Introduction
Chronic kidney disease (CKD) is now clearly recognized as a public health problem worldwide. Patients with CKD display a substantial increase in end‐stage renal disease (ESRD) and cardiovascular disease (CVD).1 Moreover, the prognosis of CVD in CKD is extremely poor.2 Understanding the pathophysiology of CVD in CKD might help to develop treatment strategies to reduce its morbidity and mortality. Traditional cardiovascular risk factors for the general population, such as diabetes mellitus, high blood pressure, and dyslipidemia, are more common in patients with CKD, but cannot entirely explain the increased cardiovascular risk.3 Compelling evidence suggests that the uremic milieu itself plays a critical role in the development and progression of CVD.4 Various solutes that would normally be excreted by the kidneys accumulate in patients with CKD. These solutes are called uremic toxins when they interact negatively with biological functions and contribute to the uremic syndrome. Of note, the gut microbiota is markedly altered in CKD. Fermentation of protein and amino acids by certain gut microbiota results in the generation of different toxic metabolites that are absorbed into the circulation and are retained in CKD. Indoxyl sulfate, indole‐3 acetic acid, p‐cresyl sulfate, trimethylamine N‐oxide, and phenylacetylglutamine are uremic toxins derived from dietary protein metabolism by gut microbiota (Table 1) that have been associated with CVD in patients with CKD (Table 2).5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Indoxyl sulfate is among the most representative gut‐derived uremic toxins and has emerged as an important therapeutic target. In this review, we will summarize recent advances in understanding the association between gut‐derived uremic toxins and CVD in CKD, with special emphasis on the pathogenic role of indoxyl sulfate.
Table 1.
Gut‐Derived Uremic Toxins That Have Been Associated With CVD in Patients With CKD
| Name | Source | Class |
|---|---|---|
| Indoxyl sulfate | Tryptophan | Protein‐bound |
| Indole‐3 acetic acid | Tryptophan | Protein‐bound |
| p‐cresyl sulfate | Phenylalanine, tyrosine | Protein‐bound |
| Trimethylamine N‐oxide | Choline, l‐carnitine, PTC | Water‐soluble |
| Phenylacetylglutamine | Phenylalanine | Water‐soluble |
CKD indicates chronic kidney disease; CVD, cardiovascular disease; PTC, phosphatidylcholine.
Table 2.
Summary of Studies Relating Gut‐Derived Uremic Toxins to CVD in Patients With CKD
| Reference | Patients | CVD |
|---|---|---|
| Indoxyl sulfate | ||
| 2009 Barreto et al5 | CKD | Vascular calcification, vascular stiffness, mortality |
| 2012 Lin et al6 | CKD | Cardiovascular events |
| 2015 Cao et al7 | HD | Congestive heart failure |
| 2016 Wu et al8 | HD | Vascular access thrombosis |
| Indole‐3 acetic acid | ||
| 2015 Dou et al9 | CKDa | Cardiovascular events and mortality |
| P‐cresyl sulfate | ||
| 2006 Bammens et al10 | HD | Mortality |
| 2008 Meijers et al11 | HD | Cardiovascular events |
| 2010 Meijers et al12 | CKD | Cardiovascular events |
| 2010 Liabeuf et al13 | CKD | Mortality |
| 2010 Lin et al14 | HD | Cardiovascular events |
| 2012 Wu et al15 | HDb | Cardiovascular and all‐cause mortality |
| 2014 Poesen et al16 | CKD | Cardiovascular events |
| Trimethylamine N‐oxide | ||
| 2016 Stubbs et al17 | CKDa | Coronary atherosclerosis |
| Phenylacetylglutamine | ||
| 2016 Poesen et al18 | CKD | Cardiovascular events and mortality |
CKD indicates chronic kidney disease; CVD, cardiovascular disease; HD, hemodialysis.
Including dialyzed CKD patients.
Elderly HD patients (>65 years old).
