counteracts the expression of proinflammatory cytokines in mycobacteria‐infected murine macrophages.8 Consistent with these findings, our study showed a significant reduction in the mRNA, serum and immunoreactivity levels of multiple proinflammatory cytokines in DKK3‐deficient mice. In addition, activation of the NF‐κB signaling pathway, a master regulator of inflammatory responses and the development of atherosclerosis, was inhibited via DKK3 ablation. A previous study suggests that the migration of human monocytes is impaired, and VCAM‐1 expression is downregulated in response to Wnt/β‐catenin activation.43, 44 As expected, our results revealed decreased ICAM‐1 and VCAM‐1 expression as well as macrophage infiltration in DKK3−/−ApoE−/− mice. Collectively, our results indicate that activation of the Wnt/β‐catenin pathway resulting from DKK3 deficiency partially inhibits the development of atherosclerosis.
In conclusion, the present study demonstrated that the absence of DKK3 protects against atherogenesis in a manner highly dependent on the role of this protein in macrophages, as confirmed by bone marrow transplantation. In particular, DKK3 accelerates the pathological process and promotes plaque accumulation via the inflammatory response. The underlying mechanism of the proatherosclerotic role of DKK3 partially involves the activation of the Wnt/β‐catenin pathway. These data underscore that inhibition of DKK3 and activation of the Wnt/β‐catenin signaling pathway may have therapeutic value for the resolution of inflammation in atherosclerosis.
Sources of Funding
This work was supported by grants from the National Science Fund for Distinguished Young Scholars (No. 81425005), the Key Project of the National Natural Science Foundation (No. 81330005), the National Natural Science Foundation of China (No. 81370209; No. 81370365; No. 81270184; No. 31371481), National Science and Technology Support Project (Nos. 2013YQ030923‐05, 2014BAI02B01, 2015BAI08B01 and 2016YFF0101500).
Disclosures
None.
Supporting information
Figure S1. Expression of DKK3 in endothelial cell and vascular smooth muscle cells.
Acknowledgments
We thank Dr Takahisa Furukawa (Osaka Bioscience Institute, Osaka, Japan) for providing DKK3 KO mice. We also acknowledge the valuable technological assistance that Cheng Du, Qiao‐Fang Wei, Meng‐lin Chao, and Ling Yang provided for this study.
(J Am Heart Assoc. 2017;6:e004690. DOI: 10.1161/JAHA.116.004690.)
Contributor Information
Hao Xia, Email: xiahao1966@163.com.
Hongliang Li, Email: lihl@whu.edu.cn.
References
- 1. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 2. Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. [DOI] [PubMed] [Google Scholar]
- 4. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. [DOI] [PubMed] [Google Scholar]
- 5. Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. [DOI] [PubMed] [Google Scholar]
- 7. Nelson WJ, Nusse R. Convergence of Wnt, β‐catenin, and cadherin pathways. Science. 2004;303:1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria‐induced inflammatory responses. Eur J Cell Biol. 2011;90:553–559. [DOI] [PubMed] [Google Scholar]
- 9. Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta‐catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–169. [DOI] [PubMed] [Google Scholar]
- 10. Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura RE, Hunter DD, Yi H, Brunken WJ, Hackam AS. Identification of two novel activities of the Wnt signaling regulator Dickkopf 3 and characterization of its expression in the mouse retina. BMC Cell Biol. 2007;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang ZR, Dong WG, Lei XF, Liu M, Liu QS. Overexpression of Dickkopf‐3 induces apoptosis through mitochondrial pathway in human colon cancer. World J Gastroenterol. 2012;18:1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H, Huh NH. Adenovirus‐mediated overexpression of REIC/Dkk‐3 selectively induces apoptosis in human prostate cancer cells through activation of c‐Jun‐NH2‐kinase. Cancer Res. 2005;65:9617–9622. [DOI] [PubMed] [Google Scholar]
