Abstract
Background
Experimental studies have demonstrated that lead and cadmium have direct toxic effects on the myocardium, but the few human studies are limited by design, assessment of exposure, and use of heart failure as a late‐stage endpoint.
Methods and Results
In a prospective population study, we studied the association of left ventricular (LV) function with blood lead (BPb) and 24‐hour urinary cadmium (UCd). In 179 participants randomly recruited from a Flemish population (50.3% women; mean age 39.1 years), geometric mean BPb and UCd at enrollment (1985‐2000) were 0.20 μmol/L and 6.1 nmol, respectively. We assessed systolic and diastolic LV function 11.9 years (median) later (2005‐2010) by using Doppler imaging of the transmitral blood flow and the mitral annular movement and speckle tracking. In multivariable‐adjusted linear regression, LV systolic function decreased with BPb. For a doubling of exposure, estimates were −0.392% for global longitudinal strain (P=0.034), −0.618% and −0.113 s−1 for regional longitudinal strain (P=0.028) and strain rate (P=0.008), and −0.056 s−1 for regional radial strain rate (P=0.050). Regional longitudinal strain rate (−0.066 s−1, P=0.009) and regional radial strain (−2.848%, P=0.015) also decreased with UCd. Models including both exposure indexes did not allow differentiating whether LV dysfunction was predominately related to BPb or UCd. Diastolic LV function was not associated with BPb or UCd (P≥0.159).
Conclusions
Although effect sizes were small, our results suggest that environmental exposure to lead, cadmium, or both might be a risk factor for systolic LV dysfunction, a condition often proceeding to heart failure.
Keywords: cadmium, heart failure, lead, population science, systolic function
Subject Categories: Epidemiology, Cardiovascular Disease, Heart Failure
Introduction
Heart failure is a major public health problem with a prevalence of nearly 6 million in the United States and more than 23 million worldwide.1 Heart failure starts with risk factors for left ventricular dysfunction, progresses to asymptomatic changes in cardiac structure and function, and then evolves into clinically overt disease. Diastolic heart failure, also referred to as heart failure with normal ejection fraction, accounts for 40% to 50% of all cases.2 Due to the demographic transition, the incidence of heart failure rises exponentially with age.1 Identification of environmental triggers of left ventricular dysfunction is therefore of major importance.
In addition to potential secondary effects mediated via hypertension3, 4, 5 or renal dysfunction,6, 7 experimental studies have demonstrated that lead and cadmium8, 9, 10 have direct toxic effects on the myocardium. The currently available human evidence linking cardiac dysfunction to exposure to these metals originates from case‐control reports11, 12 or cross‐sectional studies,13 all involving lead‐exposed workers.11, 12, 13 A report from the second National Health and Nutrition Examination Survey (NHANES) showed an association between ECG left ventricular hypertrophy and the blood lead concentration.14 Population studies linking left ventricular function to environmental cadmium exposure focused on heart failure in its terminal stage15, 16, 17 or on a composite endpoint, including heart failure.18, 19 Furthermore, in these population studies the internal cadmium burden was estimated from spot urines16, 18 or blood cadmium.15, 16 No study differentiated between systolic and diastolic left ventricular dysfunction using modern echocardiographic techniques.20 To bridge these knowledge gaps, we related in a prospective population study systolic and diastolic echocardiographic left ventricular function (2005‐2010) to baseline measurements of blood lead and 24‐hour urinary cadmium (1985‐2000).
Methods
Study Population
The Cadmium in Belgium (CadmiBel) study (1985‐1989)21 included 1107 Flemish participants randomly recruited from 10 districts in northeastern Belgium.7 The participation rate was 78%.7 The geometric mean cadmium concentration in the soil sampled from 85 kitchen gardens was 5.3 mg/kg (5th‐95th percentile interval, 1.4‐18.9) in 6 districts, which bordered on 3 zinc/cadmium smelters, and 0.9 mg/kg (0.4‐1.6) in 4 districts that were more than 10 km away from the smelters.22 The participants of the 10 districts had similar characteristics apart from exposure to cadmium.7, 23 From 1991 to 1995, participants were invited for follow‐up in the Public Health and Environmental Exposure to Cadmium Study (PheeCad).3 From 1996 until 2004, 1930 family members of the former participants (1985‐1990) were enrolled.24 At the time of recruitment, inhabitants of the catchment area were white Flemish. The 2005‐2010 cycle of fieldwork included an echocardiographic examination. Of 3037 former participants, 1208 were reinvited by letter, of whom 26 had died, 27 were severely ill, and 100 had moved out of the area. Of the remaining 1055 subjects, 828 renewed consent (participation rate 78.5%).
Study participants eligible for the current analysis had to have their blood lead and 24‐hour urinary cadmium measured during at least 1 study period and to have undergone an echocardiographic assessment of systolic and diastolic left ventricular function. Of 210 participants complying with these eligibility criteria, we excluded 31 from analysis because of insufficient quality of the echocardiographic images (n=22) or because of previous myocardial infarction (n=3) or severe valvular disease (n=6). Of the remaining 179 participants, 70 had a repeat exposure measurement prior to the echocardiographic examination. Compared with the 649 participants who underwent echocardiography but were not included in our analyses, those analyzed had similar distributions of sex (51.0% vs 50.3% women; P=0.864), age (51.2 versus 51.1 years; P=0.910), and body mass index (26.6 vs 26.5 kg/m2; P=0.979). However, analyzed participants had a lower systolic blood pressure (130.2 vs 126.8 mm Hg; P=0.022) with a higher prevalence of smokers (18.6% vs 26.8%; P=0.016) and drinkers consuming alcohol in excess of 5 g per day (38.7% vs 44.7%; P=0.008). The other cardiovascular risk factors, such as diastolic blood pressure, total and high‐density lipoprotein (HDL) cholesterol, serum creatinine, and plasma glucose were equally distributed between the 2 groups (P≥0.054).
The study complied with the Helsinki Declaration for Investigation of Human Subjects.25 The Ethics Committee of the University of Leuven, Belgium, approved the study and follow‐up of the participants. Participants gave or renewed written informed consent at each contact.
Measurements at Baseline
Study nurses administered a questionnaire inquiring into each participant's occupational exposure to heavy metals. Participants collected 24‐hour urine samples in wide‐neck polyethylene containers for measurement of volume, cadmium, and creatinine. Within 2 weeks of the urine collection, a venous blood sample was obtained. Lead and cadmium were measured by electrothermal atomic absorption spectrometry with a stabilized‐temperature platform furnace and Zeeman background correction.21 The external quality‐control program did not show any time trend in the accuracy of the lead3 or cadmium4 measurements. The detection limits for blood lead and the urinary cadmium concentration were 0.08 μmol/L6 and 0.89 nmol/L26 for the 1985‐1995 interval and thereafter 0.024 μmol/L and 0.18 nmol/L, respectively. We set 1 level below the 1985‐1995 blood lead threshold as the detection limit. We used the baseline measurements of the heavy metals as primary exposure variable in all participants or the average of the baseline (1985‐2000) and the follow‐up measurement (1991‐2004) in 70 participants with repeat assessment of the exposure variables.
