Abstract
Background
Clinical guidelines recommend using predicted atherosclerotic cardiovascular disease (ASCVD) risk to inform treatment decisions. The objective was to compare the contribution of changes in modifiable risk factors versus aging to the development of high 10‐year predicted ASCVD risk.
Methods and Results
A prospective follow‐up was done of the Jackson Heart Study, an exclusively black cohort at visit 1 (2000–2004) and visit 3 (2009–2012). Analyses included 1115 black participants without high 10‐year predicted ASCVD risk (<7.5%), hypertension, diabetes mellitus, or ASCVD at visit 1. We used the Pooled Cohort equations to calculate the incidence of high (≥7.5%) 10‐year predicted ASCVD risk at visit 3. We recalculated the percentage with high 10‐year predicted ASCVD risk at visit 3 assuming each risk factor (age, systolic blood pressure, antihypertensive medication use, diabetes mellitus, smoking, total and high‐density lipoprotein cholesterol), one at a time, did not change from visit 1. The mean age at visit 1 was 45.2±9.5 years. Overall, 30.9% (95% CI 28.3–33.4%) of participants developed high 10‐year predicted ASCVD risk. Aging accounted for 59.7% (95% CI 54.2–65.1%) of the development of high 10‐year predicted ASCVD risk compared with 32.8% (95% CI 27.0–38.2%) for increases in systolic blood pressure or antihypertensive medication initiation and 12.8% (95% CI 9.6–16.5%) for incident diabetes mellitus. Among participants <50 years, the contribution of increases in systolic blood pressure or antihypertensive medication initiation was similar to aging.
Conclusions
Increases in systolic blood pressure and antihypertensive medication initiation are major contributors to the development of high 10‐year predicted ASCVD risk in blacks, particularly among younger adults.
Keywords: blacks, atherosclerotic cardiovascular disease risk, blood pressure, hypertension, population
Subject Categories: Cardiovascular Disease, Race and Ethnicity, High Blood Pressure, Risk Factors, Hypertension
Introduction
Several clinical practice guidelines recommend using predicted atherosclerotic cardiovascular disease (ASCVD) risk to inform treatment initiation decisions.1, 2, 3 Age is a major nonmodifiable risk factor for ASCVD and a component of virtually all ASCVD risk prediction equations.4, 5 Predicted ASCVD risk is also determined by modifiable risk factors. For example, the Pooled Cohort risk equations from the American College of Cardiology/American Heart Association use age, race, sex, systolic blood pressure (SBP), antihypertensive medication use, diabetes mellitus, cigarette smoking, and total and high‐density lipoprotein (HDL) cholesterol to estimate 10‐year predicted ASCVD risk.5
Identifying modifiable risk factors with the largest relative contribution to the development of high ASCVD risk may inform which of these factors to target for primordial prevention and population health management.6 This is particularly important for blacks, a population with a higher burden of modifiable risk factors including hypertension and diabetes mellitus and a higher average 10‐year predicted ASCVD risk compared to other race/ethnic groups in the United States.7, 8, 9 Therefore, we identified the contribution of changes in modifiable risk factors, independent of aging, on developing high 10‐year predicted ASCVD risk (≥7.5%) in the Jackson Heart Study (JHS), a cohort comprising exclusively blacks. As aging is an important, nonmodifiable ASCVD risk factor, we contrasted changes in the modifiable risk factors versus the contribution of aging over the study period. Because high 10‐year predicted ASCVD risk increases with age, we conducted secondary analyses for younger (age <50 years) and older (age ≥50 years) adults, separately.8
Methods
Study Population
The JHS was designed to examine cardiovascular disease risk in black men and women.10, 11 The study enrolled 5306 blacks in Jackson, MS between 2000 and 2004. Participants completed study visits at baseline in 2000–2004 (visit 1) and during follow‐up in 2005–2008 (visit 2) and 2009–2012 (visit 3). The current analysis was restricted to 1987 JHS participants who did not have a history of ASCVD, hypertension, or diabetes mellitus at visit 1. We excluded participants who did not attend visit 3 (n=433) and those with missing data needed to calculate 10‐year predicted ASCVD risk at visit 1 or visit 3 using the Pooled Cohort risk equations (n=218). Additionally, we excluded 221 participants with high 10‐year predicted ASCVD risk, defined as ≥7.5%, at visit 1. The remaining 1115 JHS participants were analyzed (Figure 1). The protocol for the JHS was approved by the institutional review boards of the University of Mississippi Medical Center, Jackson State University, and Tougaloo College. All participants provided written informed consent. The analysis of JHS data for the current study was approved by the institutional review board at the University of Alabama at Birmingham.
Figure 1.