- 14. Yu J, Tao Q, Cheng YY, Lee KY, Ng SS, Cheung KF, Tian L, Rha SY, Neumann U, Rocken C, Ebert MP, Chan FK, Sung JJ. Promoter methylation of the Wnt/β‐catenin signaling antagonist Dkk‐3 is associated with poor survival in gastric cancer. Cancer. 2009;115:49–60. [DOI] [PubMed] [Google Scholar]
- 15. Xiang T, Li L, Yin X, Zhong L, Peng W, Qiu Z, Ren G, Tao Q. Epigenetic silencing of the WNT antagonist Dickkopf 3 disrupts normal Wnt/β‐catenin signalling and apoptosis regulation in breast cancer cells. J Cell Mol Med. 2013;17:1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roman‐Gomez J, Jimenez‐Velasco A, Agirre X, Castillejo JA, Navarro G, Barrios M, Andreu EJ, Prosper F, Heiniger A, Torres A. Transcriptional silencing of the Dickkopfs‐3 (Dkk‐3) gene by CpG hypermethylation in acute lymphoblastic leukaemia. Br J Cancer. 2004;91:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurose K, Sakaguchi M, Nasu Y, Ebara S, Kaku H, Kariyama R, Arao Y, Miyazaki M, Tsushima T, Namba M, Kumon H, Huh NH. Decreased expression of REIC/Dkk‐3 in human renal clear cell carcinoma. J Urol. 2004;171:1314–1318. [DOI] [PubMed] [Google Scholar]
- 18. Tsuji T, Nozaki I, Miyazaki M, Sakaguchi M, Pu H, Hamazaki Y, Iijima O, Namba M. Antiproliferative activity of REIC/Dkk‐3 and its significant down‐regulation in non‐small‐cell lung carcinomas. Biochem Biophys Res Commun. 2001;289:257–263. [DOI] [PubMed] [Google Scholar]
- 19. Hsieh SY, Hsieh PS, Chiu CT, Chen WY. Dickkopf‐3/REIC functions as a suppressor gene of tumor growth. Oncogene. 2004;23:9183–9189. [DOI] [PubMed] [Google Scholar]
- 20. Lu KH, Tounsi A, Shridhar N, Kublbeck G, Klevenz A, Prokosch S, Bald T, Tuting T, Arnold B. Dickkopf‐3 contributes to the regulation of anti‐tumor immune responses by mesenchymal stem cells. Front Immunol. 2015;6:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monaghan AP, Kioschis P, Wu W, Zuniga A, Bock D, Poustka A, Delius H, Niehrs C. Dickkopf genes are co‐ordinately expressed in mesodermal lineages. Mech Dev. 1999;87:45–56. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Liu Y, Zhu XH, Zhang XD, Jiang DS, Bian ZY, Zhang XF, Chen K, Wei X, Gao L, Zhu LH, Yang Q, Fan GC, Lau WB, Ma X, Li H. Dickkopf‐3 attenuates pressure overload‐induced cardiac remodelling. Cardiovasc Res. 2014;102:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bao MW, Cai Z, Zhang XJ, Li L, Liu X, Wan N, Hu G, Wan F, Zhang R, Zhu X, Xia H, Li H. Dickkopf‐3 protects against cardiac dysfunction and ventricular remodelling following myocardial infarction. Basic Res Cardiol. 2015;110:25. [DOI] [PubMed] [Google Scholar]
- 24. Xie L, Wang PX, Zhang P, Zhang XJ, Zhao GN, Wang A, Guo J, Zhu X, Zhang Q, Li H. DKK3 expression in hepatocytes defines susceptibility to liver steatosis and obesity. J Hepatol. 2016;65:113–124. [DOI] [PubMed] [Google Scholar]
- 25. Wang PX, Zhang XJ, Luo P, Jiang X, Zhang P, Guo J, Zhao GN, Zhu X, Zhang Y, Yang S, Li H. Hepatocyte TRAF3 promotes liver steatosis and systemic insulin resistance through targeting TAK1‐dependent signalling. Nat Commun. 2016;7:10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li HL, Wang AB, Zhang R, Wei YS, Chen HZ, She ZG, Huang Y, Liu DP, Liang CC. A20 inhibits oxidized low‐density lipoprotein‐induced apoptosis through negative Fas/Fas ligand‐dependent activation of caspase‐8 and mitochondrial pathways in murine RAW264.7 macrophages. J Cell Physiol. 2006;208:307–318. [DOI] [PubMed] [Google Scholar]
- 27. Ni W, Egashira K, Kitamoto S, Kataoka C, Koyanagi M, Inoue S, Imaizumi K, Akiyama C, Nishida KI, Takeshita A. New anti‐monocyte chemoattractant protein‐1 gene therapy attenuates atherosclerosis in apolipoprotein E‐knockout mice. Circulation. 2001;103:2096–2101. [DOI] [PubMed] [Google Scholar]