Echocardiography at Follow‐Up
A detailed description of the protocol for echocardiography is available in previous publications.27, 28, 29 All echocardiographic images were obtained with a Vivid7 Pro device (GE Vingmed, Horten, Norway) interfaced with a 2.5‐ to 3.5‐MHz phased‐array probe according to current guidelines.20 For postprocessing, at least 5 cardiac cycles with a simultaneously acquired ECG signal were digitally stored.
Left Ventricular Structure
The internal left ventricular diameter and interventricular septal and posterior wall thicknesses were measured at end‐diastole from the 2‐dimensionally guided M‐mode tracings or from correctly oriented 2‐dimensional images when optimal orientation of M‐mode ultrasound beam could not be achieved. End‐diastolic left ventricular dimensions were used to calculate left ventricular mass by an anatomically validated formula.20 Left ventricular hypertrophy was a left ventricular mass indexed to body surface area with the threshold set at 95 g/m2 in women and 115 g/m2 in men.30 Relative wall thickness was the average of the interventricular and posterior wall thickness divided by the end‐diastolic diameter.
Systolic Left Ventricular Function
Left ventricular ejection fraction was computed from the end‐systolic and end‐diastolic volumes measured from the apical 4‐chamber view, using the Simpson method.20 To measure 2‐dimensional global longitudinal strain, we selected at least 2 cardiac cycles with optimal image quality, and we used the Q‐Analysis of EchoPac BT113 software (GE Vingmed, Horten, Norway).29 We manually traced the endocardial border with the point‐and‐click option at the end‐systolic frame of the 2‐dimensional images from the apical 4‐chamber view. The software with default settings, including data smoothing, automatically tracked the myocardial speckle motion, creating basal, middle, and apical regions of interest. Whenever required for optimal visualization, we made manual adjustments afterward of the automated segmental tracking results. We excluded images from analysis if wall tracking was insufficient in 2 or more segments. The software then generates the peak systolic endocardial, midwall, and epicardial global strain values. We carried global longitudinal strain at the midwall further into our statistical analysis.29
To assess regional strain, we also recorded high‐intensity myocardial velocity signals using tissue Doppler imaging (TDI) at a rate of at least 190 frames per second while adjusting the imaging angle to ensure parallel alignment of the ultrasound beam with the myocardial segment of interest. The Nyquist limit was set as low as possible to avoid aliasing. We positioned the TDI sample volume in the interrogated wall at the level of the posterior tendinous chords and extracted the strain and strain rate curves using the in‐house‐developed SPEQLE software, version 4.6.2.27 The software allows M‐mode tracking of the myocardium to ensure that the sample volume is maintained at the same position throughout the cardiac cycle. We derived peak radial strain rate from the spatial velocity gradient over a computational segment of 5 mm in the inferolateral wall and peak longitudinal strain rate over 10‐mm‐long segments in the inferolateral and inferior walls. With the ECG and blood flow velocity through the aortic valve providing time reference signals, the end‐systolic strain was the integral of mean strain rate over time. Henceforth we refer to peak systolic strain rate and end‐systolic strain as strain rate and strain, which were averages of 3 cardiac cycles. For longitudinal strain and strain rate, we averaged measurements obtained at the inferolateral and inferior walls.27
Diastolic Left Ventricular Function
We measured peak early (E) and peak late (A) diastolic velocity of the transmitral blood flow and peak early (e′) diastolic mitral annular velocities from tissue Doppler recordings at the septal, lateral, inferior, and posterior acquisition sites, which were averaged for analysis.28 We computed the E/e′ ratio as an index of left ventricular filling pressure.20 For analysis, we averaged the indexes of diastolic function over 3 cardiac cycles.
Other Follow‐Up Measurements
After participants had rested for 5 minutes in the sitting position, nurses obtained 5 consecutive auscultatory blood pressure readings (phase V diastolic pressure) using mercury sphygmomanometers fitted with an appropriately sized cuff. The 5 readings were averaged for analysis. Hypertension was a blood pressure of at least 140 mm Hg systolic or 90 mm Hg diastolic or use of antihypertensive drugs. Mean arterial pressure was diastolic blood pressure plus one‐third of the difference between systolic and diastolic blood pressure. Body mass index was weight in kilograms divided by the square of height in meters. The waist‐to‐hip ratio was waist circumference divided by hip circumference. The study nurses administered a questionnaire providing information on each participant's recent medical history, smoking and drinking habits, and intake of medications. Venous blood samples, obtained after at least 6 hours fasting, were analyzed for plasma glucose and serum creatinine, total and HDL cholesterol, and γ‐glutamyltransferase as an index of alcohol intake. Diabetes mellitus was a self‐reported diagnosis, a fasting plasma glucose level of at least 126 mg/dL, or use of antidiabetic agents.31
Statistical Analysis
For database management and statistical analysis, we used SAS software version 9.4 (SAS Institute Inc, Cary, NC). Departure from normality was evaluated by Shapiro‐Wilk statistic. Skewness and kurtosis were computed as the third and fourth moments about the mean divided by the cube of the standard deviation. The variables that required a logarithmic transformation to approximate the normal distribution included blood lead, 24‐hour urinary cadmium, and serum γ‐glutamyltransferase. For logarithmically transformed variables, we reported central tendency and spread of data as geometric mean and interquartile range, respectively. Significance was a 2‐tailed α‐level of 0.05 or less. Means and proportions were compared using a large sample z‐test and Fisher exact test, respectively.
We assessed the association of left ventricular structure and function with the biomarkers of exposure using multivariable linear regression. We adjusted the analyses for covariables of possible physiological relevance27, 29, 32 measured at the time of echocardiography, including sex, age, mean arterial pressure, heart rate, body mass index, fasting plasma glucose, total‐to‐HDL cholesterol ratio, serum creatinine, γ‐glutamyltransferase (as index of alcohol intake), and smoking. In all models we also accounted for treatment with diuretics (thiazides, loop diuretics, and aldosterone antagonists), inhibitors of the renin‐angiotensin system (β‐blockers, angiotensin‐converting enzyme inhibitors, and angiotensin type‐1 receptor antagonists), and vasodilators (calcium‐channel blockers and α‐blockers). In participants with baseline and follow‐up measurements of the heavy metals, we also computed the size of the associations between left ventricular function and exposure corrected for regression dilution bias, using the quantile approach.33, 34 Correcting for regression dilution bias does not affect the significance of the associations but provides more accurate estimates of their true value.33, 34
Results
Biomarkers of Exposure
At enrollment (1985‐2000), geometric means of the exposure biomarkers were 0.20 μmol/L (4.19 μg/dL) for blood lead (interquartile range 0.12‐0.38 μmol/L; 5th‐95th percentile interval 0.07‐0.58 μmol/L) and 6.1 nmol (0.69 μg) for 24‐hour urinary cadmium (interquartile range 3.66‐9.34 nmol; 5th‐95th percentile interval 1.81‐23.3 nmol). The logarithmically transformed distributions of the exposure biomarkers did not deviate from normality (Figure 1). None of the participants reported potential exposure to heavy metals at work or during leisure time.
