Abstract
Background
Thrombus migration (TM) in intracranial vessels during ischemic stroke has been reported in the form of case reports, but its incidence, impact on the technical success of subsequent endovascular thrombectomy and patients' outcome have never been studied systematically.
Methods and Results
Retrospective analysis was done of 409 patients with isolated middle cerebral artery occlusions treated with endovascular thrombectomy. TM was observed (1) by analyzing discrepancies between computed tomographic angiography and digital subtraction angiography and (2) by comparing infarct pattern in the striatocapsular region with exact, angiographically assessed thrombus location within the M1‐segment and the involvement of the middle cerebral artery perforators. Preinterventional infarction of discrepant regions (infarction in regions supplied by more proximal vessels than those occluded by the clot) was ensured by carefully reviewing available preinterventional multimodal imaging. Adequate imaging inclusion criteria were met by 325 patients. Ninety‐seven patients showed signs of TM (26 with direct evidence, 71 with indirect evidence). There was no difference in the frequency of preinterventional intravenous recombinant tissue plasminogen activator administration between patients with TM and those without (63.9% vs 64.9%, P=0.899). TM was associated with lower rates of complete reperfusion (Thrombolysis in Cerebral Infarction score 3) (adjusted odds ratio 0.400, 95% CI 0.226‐0.707). Subsequently, preinterventional TM was associated with lower rates of substantial neurologic improvement (adjusted odds ratio 0.541, 95% CI 0.309‐0.946).
Conclusions
Preinterventional TM does not seem to be facilitated by intravenous recombinant tissue plasminogen activator and often occurs spontaneously. However, TM is associated with the risk of incomplete reperfusion in subsequent thrombectomy, suggesting increased clot fragility. Occurrence of TM may thereby have a substantial impact on the outcome of endovascularly treated stroke patients.
Keywords: embolic stroke, endovascular recanalization, ischemic stroke, thrombectomy, thrombus, thrombus migration
Subject Categories: Cerebrovascular Disease/Stroke, Cerebrovascular Procedures, Ischemic Stroke, Revascularization
Introduction
Endovascular thrombectomy (ET) has evolved as the standard of care for patients suffering from acute ischemic stroke due to large vessel occlusions in the anterior circulation.1, 2, 3, 4, 5 Timely6 and complete reperfusion7, 8 is considered a key factor associated with salvaging the tissue at risk and good clinical outcomes. However, because of their different vascular architecture, not all regions supplied by the middle cerebral artery (MCA) are equally salvageable by ET.9, 10, 11 Thrombi occluding the orifices of the lenticulostriate arteries have been shown to almost inevitably lead to striatocapsular infarction despite successful recanalization,11 likely because they lack a collateral blood flow.12 This is of clinical relevance, as more proximal M1 occlusions are associated with worse clinical outcome.13 Moreover, striatocapsular infarcts are often larger than might have been expected from the exact location of the thrombus (eg, involving the whole striatocapsular region despite the fact that the occlusion is confined to the lateral perforator group or even spares all perforators).11, 14, 15 Three hypotheses have to be taken into account. First, secondary tissue damage that has arisen from either inflammatory tissue damage16, 17 or delayed extrafocal degeneration18, 19 may be the causal factor explaining these discrepancies. Second, occlusion of the perforator orifices during the course of thrombectomy has to be considered. Third and most likely, the “excess” infarction is related to thrombus migration (TM), ie, caused by a migrating thrombus that had blocked more proximal lenticulostriate arteries only temporarily but for a sufficiently long time to induce irreversible infarction in the dependent striatocapsular subterritories already prior to ET.