Gut‐Derived Uremic Toxins in CKD
Uremic toxins are traditionally classified, according to the physicochemical characteristics affecting their clearance during dialysis, into the small water‐soluble molecules (molecular weight [MW] <500 Da), the larger “middle molecules” (MW >500 Da), and the protein‐bound molecules.19 Alternatively, uremic toxins may be classified according to their site of origin. Although endogenous metabolism is the prime contributor to the internal milieu, a significant fraction of uremic retention solutes originates from the intestinal tract. Aronov et al demonstrated, by using high‐resolution mass spectrometry, an absence or reduced plasma levels of >30 uremic solutes in hemodialysis patients with a colectomy compared to those with an intact colon.20 Indeed, loss of renal function is not the sole reason for high serum concentrations of these uremic retention solutes. There is increasing interest in the gut microbiota as a relevant source of uremic toxins, because CKD profoundly alters the gut microenvironment and has been associated with a distinct gut microbial composition and metabolism. Poesen et al studied fecal metabolite profiles of hemodialysis and healthy individuals and observed a clear discrimination with 81 fecal volatile organic compounds detected at significantly different levels in the 2 groups.21 Vaziri et al further showed a marked difference in the abundance of 190 microbial operational taxonomic units between hemodialysis patients and healthy controls,22 with significant expansion of bacterial families possessing urease, uricase, and indole and p‐cresol forming enzymes in hemodialysis patients.23 Indole is produced by intestinal bacteria as a degradation product of the amino acid, tryptophan, and is subsequently absorbed and metabolized in the liver to indoxyl sulfate, the prototype of protein‐bound uremic toxins. Increased intestinal concentration of uremic toxins associated with CKD leads to disruption of intestinal barrier integrity and translocation of bacterial components and metabolites, which triggers intestinal and subsequent systemic inflammation and could further accelerate the progression of CKD.24
Clinical Studies of Cardiovascular Toxicity of Indoxyl Sulfate in CKD
Indoxyl sulfate accumulates in CKD, circulating mostly bound to albumin, and cannot be sufficiently removed by means of conventional dialysis. In addition to involvement in the progression of CKD,25 a growing number of clinical studies supports the idea that indoxyl sulfate may also contribute to CVD in the CKD and ESRD population. Barreto et al had previously demonstrated that serum levels of indoxyl sulfate were positively and significantly associated with aortic calcification and pulse wave velocity (PWV) in patients with different stages of CKD.5 Moreover, the highest indoxyl sulfate tertile was a powerful predictor of overall and cardiovascular mortality. Additionally, Lin et al determined that serum indoxyl sulfate level was a valuable marker in predicting cardiovascular events in patients with advanced CKD.6 Recently, Cao et al conducted a prospective study to investigate the relationship between indoxyl sulfate and heart failure in patients with ESRD on hemodialysis. After a median follow‐up of 48 months, high plasma indoxyl sulfate was associated with a higher risk of first heart failure event. The results remained significant after adjusting for both conventional and unconventional risk factors.7 In our recent study,8 hemodialysis patients undergoing angioplasty for access dysfunction were prospectively enrolled over a 3‐year period, with 175 and 131 of the enrolled patients having arteriovenous grafts (AVGs) and arteriovenous fistulas, respectively. After a median follow‐up of 32 months, 86% of patients had symptomatic restenosis, 50% had access thrombosis, and 8% had access failure. Patients with AVG thrombosis after endovascular interventions had higher serum levels of free and total indoxyl sulfate. Moreover, absolute and tertiles of free indoxyl sulfate levels independently predicted AVG thrombosis. Therefore, indoxyl sulfate may be involved in the development and progression of CVD in patients with CKD.
Basic Studies of Cardiovascular Toxicity of Indoxyl Sulfate in CKD
By virtue of its proinflammatory and pro‐oxidant properties,4 indoxyl sulfate is implicated in the pathogenesis of at least 6 phenotypes of CVD in CKD (Figure 1), including atherosclerosis,26, 27, 28, 29 arteriosclerosis,30, 31, 32, 33 congestive heart failure,34, 35, 36 arrhythmia,37, 38, 39 vascular access thrombosis,40, 41 and peripheral arterial disease (PAD).42, 43
Figure 1.
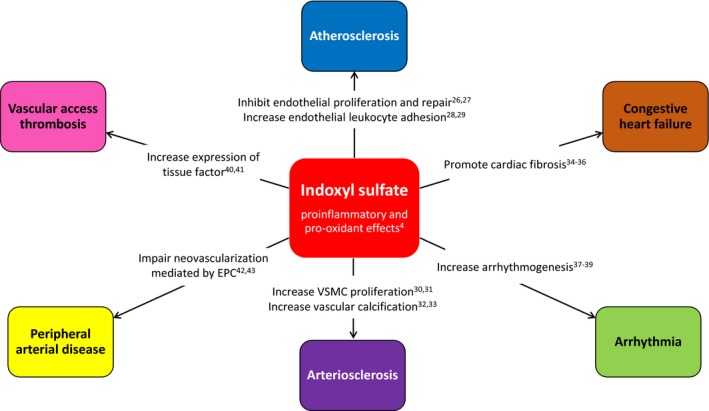
Role of indoxyl sulfate in the pathogenesis of various forms of cardiovascular disease in chronic kidney disease. EPC indicates endothelial progenitor cell; VSMC, vascular smooth muscle cell.
Atherosclerosis
It had been recognized early after the introduction of maintenance hemodialysis that atherosclerosis was the major cause of CVD in patients with CKD and that its progression, based on observations of long‐term survivors on hemodialysis, was accelerated by the uremic state.44 Endothelial dysfunction is considered an early marker for atherosclerosis. Oxidative stress and nitric oxide (NO) deficiency play an important role in endothelial dysfunction observed in CKD. Tumur et al have shown in vitro that indoxyl sulfate inhibits NO production and cell viability by inducing free radicals through induction of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in human vascular endothelial cells (HUVECs).26 Furthermore, Dou et al demonstrated that indoxyl sulfate inhibits the proliferation and wound repair of HUVECs.27 Finally, several reports have described that indoxyl sulfate upregulates endothelial expression of adhesion molecules and enhances leukocyte endothelial interactions in vitro and in vivo.28, 29 These findings suggest that indoxyl sulfate may be responsible, at least in part, for atherosclerosis in CKD by inducing inflammation and endothelial dysfunction.