Figure 1.
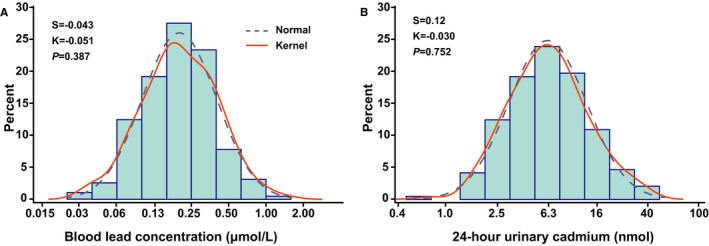
Logarithmically transformed distributions of blood lead (A) and 24‐hour urinary cadmium excretion (B) measured at baseline (1985‐2000). S and K are the coefficients of skewness and kurtosis. The P‐values are for departure of the actually observed distribution (full line) from normality (dashed line) according to Shapiro‐Wilk statistics.
After a median interval of 5.3 years (interquartile range 4.9‐5.7 years; 5th‐95th percentile interval 4.6‐6.1 years), 70 participants (39.1%) had their exposure biomarkers remeasured (1991‐2004). From the first to the second assessment, blood lead dropped by 35.7% (95% confidence interval 30.3% to 41.0%; P<0.001) with a similar tendency for 24‐hour urinary cadmium (5.5%; 95% confidence interval −3.5% to 14.6%; P=0.236).
Characteristics of Participants
All 179 participants were white Europeans, of whom 90 (50.3%) were women. Age averaged 39.1 years (interquartile range 29.8‐49.6 years; 5th‐95th percentile interval 17.0‐62.5 years) at enrollment and at the time of the echocardiographic examination 52.1 years (interquartile range 41.4‐60.6 years; 5th‐95th percentile interval 23.0‐74.1 years). The median interval between enrollment and echocardiography (2005‐2010) was 11.9 years (interquartile range 6.3‐19.9 years; 5th‐95th percentile interval 5.2‐22.3 years).
Table 1 lists the characteristic of the participants at the time of echocardiography by the medians of the distributions of the exposure biomarkers. In regard to blood lead and 24‐hour urinary cadmium, people with high compared with low exposure were 14.9 and 18.4 years older. More advanced age was associated with a higher cardiovascular risk profile as exemplified by higher mean values of body mass index, waist‐to‐hip ratio, systolic and diastolic blood pressures, and prevalence of hypertension and treated hypertension. In the whole study population the number of patients on diuretics, inhibitors of the renin‐angiotensin system, and vasodilators amounted to 9 (5.0%), 31 (17.3%), and 8 (4.5%), respectively. Among these patients, 30 (16.8%) were on monotherapy with a single drug class, whereas 9 (5.0%) were on combination therapy.
Table 1.
Characteristics of Participants (2005‐2010) by Median Blood Lead and 24‐Hour Urinary Cadmium (1985‐2000)
| Characteristic | Blood Lead (μmol/L) | 24‐Hour Urinary Cadmium (nmol) | ||
|---|---|---|---|---|
| Limits | <0.180 | ≥0.180 | <6.08 | ≥6.08 |
| Number of participants (%) | ||||
| All participants in category | 90 | 89 | 90 | 89 |
| Women | 47 (52.2) | 43 (48.3) | 47 (52.2) | 43 (48.3) |
| Smokers | 22 (23.4) | 27 (30.3) | 28 (31.1) | 20 (22.5) |
| Drinking ≥5 g alcohol per day | 46 (51.1) | 34 (38.2) | 43 (47.8) | 37 (41.6) |
| Hypertension | 16 (17.8) | 40 (44.9)§ | 18 (20.0) | 38 (42.7)† |
| Antihypertensive treatment | 11 (12.2) | 28 (31.5)† | 13 (14.4) | 26 (29.2)* |
| Diabetes mellitus | 2 (2.2) | 2 (2.2) | 2 (2.2) | 2 (2.2) |
| Mean (SD) of characteristic | ||||
| Age, y | 43.7 (14.3) | 58.6 (9.7)§ | 41.9 (12.2) | 60.3 (9.6)§ |
| Body mass index, kg/m2 | 25.7 (3.6) | 27.4 (4.4)† | 25.1 (4.0) | 28.0 (3.7)§ |
| Waist‐to‐hip ratio | 0.86 (0.08) | 0.89 (0.09)† | 0.86 (0.08) | 0.89 (0.09)† |
| Office blood pressure | ||||
| Systolic pressure, mm Hg | 123.2 (13.0) | 130.5 (16.1)† | 122.5 (13.3) | 131.2 (15.5)§ |
| Diastolic pressure, mm Hg | 77.4 (8.3) | 80.4 (9.3)* | 77.4 (9.0) | 80.4 (8.6)* |
| Mean arterial pressure, mm Hg | 92.6 (8.9) | 97.1 (10.2)† | 92.4 (9.6) | 97.3 (9.5)‡ |
| Heart rate, beats per minute | 60.1 (9.2) | 62.0 (8.6) | 61.0 (9.7) | 61.1 (8.2) |
| Biochemical data | ||||
| Total cholesterol, mmol/L | 5.17 (1.11) | 5.47 (0.92)* | 5.17 (1.05) | 5.46 (0.99) |
| HDL cholesterol, mmol/L | 1.44 (0.35) | 1.48 (0.40) | 1.49 (0.35) | 1.43 (0.40) |
| Total/HDL cholesterol ratio | 3.73 (0.96) | 3.91 (1.05) | 3.62 (0.94) | 4.02 (1.04)† |
| Plasma glucose, mmol/L | 4.82 (0.68) | 4.87 (0.50) | 4.73 (0.64) | 4.96 (0.53)* |
| Serum creatinine, μmol/L | 82.0 (14.0) | 85.2 (14.7) | 81.9 (13.9) | 85.3 (14.7) |
| γ‐glutamyltransferase, units/L | 20 (14‐28) | 24 (18‐32) | 20 (14‐28) | 24 (18‐35)* |
Hypertension was a blood pressure of ≥140 mm Hg systolic or ≥90 mm Hg diastolic or use of antihypertensive drugs. Diabetes mellitus was a fasting plasma glucose level of ≥7.0 mmol/L or use of antidiabetic agents. For γ‐glutamyltransferase, values are geometric mean (interquartile range). HDL indicates high‐density lipoprotein cholesterol.