- 28. Ji YX, Zhang P, Zhang XJ, Zhao YC, Deng KQ, Jiang X, Wang PX, Huang Z, Li H. The ubiquitin E3 ligase TRAF6 exacerbates pathological cardiac hypertrophy via TAK1‐dependent signalling. Nat Commun. 2016;7:11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deng KQ, Wang A, Ji YX, Zhang XJ, Fang J, Zhang Y, Zhang P, Jiang X, Gao L, Zhu XY, Zhao Y, Gao L, Yang Q, Zhu XH, Wei X, Pu J, Li H. Suppressor of IKKε is an essential negative regulator of pathological cardiac hypertrophy. Nat Commun. 2016;7:11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang DS, Wei X, Zhang XF, Liu Y, Zhang Y, Chen K, Gao L, Zhou H, Zhu XH, Liu PP, Bond Lau W, Ma X, Zou Y, Zhang XD, Fan GC, Li H. IRF8 suppresses pathological cardiac remodelling by inhibiting calcineurin signalling. Nat Commun. 2014;5:3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenfeld ME, Carson KG, Johnson JL, Williams H, Jackson CL, Schwartz SM. Animal models of spontaneous plaque rupture: the holy grail of experimental atherosclerosis research. Curr Atheroscler Rep. 2002;4:238–242. [DOI] [PubMed] [Google Scholar]
- 32. Karamariti E, Margariti A, Winkler B, Wang X, Hong X, Baban D, Ragoussis J, Huang Y, Han JD, Wong MM, Sag CM, Shah AM, Hu Y, Xu Q. Smooth muscle cells differentiated from reprogrammed embryonic lung fibroblasts through DKK3 signaling are potent for tissue engineering of vascular grafts. Circ Res. 2013;112:1433–1443. [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Karamariti E, Simpson R, Wang W, Xu Q. Dickkopf homolog 3 induces stem cell differentiation into smooth muscle lineage via ATF6 signalling. J Biol Chem. 2015;290:19844–19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. [DOI] [PubMed] [Google Scholar]
- 35. Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. [DOI] [PubMed] [Google Scholar]
- 36. van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. [DOI] [PubMed] [Google Scholar]
- 37. Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β‐catenin–TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mill C, George SJ. Wnt signalling in smooth muscle cells and its role in cardiovascular disorders. Cardiovasc Res. 2012;95:233–240. [DOI] [PubMed] [Google Scholar]
- 39. Sarzani R, Salvi F, Bordicchia M, Guerra F, Battistoni I, Pagliariccio G, Carbonari L, Dessi‐Fulgheri P, Rappelli A. Carotid artery atherosclerosis in hypertensive patients with a functional LDL receptor‐related protein 6 gene variant. Nutr Metab Cardiovasc Dis. 2011;21:150–156. [DOI] [PubMed] [Google Scholar]
- 40. Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson‐Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Magoori K, Kang MJ, Ito MR, Kakuuchi H, Ioka RX, Kamataki A, Kim DH, Asaba H, Iwasaki S, Takei YA, Sasaki M, Usui S, Okazaki M, Takahashi S, Ono M, Nose M, Sakai J, Fujino T, Yamamoto TT. Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor‐related protein 5 and apolipoprotein E. J Biol Chem. 2003;278:11331–11336. [DOI] [PubMed] [Google Scholar]
- 42. Go GW. Low‐density lipoprotein receptor‐related protein 6 (LRP6) is a novel nutritional therapeutic target for hyperlipidemia, non‐alcoholic fatty liver disease, and atherosclerosis. Nutrients. 2015;7:4453–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malhotra S, Kincade PW. Canonical Wnt pathway signaling suppresses VCAM‐1 expression by marrow stromal and hematopoietic cells. Exp Hematol. 2009;37:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tickenbrock L, Schwable J, Strey A, Sargin B, Hehn S, Baas M, Choudhary C, Gerke V, Berdel WE, Muller‐Tidow C, Serve H. Wnt signaling regulates transendothelial migration of monocytes. J Leukoc Biol. 2006;79:1306–1313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression of DKK3 in endothelial cell and vascular smooth muscle cells.


