Abstract
Background
We investigated national trends in volume and outcomes of percutaneous coronary angioplasty (PCI), coronary artery bypass grafting (CABG), and ischemic heart disease–related mortality in Israel.
Methods and Results
Using International Classification of Diseases 9th and 10th revision codes, we linked 5 Israeli national databases, including the Israel Center for Disease Control National PCI and CABG Registries, the Ministry of Health Hospitalization Report, the Center of Bureau of Statistics, and the Ministry of Interior Mortality Report, to assess the annual PCI and CABG volume, procedural mortality, comorbidities, and ischemic heart disease‐related mortality between 2002 and 2014. Trends over time were analyzed using linear regression, assuming a Poisson distribution. A total of 298 390 revascularization procedures (PCI: 255 724, CABG: 42 666) were performed during the study period. PCI volume increased by 9% from 2002 to 2008 (387.4/100 000 to 423.2/100 000), steadily decreasing by 10.5% to 378.5/100 000 in 2014 (P=0.70 for the trend). CABG volume decreased by 59% (109.0/100 000 to 45.2/100 000) from 2002 to 2013, leveling at 46.4/100 000 (P<0.0001). PCI/CABG ratio increased from 3.6 in 2002 to 8.5 in 2013, slightly decreasing to 8.2 by 2014 (P<0.0001). In‐hospital procedural mortality remained stable (PCI: 1.2–1.6%, P=0.34, CABG: 3.7–4.4%, P=0.29) despite a significant change in patient clinical profile. During the course of the study, ischemic heart disease‐related mortality decreased by 46% (84.6–46/100 000, P<0.001).
Conclusions
We observed a dramatic change in coronary revascularization procedures type and volume, and a marked decrease in ischemic heart disease‐related mortality in Israel. The reasons for the observed changes remain unclear and need to be further investigated.
Keywords: coronary disease, population, revascularization, survival
Subject Categories: Cardiovascular Disease, Cardiovascular Surgery, Catheter-Based Coronary and Valvular Interventions, Mortality/Survival
Introduction
Ischemic heart disease (IHD) remains among the leading causes of mortality around the globe.1, 2 The treatment modalities available for patients with IHD include medical management (optimal medical therapy) and coronary revascularization performed either percutaneously (percutaneous coronary intervention [PCI]) or surgically (coronary artery bypass grafting [CABG]). The choice among the various options for the individual patient is influenced patient, physician, and institutional factors. Patient factors include the clinical scenario, ie, the acuity, type and severity of symptoms, comorbidities, and coronary anatomy. Physician‐related factors include specialty, experience, expertise, and natural bias. Institutional factors consist of availability of infrastructure such as cardiac catheterization laboratory and cardiac surgery programs, as well as institutional policies with regards to evidence‐based medicine and cost‐effective practice.3, 4
The guidelines for treatment of IHD developed by the various cardiovascular professional societies provide clinicians with a useful tool in selecting the optimal treatment for the individual patient.5 More recently, appropriate use criteria were defined and updated in an effort to reduce the impact of physician bias, to promote the “heart team” concept, and to decrease the impact of institutional financial pressure on clinical decisions.6
The impact of practice guidelines and appropriateness criteria on actual decision making is a subject of ongoing investigations.4 Several studies have previously shown marked changes in type, volume, and outcomes of coronary revascularization procedures during the past 2 decades using global, national, or regional data sets.2, 3, 4, 7, 8, 9, 10 The aim of the present study was to investigate national‐level trends in coronary revascularization and IHD‐related mortality in Israel.
Methods
Data Sources
Using the 9th and 10th revisions of the International Classification of Diseases (ICD‐9 and ICD‐10) codes, we linked information derived from 5 national data sets: the Israel Center for Disease Control (ICDC) National PCI and CABG Registries, the Ministry of Health Hospitalization Report, the Center of Bureau of Statistics, and the Ministry of Interior Mortality Report. The ICDC national aggregatory PCI and CABG registries are based on a mandatory institutional reporting of the number and type of procedures using ICD‐9 coding. From 2002 to 2007 data were retrospectively retrieved and submitted to the ICDC. From 2008 forward, the reporting is prospective on a monthly basis. The Ministry of Health Hospitalization Report is a repository containing deidentified information on all hospital admissions with regards to clinical diagnoses and procedures. The Center of Bureau of Statistics is a governmental body within the office of the Israeli Prime Minister. Demographic and healthcare‐related data are collected and cross‐checked using multiple sources within the Ministry of Health, Ministry of Interior, and the Ministry of Finance. The Ministry of Interior Mortality Report is based on mandatory reporting of each death to the Ministry of Health using death certificates. The reports contain ICD‐10–based causes of death. The ICDC uses a unique coding system based on Israeli Identification Numbers to link the data sources and associate medical diagnoses and type of procedure with outcomes such as mortality and length of hospital stay, keeping the data deidentified. Since we retrospectively collected deidentified data obtained from administrative databases, the institutional review board waived the need for informed consent.