Significance of the between‐group differences: *P≤0.05; † P≤0.01; ‡ P≤0.001; § P<0.0001.
Left Ventricular Structure
In line with the age distribution and the higher cardiovascular risk profile, people with higher exposure at enrollment (1985‐2000) had an increased left ventricular mass index and higher relative wall thickness (Table 2). However, in multivariable‐adjusted analyses (Table 3), the associations of left ventricular mass index, end‐diastolic diameter, and relative wall thickness with the biomarkers of exposure did not retain significance (P≥0.072). The only exception was a marginal increase in the left ventricular end‐diastolic internal diameter by 0.086 cm (P=0.035) for a doubling of the 24‐hour urinary cadmium excretion.
Table 2.
Echocardiographic Measurements (2005‐2010) by Medians of the Exposure Biomarkers (1985‐2000)
| Characteristic | Blood Lead (μmol/L) | 24‐Hour Urinary Cadmium (nmol) | ||
|---|---|---|---|---|
| Limits | <0.180 | ≥0.180 | <6.08 | ≥6.08 |
| Participants in category, number | 90 | 89 | 90 | 89 |
| Left ventricular structure | ||||
| Mass index, g/m2 | 84.0 (16.9) | 93.0 (19.50)† | 81.5 (12.9) | 95.5 (21.0)§ |
| Hypertrophy, n (%) | 5 (5.6) | 18 (20.2)† | 2 (2.2) | 21 (23.6)§ |
| End‐diastolic diameter, cm | 5.05 (0.46) | 4.99 (0.43) | 5.01 (0.42) | 5.04 (0.47) |
| Relative wall thickness | 0.35 (0.06) | 0.38 (0.05)§ | 0.34 (0.04) | 0.39 (0.06)§ |
| Systolic function | ||||
| Ejection fraction, % | 67.7 (6.9) | 69.1 (7.7) | 67.3 (6.9) | 69.5 (7.8) |
| Global longitudinal strain, % | 20.0 (2.4) | 18.9 (2.6)† | 19.5 (2.4) | 19.5 (2.8) |
| Regional longitudinal strain, % | 22.9 (3.2) | 21.0 (3.9)‡ | 22.5 (3.0) | 21.4 (4.2)* |
| Regional longitudinal strain rate, s−1 | 1.34 (0.28) | 1.24 (0.24)† | 1.32 (0.23) | 1.25 (0.29) |
| Regional radial strain, % | 60.3 (12.2) | 55.0 (12.7)† | 61.2 (12.3) | 54.0 (12.0)‡ |
| Regional radial strain rate, s−1 | 3.37 (0.68) | 3.09 (0.73)† | 3.44 (0.75) | 3.02 (0.62)‡ |
| Diastolic function | ||||
| E peak, cm/s | 78.6 (15.9) | 72.4 (14.8)† | 80.2 (15.9) | 70.8 (14.0)§ |
| E/A ratio | 1.50 (0.51) | 1.04 (0.32)§ | 1.51 (0.48) | 1.03 (0.33)§ |
| e′, cm/s | 13.1 (3.5) | 9.9 (2.7)§ | 13.5 (3.2) | 9.5 (2.5)§ |
| E/e′ ratio | 6.29 (1.72) | 7.71 (2.26)§ | 6.18 (1.57) | 7.83 (2.29)§ |
Values are mean (SD) except for left ventricular hypertrophy, for which number of participants (%) are given. Due to image quality, global longitudinal strain and radial strain were available in 173 and 166 participants; the number of participants with exposure measurements </≥ median was 90/83 and 86/80 for blood lead, respectively, and 87/86 and 86/80 for 24‐hour urinary cadmium, respectively.
Significance of the between‐group differences: *P≤0.05; † P≤0.01; ‡ P≤0.001, § P<0.0001.
Table 3.
Multivariable‐Adjusted Associations of Left Ventricular Structure (2005‐2010) With the Exposure Biomarkers (1985‐2000) in 179 Participants
| Characteristic | Biomarker | Estimate (95% Confidence Interval) | P Value |
|---|---|---|---|
| Left ventricular mass index, g/m2 | Blood lead | −1.399 (−4.504 to 1.707) | 0.375 |
| Urinary cadmium | 2.934 (−0.623 to 6.491) | 0.105 | |
| End‐diastolic diameter, cm | Blood lead | −0.064 (−0.134 to 0.006) | 0.072 |
| Urinary cadmium | 0.086 (0.006 to 0.167) | 0.035 | |
| Relative wall thickness | Blood lead | 0.0065 (−0.0031 to 0.0162) | 0.185 |
| Urinary cadmium | 0.0032 (−0.008 to 0.0143) | 0.578 |
HDL indicates high‐density lipoprotein. Associations were adjusted for sex, age, mean arterial pressure, heart rate, body mass index, fasting plasma glucose, total‐to‐HDL cholesterol ratio, serum creatinine, γ‐glutamyltransferase, smoking, and antihypertensive treatment (by drug class) and express the effect size for a doubling of the exposure biomarker.
Systolic Left Ventricular Function
In unadjusted analyses dichotomized by the median of blood lead (Table 2), left ventricular global longitudinal strain (P=0.004), regional longitudinal strain (P<0.001) and strain rate (P=0.002), and radial strain (P=0.006) and strain rate (P=0.009) all decreased with higher lead exposure. In similar analyses (Table 2), only regional longitudinal strain (P=0.048) and regional radial strain (P<0.001) and strain rate (P<0.001) decreased with higher 24‐hour urinary cadmium. There were no differences (P≥0.094) in left ventricular ejection fraction associated with the biomarkers of exposure (Table 2). Multivariable‐adjusted analyses with effect sizes expressed for a doubling of the exposure biomarkers confirmed these findings (Table 4). As in the unadjusted analyses (Table 2), the multivariable‐adjusted relations of left ventricular ejection fraction with the exposure biomarkers were far from significant (P≥0.689). Global longitudinal strain (−0.392%, P=0.034), regional longitudinal strain (−0.618%, P=0.028), and strain rate (−0.056 s−1, P=0.008), and regional radial strain rate (−0.113 s−1, P=0.050) all decreased with blood lead concentration at enrollment (1985‐2000) with the exception of regional radial strain (P=0.062). Regional longitudinal strain rate (−0.066 s−1, P=0.009) and radial strain (−2.848%, P=0.015) decreased with 24‐hour urinary cadmium, whereas the associations of global longitudinal strain, regional longitudinal strain, and radial strain rate with 24‐hour urinary cadmium were not significant (P≥0.071). Table 4 additionally provides estimates of the association sizes corrected for regression dilution bias. In models adjusted as before that included both indexes of exposure, the associations of regional longitudinal and radial strain and strain rate weakened to a nonsignificant level (Figure 2). The correlation coefficient between blood lead and 24‐hour urinary cadmium was 0.49, but the variance inflation factors for collinearity between the 2 exposure measures were 2.2 or less. Finally, sensitivity analyses, in which we averaged the biomarkers of exposure in 70 participants with duplicate measurements, from which we excluded participants on antihypertensive drug treatment, or with additional adjustment for the time interval between echocardiography and the assessment of the exposure, were confirmatory.