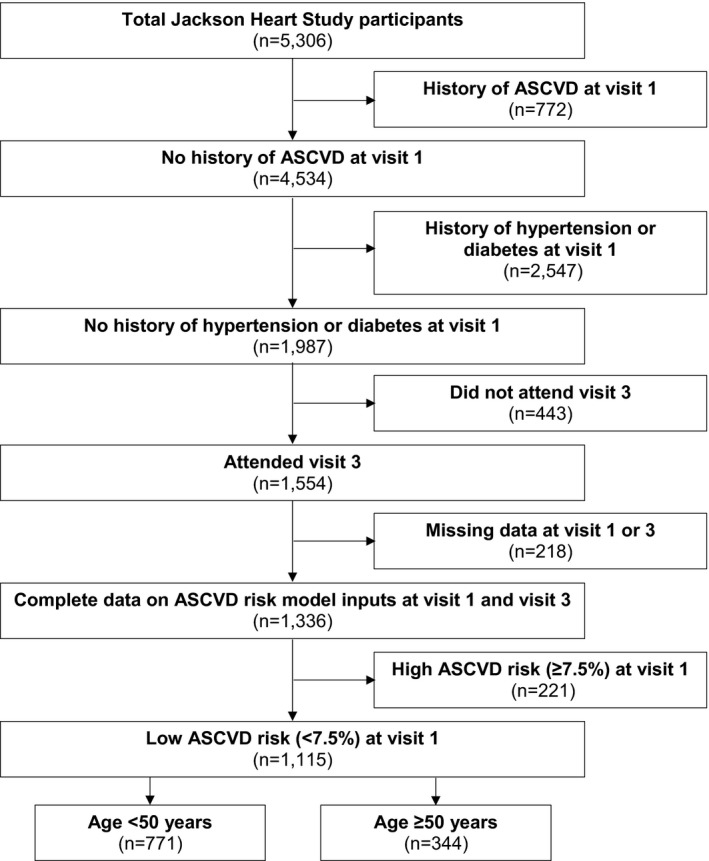
Flowchart showing the eligibility criteria applied to participants in the Jackson Heart Study. ASCVD indicates atherosclerotic cardiovascular disease.
Data Collection
Data collected through interview‐administered questionnaires at visit 1 and visit 3 included age, sex, education, antihypertensive medication use, and history of ASCVD defined by a prior myocardial infarction, stroke, or coronary, carotid, or peripheral revascularization procedure. Use of statin and antidiabetes mellitus medication was collected through a pill bottle review. Specifically, participants were asked to bring all medications they took in the past 2 weeks to the study visit. Drug names were recorded by trained staff. If a participant did not bring all of their medications, a complete medication list was obtained by a postexamination phone call to the participant or their pharmacy. Data on smoking status was collected at visit 1 and every 6 months thereafter during follow‐up via phone call. Smoking status at visit 3 was determined based on the 6‐month phone call closest to visit 3.
Blood pressure was measured 2 times during each visit following a standardized protocol. The 2 blood pressure measurements were averaged for analysis. Blood pressure was measured using a random‐zero sphygmomanometer (Hawksley and Sons Ltd., Lancing, UK) at visit 1 and a semiautomatic oscillometric device (Omron HEM‐907XL, Omron Healthcare Inc., Lake Forest, IL) at visit 3. As described previously, the random‐zero blood pressure measurements were calibrated to the semiautomated device using robust regression.12 Hypertension was defined as SBP ≥140 mm Hg, diastolic blood pressure (DBP) ≥90 mm Hg, or self‐reported antihypertensive medication use.
Fasting blood samples were collected during visits 1 and 3. Serum glucose was measured using a glucose oxidase method on a Vitros 250 or 950, Ortho‐Clinical Diagnostics analyzer. Hemoglobin A1c was measured using a TOSOH high‐performance liquid chromatography system. Triglycerides and total and HDL cholesterol were measured using standard techniques.13 Low‐density lipoprotein cholesterol was calculated using the Friedewald equation.14 Diabetes mellitus was defined by self‐reported history with current use of oral antidiabetes mellitus medication or insulin, fasting glucose ≥126 mg/dL, or hemoglobin A1c ≥6.5%.
10‐Year Predicted ASCVD Risk
We calculated 10‐year predicted ASCVD risk for black men and women at visits 1 and 3 using the Pooled Cohort risk equations (Table S1).5 These equations have been shown to have good calibration and discrimination in several cohorts including the JHS.5, 15, 16 The sex‐ and race‐specific Pooled Cohort risk equations include the following risk factors: age, cigarette smoking, SBP, antihypertensive medication use, diabetes mellitus, and total and HDL cholesterol. We defined a 10‐year predicted ASCVD ≥7.5% as high; this is the level that is used to denote consideration of statin initiation for primary prevention of ASCVD in the American College of Cardiology/American Heart Association Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults.3
Statistical Analysis
We calculated the distribution of 10‐year predicted ASCVD risk at visits 1 and 3. Participant characteristics at visits 1 and 3 were calculated for the overall population and for those with and without high ASCVD risk at visit 3, separately. Two‐sample t tests and χ2 tests, as appropriate, were used to compare the characteristics for participants who did and did not develop high ASCVD risk by visit 3.