Data Collected
Procedural volume
The annual procedural volumes of CABG and PCI were obtained from the CABG and PCI National Registries residing within the ICDC of the Ministry of Health. Annual volumes were derived from mandatory institutional reports submitted to the ICDC by each institution performing any revascularization procedure. During the study period, 12 institutions performed both CABG and PCI. Eleven institutions performed PCI only. The codes used to identify CABG were 36.10 to 36.17 and 36.19. The codes used to identify PCI were 36.09, 00.66, 36.06, and 36.07. We collected the total number of revascularization procedures rather than the number of patients or admissions.
Patient clinical profile and procedural outcomes
Patients demographics, comorbidities, and in‐hospital procedural mortality were obtained from the Ministry of Health Hospitalization Reports. The ICD‐9 used to identify comorbidities included the following: hypertension: 401 and 402; hyperlipidemia: 272; diabetes mellitus: 250; cerebrovascular disease: 430 to 438 (any form of intra cranial hemorrhage, occlusion or stenosis of intracerebral arteries, occlusion or stenosis of precerebral arteries [carotid and vertebral], transient ischemic attack, reversible ischemic neurological deficit, and cerebrovascular accident); peripheral vascular disease: 443; renal failure: 584, 585, and 586; chronic obstructive pulmonary disease: 415 to 416; myocardial infarction: 410; angina pectoris: 413; acute coronary syndrome: 411.1 (previously coded unstable angina pectoris); and congestive heart failure: 428. Length of hospital stay and procedural mortality rates were derived from the hospitalization reports. Mortality rates were cross‐checked and confirmed using the Ministry of Interior Mortality Reports.
IHD‐related mortality
Annual rates of IHD‐related mortality were obtained from two data sets: the Central Bureau of Statistics and the Ministry of Interior Mortality Reports. The ICD‐10 codes used to identify IHD‐related mortality were I20 to I25 and I46.0 to 146.9.
Statistical Analysis
Categorical data were expressed as absolute numbers with percentages. Continuous variables were expressed as median and interquartile range (IQR). Annual rates for PCIs and CABGs were referenced to the overall population of Israel each year between 2002 and 2014 available from the Israeli Central Bureau of Statistics. Institutional PCI/CABG ratios were compared using Kruskal–Wallis analysis. Trends over time were calculated using linear regression, Poisson regression model, and annual percent change. SPSS for Windows, version 22 (IBM Corp, Armonk, NY), was used for all calculations.
Results
Volume and Type of Coronary Revascularization Procedures
From 2002 to 2014 the population of Israel increased by 26% from 6 600 000 to 8 345 000 people. A total of 298 390 revascularization procedures (PCI: 255 724, CABG: 42 666) were performed during this period. Although the absolute annual number of revascularization increased by ≈8% (from 21 675 to 23 421), when corrected for the population size, we observed a 14% decline in the total volume of revascularization procedures nationally from 496.3/100 000 in 2002 to 424.8/100 000 in 2014 (P<0.0001 for trend; Table 1, Figure 1).
Table 1.
Annual Rates of Coronary Revascularization Procedures in Israel
| Year | CABG Surgery | PCI | Total | PCI/CABG | |||
|---|---|---|---|---|---|---|---|
| No. | Rate per 100 000 | No. | Rate per 100 000 | No. | Rate per 100 000 | Ratio | |
| 2002 | 4759 | 109.0 | 16 916 | 387.4 | 21 675 | 496.3 | 3.6 |
| 2003 | 4125 | 92.6 | 17 482 | 392.6 | 21 607 | 485.2 | 4.2 |
| 2004 | 4044 | 89.1 | 18 008 | 396.9 | 22 052 | 486.0 | 4.5 |
| 2005 | 3751 | 81.2 | 19 036 | 411.9 | 22 787 | 493.0 | 5.1 |
| 2006 | 3810 | 80.9 | 19 737 | 419.3 | 23 547 | 500.2 | 5.2 |
| 2007 | 3352 | 69.9 | 19 823 | 413.6 | 23 175 | 483.5 | 5.9 |
| 2008 | 3125 | 64.1 | 20 645 | 423.2 | 23 770 | 487.3 | 6.6 |
| 2009 | 2916 | 57.9 | 20 160 | 400.0 | 23 076 | 457.9 | 6.9 |
| 2010 | 2749 | 53.6 | 20 757 | 404.7 | 23 506 | 458.3 | 7.6 |
| 2011 | 2605 | 49.9 | 20 953 | 401.6 | 23 558 | 451.5 | 8.0 |
| 2012 | 2431 | 45.8 | 20 659 | 388.9 | 23 090 | 434.7 | 8.5 |
| 2013 | 2444 | 45.2 | 20 682 | 382.2 | 23 126 | 427.3 | 8.5 |
| 2014 | 2555 | 46.4 | 20 866 | 378.5 | 23 421 | 424.8 | 8.2 |
| P for trend | <0.0001 | 0.7 | <0.0001 | <0.0001 | |||
CABG indicates coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Figure 1.