Table 4.
Multivariable‐Adjusted Associations of Systolic Left Ventricular Function (2005‐2010) With the Exposure Biomarkers (1985‐2000)
| Characteristic (Number of Participants) | Biomarker | Estimate (95% Confidence Interval) | P Value | |
|---|---|---|---|---|
| Uncorrected | Corrected | |||
| Ejection fraction, % (n=179) | Blood lead | 0.150 (−1.019 to 1.320) | 0.190 (−1.293 to 1.675) | 0.800 |
| Urinary cadmium | −0.283 (−1.628 to 1.116) | −0.327 (−1.942 to 1.288) | 0.689 | |
| Global longitudinal strain, % (n=173) | Blood lead | −0.392 (−0.753 to −0.030) | −0.497 (−0.957 to −0.038) | 0.034 |
| Urinary cadmium | −0.136 (−0.579 to 0.308) | −0.157 (−0.669 to 0.355) | 0.547 | |
| Regional longitudinal strain, % (n=179) | Blood lead | −0.618 (−1.167 to −0.068) | −0.784 (−1.482 to −0.087) | 0.028 |
| Urinary cadmium | −0.609 (−1.271 to 0.053) | −0.702 (−1.467 to 0.061) | 0.071 | |
| Regional longitudinal strain rate, s−1 (n=179) | Blood lead | −0.056 (−0.097 to −0.015) | −0.071 (−0.124 to −0.019) | 0.008 |
| Urinary cadmium | −0.066 (−0.115 to −0.017) | −0.076 (−0.133 to −0.019) | 0.009 | |
| Regional radial strain, % (n=166) | Blood lead | −1.825 (−3.740 to 0.090) | −2.316 (−4.748 to −0.115) | 0.062 |
| Urinary cadmium | −2.848 (−5.134 to −0.561) | −3.287 (−5.927 to −0.648) | 0.015 | |
| Regional radial strain rate, s−1 (n=166) | Blood lead | −0.113 (−0.226 to −0.0002) | −0.143 (−0.287 to −0.0002) | 0.050 |
| Urinary cadmium | −0.117 (−0.254 to 0.019) | −0.135 (−0.292 to 0.022) | 0.092 | |
HDL indicates high‐density lipoprotein. Associations were adjusted for sex, age, mean arterial pressure, heart rate, body mass index, fasting plasma glucose, total‐to‐HDL cholesterol ratio, serum creatinine, γ‐glutamyltransferase, smoking, and antihypertensive treatment (by drug class) and express the effect size for a doubling of the exposure biomarker. Uncorrected and corrected indicate whether association sizes were corrected for regression dilution bias using the quantile method (see Statistical Methods).
Figure 2.
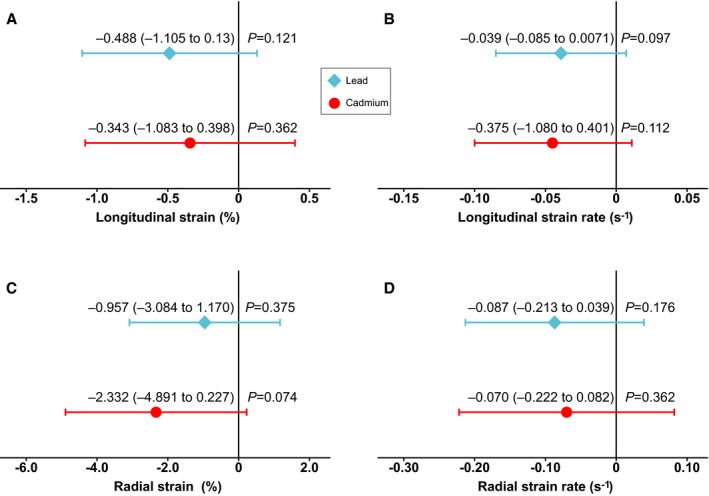
Associations of regional longitudinal (A and B) and radial (C and D) left ventricular strain and strain rate with blood lead and 24‐hour urinary cadmium. Both biomarkers were simultaneously entered into the models. Associations were adjusted for sex, age, mean arterial pressure, heart rate, body mass index, fasting plasma glucose, total‐HDL cholesterol ratio, serum creatinine, γ‐glutamyltransferase, smoking, and antihypertensive treatment (by drug class) and express the effect size for a doubling of the exposure biomarker. Horizontal bars indicate the 95% confidence interval. Measurements of longitudinal and radial strain were available in 179 and 166 participants, respectively. HDL indicates high‐density lipoprotein.
Diastolic Left Ventricular Function
In unadjusted analyses (Table 2), the echocardiographic indexes capturing left ventricular diastolic function decreased with higher exposure. However, in view of their strong age dependency, these associations disappeared in multivariable analyses adjusted as before (P≥0.159; Table 5).
Table 5.
Multivariable‐Adjusted Association of Diastolic Left Ventricular Function (2005‐2010) With the Exposure Biomarkers (1985‐2004) in 179 Participants
| Characteristic | Biomarker | Estimate (95% Confidence Interval) | P Value |
|---|---|---|---|
| E peak, cm/s | Blood lead | 1.308 (−1.120 to 3.736) | 0.289 |
| Urinary cadmium | −1.309 (−4.107 to 1.490) | 0.357 | |
| E/A ratio | Blood lead | −0.036 (−0.085 to 0.014) | 0.159 |
| Urinary cadmium | 0.010 (−0.048 to 0.068) | 0.734 | |
| e′ peak, cm/s | Blood lead | −0.188 (−0.494 to 0.118) | 0.228 |
| Urinary cadmium | −0.017 (−0.371 to 0.337) | 0.925 | |
| E/e′ ratio | Blood lead | 0.172 (−0.133 to 0.477) | 0.267 |
| Urinary cadmium | −0.065 (−0.417 to 0.287) | 0.716 |
E/A is the ratio of the peak early to the peak late diastolic velocity of the transmitral blood flow. Associations were adjusted for sex, age, mean arterial pressure, heart rate, body mass index, fasting plasma glucose, total‐to‐HDL cholesterol ratio, serum creatinine, γ‐glutamyltransferase, smoking, and antihypertensive treatment (by drug class) and express the effect size for a doubling of the exposure biomarker. HDL indicates high‐density lipoprotein.