We calculated the mean 10‐year predicted ASCVD risk at visits 1 and 3 and the change in ASCVD risk between these visits. We recalculated the mean 10‐year predicted ASCVD risk at visit 3, assuming each risk factor in the Pooled Cohort risk equations did not change from visit 1. For example, the mean 10‐year predicted ASCVD risk at visit 3 was calculated using participants' ages at visit 1 and all other risk factors from visit 3. This was repeated assuming (1) SBP and antihypertensive medication use, (2) current smoking, (3) diabetes mellitus, and (4) total and HDL cholesterol, one at a time, did not change between visits 1 and 3. The increase in 10‐year predicted ASCVD risk attributable to aging and, separately, change in each risk factor was calculated as the observed 10‐year predicted ASCVD risk at visit 3 minus the recalculated 10‐year predicted ASCVD risk, assuming each risk factor had not changed from visit 1 (Figure S1, Equation 1). This was also calculated as a percentage of the overall change in 10‐year predicted ASCVD risk between visits 1 and 3 and as a ratio of the change in risk attributable to aging (Figure S1, Equations 2 and 3, respectively). We recalculated the percentage of participants with high 10‐year predicted ASCVD risk (≥7.5%) at visit 3 assuming age and each modifiable risk factor in the Pooled Cohort risk equations, one at a time, did not change from visit 1. We also calculated the percent of the observed high 10‐year predicted ASCVD risk at visit 3 that was attributable to participants aging and changes in modifiable risk factors, overall and as a ratio of high 10‐year predicted ASCVD risk solely because of participants aging (Figure S1, Equations 4–6). A 1000‐iteration bootstrap was used to calculate 95% CI for the amount of the change in mean 10‐year predicted ASCVD risk and prevalence of high 10‐year predicted ASCVD risk attributable to aging and changes in modifiable risk factors.17 Because the Pooled Cohort risk equations were derived among adults ≥40 years of age, we performed a sensitivity analysis restricted to JHS participants who were ≥40 years of age at visit 1 (n=798). Analyses were repeated within subgroups of age defined by <50 years and ≥50 years. All analyses were conducted using SAS software, Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
The median time between visits 1 and 3 was 8.0 years (range 6.4–11.8 years). Among the 1115 JHS participants meeting the inclusion criteria, 345 (30.9%) developed a high 10‐year predicted ASCVD risk at visit 3 (Figure 2). At visit 1, participants who developed versus those who did not develop a high 10‐year predicted ASCVD risk at visit 3 were older, more likely to be male, have less than a high school education, smoke cigarettes, and take statins (Table 1, top panel). Those who developed high 10‐year predicted ASCVD risk also had a higher mean SBP, DBP, and total and low‐density lipoprotein cholesterol at visit 1 than their counterparts who did not develop high 10‐year predicted ASCVD risk at visit 3. At visit 3, 41.5% and 9.4% of participants had developed hypertension and diabetes mellitus, respectively (Table 1, bottom panel).
Figure 2.
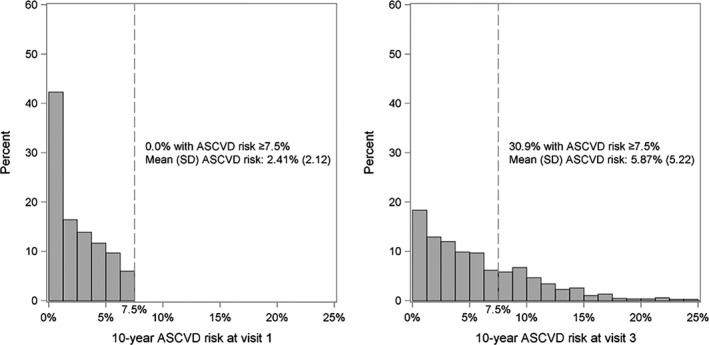
Distribution of 10‐year ASCVD risk over an 8‐year time period (visit 1–visit 3) in the overall population (n=1115). ASCVD indicates atherosclerotic cardiovascular disease.
Table 1.
Participant Characteristics at Visit 1 (Top Panel) and Visit 3 (Bottom Panel), Overall and by 10‐Year Predicted ASCVD Risk at Visit 3 (n=1115)
| All Participants (N=1115) | 10‐Year ASCVD Risk at Visit 3 | |||
|---|---|---|---|---|
| <7.5% (N=770) | ≥7.5% (N=345) | P Value | ||
| Characteristics at visit 1 | ||||
| Age, y | 45.2 (9.5) | 41.6 (8.0) | 53.0 (7.6) | <0.001 |
| Age group, y | ||||
| <30 | 70 (6.3) | 69 (9.0) | 1 (0.3) | <0.001 |
| 30 to 39 | 247 (22.2) | 235 (30.5) | 12 (3.5) | |
| 40 to 49 | 454 (40.7) | 348 (45.2) | 106 (30.7) | |
| 50 to 59 | 266 (23.9) | 114 (14.8) | 152 (44.1) | |
| ≥60 | 78 (7.0) | 4 (0.5) | 74 (21.4) | |
| Male sex | 375 (33.6) | 207 (26.9) | 168 (48.7) | <0.001 |
| Less than high school education | 66 (5.9) | 35 (4.5) | 31 (9.0) | 0.006 |
| Current smoker | 105 (9.4) | 57 (7.4) | 48 (13.9) | <0.001 |