Arteriosclerosis
Although atherosclerosis clearly contributes to the high prevalence of CVD morbidity and mortality in patients with CKD, it is often accompanied by the involvement of nonatherosclerotic processes such as arteriosclerosis, which has the principal consequences of left ventricular hypertrophy and altered coronary perfusion.45 Aging is associated with degeneration and sclerosis of the medial layer of large arteries. Arteriosclerosis in CKD is mainly characterized by premature arterial aging with diffuse thickening and stiffening of arterial walls. Arteriosclerosis, as determined by aortic PWV, is a strong independent predictor of all‐cause, and mainly cardiovascular, mortality in patients with ESRD undergoing hemodialysis.46
Vascular smooth muscle cell (VSMC) is the sole cell type of the medial layer of the vascular wall. VSMC proliferation and medial calcification are responsible for arterial stiffness in CKD. VSMC proliferation also causes intimal hyperplasia and arterial stenotic lesions, which contribute to the development of atherosclerosis. In a study evaluating the effect of indoxyl sulfate on VSMC proliferation, Yamamoto et al described that indoxyl sulfate directly stimulates rat VSMC proliferation and activates mitogen‐activated protein kinase in vitro.30 Using human aortic smooth muscle cells (HASMCs), Muteliefu et al reported that indoxyl sulfate significantly promotes the proliferation of HASMCs, in a concentration‐dependent manner, by inducing oxidative stress.31 Additionally, Adijiang et al demonstrated in vivo that indoxyl sulfate promotes aortic calcification and aortic wall thickening in hypertensive rats.32 Muteliefu et al further demonstrated in vitro that indoxyl sulfate induces free radicals, such as superoxide, by upregulating NADPH oxidase, especially NADPH oxidase 4 (Nox4).33 Free radicals derived from Nox4 are important for the induction of transdifferentiation of HASMCs into cells with a more‐osteoblastic phenotype.
Congestive Heart Failure
Epidemiological studies show that sudden cardiac death, arrhythmia, or congestive heart failure are more‐prominent causes of cardiovascular death than coronary artery disease in patients with ESRD.47 This finding suggests that the mechanistic processes driving CVD in CKD may differ from those in the general population. “Uremic cardiomyopathy” is pathologically composed of interstitial fibrosis, microvessel disease with a selective capillary deficit, and wall thickening of intramyocardial arterioles in the hypertrophied heart.48 By using isolated neonatal rat cardiac myocytes and fibroblasts as well as a human leukemia monocytic cell line, Lekawanvijit et al demonstrated in vitro that indoxyl sulfate has proinflammatory, profibrotic, and prohypertrophic effects, indicating that indoxyl sulfate might adversely contribute to cardiac remodeling in CKD.34 Furthermore, reduction of indoxyl sulfate with AST‐120, an oral charcoal adsorbent, was accompanied by reduced left ventricular fibrosis and transforming growth factor (TGF)‐β and phosphorylated nuclear factor kappa B (NF‐κB) protein expression in subtotal‐nephrectomized rats in a blood‐pressure–independent manner.35 Fujii et al also showed that administration of AST‐120 leads to reduction of oxidative stress, cardiac fibrosis, and left ventricular hypertrophy in a subtotal nephrectomy rat model.36 Taken together, the cardiac profibrotic effect of indoxyl sulfate is likely to be driven by the oxidative stress/NF‐κB/TGF‐β pathway through NADPH oxidases.
Arrhythmia
Sudden cardiac death is the leading cause of death in dialysis patients, accounting for 20% to 30% of all deaths in this population.47 Although a proportion of sudden cardiac death events could be caused by acute coronary ischemia, the overall incidence of sudden cardiac death is much greater than the incidence of coronary events,49 probably suggesting a primary increase in the risk of fatal ventricular arrhythmias in patients with ESRD.50 Several factors, including uremia‐specific factors that arise from retained uremic toxins, could predispose patients with CKD to a higher risk of ventricular arrhythmias. In a recent study, Tang et al showed, in a cohort of early CKD patients, that serum indoxyl sulfate level was independently associated with prolonged QTc interval. In vitro, the arrhythmogenic effect of indoxyl sulfate was shown through inhibition of the H9c2 cardiomyocyte Ik channel.37 As CKD progresses, the proarrhythmic effect of indoxyl sulfate may be enhanced in conjunction with a sustained increase in sympathetic tone,51 left ventricular hypertrophy,52 and fluid and electrolyte imbalance.53 Therefore, the observations by Tang et al are interesting and warrant further investigation.
Atrial fibrillation (AF), the most common clinical arrhythmia, causes significant cardiovascular morbidity and mortality attributed to congestive heart failure and stroke. Reported estimates of the prevalence of AF in nondialysis CKD patients range from 8% to 18%, compared to 0.4% to 1.0% in the general population.54 Using data from a large, population‐based study, Alonso et al reported that impaired kidney function and presence of albuminuria were strongly associated with the incidence of AF independently of other cardiovascular risk factors.55 CKD may increase the risk of AF because of poor blood pressure control and expansion of extracellular fluid,56 leading to left ventricular hypertrophy and, eventually, atrial stretch and fibrosis. In addition, an upregulated renin‐angiotensin‐aldosterone system in CKD causes fibrosis and electric remodeling in the atrium,57 providing a substrate for the development of AF. Finally, the cardiac profibrotic effect of indoxyl sulfate may alter atrial electrical conduction and excitability. Indoxyl sulfate has been proven to induce arrhythmogenesis through oxidative stress in pulmonary veins, sinoatrial nodes, and left atria isolated from rabbit hearts.38 Recently, Aoki et al showed, in 5 of 6 nephrectomized rats, that indoxyl sulfate contributes to the increased inducibility of AF, which is significantly attenuated by AST‐120 treatment.39 Therefore, strategies for the prevention of AF will have to consider indoxyl sulfate as a novel risk factor for AF, in addition to other well‐established risk factors in CKD.