So far, TM has been described in a small cohort study in which reactive hyperemia in the MCA territory has been interpreted as a sign of TM15 or in studies in which sequential transcranial Doppler was performed in patients suffering from intracranial large vessel occlusions.20, 21 Further, TM has been witnessed and reported anecdotally in the form of case reports during diagnostic or therapeutic angiography or sequential CTAs.22, 23, 24, 25 However, neither its incidence nor its dependency on the administration of intravenous (IV) recombinant tissue plasminogen activator (rtPA) or its relevance for ET has been studied systematically. This study aims to clarify whether a discrepancy between thrombus location and infarct size in the striatocapsular region supplied by the MCA perforators is possibly due to TM prior to ET. Further, we hypothesized that the occurrence of TM is a sign of thrombus instability and consequently has impact on the technical success of subsequent ET.
Methods
Study Population
A retrospective analysis of a prospective database including all consecutive patients subjected to digital subtraction angiography (DSA) with the intention to treat an isolated MCA occlusion between January 2007 and June 2016 (n=409) was carried out. We excluded patients with imaging inadequate to assess infarct extent (n=72) and patients with any other clinical condition that might interfere with clinical assessment of infarct severity (n=12, see study flowchart in Figure 1). The final study population consisted of 325 patients (297 M1 occlusions, 28 M2 occlusions). Baseline characteristics and risk factors were evaluated by reviewing patients' clinical records. All procedures were in accord with the 1964 declaration of Helsinki and its later amendments and approved by the local ethics committee. Written informed consent of individual patients for this anonymized retrospective study was waived according to institutional guidelines.
Figure 1.
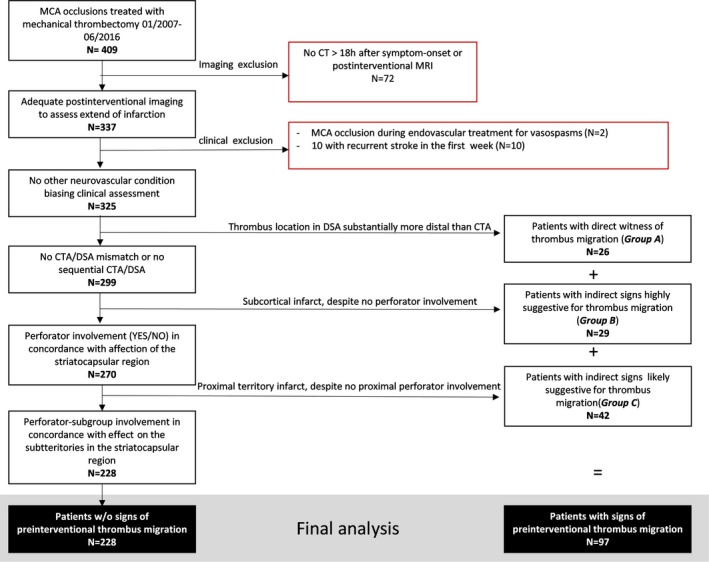
Study flowchart. Stepwise exclusion as well as classification of patients with signs of thrombus migration are displayed. Examples of thrombus migration of the respective groups A, B, and C are shown in Figures 2, 3 through 4. CT, computed tomography; CTA, computed tomographic angiography; DSA, digital subtraction angiography; MCA, middle cerebral artery; MRI, magnetic resonance imaging; w/o, without.
Endovascular Treatment
Concordant with our institutional standard operating procedures, all stroke patients with CTA‐proven large vessel occlusion in the anterior circulation were eligible for ET if the groin puncture could be performed within 6 hours after symptom onset and if the infarct on preinterventional noncontrast computed tomography (CT) did not exceed one‐third of the MCA territory. Our center did not apply an age limit. Preinterventional IV rtPA was administered as a “bridging approach” if there were no contraindications (n=210; 64.6%). Procedures were performed under general anesthesia or conscious sedation. See Data S1 and S2 for device choice throughout the study period and device impact on rates of successful recanalizations. In total, 7 cases with spontaneous or IV rtPA‐related partial or complete recanalizations as revealed by first intracranial DSA runs were observed throughout the study period. In these cases only diagnostic angiography was performed. Those cases were included in the analysis regarding factors associated with thrombus migration but were excluded for the analysis with regard to impact on technical success of thrombectomy because no maneuvers were performed.