| BMI, kg/m2 | 30.8 (7.2) | 31.0 (7.4) | 30.5 (6.8) | 0.32 |
| SBP, mm Hg | 116.7 (10.2) | 115.2 (10.1) | 120.1 (9.6) | <0.001 |
| DBP, mm Hg | 74.1 (7.2) | 73.5 (7.0) | 75.4 (7.4) | <0.001 |
| Total cholesterol, mg/dL | 193.3 (37.7) | 188.9 (36.9) | 203.1 (37.7) | <0.001 |
| HDL cholesterol, mg/dL | 51.6 (13.3) | 52.1 (13.1) | 50.5 (13.6) | 0.06 |
| LDL cholesterol, mg/dL | 124.3 (35.7) | 120.8 (35.2) | 132.2 (35.5) | <0.001 |
| Statin use | 19 (1.8) | 6 (0.8) | 13 (4.1) | <0.001 |
| Characteristics at visit 3 | ||||
| Age, y | 53.2 (9.4) | 49.7 (7.9) | 61.1 (7.5) | <0.001 |
| Age group, y | ||||
| <30 | 7 (0.6) | 7 (0.9) | 0 (0.0) | <0.001 |
| 30 to 39 | 81 (7.3) | 79 (10.3) | 2 (0.6) | |
| 40 to 49 | 316 (28.3) | 293 (38.1) | 23 (6.7) | |
| 50 to 59 | 439 (39.4) | 316 (41.0) | 123 (35.7) | |
| 60 to 69 | 222 (19.9) | 73 (9.5) | 149 (43.2) | |
| ≥70 | 50 (4.5) | 2 (0.3) | 48 (13.9) | |
| Male sex | 375 (33.6) | 207 (26.9) | 168 (48.7) | <0.001 |
| Less than high school education | 64 (5.8) | 32 (4.2) | 32 (9.4) | 0.001 |
| Current smoker | 100 (9.0) | 46 (6.0) | 54 (15.7) | <0.001 |
| BMI, kg/m2 | 32.1 (7.3) | 32.5 (7.8) | 31.1 (6.2) | 0.002 |
| SBP, mm Hg | 121.2 (14.2) | 118.2 (12.7) | 128.0 (14.9) | <0.001 |
| DBP, mm Hg | 76.3 (10.0) | 75.7 (9.4) | 77.7 (10.9) | 0.002 |
| Antihypertensive medication | 355 (31.8) | 182 (23.6) | 173 (50.1) | <0.001 |
| Hypertension | 463 (41.5) | 241 (31.3) | 222 (64.3) | <0.001 |
| Diabetes mellitus | 105 (9.4) | 28 (3.6) | 77 (22.3) | <0.001 |
| Total cholesterol, mg/dL | 199.7 (37.2) | 196.3 (35.7) | 207.4 (39.3) | <0.001 |
| HDL cholesterol, mg/dL | 58.0 (15.3) | 58.6 (15.5) | 56.5 (14.9) | 0.04 |
| LDL cholesterol, mg/dL | 123.9 (34.0) | 120.9 (32.7) | 130.5 (36.0) | <0.001 |
| Statin use | 133 (13.1) | 73 (10.5) | 60 (18.8) | <0.001 |
Numbers in the table are mean (SD) or n (percentage). ASCVD indicates atherosclerotic cardiovascular disease; BMI, body mass index; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
The mean 10‐year predicted ASCVD risk increased from 2.41% (95% CI 2.28%, 2.53%) at visit 1 to 5.87% (95% CI 5.57%, 6.18%) at visit 3 (Table 2). When calculating 10‐year predicted ASCVD risk at visit 3 using participants' ages at visit 1 and all other risk factor levels from visit 3, the mean 10‐year predicted ASCVD risk was 3.45% (95% CI 3.24%, 3.65%). Aging alone accounted for 69.8% (95% CI 67.5%, 72.1%) of the overall increase in 10‐year predicted ASCVD risk between visits 1 and 3. Change in SBP and initiation of antihypertensive medication and incident diabetes mellitus accounted for 35.3% (95% CI 32.4%, 38.0%) and 17.3% (95% CI 14.2%, 20.5%), respectively, of the overall increase in mean 10‐year predicted ASCVD risk.
Table 2.
Increase in Mean 10‐Year Predicted ASCVD Risk Between Visits 1 and 3, Overall and Attributable to Aging and Changes in Modifiable Risk Factors
| Risk Factor | Mean 10‐Year Predicted ASCVD Risk (95% CI) | Increase in 10‐Year ASCVD Risk (Visit 3—Visit 1) Because of Aging and Changes in Each Risk Factor | ||
|---|---|---|---|---|
| Increasea (95% CI) | Percentage of Overall Increase (95% CI) | Ratio as Compared to Aging (95% CI) | ||
| Observed risk at visit 1 | 2.41 (2.28, 2.53) | … | … | … |
| Observed risk at visit 3 | 5.87 (5.57, 6.18) | … | … | … |
| Overall increase (visit 3 minus visit 1) | 3.46 (3.25, 3.69) | … | 100 (reference) | … |
| Risk factor at visit 1 used for prediction | ||||
| Age | 3.45 (3.24, 3.65) | 2.42 (2.32, 2.54) | 69.8 (67.5, 72.1) | 100 (reference) |
| SBP or initiation of antihypertensive medication | 4.65 (4.41, 4.89) | 1.22 (1.09, 1.37) | 35.3 (32.4, 38.0) | 50.6 (45.3, 55.7) |
| Smoking | 5.88 (5.58, 6.17) | −0.01 (−0.07, 0.06) | b | b |
| Diabetes mellitus | 5.27 (5.02, 5.53) | 0.60 (0.48, 0.74) | 17.3 (14.2, 20.5) | 24.8 (20.1, 29.8) |
| Total and HDL cholesterol | 6.10 (5.79, 6.42) | −0.23 (−0.29, −0.16) | b | b |
Bootstrapping was used to quantify the 95% CIs of the 10‐year predicted ASCVD risk estimates. The percentages in the column labeled “Percentage of overall increase (95% CI)” do not add up to 100% because they are not mutually exclusive. ASCVD indicates atherosclerotic cardiovascular disease; HDL, high‐density lipoprotein; SBP, systolic blood pressure.