Discussion
The key findings of our current study can be summarized as follows: (1) higher blood lead concentrations at baseline predicted impaired systolic left ventricular function a decade later; (2) regional longitudinal strain rate and regional radial strain at follow‐up were lower if the baseline 24‐hour urinary cadmium excretion was higher; and (3) diastolic left ventricular function was not associated with exposure to lead or cadmium. Mean values for regional longitudinal strain and strain rate were 22.0% and 1.28 s−1, and 57.7% and 3.24 s−1 for regional radial strain and strain rate (Table 2). A doubling of the exposure biomarkers, as shown in Table 3, was associated with decreases in the longitudinal and radial strain and strain rate by ~3% to 4%. However, the distributions of blood lead and 24‐hour urinary cadmium from the low to high end showed a 100‐fold increase in exposure. From this viewpoint, the observed changes in left ventricular systolic function were clinically meaningful. Furthermore, a recent systematic review35 highlighted that left ventricular ejection fraction does not accurately capture systolic function in people with a value within the normal range (>45%) and that therefore its use biases results to a null outcome. This may explain the absence of any association between left ventricular ejection fraction and the biomarkers of exposure in our current study.
Experimental studies8, 9, 10 provided the scientific background for searching for association between left ventricular function and exposure to lead or cadmium in humans and suggested possible mechanisms for direct cardiotoxic effects. Kopp and co‐workers studied female hooded rats fed 5 ppm cadmium, 5 ppm lead, or both via drinking water for 15 months.8, 9 Growth and food and water intake were comparable with those of controls. Perfused hearts of exposed animals showed depressed myocardial contractility and reduced positive inotropic responsiveness to isoproterenol.8 The depressed myocardial contractility was not due to altered β‐receptor function but to depressed phosphorylation of the myocardial contractile proteins.8 In anesthetized rats exposed to cadmium and lead for 20 months according to a similar protocol, cadmium selectively slowed conduction proximal to the His bundle, whereas lead alone or combined with cadmium impaired conductivity distal to the His bundle.9 Phosphorus‐31 nuclear magnetic resonance spectroscopy revealed depressed high‐energy phosphate and glycerol‐3‐phosphorylcholine concentrations in hearts of cadmium‐exposed rats.9 In perfused rat hearts,10 contractility and the glycerol‐3‐phosphorylcholine content of the myocardium changed with the amount of calcium in the perfusate (0.9‐5 mmol/L). The combination of 5.0 mmol/L calcium with 0.3 or 30 μmol/L lead resulted in significant disturbances in phosphoglyceride, glycolytic, and high‐energy phosphate pathways.10 These animal experiments8, 9, 10 produced evidence for direct pathophysiologic changes in the absence of overt heavy‐metal toxicity, the main mechanisms being slowing conductivity in the heart and dysregulation of energy metabolism. In addition, the cardiotoxic effects of lead might be partially related to interference with calcium‐dependent processes.10 At the other end of the spectrum, autopsy studies of children who died from lead poisoning revealed myocarditis characterized with interstitial fibrosis.36
To our knowledge, few studies in workers addressed the possible association of left ventricular structure or function with exposure to heavy metals. None of these worker studies addressed cadmium exposure. In 105 laborers, left ventricular mass index increased across thirds of the distributions of the cumulative blood lead level and the time‐weighted average blood lead, but these associations lost significance in adjusted analyses of exposure analyzed as a continuous variable.13 In a Polish study of 88 exposed workers and 55 controls,11 blood lead levels averaged 30.4 μg/dL (1.47 μmol/L) and 12.2 μg/dL (0.59 μmol/L), respectively. In the group exposed to lead the end‐diastolic left ventricular internal diameter was 6% higher with a 3% decreased ejection fraction and an 11% raised left ventricular mass index.11 In another case‐control study the transmitral E peak velocity and E/A ratio of 69 exposed workers were significantly lower than those in 38 controls,12 suggesting impaired left ventricular function.
Turning to population studies, the available data relating heart failure to exposure to heavy metals are confined to cadmium. In a 15‐year follow‐up study on the population of the heavily cadmium‐polluted Kakehashi River basin in Ishikawa Prefecture, Japan, the standardized mortality rate of heart failure was higher in inhabitants with renal tubular dysfunction, as evidenced by a urinary concentration of retinol‐binding protein or β2‐microglobulin exceeding 4 μg/L or 1 mg per gram creatinine, respectively.37, 38 An analysis of the 1999‐2006 NHANES data included 12 049 participants, aged 30 years and older, of whom 471 reported a history of heart failure.16 In multivariable‐adjusted analyses a 50% higher blood or urinary cadmium concentration was associated with a 48% (95% CI 17% to 87%) and 12% (95% CI 3% to 20%) higher odds of self‐reported prevalent heart failure.16 Among 3348 Native Americans aged 45 to 74 years enrolled in the Strong Heart Study, the urinary cadmium level at baseline (1989‐1991) averaged 0.94 μg (8.0 nmol) per gram creatinine.17 Over follow‐up until December 2008, 328 participants developed heart failure. The hazard ratio contrasting the 80th with the 20th percentile of urinary cadmium was 1.39 (95% CI 1.01‐1.94).17 The Malmö Diet and Cancer Study15 enrolled 4378 Swedes. Over 17 years of follow‐up, 143 participants (47% women) were hospitalized for new‐onset heart failure. The multivariable‐adjusted hazard ratio contrasting the top vs the lowest fourth of the blood cadmium distribution was 1.95 (95% CI 1.02‐3.71). None of the aforementioned studies in workers or populations differentiated between systolic and diastolic dysfunction. All population studies15, 17, 19, 37, 38 focused on heart failure, the end stage of a long‐lasting process with varying etiology.