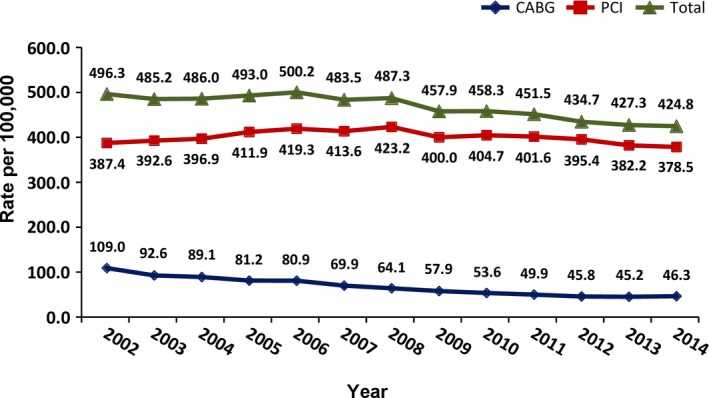
National trends in coronary revascularization procedures in Israel between 2002 and 2014. Total revascularization volume: P<0.0001; percutaneous coronary intervention (PCI): P=0.70; and coronary artery bypass grafting (CABG): P<0.0001.
The volume of PCI increased by 9% from 2002 to 2008 (387.4/100 000 to 423.2/100 000) and then steadily declined by 10.5% to 378.5/100 000 in 2014 (P=0.7; Table 1, Figure 1). The volume of CABG decreased by 59% (109.0/100 000 to 45.2/100 000) from 2002 to 2013, leveling off during the last year of the study to 46.3/100 000 (P<0.0001; Table 1, Figure 1).
The PCI/CABG ratio increased from 3.6 in 2002 to 8.5 in 2013, slightly decreasing to 8.2 by 2014 (P<0.0001; Figure 2A). There was a marked institutional variability with regards to procedural volume and the PCI/CABG ratio ranging from a median of 2.1 (IQR=1.8–2.4) to 9.9 (IQR=7.3–12.3) (Table 2, Figure 2B). We could not obtain the PCI/CABG ratio for the institutions performing PCI only. Revascularization procedures performed on patients who underwent diagnostic catheterization at an institution with no cardiac surgery program and referred for CABG elsewhere, were counted within the institution performing the CABG. This limitation skewed the institutional PCI/CABG ratio to be lower.
Figure 2.
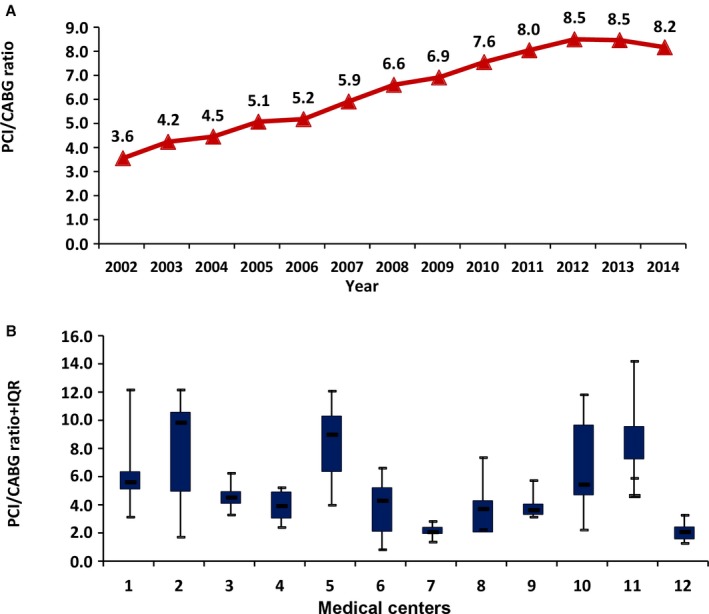
National trends in percutaneous coronary intervention/coronary artery bypass grafting (PCI/CABG) ratio in Israel between 2002 and 2014. A, P<0.0001. Median (triangles) and interquartile range (IQR; quadrangles) minimum and maximum values of PCI/CABG ratio of the 12 institutions who performed both PCI and CABG during the study period. B, P<0.001.
Table 2.