The overall increase in 10‐year predicted ASCVD risk attributable to aging and the change in each risk factor, separately, calculated as the 10‐year predicted ASCVD risk at visit 3 minus the recalculated 10‐year predicted ASCVD risk at visit 3, assuming the risk factor in the row had not changed from visit 1. The equations used to calculate percentage of overall increase and the ratio as compared to aging are shown in Figure S1.
Smoking and cholesterol did not contribute to the increase in mean 10‐year predicted ASCVD risk increase. See column labeled “Increase* (95% CI)”.
Overall, 30.9% of JHS participants included in the analysis developed a high 10‐year predicted ASCVD risk at visit 3 (Table 3). Assuming no change in participants' ages from visit 1, 12.5% (95% CI 10.5%, 14.4%) of participants would have a high 10‐year ASCVD risk at visit 3. Aging alone accounted for 59.7% (95% CI 54.2%, 65.1%) of the development of a high 10‐year predicted ASCVD risk between visits 1 and 3. Assuming no change in SBP or initiation of antihypertensive medication by visit 3, 20.8% (95% CI 18.5%, 23.1%) of participants would still have developed a high 10‐year predicted ASCVD risk. Changes in SBP and antihypertensive medication initiation accounted for 32.8% (95% CI 27.0%, 38.2%) of the overall percentage of participants developing high 10‐year predicted ASCVD risk at visit 3. The incidence of diabetes mellitus accounted for a smaller percentage of the development of high 10‐year predicted ASCVD risk, and changes in smoking and total and HDL cholesterol did not account for any of the development of high 10‐year predicted ASCVD risk. Results were similar when the analysis was restricted to participants ≥40 years of age at visit 1 (Tables S2 and S3).
Table 3.
Percentage of Participants Developing High Predicted ASCVD Risk Between Visits 1 and 3, Overall, and Attributable to Aging and Modifiable Risk Factors (n=1115)
| High 10‐Year Predicted ASCVD Risk (95% CI) | High 10‐Year ASCVD Risk Because of Individual Risk Factors | |||
|---|---|---|---|---|
| Percent (95% CI) | Percent Over Observed High Risk (95% CI) | Ratio as Compared to Aging (95% CI) | ||
| Observed | ||||
| Visit 1 | 0.0 | … | … | … |
| Visit 3 | 30.9 (28.3, 33.4) | … | … | … |
| Overall increase (visit 3 minus visit 1) | 30.9 (28.3, 33.4) | … | 100 (reference) | … |
| Risk factor at visit 1 used for prediction | ||||
| Age | 12.5 (10.5, 14.4) | 18.5 (16.3, 20.9) | 59.7 (54.2, 65.1) | 100 (reference) |
| SBP or initiation of antihypertensive medication | 20.8 (18.5, 23.1) | 10.1 (8.2, 12.1) | 32.8 (27.0, 38.2) | 54.9 (45.9, 63.8) |
| Smoking | 31.3 (28.7, 33.8) | −0.4 (−1.1, 0.1) | a | a |
| Diabetes mellitus | 27.0 (24.3, 29.2) | 3.9 (2.8, 5.0) | 12.8 (9.6, 16.5) | 21.4 (15.7, 27.4) |
| Total and HDL cholesterol | 31.7 (28.8, 34.3) | −0.8 (−2.0, 0.3) | a | a |
The equations used to calculate the percent over observed high risk and the ratio as compared to aging are shown in Figure S1. Bootstrapping was used to quantify the 95% CIs of the percentage developing a high 10‐year ASCVD predicted risk. High predicted ASCVD risk is defined at ≥7.5%. The percentages in the column labeled “Percent over observed high risk (95% CI)” do not add up to 100% because they are not mutually exclusive. ASCVD indicates atherosclerotic cardiovascular disease; HDL, high‐density lipoprotein; SBP, systolic blood pressure.
Percentage of overall change because of smoking and cholesterol not contributing to the development of high 10‐year predicted ASCVD risk.
Change in 10‐Year ASCVD Risk by Age
Among 771 participants <50 years of age at visit 1, 119 (15.4%) developed high 10‐year predicted ASCVD risk at visit 3 compared with 226 of 344 (65.7%) participants ≥50 years of age at visit 1 (Figure S2). Characteristics of participants who developed and did not develop a high 10‐year predicted ASCVD risk at visit 3 by age category are provided in Table S4 (participants <50 years of age) and Table S5 (participants ≥50 years of age).
Compared with the other modifiable risk factors, increasing SBP and initiation of antihypertensive medication accounted for a larger amount of the increase in mean 10‐year predicted ASCVD risk between visits 1 and 3 among both participants <50 and ≥50 years of age (Table S6). Assuming no change in SBP and no initiation of antihypertensive medication by visit 3, 6.4% (95% CI 4.5%, 7.9%) and 53.2% (95% CI 47.7%, 58.4%) of participants <50 years and ≥50 years, respectively, would have developed a high 10‐year predicted ASCVD risk (Table S7). Among participants <50 years of age, increasing SBP and antihypertensive medication initiation accounted for 58.8% (95% CI 49.2%, 68.8%) of the development of high 10‐year predicted ASCVD risk, which was similar to the contribution of aging alone (65.5% [95% CI 56.7%, 73.6%]). Among participants ≥50 years of age, aging alone accounted for 56.6% (95% CI 50.5%, 63.5%), with each modifiable risk factor contributing less than 20% of the development of high 10‐year predicted ASCVD risk at visit 3.