Compared with the literature, our current study stands out because of its prospective design, because it focused on the earliest stages of left ventricular dysfunction rather than on overt heart failure, an end‐stage syndrome covering a wide range of etiologies, because it applied state‐of‐the‐art echocardiographic techniques, and because it differentiated between systolic and diastolic left ventricular function. Moreover, animal studies showing evidence for direct cardiotoxic effects in the absence of overt heavy‐metal toxicity8, 9 and the high prevalence of asymptomatic left ventricular dysfunction in the general population28, 29, 39 justified our approach. Decreased global left ventricular strain is a strong predictor of cardiovascular mortality.29 Notwithstanding these strong points, our study should be interpreted within the context of its possible limitations. First, our observational study does not allow inferring causality for lead or cadmium as instigators of left ventricular dysfunction. However, several of the Bradford‐Hill criteria are fulfilled, including temporality, a dose‐effect association, and plausibility.40 Second, many of our baseline data (39.1%) were collected from 1985 until 1989. In this period, blood lead averaged 0.42 μmol/L (8.72 μg/dL), and 24‐hour urinary cadmium 10.6 nmol (1.19 μg). In participants with repeat assessment of their exposure, blood lead dropped by 35.7% over ~5 years. Along similar lines, in the United States NHANES documented a progressive decline in the geometric blood lead concentration over time. Among adults, mean blood lead levels decreased from 0.63 μmol/L (13.1 μg/dL) in NHANES II (1976‐1980)41, 42 to 0.13 μmol/L (2.76 μg/dL) in NHANES III (1988‐1994)43 and next to 0.07 μmol/L (1.51 μg/dL) in NHANES IV (2003‐2010).44 Thus, in the developed world current exposure levels are substantially lower than noticed in our study. Whether, at these lower exposure levels, the risk of left ventricular dysfunction remains elevated is unknown. Third, exposure to lead and cadmium often coexist. Our current analyses did not allow us to differentiate among lead, cadmium, and both combined as being predominantly associated with regional systolic left ventricular function. However, global longitudinal strain was associated only with the blood lead concentration and not with 24‐hour urinary cadmium. The most consistent association was therefore with blood lead. Fourth, our multivariable‐adjusted models accounted for a large number of variables with known physiological relevance. However, as in all observational studies, we cannot exclude residual confounding by factors, either unmeasured or not entered into the models. Finally, our analyses included only the 21.6% of people who underwent an echocardiographic examination.
Conclusions
Although the effect sizes in our study were small, environmental exposure to lead, cadmium, or both might be a risk factor for systolic left ventricular dysfunction and in the end for heart failure. Our current study is a first attempt to address the association of systolic and diastolic left ventricular function with low‐level exposure to heavy metals, as it is still being observed in Western industrialized countries and most developing nations. Experimental research should clarify the cardiotoxic mechanisms of lead and cadmium. Furthermore, our current findings need replication in prospective population studies, in particular at exposure levels lower than those seen in the present study. In the meantime, reducing exposure and sanitization of contaminated soils, where needed, are of primary importance. However, given the long half‐lives of lead and cadmium in the human body, preventing exposure might not be sufficient once exposure has occurred. Health care providers should be made aware of the risks associated with environmental exposure to cardiotoxic agents. Although nowadays stringent regulations protect workers, the same does not always hold true for populations living in historically polluted regions, such as, for instance, part of the catchment area of our current study.
Sources of Funding
The European Union (grants HEALTH‐FP7‐278249‐EUMASCARA, HEALTH‐F7‐305507 HOMAGE, and the European Research Council Advanced Researcher Grant 2011‐294713‐EPLORE and Proof‐of‐Concept Grant 713601‐uPROPHET) gave support to the Studies Coordinating Centre, Leuven, Belgium. The Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13 and G.0880.13) also supported the FLEMENGHO study.
Disclosures
None.
Acknowledgments
The authors gratefully acknowledge the contribution of the nurses working at the examination center (Linda Custers, Marie‐Jeanne Jehoul, Daisy Thijs, and Hanne Truyens) and the clerical staff at the Studies Coordinating Centre (Vera De Leebeeck and Renilde Wolfs).
(J Am Heart Assoc. 2017;6:e004692. DOI: 10.1161/JAHA.116.004692.)
References
- 1. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Staessen JA, Roels H, Fagard R; for the PheeCad Investigators . Lead exposure and conventional and ambulatory blood pressure. A prospective population study. JAMA. 1996;275:1563–1570. [PubMed] [Google Scholar]
- 4. Staessen JA, Kuznetsova T, Roels HA, Emelianov D, Fagard R; for the Public Health and Environmental Exposure to Cadmium Study Group . Exposure to cadmium and conventional and ambulatory blood pressures in a prospective population study. Am J Hypertens. 2000;13:146–156. [DOI] [PubMed] [Google Scholar]
- 5. Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An epidemiological re‐appraisal of the association between blood pressure and lead: a meta‐analysis. J Hum Hypertens. 2002;16:123–131. [DOI] [PubMed] [Google Scholar]
- 6. Staessen JA, Lauwerys RR, Buchet JP, Bulpitt CJ, Rondia D, Vanrenterghem Y, Amery A; the Cadmibel Study Group . Impairment of renal function with increasing blood lead concentrations in the general population. N Engl J Med. 1992;327:151–156. [DOI] [PubMed] [Google Scholar]
- 7. Staessen JA, Lauwerys RR, Ide G, Roels HA, Vyncke G, Amery A. Renal function and historical environmental cadmium pollution from zinc smelters. Lancet. 1994;343:1523–1527. [DOI] [PubMed] [Google Scholar]
- 8. Kopp SJ, Bárány M, Erlanger M, Perry EF, Perry HM. The influence of chronic low‐level cadmium and/or lead feeding on myocardial contractility related to phosphorylation of cardiac myofibrillar proteins. Toxicol Appl Pharmacol. 1980;54:48–56. [DOI] [PubMed] [Google Scholar]
- 9. Kopp SJ, Perry HM Jr, Glonek T, Erlanger M, Perry EF, Barany M, D'Agrosa LS. Cardiac physiologic‐metabolic changes after chronic low‐level heavy metal feeding. Am J Physiol. 1980;239:H22–H30. [DOI] [PubMed] [Google Scholar]
- 10. Prentice RC, Kopp SJ. Cardiotoxicity of lead at various perfusate calcium concentrations: functional and metabolic responses of the perfused rat heart. Toxicol Appl Pharmacol. 1985;81:491–501. [DOI] [PubMed] [Google Scholar]
- 11. Kasperczyk S, Przywara‐Chowaniec B, Kasperczyk A, Rykaczewska‐Czerwinska M, Wodniechi J, Birkner E, Dziwisz M, Krauze‐Wielicka M. Function of heart muscle in people chronically exposed to lead. Ann Agric Environ Med. 2005;12:207–210. [PubMed] [Google Scholar]