Vascular Access Thrombosis
Vascular access dysfunction continues to be a major source of morbidity and mortality in hemodialysis patients. Thrombosis is the most common cause of AVG dysfunction. Although thrombosis may develop secondary to intimal hyperplasia at the venous anastomosis, it can also develop in the absence of any demonstrable structural abnormalities. However, few data are available on the mechanisms involved in the later event. Indoxyl‐sulfate–induced hypercoagulability may play a role in the pathogenesis of the prothrombotic state. The results of our recent study have demonstrated that serum indoxyl sulfate is an independent predictor for dialysis AVG thrombosis after endovascular interventions.8 Accumulating evidence has shown that indoxyl sulfate increases endothelial cell and VSMC productions of tissue factor (TF).40, 41 TF is a crucial mediator of injury‐related thrombosis. Using a model of the de‐endothelialized, postinterventional state, Chitalia et al exposed human VSMCs (pretreated with uremic serum obtained from ESRD patients on hemodialysis) to coronary‐like blood flow.40 They found that uremic serum significantly upregulates VSMC TF levels and is profoundly thrombogenic. Recently, Gondouin et al have also demonstrated that both circulating TF concentration and activity were elevated and were positively correlated with plasma indoxyl sulfate in patients with CKD.41 These findings suggest that indoxyl sulfate should be considered as a biomarker or risk factor for postinterventional vascular access thrombosis in patients with ESRD on hemodialysis.58
Peripheral Arterial Disease
Patients with CKD have an increased prevalence of PAD.59 Unfortunately, PAD in CKD is a challenging problem because it is associated with an increased risk of mortality and morbidity, including limb amputation.60 Moreover, outcomes of revascularization for critical limb ischemia in patients with CKD are poor.61 Risk factors for PAD among patients with CKD are not fully understood, but probably include both conventional and uremia‐associated risk factors. Jacob et al have shown, in a rat model of subtotal nephrectomy and hindlimb ischemia (HI), that CKD impairs angiogenesis and limb perfusion.62 Angiogenesis, defined as the sprouting of new blood vessels from preexisting vascular structures, is an essential physiological process for tissue ischemia. Indoxyl sulfate has been shown to inhibit endothelial proliferation and wound repair by increasing free radical production.27 Neovascularization involves not only the proliferation of local endothelial cells, but also circulating endothelial progenitor cells (EPCs) derived from bone marrow. In an ischemic microenvironment, ischemia‐induced hypoxia‐inducible factor (HIF)‐1α, vascular endothelial growth factor (VEGF), and NO are known to be essential for EPC mobilization, homing, and angiogenesis.63, 64
Indoxyl sulfate has been shown to have direct effects on EPCs through NO‐dependent mechanisms both in vitro and in vivo.65 In a recent study, we aimed to elucidate whether indoxyl sulfate could impede neovascularization in ischemic hindlimbs through modulating EPC function in CKD.42 Subtotal nephrectomy was undertaken in 8‐week‐old male C57BL/6 mice, which were then divided into 3 groups including the vehicle group, the indole group, and the indole plus AST‐120 group. Eight weeks later, unilateral HI surgery was introduced in all animals. At week 2 and week 4 after HI surgery, blood flow recovery, as detected by laser Doppler, was significantly impeded in the indole group compared to the vehicle group, but was improved in the indole plus AST‐120 group (Figure 2). In parallel, circulatory Sca1/Flk‐1‐positive EPCs and the density of CD31+ capillary neovessels in muscle of ischemic limbs after HI surgery were significantly reduced in the indole group compared to those in the vehicle group. Similarly, the reductions were significantly reversed in the indole plus AST‐120 group.
Figure 2.
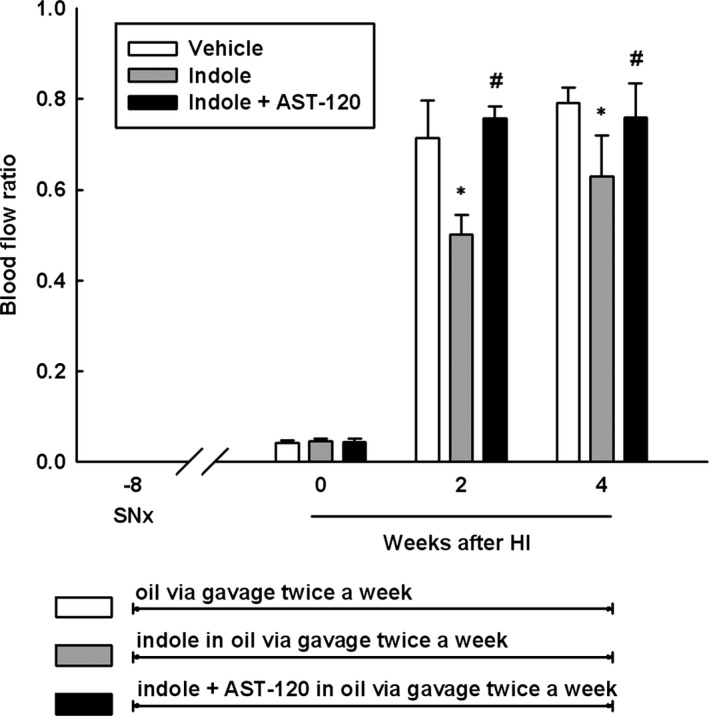
Effects of indoxyl sulfate on ischemia‐induced neovascularization in SNx mice. SNx mice were divided into 3 groups: the vehicle group, the indole group, and the indole+AST‐120 group. Blood perfusion in ischemic hindlimb was measured immediately after, and 2 and 4 weeks after, HI surgery by laser Doppler. Results are means±SEM. *P<0.05 versus vehicle group; # P<0.05 versus indole group; n=5 in each group. HI indicates hindlimb ischemia; SNx, subtotal nephrectomy.