Institutional PCI/CABG Ratioa
| Year Institution | PCI/CABG Ratio | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Median | IQR | |
| 2002 | 3.2 | 1.8 | 4.7 | 3.6 | 4.0 | 0.9 | 1.4 | 2.3 | 3.2 | 2.3 | 6.0 | 1.4 | 2.7 | 2.1 |
| 2003 | 4.5 | 2.0 | 4.9 | 4.9 | 4.8 | 1.5 | 1.6 | 2.6 | 3.5 | 4.7 | 6.0 | 1.6 | 4.0 | 3.0 |
| 2004 | 5.1 | 3.0 | 4.2 | 2.9 | 5.7 | 1.5 | 1.7 | 3.7 | 3.8 | 4.6 | 5.9 | 1.7 | 3.7 | 2.1 |
| 2005 | 4.6 | 5.0 | 5.4 | 2.4 | 8.4 | 2.1 | 1.8 | 3.3 | 5.8 | 5.1 | 7.3 | 1.6 | 4.8 | 3.1 |
| 2006 | 5.4 | 6.6 | 6.0 | 2.7 | 8.4 | 3.1 | 2.0 | 3.2 | 3.6 | 4.7 | 7.3 | 1.3 | 4.1 | 3.2 |
| 2007 | 5.8 | 9.6 | 6.3 | 3.1 | 6.4 | 3.8 | 2.0 | 4.0 | 3.3 | 4.9 | 10.0 | 1.7 | 4.5 | 3.1 |
| 2008 | 5.5 | 9.9 | 3.3 | 4.9 | 9.3 | 5.2 | 2.4 | 2.8 | 3.6 | 5.4 | 10.0 | 2.1 | 5.0 | 3.2 |
| 2009 | 5.8 | 10.6 | 3.6 | 4.9 | 9.9 | 5.2 | 2.1 | 3.7 | 3.3 | 7.4 | 8.5 | 2.4 | 5.1 | 4.2 |
| 2010 | 8.0 | 11.0 | 4.2 | 5.0 | 10.3 | 6.7 | 2.2 | 4.0 | 3.2 | 8.9 | 12.3 | 2.1 | 5.8 | 5.5 |
| 2011 | 8.5 | 12.2 | 4.1 | 5.3 | 10.4 | 6.4 | 2.1 | 6.0 | 4.0 | 11.9 | 11.9 | 2.4 | 6.2 | 6.6 |
| 2012 | 5.8 | 9.8 | 4.3 | 4.6 | 9.0 | 6.6 | 2.7 | 7.4 | 4.4 | 11.3 | 14.2 | 3.3 | 6.2 | 4.8 |
| 2013 | 10.2 | 10.9 | 4.9 | 3.9 | 11.0 | 5.0 | 2.5 | 4.9 | 3.8 | 11.0 | 13.8 | 3.0 | 4.9 | 7.1 |
| 2014 | 8.2 | 10.3 | 4.0 | 3.9 | 12.1 | 4.3 | 2.9 | 4.3 | 4.3 | 9.6 | 12.3 | 2.8 | 4.3 | 5.9 |
| Median | 5.7 | 9.8 | 4.3 | 3.9 | 9.0 | 4.3 | 2.1 | 3.7 | 3.6 | 5.4 | 9.9 | 2.1 | ||
| IQR | 2.9 | 5.6 | 0.8 | 1.8 | 3.9 | 3.1 | 0.6 | 1.1 | 0.7 | 4.9 | 5.0 | 0.8 | ||
IQR indicates interquartile range.
For the 12 institutions performing both coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI).
Patient Clinical Profile and Procedural Outcomes
We observed variable trends with respect of associated comorbidities in patients undergoing both types of coronary revascularization procedures (Table 3). While the prevalence of some of the risk factors such as renal failure, chronic obstructive pulmonary disease and prior myocardial infarction, hypertension, and diabetes mellitus increased, other factors such as peripheral vascular disease declined or remained stable (heart failure).
Table 3.
Prevalence of Comorbidities of Patients Undergoing Coronary Revascularization
| Comorbidities | Coronary Revascularization Procedures | |||
|---|---|---|---|---|
| 2002 N=20 826, No. (%) | 2012 N=21 461, No. (%) | 2014 N=21 728, No. (%) | P of Trend | |
| Renal failure | 1356 (6.5) | 1989 (9.3) | 2284 (10.5) | 0.09 |
| Heart failure | 1981 (9.5) | 2328 (10.9) | 2625 (12.1) | 0.21 |
| Hypertension | 9995 (48.0) | 10 103 (47.1) | 11 192 (51.5) | 0.69 |
| Diabetes mellitus | 6193 (29.7) | 6918 (32.2) | 7466 (34.4) | 0.21 |
| Dyslipidemia | 10 931 (52.5) | 11 395 (53.1) | 12 393 (57.0) | 0.49 |
| Peripheral vascular disease | 1324 (6.4) | 955 (4.5) | 950 (4.4) | 0.07 |
| Cerebrovascular disease | 1103 (5.3) | 963 (4.5) | 963 (4.4) | 0.03 |
| Acute coronary syndrome | 3809 (18.3) | 2712 (12.6) | 2584 (11.9) | 0.04 |
| Myocardial infarction | 6278 (30.2) | 8183 (38.1) | 8902 (41.0) | 0.07 |
| Obstructive pulmonary disease | 235 (1.1) | 456 (2.1) | 534 (2.5) | 0.08 |
In‐hospital procedural mortality (Figure 3A) was higher after CABG and remained stable after both types of revascularization procedures (PCI: 1.2–1.6%, P=0.34; CABG: 3.7–4.3%, P=0.3) during the study period. Length of hospital stay (Figure 3B) decreased after PCI from a mean of 4.9±1.9 days (median 5.2 days) in 2002 to a mean of 3.7±1.5 days (median 3.8 days) in 2014 (P<0.001). In contrast, length of stay markedly increased after CABG from 10.0±3.9 days (median 9.8 days) in 2002 to a mean of 14.1±4.5 days (median 14.5 days) in 2014 (P<0.0001).
Figure 3.