Image Analysis
Technical Success of Thrombectomy
Thrombolysis in Cerebral Infarction (TICI)‐graded recanalization success of ET was assessed by 2 neuroradiologists independently, with TICI‐2b defined as reperfusion of more than 66% of the initially involved territory, according to the original TICI scale.26 In cases of discrepancies a consensus read was performed (see Kleine et al8 for interrater agreement). Successful recanalization was defined as ≥TICI‐2b. First‐pass recanalization was defined if ET was terminated after 1 maneuver (1 aspiration or 1 stent‐retriever retrieval).
Definition of TM
Nearly all patients with an ischemic stroke in the anterior circulation had a thrombus movement or dislocation prior to its being “stuck” in the MCA (originating from either an internal carotid artery plaque or an atrial thrombus). However, the term “thrombus migration” in this case refers to a totally distinct pathophysiological process because either it describes how an atrial thrombus starts migrating into the aortic arch or it describes a process related to plaque rupture (eg, of an internal carotid artery plaque). We have defined thrombus migration as “secondly” occurring downstream migration in a vessel just as large as the thrombus diameter itself. Hence, the thrombus was stuck in the first place and started migrating again afterward.
Direct Evidence for TM
Preinterventional CT angiography (CTA) images were available in 221 cases (68.0%). A substantial difference between thrombus location on CTA images and thrombus location as revealed by first DSA runs was classified as direct evidence for TM (migration group A, see Figure 2).
Figure 2.
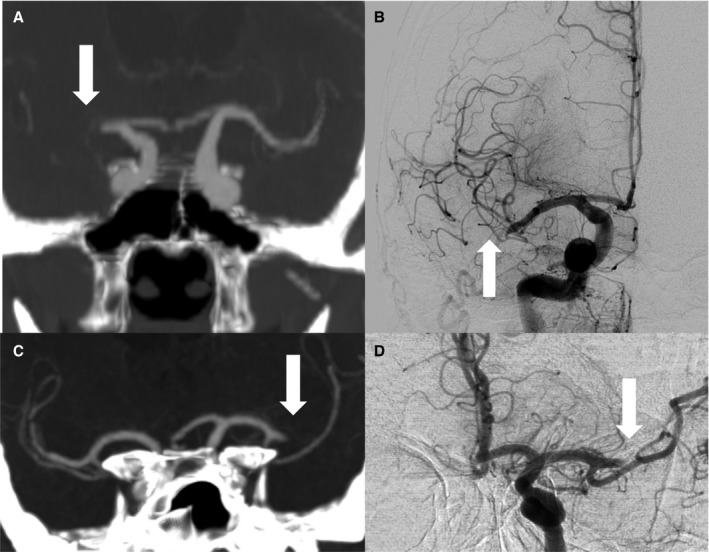
Thrombus migration vs stable clot position on sequential CTA/DSA. Upper row (A+B), case with direct evidence of thrombus migration; (A) coronary CTA reformation with 10 mm maximum intensity projection shows proximal MCA occlusion on the right side (arrow); (B) initial DSA runs before recanalization revealed significant thrombus dislocation (see arrow) to the distal M1‐segment/Truncus inferior (migration group A); lower row (C+D), case with stable clot position; (C) coronary CTA reformation with 10 mm maximum intensity projection displays proximal thrombus end after MCA bifurcation (arrow); (D) unchanged thrombus position on first DSA runs before recanalization (arrow). CTA, computed tomographic angiography; DSA, digital subtraction angiography; MCA, middle cerebral artery.
Indirect Evidence for TM
Involvement of the MCA perforators (medial, middle, and lateral group, respectively) and the infarction of their respective supplied territory were classified as described in detail before (see Kleine et al for reliability and illustrations11). Patients with infarction of any lenticulostriate artery territory, even if CTA and DSA revealed that the occlusion spared all MCA perforator groups, were classified as indirect evidence for TM (migration group B, see Figure 3). Additionally, patients in whom the infarct involved more proximal parts of the lenticulostriate territory than expected from the exact thrombus location were also classified as indirect evidence for thrombus migration (eg, infarct involves all proximal territories despite the occlusion being confined to the lateral perforator group, group C, see Figure 4).