In the same study, we first showed in vitro and in vivo that indoxyl sulfate suppresses EPC angiogenic function by inhibiting hypoxia‐induced HIF‐1α activation and consecutive interleukin (IL)‐10 and VEGF synthesis.42 The toxic effects of a variety of environmental contaminants, such as dioxins, are mediated by the aryl hydrocarbon receptor (AhR), for which indoxyl sulfate is an endogenous ligand. AhR requires aryl hydrocarbon receptor nuclear translocator (ARNT) to regulate gene expression. ARNT, also called HIF‐1β, is also required by HIF‐1α to enhance the expression of various genes in response to hypoxia. Therefore, AhR activation by indoxyl sulfate suppresses accumulation of the HIF‐1α‐ARNT complex in the nucleus in inverse proportion to the increase in amount of the nuclear AhR‐ARNT complex, resulting in inhibition of HIF‐1α activity.43 Recent evidence suggests that the adverse cardiovascular effects of indoxyl sulfate could be mediated by AhR activation, in a “dioxin‐like” effect.66 Together, by inducing endothelial dysfunction and TF expression, indoxyl sulfate favors atherosclerosis and thrombosis, which, in turn, results in limb ischemia. In the presence of tissue hypoxia, however, indoxyl sulfate further decreases the proangiogenic functions of EPCs by suppressing the HIF‐1α/IL‐10/VEGF pathway, leading to impaired neovascularization and thus progression of PAD. Our results strongly support the concept that therapies targeting indoxyl sulfate could be an alternative approach for PAD in patients with CKD.
Approaches to Decrease Gut‐Derived Uremic Toxin Production
Given that the gut microbiota generates an important portion of uremic toxins, modification of gut microenvironment and microbial composition represents a promising therapeutic target to decrease the generation of uremic toxins and their adverse cardiovascular effects. Interventions aimed at preventing gut‐derived uremic toxin production and absorption can be divided into 3 main approaches: dietary protein restriction, maintenance of gut symbiosis, and oral sorbents.67 Encouraging results, in terms of decreased indoxyl sulfate levels and cardiovascular protective effects, have been shown in several clinical studies in patients with CKD (Table 3).68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 Additional adequately powered randomized, controlled trials are required to determine whether indoxyl‐sulfate–lowering therapy reduces the risk of CVD in CKD.79, 80
Table 3.
Indoxyl Sulfate Lowering and Cardiovascular Effects in Studies Targeting on Gut‐Derived Uremic Toxins in Patients With CKD
| Reference | Patients | Intervention | Effects |
|---|---|---|---|
| Dietary protein restriction | |||
| 2013 Marzocco et al68 | CKD | Very low protein diet | ↓ Indoxyl sulfate |
| Maintenance of gut symbiosis | |||
| 1996 Hida et al69 | HD | Probiotics | ↓ Indoxyl sulfate |
| 2003 Takayama et al70 | HD | Probiotics | ↓ Indoxyl sulfate |
| 2014 Sirich et al71 | HD | Prebiotics | ↓ Indoxyl sulfate |
| Oral sorbents | |||
| 1997 Niwa et al72 | CKD | AST‐120 | ↓ Indoxyl sulfate |
| 2006 Schulman et al73 | CKD | AST‐120 | ↓ Indoxyl sulfate |
| 2004 Nakamura et al74 | CKD | AST‐120 | ↓ Carotid IMT, ↓ PWV |
| 2010 Shibahara et al75 | CKDa | AST‐120 |
↓ Edema, ↓ ANP, ↓ CT ratio ↓ Length of hospital stay |
| 2011 Yu et al76 | CKD | AST‐120 |
↓ Indoxyl sulfate, ↓ oxidative stress ↓ Endothelial dysfunction |
| 2011 Nakai et al77 | CKD | AST‐120 | ↓ Left ventricular concentric change |
| 2013 Goto et al78 | CKD | AST‐120 | ↓ Abdominal aortic calcification |
ANP indicates atrial natriuretic peptide; CKD, chronic kidney disease; CT, cardiothoracic; HD, hemodialysis; IMT, intima‐media thickness; PWV, pulse wave velocity.
CKD patients with congestive heart failure.
Conclusions
Patients with CKD are strongly predisposed to the development CVD. Evidence is emerging that besides the traditional risk factors, uremia‐specific risk factors that arise from the accumulation of gut‐derived uremic toxins, such as indoxyl sulfate, may be directly responsible for the pathogenesis of CVD in CKD. These pathophysiological effects explain the association between indoxyl sulfate concentration and the increased risk of CVD and mortality in patients with CKD. Therefore, strategies designed to reduce the serum levels of indoxyl sulfate may be considered as a novel therapeutic approach in improving the outcomes of CVD associated with CKD.