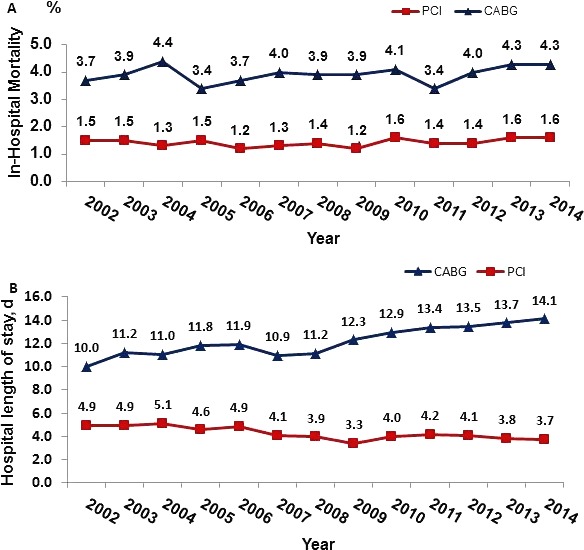
National trends in procedural mortality (A) and length of hospital stay (B) after percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) in Israel between 2002 and 2014. CABG mortality: P=0.29; PCI mortality: P=0.34; CABG hospital stay: P<0.0001; and PCI hospital stay: P<0.001.
IHD‐Related Mortality
To assess the national rates of IHD‐related mortality, we summed the mortality rates after acute ST‐ or non–ST‐segment elevation myocardial infarction, other types of acute coronary syndrome, and sudden cardiac death. During the study period, annual IHD‐related mortality decreased by 46% (84.6/100 000 to 46/100 000, P<0.0001; Figure 4).
Figure 4.
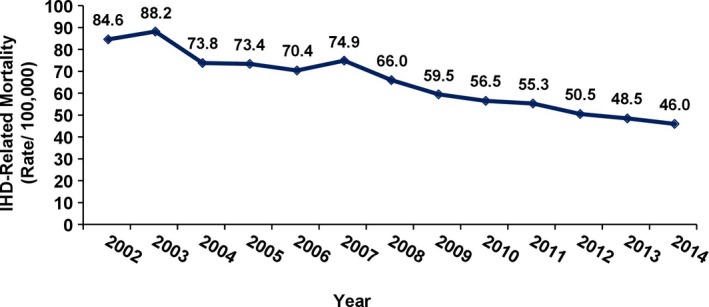
Ischemic heart disease (IHD)–related mortality in Israel between 2002 and 2014 (P<0.0001).
Discussion
The first major observation in our study was a significant decline in the total number of coronary revascularization procedures. This is in line with other reports from the United States, Australia, and the Organization for Economic Cooperation and Development (OECD)—an organization of 35 countries from North and South America, Europe, and Asia with a mission to promote policies that will improve the economic and social well‐being [including health] of people around the world.).2, 3, 4, 7, 8, 9, 10 Three major factors may account for this phenomenon. The first one is the accumulating data that in select groups of patients with IHD, optimal medical treatment yields equivalent, or at times superior, outcomes compared with PCI.11, 12 The COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial randomized 2287 patients with objective evidence of significant myocardial ischemia and coronary disease to optimal medical treatment or PCI. PCI did not reduce the risk of death, myocardial infarction, or other major cardiovascular events when added to optimal medical therapy.11 In a more recent meta‐analysis of 100 trials in 95 553 patients with 262 090 patient years, PCI using newer‐generation drug‐eluting stents was associated with a decreased need for revascularization but had no effect on patient survival or adverse cardiac events. In contrast, CABG reduced the risk of death, myocardial infarction, and subsequent myocardial revascularization compared with medical treatment.12 The second factor is the increasing awareness that objective assessment of the hemodynamic significance of coronary artery stenosis using tests such as functional flow reserve is a key to determine whether any form of revascularization is indicated.13 The third factor is the introduction of appropriateness criteria for coronary revascularization as a tool to prevent overuse or underuse of PCI.6 In a study of 2.7 million PCIs from 766 hospitals, Desai and colleagues4 showed that adherence to the criteria was associated with a significant decline in the total number of PCIs performed, particularly those deemed inappropriate or with borderline indication.
The second major observation in our study was the dramatic shift from CABG to PCI as the revascularization of choice. This is in line with other reports published during the past 2 decades.2, 3, 4, 7, 8, 9, 10 The marked increase in the national PCI to CABG ratio from 3.6 to 8.5 in the early period of our study reflects a dramatic shift in practice of cardiovascular specialists in Israel. This is among the highest value reported at a regional or national level, and is substantially higher compared with that reported in the United States and Australia and the average OECD rate.2 On a national level, Spain, France, Estonia, and Korea are countries with a similar or slightly higher PCI/CABG ratio compared with Israel. In the United States, only a few single institutions in New York City reported similar PCI to CABG rates.2, 3, 7, 14
The widespread adoption of PCI as the coronary revascularization of choice is related to a combination of objective and subjective factors. Compared with CABG, PCI is less invasive, can be performed much faster in urgent or emergent situations, and requires less infrastructure. Introduction of drug‐eluting stents, improving catheter and imaging techniques and technologies, and increased experience resulted in an exponential use of PCI as the preferred approach with improved early and mid‐term outcomes. However, in recent years, it has become apparent that CABG should remain the revascularization procedure of choice in specific patient populations such as those with diabetes mellitus and those with complex left main disease, 2‐vessel disease with proximal left anterior descending coronary artery disease, or severe 3‐vessel disease. Five‐year data from prospective randomized trials including the SYNTAX (Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery), BARI 2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes), and FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) trials, large “real‐world” registries and meta‐analyses showed that in these patient populations, CABG is superior to PCI with regards to midterm survival, incidence of major adverse cardiac events, and rates of repeat revascularization.15, 16, 17, 18, 19, 20
Despite the unequivocal evidence reflected in professional cardiovascular societies guidelines for revascularization,5 subjective physician, institutional, and patient factors continue to play a major role in the bias towards a specific type of revascularization procedure. Physician‐related factors include unfamiliarity with the most recent data and updated guidelines, operator bias, and conflicts of interest inherent to the setting where the same operator performs the diagnostic and therapeutic procedures. Institutional factors include infrastructure, policies, and financial considerations. Finally, in line with the ethical principle of patient autonomy, patient natural inclination towards a less invasive procedure and culture‐related preferences play a central role. We believe that this array of factors has led to disproportional use of PCI over CABG in Israel, often not in accordance with practice guidelines and appropriateness criteria.