Discussion
In the current prospective study of blacks with a mean age of 45 years and 10‐year predicted ASCVD risk <7.5%, ≈1 out of 3 participants developed a 10‐year predicted ASCVD risk ≥7.5% over an 8‐year follow‐up period. Aging was the largest contributor, accounting for ≈60% of the development of high 10‐year predicted ASCVD risk. Increases in SBP or initiation of antihypertensive medication was the modifiable risk factor that contributed the highest proportion to the development of high 10‐year predicted ASCVD risk, especially among participants <50 years of age at baseline. In participants age <50 years, the contribution of increases in SBP or initiation of antihypertensive medication to 10‐year predicted ASCVD risk was similar to the contribution of aging alone. Incident diabetes mellitus and changes in smoking status and total and HDL cholesterol had a smaller contribution to the development of high 10‐year predicted ASCVD risk.
In the current study, ASCVD risk increased substantially over 8 years of follow‐up and aging accounted for more than two thirds of this increase. This finding indicates that many people will develop high ASCVD risk simply by aging alone, potentially becoming candidates for treatment with a statin or aspirin.1, 3 It has been previously shown that all adults will eventually develop a 10‐year predicted ASCVD risk ≥7.5% despite maintaining an optimal risk factor profile.18 For example, among adults with optimal modifiable risk factor levels (ie, no diabetes mellitus and not a smoker, total and HDL cholesterol of 170 and 50 mg/dL, respectively, and SBP of 110 mm Hg without antihypertensive medication use), the development of high ASCVD risk will occur at ages 65, 70, and 75 years for non‐Hispanic white men, black men and women, and non‐Hispanic white women, respectively.18 However, as aging did not explain all of the increase in ASCVD risk in the current study, preventing modifiable risk factors will delay the development of high ASCVD risk.
Hypertension is a well‐established and modifiable ASCVD risk factor that disproportionately affects blacks.7, 19, 20 Blacks have higher mean SBP beginning in childhood and develop hypertension earlier in life compared with other race/ethnic groups in the United States.19, 21, 22 Over the 8 years of follow‐up in the current study, the mean SBP increased by ≈5 mm Hg and 41.5% of participants developed hypertension. These changes accounted for a substantial proportion of the increase in mean 10‐year predicted ASCVD risk and over half of the participants with high 10‐year predicted ASCVD risk at visit 3 had also developed hypertension. In the current study, the impact of increasing SBP and incident hypertension was greater among blacks <50 years at baseline, and this effect was similar in size to the contribution from aging alone. Results from the current analysis suggest that preventing increases in mean SBP and incident hypertension in young blacks could reduce the mean 10‐year predicted ASCVD risk by a magnitude similar to the effect of aging alone.
Diabetes mellitus is a well‐established ASCVD risk factor and has a high prevalence in blacks.23, 24 In the current prospective study, ≈10% of participants developed diabetes mellitus. At the population level, incident diabetes mellitus contributed less than changes in SBP or initiation of antihypertensive medication to the development of high 10‐year predicted ASCVD risk. As most people with diabetes mellitus have high ASCVD risk, it remains an important target for primary prevention of ASCVD.25 Furthermore, there are common behavioral determinants of both hypertension and diabetes mellitus including diet and physical activity.26, 27 Interventions focused on these health behaviors have the potential to reduce both increases in SBP and the development of diabetes mellitus.
Smoking and blood cholesterol levels are well‐established ASCVD risk factors.28, 29 However, changes in these risk factors did not contribute to the development of high 10‐year predicted ASCVD risk among blacks in the current analysis. In JHS, the percentage of participants who were smokers did not change substantially throughout the study period. This is consistent with data indicating that very few individuals start smoking after the age of 25 years.30 Participants who developed high 10‐year ASCVD risk were more likely to be smokers at baseline compared with those who did not develop high 10‐year ASCVD risk. This suggests that population‐wide benefits may be present with smoking cessation interventions. Similarly, participants who developed high 10‐year ASCVD risk had higher total and lower HDL cholesterol levels at visit 1 compared to their counterparts who did not develop high risk. Although levels of total cholesterol increased during follow‐up, levels of HDL cholesterol also increased. This may explain the lack of contribution of changes in cholesterol levels to the incidence of high ASCVD risk because high HDL cholesterol is associated with a lower ASCVD risk.31 Also, increases in total cholesterol during follow‐up may have been attenuated because of statin initiation. Statin use is not incorporated into ASCVD risk prediction equations, and the accuracy of these equations among individuals taking statins has not been well investigated.
There are many reasons why it is important to prevent the development of high 10‐year predicted ASCVD risk. Prior studies have reported that most ASCVD events occur among people with 10‐year predicted ASCVD risk ≥7.5%.15, 32 Also, predicted ASCVD risk is increasingly being used in clinical practice guidelines.1, 2, 3 For example, guideline recommendations for the use of statins and aspirin for primary prevention of ASCVD are, in part, based on an individual's 10‐year predicted ASCVD risk.1, 3 Therefore, preventing the development of high 10‐year predicted ASCVD risk through targeting modifiable risk factors could delay medication initiation. In turn, this could reduce medication costs, polypharmacy and risk of adverse events, and contribute to increased quality of life into older ages.