Sources of Funding
This work was supported by grants from the Novel Bioengineering and Technological Approaches to Solve Two Major Health Problems in Taiwan sponsored by the Taiwan Ministry of Science and Technology Academic Excellence Program (MOST 105‐2633‐B‐009‐003) and Research Project (MOST 103‐2314‐B‐005‐MY2 and MOST 105‐2314‐B‐014‐MY3), the Ministry of Science and Technology, ROC; Foundation for Poison Control; and Research Project (TCRD‐TPE‐103‐RT‐4 and TCRD‐TPE‐104‐RT‐4), Taipei Tzu Chi Hospital, Taiwan.
Disclosures
None.
J Am Heart Assoc. 2017;6:e005022 doi: 10.1161/JAHA.116.005022.
References
- 1. Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF. All‐cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462293 adults in Taiwan. Lancet. 2008;371:2173–2182. [DOI] [PubMed] [Google Scholar]
- 2. Levin A, Foley RN. Cardiovascular disease in chronic renal insufficiency. Am J Kidney Dis. 2000;36:S24–S30. [DOI] [PubMed] [Google Scholar]
- 3. Longenecker JC, Coresh J, Powe NR, Levey AS, Fink NE, Martin A, Klag MJ. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13:1918–1927. [DOI] [PubMed] [Google Scholar]
- 4. Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p‐cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25:1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uremic Toxin Work Group (EUTox) . Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin CJ, Liu HL, Pan CF, Chuang CK, Jayakumar T, Wang TJ, Chen HH, Wu CJ. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch Med Res. 2012;43:451–456. [DOI] [PubMed] [Google Scholar]
- 7. Cao XS, Chen J, Zou JZ, Zhong YH, Teng J, Ji J, Chen ZW, Liu ZH, Shen B, Nie YX, Lv WL, Xiang FF, Tan X, Ding XQ. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin J Am Soc Nephrol. 2015;10:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu CC, Hsieh MY, Hung SC, Kuo KL, Tsai TH, Lai CL, Chen JW, Lin SJ, Huang PH, Tarng DC. Serum indoxyl sulfate associates with post‐angioplasty thrombosis of dialysis grafts. J Am Soc Nephrol. 2016;27:1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dou L, Sallée M, Cerini C, Poitevin S, Gondouin B, Jourde‐Chiche N, Fallague K, Brunet P, Calaf R, Dussol B, Mallet B, Dignat‐George F, Burtey S. The cardiovascular effect of the uremic solute indole‐3 acetic acid. J Am Soc Nephrol. 2015;26:876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y. Free serum concentrations of the protein‐bound retention solute p‐cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–1087. [DOI] [PubMed] [Google Scholar]
- 11. Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P. Free p‐cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008;73:1173–1180. [DOI] [PubMed] [Google Scholar]
- 12. Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P. p‐Cresol and cardiovascular risk in mild‐to‐moderate kidney disease. Clin J Am Soc Nephrol. 2010;5:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA. Free p‐cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant. 2010;25:1183–1191. [DOI] [PubMed] [Google Scholar]
- 14. Lin CJ, Wu CJ, Pan CF, Chen YC, Sun FJ, Chen HH. Serum protein‐bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol Dial Transplant. 2010;25:3693–3700. [DOI] [PubMed] [Google Scholar]
- 15. Wu IW, Hsu KH, Hsu HJ, Lee CC, Sun CY, Tsai CJ, Wu MS. Serum free p‐cresyl sulfate levels predict cardiovascular and all‐cause mortality in elderly hemodialysis patients–a prospective cohort study. Nephrol Dial Transplant. 2012;27:1169–1175. [DOI] [PubMed] [Google Scholar]
- 16. Poesen R, Viaene L, Verbeke K, Augustijns P, Bammens B, Claes K, Kuypers D, Evenepoel P, Meijers B. Cardiovascular disease relates to intestinal uptake of p‐cresol in patients with chronic kidney disease. BMC Nephrol. 2014;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA, Yu AS. Serum trimethylamine‐N‐oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poesen R, Claes K, Evenepoel P, de Loor H, Augustijns P, Kuypers D, Meijers B. Microbiota‐derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J Am Soc Nephrol. 2016;27:3479–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A; European Uremic Toxin Work Group . Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW. Colonic contribution to uremic solutes. J Am Soc Nephrol. 2011;22:1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poesen R, Windey K, Neven E, Kuypers D, De Preter V, Augustijns P, D'Haese P, Evenepoel P, Verbeke K, Meijers B. The influence of CKD on colonic microbial metabolism. J Am Soc Nephrol. 2016;27:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. [DOI] [PubMed] [Google Scholar]
- 23. Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease‐ and uricase‐containing, indole‐ and p‐cresol‐forming and contraction of short‐chain fatty acid‐producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS. p‐Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tumur Z, Niwa T. Indoxyl sulfate inhibits NO production and cell viability by inducing oxidative stress in vascular endothelial cells. Am J Nephrol. 2009;29:551–557. [DOI] [PubMed] [Google Scholar]