In agreement with other reports, our study showed a wide institutional variation in practice with PCI to CABG ratios ranging from 2.1 to 9.5.2, 3, 4, 14 This observation suggests that institutional policies, financial pressures, heterogeneous infrastructure, patterns of practice, and cardiology‐cardiac surgery level of collaboration play a major role in selecting the type of revascularization procedure. It also underscores the importance of the heart team concept to ensure a more structured, uniform, and nonbiased process.21 We believe that different levels of implementation of the heart team concept may explain the wide institutional range of PCI to CABG ratio.
A unique observation in the present study is the shift of the practice pendulum back towards CABG in the final 3 years of the study. The volume of CABG procedures leveled off and even slightly increased at the same time that PCI procedural volume continued to decline, resulting in a PCI to CABG ratio of 8.2 at the final year of the study. Our study was not designed to investigate the causes of the observed changes in practice. An effective way to examine the cause and effect of such changes in practice would be to establish an IHD‐specific (rather than procedural‐specific) national clinical database similar to that recently accomplished in the Netherlands.22 A comprehensive collection of data on the 3 treatment modalities of IHD—optimal medical therapy, PCI and CABG––including long‐term outcomes, would be a very powerful tool to investigate these questions. We hypothesize that the accumulating level A data derived from randomized trials involving large numbers of patients with long‐term follow‐up, familiarity with the appropriate use criteria and current guidelines, and perhaps implementation of the heart team concept in a larger proportion of patients may account for these changes. Our observation is similar to that described by Bangalore and colleagues23 who assessed the impact of the COURAGE and BARI 2D trials on more than 8.1 million PCIs.
The clinical profile of the patients in this study was typical of patients undergoing coronary revascularization in the current era. The procedural mortality of CABG and PCI remained relatively stable, despite the high proportion of patients with significant comorbidities. The raw mortality rates for CABG and PCI are somewhat higher compared withthose reported by the American Society of Thoracic Surgeons and the National Cardiovascular Data Registry.24, 25 However, it is difficult to compare outcomes without a valid and reliable model of risk‐adjustment. Furthermore, we reported in‐hospital mortality. It has been clearly established that 30‐ or even 120‐day mortality rates are more accurate quality measures.26, 27 Interestingly, we observed a significant increase in hospital length of stay after CABG and a significant decrease after PCI. The causes of these trends are unclear. We speculate that a change in the risk profile of patients referred for CABG may account for this observation. In line with other reports, over time, patients undergoing CABG in Israel were older, sicker, and have more advanced coronary disease.8, 25 The length of hospital stay after both procedures were longer compared with those reported by the Society of Thoracic Surgeons and the National Cardiovascular Data Registry.28 Similar to mortality, it is impossible to reliably compare our outcomes with any benchmark without using validated length‐of‐stay prediction models. A fundamental difference between Israel and the United States is the discharge destination. The majority of patients in Israel are discharged to home, whereas a significant proportion of patients in the United States are discharged to extended‐care facilities.28, 29
Parallel to the dramatic change in the volume and type of coronary revascularization procedure, we observed a 46% decline in IHD‐related mortality in Israel during the study period—from 84.6 per 100 000 to 46 per 100 000. This absolute rate, as well as the steepness of decline are among the best in the OECD, and substantially better compared with the United States.2 As a result, IHD moved to be the second most common cause of death in Israel, being replaced by cancer.30 Our study was not designed to assess the causes of the decline in IHD‐related mortality. More effective primary and secondary prevention of atherosclerosis including dietary and lifestyle changes, use of statins, angiotensin‐converting enzyme inhibitors, and antidiabetic medications, as well as decreasing incidence and mortality after acute myocardial infarction may all account for the marked decline in IHD‐related mortality we observed. For example, the rate of smoking in adults has dropped by ≈50% since 1980 and is now only 19.7%.31 Similarly, the 7‐day, 30‐day, and 1‐year mortality rates after acute myocardial infarction in Israel dropped by more than 50% between 2000 and 2011.32 The relationship between the observed dramatic national changes in coronary revascularization procedural volume and outcomes and IHD mortality remains to be further investigated.