Healthcare reform in the United States has placed increasing emphasis on population health management.33 Population health managers are often challenged with deciding on which interventions to implement, and who should receive them, to optimize the health of the overall population. The current study suggests that developing high ASCVD risk could be averted for a substantial percentage of blacks through interventions that prevent increases in SBP and the need for initiation of antihypertensive medication. These may include physical activity, limiting sodium and alcohol intake, weight loss, and the Dietary Approaches to Stop Hypertension (DASH) diet, which have all been shown to lower blood pressure.34, 35
The current study has several strengths. These include the prospective study design, a large sample of blacks, and the use of standardized protocols to measure ASCVD risk factors, blood pressure, and other components of the Pooled Cohort risk equations at 2 time points nearly 8 years apart. The JHS is a community‐based sample of exclusively blacks in 3 counties surrounding Jackson, MS. Therefore, the results may not be generalizable to other race/ethnic groups or geographic regions.
In summary, ≈1 out of 3 blacks in the JHS developed a high 10‐year predicted ASCVD risk over a median follow‐up of 8 years. Increases in SBP and initiation of antihypertensive medication were the modifiable risk factors accounting for the largest percentage of the development of high ASCVD risk. Among adults <50 years, increases in SBP or initiation of antihypertensive medication contributed to the development of high ASCVD risk similar to aging 8 years. These results support the need for the development and implementation of population health management interventions to reduce the incidence of high ASCVD risk among blacks by preventing increases in SBP and incident hypertension.
Sources of Funding
The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (N01‐HC‐95170); University of Mississippi Medical Center (N01‐HC‐95171); and Tougaloo College (N01‐HC‐95172). The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. This work was also supported by the NIH (R01 HL117323, K24‐HL125704) from the National Heart, Lung, and Blood Institute and the National Institute of Health, Bethesda, MD. Dr Bress was supported by the NIH 5KL2TR001065 (PI Byington/Dere) from the National Center for Advancing Translational Sciences, Bethesda, MD. Drs Bress, Colantonio, and Muntner had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Disclosures
Bress receives research support from Novartis unrelated to the current manuscript. Reynolds has received research support from Amgen and Regeneron Pharmaceuticals, Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1. Estimation of Race‐ and Gender‐Specific ASCVD Risk Using the ASCVD Pooled Cohort Risk Equations
Table S2. Increase in Mean 10‐Year Predicted ASCVD Risk (%) Between Visits 1 and 3, Overall, and Attributable to Aging and Changes in Modifiable Risk Factors Among Participants Age ≥40 Years at Visit 1 (n=798)
Table S3. Percentage of Participants ≥40 Years of Age Developing High Predicted ASCVD Risk Between Jackson Heart Study Visits 1 and 3, Overall, and Attributable to Aging and Modifiable Risk Factors
Table S4. Participant Characteristics at Visit 1 (Top Panel) and Visit 3 (Bottom Panel) Overall and by 10‐Year Predicted ASCVD Risk at Visit 3 Among Those Age <50 Years (n=771)
Table S5. Participant Characteristics at Visit 1 (Top Panel) and Visit 3 (Bottom Panel) Overall and by 10‐Year Predicted ASCVD Risk at Visit 3 Among Those Age ≥50 Years (n=344)
Table S6. Increase in Mean 10‐Year Predicted ASCVD Risk Between Visits 1 and 3, Overall, and Attributable to Aging and Changes in Modifiable Risk Factors Among Those Age <50 Years (Top Panel) and ≥50 Years (Bottom Panel)
Table S7. Percentage of Participants Age <50 Years (Top Panel) and ≥50 Years (Bottom Panel) Developing High Predicted ASCVD Risk Between Jackson Heart Study Visits 1 and 3 Attributable to Aging and Modifiable Risk Factors
Figure S1. Equations used to calculate the percentage of overall change in 10‐year predicted ASCVD risk due to risk factors and ratio as compared to aging.
Figure S2. Histograms of 10‐year ASCVD risk at visit 1 and visit 3 among participants <50 years of age (top panel, n=771) and ≥50 years of age (bottom panel, n=344).
Acknowledgments
The authors thank the participants and data collection staff of the Jackson Heart Study.
(J Am Heart Assoc. 2017;6:e005054. DOI: 10.1161/JAHA.116.005054.)