- 12. Beck B, Steinmetz‐Beck A. Echocardiographic evaluation of left ventricular function in persons with chronic professional exposure to lead [In Polish]. Adv Clin Exp Med. 2005;14:905–915. [Google Scholar]
- 13. Tepper A, Mueller C, Singal M, Sagar K. Blood pressure, left ventricular mass, and lead exposure in battery manufacturing workers. Am J Ind Med. 2001;40:63–72. [DOI] [PubMed] [Google Scholar]
- 14. Schwartz J. Lead, blood pressure, and cardiovascular disease in men and women. Environ Health Perspect. 1991;91:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borné Y, Barregard L, Persson M, Hedblad B, Fagerberg B, Engström G. Cadmium exposure and incidence of heart failure and atrial fibrillation: a population‐based prospective cohort study. BMJ Open. 2015;5:e007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peters JL, Perlstein TS, Perry MJ, McNeely E, Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ Res. 2010;110:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tellez‐Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, Silbergeld EK, Devereux RB, Navas‐Acien A. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E. Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect. 2009;117:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agarwal S, Zaman T, Tuzcu EM, Kapadia SR. Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Angiology. 2011;62:422–429. [DOI] [PubMed] [Google Scholar]
- 20. Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. A report from the American Society of Echocardiography's Guidelines and Standard Committee and the Task Force on Echocardiography in Clinical Trials. J Am Soc Echocardiogr. 2004;17:1086–1119. [DOI] [PubMed] [Google Scholar]
- 21. Lauwerys R, Amery A, Bernard A, Bruaux P, Buchet JP, Claeys F, De Plaen P, Ducoffre G, Fagard R, Lijnen P, Nick L, Roels H, Rondia D, Saint‐Remy A, Sartor F, Staessen J. Health effects of environmental exposure to cadmium: objectives, design and organization of the Cadmibel Study: a cross‐sectional morbidity study carried out in Belgium from 1985 to 1989. Environ Health Perspect. 1990;87:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hogervorst J, Plusquin M, Vangronsveld J, Nawrot T, Cuypers A, Van Hecke E, Roels HA, Carleer R, Staessen JA. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ Res. 2007;103:30–37. [DOI] [PubMed] [Google Scholar]
- 23. Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R; for the Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group . Environmental exposure to cadmium, forearm bone‐density, and risk of fractures: prospective population study. Lancet. 1999;353:1140–1144. [DOI] [PubMed] [Google Scholar]
- 24. Staessen JA, Wang JG, Brand E, Barlassina C, Birkenhäger WH, Herrmann SM, Fagard R, Tizzoni L, Bianchi G. Effects of three candidate genes on prevalence and incidence of hypertension in a Caucasian population. J Hypertens. 2001;19:1349–1358. [DOI] [PubMed] [Google Scholar]
- 25. 41st World Medical Assembly . Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Bull Pan Am Health Org. 1990;24:606–609. [Google Scholar]
- 26. Claeys F, Ducoffre G, Sartor F, Roels H. Analytical quality control of cadmium and lead in blood and cadmium in urine: results of its implementation during a five‐year epidemiological study In: Nordberg GF, Herber RFM, Alessio L, eds. Cadmium in the Human Environment: Toxicity and Carcinogenicity. Lyon: International Agency for Research on Cancer; 1992:83–92. [PubMed] [Google Scholar]
- 27. Kuznetsova T, Herbots L, Richart T, D'Hooge J, Thijs L, Fagard RH, Herregods MC, Staessen JA. Left ventricular strain and strain rate in a general population. Eur Heart J. 2008;29:2014–2023. [DOI] [PubMed] [Google Scholar]
- 28. Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, González A, Herregods MC, Fagard RH, Díez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. [DOI] [PubMed] [Google Scholar]
- 29. Kuznetsova T, Cauwenberghs N, Knez J, Yang WY, Hertbots L, D'Hooge J, Haddad F, Thijs L, Voigt JU, Staessen JA. Additive prognostic value of left ventricular systolic dysfunction in a population‐based cohort. Circ Cardiovasc Imaging. 2016;9:e004661. [DOI] [PubMed] [Google Scholar]
- 30. Marvick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, Gottdiener J, Haluska B, Ofili E, Segers P, Senior R, Tapp RJ, Zamorano JL. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). Eur Heart J Cardiovasc Imaging. 2015;16:577–605. [DOI] [PubMed] [Google Scholar]
- 31. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(suppl 1):S5–S20. [DOI] [PubMed] [Google Scholar]
- 32. Zhang ZY, Marrachelli VG, Thijs L, Yang WY, Wei FF, Monleon D, Jacobs L, Nawrot T, Verhamme P, Voigt JU, Kuznetsova T, Redón J, Staessen JA. Diastolic left ventricular function in relation to circulating metabolic biomarkers in a general population. J Am Heart Assoc. 2016;5:e002681 DOI: 10.1161/JAHA.115.002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frost C, Thompson SG. Correcting for regression dilution bias: comparison of methods for a single predictor variable. J R Stat Soc Ser A Stat Soc. 2000;163:173–189. [Google Scholar]
- 34. Gasowski J, Li Y, Kuznetsova T, Richart T, Thijs L, Grodzicki T, Clarke R, Staessen JA. Is “usual” blood pressure a proxy for 24‐hour ambulatory blood pressure in predicting cardiovascular outcomes? Am J Hypertens. 2008;21:994–1000. [DOI] [PubMed] [Google Scholar]
- 35. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta‐analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. [DOI] [PubMed] [Google Scholar]
- 36. Kline TS. Myocardial changes in lead poisoning. Am J Dis Child. 1960;99:48–54. [DOI] [PubMed] [Google Scholar]
- 37. Nishijo M, Nakagawa H, Morikawa Y, Tabata M, Senma M, Miura K, Takahara H, Kawano S, Nishi M, Mizukoshi K, Kido T, Nogawa K. Mortality of inhabitants in an area polluted by cadmium: 15 year follow up. Occup Environ Med. 1995;52:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishijo M, Morikawa Y, Nakagawa H, Tawara K, Miura K, Kido T, Ikawa A, Kobayashi E, Nogawa K. Causes of death and renal tubular dysfunction in residents exposed to cadmium in the environment. Occup Environ Med. 2006;63:545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kloch‐Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, González A, Loster M, Thijs L, Jin Y, Malyutina S, Stolarz‐Skrzypek K, Casiglia E, Díez J, Narkiewicz K, Kawecka‐Jaszcz K, Staessen JA; on behalf of the European Project on Genes in Hypertension (EPOGH) Investigators . Prevalence of diastolic left ventricular dysfunction in European populations based on cross‐validated diagnostic thresholds. Cardiovasc Ultrasound. 2012;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA. 1994;272:284–291. [PubMed] [Google Scholar]
- 42. Gartside PS. The relationship of blood lead levels and blood pressure in NHANES II: additional calculations. Environ Health Perspect. 1988;78:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–2161. [DOI] [PubMed] [Google Scholar]
- 44. Hara A, Thijs L, Asayama K, Gu YM, Jacobs L, Zhang ZY, Liu YP, Nawrot TS, Staessen JA. Blood pressure in relation to environmental lead exposure in the National Health and Nutrition Examination Survey 2003 to 2010. Hypertension. 2014;65:62–69. [DOI] [PubMed] [Google Scholar]