Study Limitations
Our study relied entirely on data derived from 5 administrative data sets using ICD‐9 and ICD‐10 codes. This methodology is associated with an inherent risk for undercoding or miscoding. In one study comparing the quality of data in an administrative database with that of a clinical, adjudicated clinical state registry, there was a 27.4% disparity in isolated CABG volume and a 9% relative difference in mortality.33 However, we believe that although the absolute numbers may not be as accurate as a clinical database, the observed trend changes in volume and outcomes are real. Furthermore, cross‐checking of data derived from entirely different sources afforded the opportunity for data validation.
Using administrative databases rather than comprehensive national clinical PCI and CABG databases limited our ability to risk‐adjust the observed procedural outcomes. This limitation prevented a more accurate and fair comparison between the two revascularization modalities, or benchmark the national outcomes against large databases such as the American Society of Thoracic Surgeons or the European Adult Cardiac Surgery Databases.
Conclusions
This study demonstrated a marked decline in the total volume of coronary revascularization procedures in Israel over a period of 13 years. There was a dramatic shift from CABG to PCI in the early phase. This trend has reversed, with the pendulum shifting slightly back towards CABG in the final years of the study. In parallel, we observed a marked improvement in IHD‐related mortality. Further studies are indicated to have a better understanding of the causes of these changes and the impact of coronary revascularization on IHD‐related mortality in Israel.
Author Contributions
Blumenfeld collected the data, performed the data analysis and interpretation, and wrote the manuscript. Na'amnih performed quality monitoring of the national registries' data, had complete access to the study data, and participated in the statistical analysis. Shapira‐Daniels, Lotan, and Shohat revised the final version of the manuscript, and Shapira participated in the study design, methodology, and data analysis and interpretation and critically revised the manuscript.
Disclosures
None.
Acknowledgments
The authors thank the cardiac surgery facilities and the interventional cardiology facilities for the monthly reports to the national registries, and the division of Information and Computing, Ministry of Health, for extracting data from the hospitalization database.
(J Am Heart Assoc. 2017;6:e004734. DOI: 10.1161/JAHA.116.004734.)
The abstract of this paper was accepted for presentation as a poster in the Annual Scientific Sessions of the American Heart Association, November 12–16 2016, in New Orleans, LA.
References
- 1. World Health Organization . Cardiovascular diseases (CVDs). Fact sheet No. 317, updated January 2015. Available at: http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed May 29, 2016.
- 2. Health at a glance‐2015. OECD indicators. Available at: http://www.oecd.org/health/health-systems/health-at-a-glance-19991312.htm. Accessed May 28, 2016.
- 3. Ko W, Tranbaugh R, Marmur JD, Supino PG, Borer JS. Myocardial revascularization in New York State: variations in the PCI‐to‐CABG ratio and their implications. J Am Heart Assoc. 2012;1:e001446 doi: 10.1161/JAHA.112.001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desai NR, Bradley SM, Parzynski CS, Nallamothu BK, Chan PS, Spertus JA, Patel MR, Ader J, Soufer A, Krumholz HM, Curtis JP. Appropriate use criteria for coronary revascularization and trends in utilization, patient selection and appropriateness of coronary interventions. JAMA. 2015;314:2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kolh P, Kurlanski P, Cremer J, Lawton J, Siepe M, Fremes S. Transatlantic editorial: a comparison between European and North American guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2016;49:1307–1317. [DOI] [PubMed] [Google Scholar]
- 6. Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spretus JA. ACC/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization. A report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society of Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology. J Am Coll Cardiol. 2009;53:530–553. [DOI] [PubMed] [Google Scholar]
- 7. Davies J; Australian Institute of Health and Welfare (AIHW) . Coronary revascularization in Australia, 2000. Available at: http://www.aihw.gov.au/publication-detail/?id=6442467493. Accessed May 29, 2016.