- 27. Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P. The uremic solutes p‐cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. [DOI] [PubMed] [Google Scholar]
- 28. Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T. Indoxyl sulfate upregulates expression of ICAM‐1 and MCP‐1 by oxidative stress‐induced NF‐kappaB activation. Am J Nephrol. 2010;31:435–441. [DOI] [PubMed] [Google Scholar]
- 29. Ito S, Osaka M, Higuchi Y, Nishijima F, Ishii H, Yoshida M. Indoxyl sulfate induces leukocyte‐endothelial interactions through up‐regulation of E‐selectin. J Biol Chem. 2010;285:38869–38875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamamoto H, Tsuruoka S, Ioka T, Ando H, Ito C, Akimoto T, Fujimura A, Asano Y, Kusano E. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006;69:1780–1785. [DOI] [PubMed] [Google Scholar]
- 31. Muteliefu G, Enomoto A, Niwa T. Indoxyl sulfate promotes proliferation of human aortic smooth muscle cells by inducing oxidative stress. J Ren Nutr. 2009;19:29–32. [DOI] [PubMed] [Google Scholar]
- 32. Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast‐specific proteins in hypertensive rats. Nephrol Dial Transplant. 2008;23:1892–1901. [DOI] [PubMed] [Google Scholar]
- 33. Muteliefu G, Enomoto A, Jiang P, Takahashi M, Niwa T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast‐specific proteins in vascular smooth muscle cells. Nephrol Dial Transplant. 2009;24:2051–2058. [DOI] [PubMed] [Google Scholar]
- 34. Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010;31:1771–1779. [DOI] [PubMed] [Google Scholar]
- 35. Lekawanvijit S, Kompa AR, Manabe M, Wang BH, Langham RG, Nishijima F, Kelly DJ, Krum H. Chronic kidney disease‐induced cardiac fibrosis is ameliorated by reducing circulating levels of a non‐dialysable uremic toxin, indoxyl sulfate. PLoS One. 2012;7:e41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fujii H, Nishijima F, Goto S, Sugano M, Yamato H, Kitazawa R, Kitazawa S, Fukagawa M. Oral charcoal adsorbent (AST‐120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant. 2009;24:2089–2095. [DOI] [PubMed] [Google Scholar]
- 37. Tang WH, Wang CP, Chung FM, Huang LL, Yu TH, Hung WC, Lu LF, Chen PY, Luo CH, Lee KT, Lee YJ, Lai WT. Uremic retention solute indoxyl sulfate level is associated with prolonged QTc interval in early CKD patients. PLoS One. 2015;10:e0119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen WT, Chen YC, Hsieh MH, Huang SY, Kao YH, Chen YA, Lin YK, Chen SA, Chen YJ. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26:203–210. [DOI] [PubMed] [Google Scholar]
- 39. Aoki K, Teshima Y, Kondo H, Saito S, Fukui A, Fukunaga N, Nawata T, Shimada T, Takahashi N, Shibata H. Role of indoxyl sulfate as a predisposing factor for atrial fibrillation in renal dysfunction. J Am Heart Assoc. 2015;4:e002023 DOI: 10.1161/JAHA.115.002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chitalia VC, Shivanna S, Martorell J, Balcells M, Bosch I, Kolandaivelu K, Edelman ER. Uremic serum and solutes increase post‐vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation. 2013;127:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gondouin B, Cerini C, Dou L, Sallée M, Duval‐Sabatier A, Pletinck A, Calaf R, Lacroix R, Jourde‐Chiche N, Poitevin S, Arnaud L, Vanholder R, Brunet P, Dignat‐George F, Burtey S. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013;84:733–744. [DOI] [PubMed] [Google Scholar]
- 42. Hung SC, Kuo KL, Huang HL, Lin CC, Tsai TH, Wang CH, Chen JW, Lin SJ, Huang PH, Tarng DC. Indoxyl sulfate suppresses endothelial progenitor cell–mediated neovascularization. Kidney Int. 2016;89:574–585. [DOI] [PubMed] [Google Scholar]
- 43. Dou L, Burtey S. The harmful effect of indoxyl sulfate on neovascularization in chronic kidney disease. Kidney Int. 2016;89:532–534. [DOI] [PubMed] [Google Scholar]
- 44. Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290:697–701. [DOI] [PubMed] [Google Scholar]
- 45. Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010;6:723–735. [DOI] [PubMed] [Google Scholar]
- 46. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end‐stage renal disease. Circulation. 1999;99:2434–2439. [DOI] [PubMed] [Google Scholar]
- 47. Herzog CA, Mangrum JM, Passman R. Sudden cardiac death and dialysis patients. Semin Dial. 2008;21:300–307. [DOI] [PubMed] [Google Scholar]
- 48. Amann K, Neimeier KA, Schwarz U, Tornig J, Matthias S, Orth SR, Mall G, Ritz E. Rats with moderate renal failure show capillary deficit in heart but not skeletal muscle. Am J Kidney Dis. 1997;30:382–388. [DOI] [PubMed] [Google Scholar]
- 49. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators . Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 50. Saravanan P, Davidson NC. Risk assessment for sudden cardiac death in dialysis patients. Circ Arrhythm Electrophysiol. 2010;3:553–559. [DOI] [PubMed] [Google Scholar]
- 51. Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–939. [DOI] [PubMed] [Google Scholar]