Figure 3.
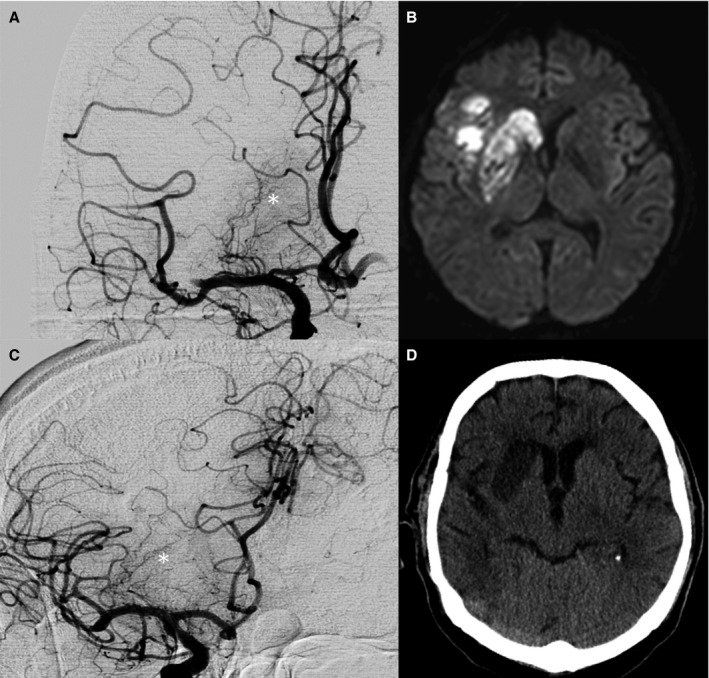
Complete striatocapsular infarcts despite a distal MCA occlusion. A and C, First DSA runs of 2 patients before endovascular thrombectomy revealed distal MCA occlusions with completely perfused MCA perforators (*). B and D, postinterventional MRI (B) and CT (D) of these patients revealed complete infarction of the lenticulostriate territory corresponding to a proximal occlusion of all perforator subgroups (migration group B). CT, computed tomography; DSA, digital subtraction angiography; MCA, middle cerebral artery; MRI, magnetic resonance imaging.
Figure 4.
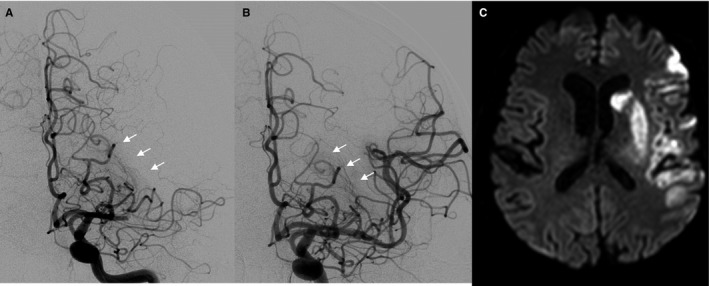
Proximal striatocapsular infarct despite an occlusion confined to the lateral perforator group. A, First DSA runs before endovascular thrombectomy was performed revealed an occlusion involving only the lateral perforator group (see white arrows in A) while the proximal and middle perforator groups were perfused; B, postinterventional DSA runs revealed reperfusion of the lateral perforator group (see white arrows in B); C, postinterventional imaging revealed a complete striatocapsular infarction corresponding to a complete occlusion of all MCA perforators (migration group C).