- 8. McNeely C, Markewell S, Vassileva C. Trends in patients characteristics and outcomes of coronary artery bypass grafting in 2000 to 2012 Medicare population. Ann Thorac Surg. 2016;102:132–138. [DOI] [PubMed] [Google Scholar]
- 9. Culler SD, Kugelmass AD, Brown PP, Reynolds MR, Simon AW. Trends in coronary revascularization procedures among Medicare beneficiaries between 2008 and 2012. Circulation. 2015;131:362–370. [DOI] [PubMed] [Google Scholar]
- 10. Yeh RW, Mauri L, Wolf ER, Romm IK, Lovett A, Shahian D, Normand SL. Population trends in rates of coronary revascularization. JAMA Intern Med. 2015;175:454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS; COURAGE Trial Research Group . Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 12. Windecker S, Stortecky S, Stefanini GG, da Costa BR, Rutjes AW, Di Nisio M, Silletta MG, Maione A, Alfonso F, Clemmensen PM, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head S, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter D, Schauerte P, Sousa Uva M, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, Kolh P, Jüni P. Revascularization versus medical treatment in patients with stable coronary disease: network meta‐analysis. BMJ. 2014;348:g3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF; FAME Study Investigators . Fractional flow reserve versus angiography for guiding percutaneous coronary angiography. N Engl J Med. 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 14. Epstein AJ, Polsky D, Yang F, Yang L, Groenveld PW. Coronary revascularization trends in the United States: 2001–2008. JAMA. 2011;305:1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Head SJ, Davierwala PM, Serruys PW, Redwood SR, Colombo A, Mack MJ, Morice MC, Holmes DR Jr, Feldman TE, Stahle E, Underwood P, Dawkins KD, Kappetein AP, Mohr FW. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three‐vessel disease: final five‐year follow‐up of the SYNTAX trial. Eur Heart J. 2014;35:2821–2830. [DOI] [PubMed] [Google Scholar]
- 16. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S III, Bertrand M, Fuster V; FREEDOM Trial Investigators . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 17. The BARI 2D Study Group . A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weintraub WS, Grau‐Sepulveda MV, Weiss JM, O'Brien SM, Peterson ED, Kolm P, Zhang Z, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Shewan CM, Garratt KN, Moussa ID, Dangas GD, Edwards FH. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sipahi L, Akay S, Dagdelen S, Blitz A, Alhan C. Coronary artery bypass grafting vs percutaneous coronary intervention and long term mortality and morbidity in multivessel disease. Meta‐analysis of randomized clinical trials of arterial grafting and stenting era. JAMA Intern Med. 2014;174:223–230. [DOI] [PubMed] [Google Scholar]
- 20. Giustino G, Mehran R. CABG surgery versus PCI in CAD—surgery strikes again! Nat Rev Cardiol. 2015;12:75–77. [DOI] [PubMed] [Google Scholar]
- 21. Head SJ, Kaul S, Mack MJ, Serruys PW, Taggart DP, Holmes DR Jr, Leon MB, Marco J, Bogers AJ, Kappetein AP. The rationale for heart‐team decision‐making for patients with stable, complex coronary artery disease. Eur Heart J. 2013;34:2510–2518. [DOI] [PubMed] [Google Scholar]
- 22. Van Veghel D, Marteijn M, de Mol B; on behalf of the Measurably Better Study Group (The Netherlands) and Advisory Board . First results of a national initiative to enable quality improvement of cardiovascular care by transparently reporting on patient‐relevant outcomes. Eur J Cardiothorac Surg. 2016;49:1660–1669. [DOI] [PubMed] [Google Scholar]
- 23. Bangalore S, Gupta N, Genereux P, Guo Y, Pancholy S, Feit F. Trend in percutaneous coronary intervention volume following the COURAGE and BARI‐2D trials: insight from over 8.1 million percutaneous interventions. Int J Cardiol. 2015;183:6–10. [DOI] [PubMed] [Google Scholar]
- 24. ElBardissi AW, Aranki SF, Sheng S, O' Brien SM, Geenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143:273–281. [DOI] [PubMed] [Google Scholar]
- 25. Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, Krone RJ, Weintraub WS, Brindis RG, Rumsfeld JS, Spertus JA; NCDR Registry Participants . Contemporary mortality risk prediction after percutaneous coronary interventions. Results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP; Society of Thoracic Surgeons Quality Measurement Task Force . The Society of Thoracic Surgery 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2–S22. [DOI] [PubMed] [Google Scholar]
- 27. Raza S, Sabik JF III, Rajeswaran J, Idrees JJ, Trezzi M, Riaz H, Javadikasgari H, Nowicki ER, Svensson LG, Blackstone EH. Enhancing the value of population‐based risk scores for institutional‐level use. Ann Thorac Surg. 2016;102:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ad N, Holmes SD, Shuman DJ, Pritchard G, Massimiano PS, Rongione AJ, Speir AM, Halpin L. Potential impact of modifiable clinical variables on length of stay after first time cardiac surgery. Ann Thorac Surg. 2015;100:2102–2108. [DOI] [PubMed] [Google Scholar]
- 29. Lazar HL, Fitzgerald CA, Ahmad T, Bao Y, Colton T, Shapira OM, Shemin RJ. Early discharge after coronary artery bypass surgery: are patients really going home earlier. J Thorac Cardiovasc Surg. 2001;121:943–950. [DOI] [PubMed] [Google Scholar]
- 30. Central Bureau of Statistics: mortality rates in Israel. Available at: http://www.cbs.gov.il/ts/databank/building_func_e.html?level_1=4. Accessed May 28, 2016.
- 31. Israeli Ministry of Health Report on Smoking. Available at: http://www.health.gov.il/publicationsfiles/smoking_2015.pdf. Accessed November 14, 2016.
- 32. Kornowski R. The ACSIS Registry and primary angioplasty following coronary bypass surgery. Catheter Cardiovasc Interv. 2011;78:537–539. [DOI] [PubMed] [Google Scholar]
- 33. Shahian DM, Silverstein T, Lovett AF, Wolf RE, Normand SLT. Comparison of clinical and administrative data sources for hospital coronary artery bypass graft surgery report cards. Circulation. 2007;115:1518–1527. [DOI] [PubMed] [Google Scholar]