References
- 1. Bibbins‐Domingo K; Force USPST . Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836–845. [DOI] [PubMed] [Google Scholar]
- 2. McCormack T, Krause T, O'Flynn N. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract. 2012;62:163–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 4. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 5. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Felt‐Lisk S, Higgins P. Exploring the Promise of Population Health Management Programs to Improve Health. Princeton, NJ: Mathematica Policy Research, Incorporated; 2011. [Google Scholar]
- 7. Carson AP, Lewis CE, Jacobs DR Jr, Peralta CA, Steffen LM, Bower JK, Person SD, Muntner P. Evaluating the Framingham hypertension risk prediction model in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2013;62:1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford ES, Giles WH, Mokdad AH. The distribution of 10‐year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004;43:1791–1796. [DOI] [PubMed] [Google Scholar]
- 9. Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sempos CT, Bild DE, Manolio TA. Overview of the Jackson Heart Study: a study of cardiovascular diseases in African American men and women. Am J Med Sci. 1999;317:142–146. [DOI] [PubMed] [Google Scholar]
- 11. Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6‐4‐17. [PubMed] [Google Scholar]
- 12. Abdalla M, Booth JN 3rd, Seals SR, Spruill TM, Viera AJ, Diaz KM, Sims M, Muntner P, Shimbo D. Masked hypertension and incident clinic hypertension among African Americans in the Jackson Heart Study. Hypertension. 2016;68:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. [DOI] [PubMed] [Google Scholar]
- 14. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15. Muntner P, Colantonio LD, Cushman M, Goff DC Jr, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd‐Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fox ER, Samdarshi TE, Musani SK, Pencina MJ, Sung JH, Bertoni AG, Xanthakis V, Balfour PC Jr, Shreenivas SS, Covington C, Liebson PR, Sarpong DF, Butler KR, Mosley TH, Rosamond WD, Folsom AR, Herrington DM, Vasan RS, Taylor HA. Development and validation of risk prediction models for cardiovascular events in black adults: the Jackson Heart Study Cohort. JAMA Cardiol. 2016;1:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 18. Karmali KN, Goff DC Jr, Ning H, Lloyd‐Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:959–968. [DOI] [PubMed] [Google Scholar]
- 19. Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle‐aged and older adults: the Multi‐Ethnic Study of Atherosclerosis. Hypertension. 2011;57:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies C . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 21. Levine DA, Lewis CE, Williams OD, Safford MM, Liu K, Calhoun DA, Kim Y, Jacobs DR Jr, Kiefe CI. Geographic and demographic variability in 20‐year hypertension incidence: the CARDIA study. Hypertension. 2011;57:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosner B, Cook NR, Daniels S, Falkner B. Childhood blood pressure trends and risk factors for high blood pressure: the NHANES experience 1988–2008. Hypertension. 2013;62:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carson AP, Tanner RM, Yun H, Glasser SP, Woolley JM, Thacker EL, Levitan EB, Farkouh ME, Rosenson RS, Brown TM, Howard G, Safford MM, Muntner P. Declines in coronary heart disease incidence and mortality among middle‐aged adults with and without diabetes. Ann Epidemiol. 2014;24:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D'Agostino RB Sr, Wilson PW, Savage PJ. Trends in cardiovascular complications of diabetes. JAMA. 2004;292:2495–2499. [DOI] [PubMed] [Google Scholar]
- 25. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 26. Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR; Writing Group of the PCRG . Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 27. Ard JD, Grambow SC, Liu D, Slentz CA, Kraus WE, Svetkey LP; Study P . The effect of the PREMIER interventions on insulin sensitivity. Diabetes Care. 2004;27:340–347. [DOI] [PubMed] [Google Scholar]
- 28. Emerging Risk Factors C , Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316:1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. United States. Public Health Service. Office of the Surgeon General and National Center for Chronic Disease Prevention and Health Promotion (U.S.). Office on Smoking and Health . Preventing Tobacco Use Among Youth and Young Adults: We Can Make the Next Generation Tobacco‐Free: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, Office on Smoking and Health; 2012. [Google Scholar]
- 31. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. [DOI] [PubMed] [Google Scholar]
- 32. Kavousi M, Leening MJ, Nanchen D, Greenland P, Graham IM, Steyerberg EW, Ikram MA, Stricker BH, Hofman A, Franco OH. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA. 2014;311:1416–1423. [DOI] [PubMed] [Google Scholar]
- 33. Sherman BW, Behling C. Beyond incentives: the impact of health care reform on employer population health management strategies. Popul Health Manag. 2014;17:67–70. [DOI] [PubMed] [Google Scholar]
- 34. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 35. Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J; National High Blood Pressure Education Program Coordinating C . Primary prevention of hypertension: clinical and public health advisory from the National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Estimation of Race‐ and Gender‐Specific ASCVD Risk Using the ASCVD Pooled Cohort Risk Equations
Table S2. Increase in Mean 10‐Year Predicted ASCVD Risk (%) Between Visits 1 and 3, Overall, and Attributable to Aging and Changes in Modifiable Risk Factors Among Participants Age ≥40 Years at Visit 1 (n=798)
Table S3. Percentage of Participants ≥40 Years of Age Developing High Predicted ASCVD Risk Between Jackson Heart Study Visits 1 and 3, Overall, and Attributable to Aging and Modifiable Risk Factors
Table S4. Participant Characteristics at Visit 1 (Top Panel) and Visit 3 (Bottom Panel) Overall and by 10‐Year Predicted ASCVD Risk at Visit 3 Among Those Age <50 Years (n=771)
Table S5. Participant Characteristics at Visit 1 (Top Panel) and Visit 3 (Bottom Panel) Overall and by 10‐Year Predicted ASCVD Risk at Visit 3 Among Those Age ≥50 Years (n=344)
Table S6. Increase in Mean 10‐Year Predicted ASCVD Risk Between Visits 1 and 3, Overall, and Attributable to Aging and Changes in Modifiable Risk Factors Among Those Age <50 Years (Top Panel) and ≥50 Years (Bottom Panel)
Table S7. Percentage of Participants Age <50 Years (Top Panel) and ≥50 Years (Bottom Panel) Developing High Predicted ASCVD Risk Between Jackson Heart Study Visits 1 and 3 Attributable to Aging and Modifiable Risk Factors
Figure S1. Equations used to calculate the percentage of overall change in 10‐year predicted ASCVD risk due to risk factors and ratio as compared to aging.
Figure S2. Histograms of 10‐year ASCVD risk at visit 1 and visit 3 among participants <50 years of age (top panel, n=771) and ≥50 years of age (bottom panel, n=344).