- 52. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 53. Bleyer AJ, Hartman J, Brannon PC, Reeves‐Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69:2268–2273. [DOI] [PubMed] [Google Scholar]
- 54. Szczech LA. Atrial fibrillation: the beat is faster than the answers. Kidney Int. 2012;81:432–433. [DOI] [PubMed] [Google Scholar]
- 55. Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;123:2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hung SC, Lai YS, Kuo KL, Tarng DC. Volume overload and adverse outcomes in chronic kidney disease: clinical observational and animal studies. J Am Heart Assoc. 2015;4:e001918 DOI: 10.1161/JAHA.115.001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. Effects of angiotensin‐converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing‐induced congestive heart failure. Circulation. 2001;104:2608–2614. [DOI] [PubMed] [Google Scholar]
- 58. Nath KA. Dialysis vascular access intervention and the search for biomarkers. J Am Soc Nephrol. 2016;27:970–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18:629–636. [DOI] [PubMed] [Google Scholar]
- 60. Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end‐stage renal disease population. Kidney Int. 1999;56:1524–1533. [DOI] [PubMed] [Google Scholar]
- 61. Casserly IP. Interventional management of critical limb ischemia in renal patients. Am J Kidney Dis. 2008;15:384–395. [DOI] [PubMed] [Google Scholar]
- 62. Jacobi J, Porst M, Cordasic N, Namer B, Schmieder RE, Eckardt KU, Hilgers KF. Subtotal nephrectomy impairs ischemia‐induced angiogenesis and hindlimb re‐perfusion in rats. Kidney Int. 2006;69:2013–2021. [DOI] [PubMed] [Google Scholar]
- 63. Dimmeler S, Dernbach E, Zeiher AM. Phosphorylation of the endothelial nitric oxide synthase at ser‐1177 is required for VEGF‐induced endothelial cell migration. FEBS Lett. 2000;477:258–262. [DOI] [PubMed] [Google Scholar]
- 64. Aicher A, Heeschen C, Mildner‐Rihm C, Urbich C, Ihling C, Technau‐Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. [DOI] [PubMed] [Google Scholar]
- 65. Wu VC, Young GH, Huang PH, Lo SC, Wang KC, Sun CY, Liang CJ, Huang TM, Chen JH, Chang FC, Chen YL, Kuo YS, Chen JB, Chen JW, Chen YM, Ko WJ, Wu KD; NSARF group . In acute kidney injury, indoxyl sulfate impairs human endothelial progenitor cells: modulation by statin. Angiogenesis. 2013;16:609–624. [DOI] [PubMed] [Google Scholar]
- 66. Sallee M, Dou L, Cerini C. The aryl hydrocarbon receptor‐activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel). 2014;6:934–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lekawanvijit S, Kompa AR, Krum H. Protein‐bound uremic toxins: a long overlooked culprit in cardiorenal syndrome. Am J Physiol Renal Physiol. 2016;311:F52–F62. [DOI] [PubMed] [Google Scholar]
- 68. Marzocco S, Dal Piaz F, Di Micco L, Torraca S, Sirico ML, Tartaglia D, Autore G, Di Iorio B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013;35:196–201. [DOI] [PubMed] [Google Scholar]
- 69. Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74:349–355. [DOI] [PubMed] [Google Scholar]
- 70. Takayama F, Taki K, Niwa T. Bifidobacterium in gastro‐resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis. 2003;41:S142–S145. [DOI] [PubMed] [Google Scholar]
- 71. Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon‐derived solutes in hemodialysis patients. Clin J Am Soc Nephrol. 2014;9:1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S. The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int. 1997;62:S23–S28. [PubMed] [Google Scholar]
- 73. Schulman G, Agarwal R, Acharya M, Berl T, Blumenthal S, Kopyt N. A multicenter, randomized, double‐blind, placebo‐controlled, dose‐ranging study of AST‐120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis. 2006;47:565–577. [DOI] [PubMed] [Google Scholar]
- 74. Nakamura T, Kawagoe Y, Matsuda T, Ueda Y, Shimada N, Ebihara I, Koide H. Oral ADSORBENT AST‐120 decreases carotid intima‐media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press Res. 2004;27:121–126. [DOI] [PubMed] [Google Scholar]
- 75. Shibahara H, Shibahara N. Cardiorenal protective effect of the oral uremic toxin absorbent AST‐120 in chronic heart disease patients with moderate CKD. J Nephrol. 2010;23:535–540. [PubMed] [Google Scholar]
- 76. Yu M, Kim YJ, Kang DH. Indoxyl sulfate‐induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol. 2011;6:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nakai K, Fujii H, Kono K, Goto S, Fukagawa M, Nishi S. Effects of AST‐120 on left ventricular mass in predialysis patients. Am J Nephrol. 2011;33:218–223. [DOI] [PubMed] [Google Scholar]
- 78. Goto S, Kitamura K, Kono K, Nakai K, Fujii H, Nishi S. Association between AST‐120 and abdominal aortic calcification in predialysis patients with chronic kidney disease. Clin Exp Nephrol. 2013;17:365–371. [DOI] [PubMed] [Google Scholar]
- 79. Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67:483–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vanholder RC, Eloot S, Glorieux GL. Future avenues to decrease uremic toxin concentration. Am J Kidney Dis. 2016;67:664–676. [DOI] [PubMed] [Google Scholar]