Time Point of Discrepant Infarction
To ensure that the discrepancies between thrombus location and infarct size were due to preinterventional infarction and did not occur periinterventionally or postinterventionally, 2 experienced senior neuroradiologists (>5 years of experience) performed in consensus a detailed image analysis for all migration group B cases using all preinterventional images available: noncontrast CT, CTA, CT perfusion, DSA. Three criteria, if available, were taken into account to ensure preinterventional infarction: (1) preinterventional demarcation of the discrepant infarct area on noncontrast CT (see Figure 5, for example); (2) reactive hyper‐ or hypoperfusion as revealed on preinterventional CTP15, 27; and (3) basal ganglia blush and/or early venous drainage on first DSA runs as a sign of irreversible infarction28, 29 (see Figure 5 for examples). Based on a synopsis of these observations, the reader ultimately dichotomized cases with discrepancies regarding thrombus location and infarct size as evidence for preinterventional infarction or no evidence for preinterventional infarction. To exclude relevant a priori bias of the readers (ie, knowing that all cases to be rated had thrombus migration, which may have induced false positive ratings), all cases were mixed with an equal number of cases without TM, and specificity of the ratings were evaluated accordingly.
Figure 5.
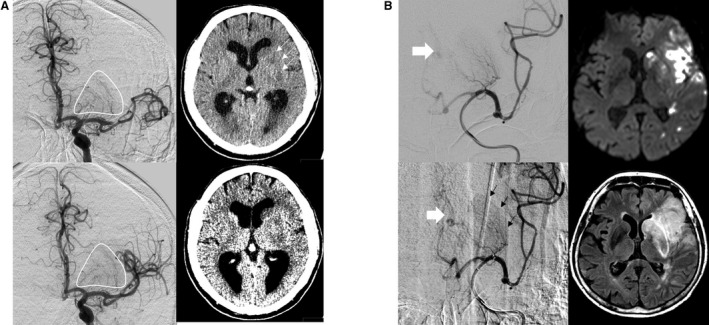
Signs of preinterventional infarction. A, Distal MCA occlusion with clear evidence of preinterventional infarction of the striatocapsular region. First DSA runs prior to endovascular treatment revealed full perfusion of all perforators (compare pre‐ and postinterventional DSA, left side upper and lower row). Preinterventional CT before DSA revealed demarcation of striatocapsular infarcts (see arrows) and hypodensities on “stroke window” settings (right side lower row). B, Case with distal MCA occlusion and clear evidence of preinterventional infarction of the striatocapsular region. First DSA runs prior to endovascular treatment revealed full perfusion of all MCA perforators but basal ganglionic blush (see black arrows, left side lower row) and early venous drainage (left side, white arrows), suggesting an already occurred infarction. Postinterventional DWI (right side upper row) and FLAIR‐sequence revealed complete striatocapsular infarct corresponding to a complete occlusion of all MCA perforators. CT, computed tomography; DSA, digital subtraction angiography; DWI, diffusion weighted imaging; FLAIR, fluid attenuation inversion recovery; MCA, middle cerebral artery.
Clinical Outcome and Classification of Hemorrhagic Transformation
Clinical outcomes were measured as NIH Stroke Scale (NIHSS) scores at the day of discharge, which were assessed by a qualified stroke neurologist. Patients who died during the acute hospital stay were assigned a score of 42. A difference between baseline NIHSS and NIHSS at discharge ≥8 or an NIHSS score at discharge ≤1 was classified as “substantial neurologic improvement,” as this outcome measure has been shown to be a very sensitive parameter for stroke treatment effects.30
Statistical Analyses
All analyses were performed using IBM SPSS Statistics, release 23.0 (IBM, Armonk, NY). Frequency counts were compared using the Fisher exact test. Non–normally distributed data were compared using the Mann‐Whitney U test, and normally distributed data sets were compared using the t test for independent samples. Logistic regression models were used to evaluate the impact of different variables on dichotomized outcome or technical success parameters. In total, 3 different logistic regression models were used (see Table S1): 1 technical (logistic regression model 1, LRM‐1), adjusting for factors that have been associated with different physical clot properties, including patient's age and time to first DSA series,31, 32 prior administration of IV rtPA33 and device choice and 2 outcome models (logistic regression model 2 and 3, LRM‐2 and LRM‐3, respectively), which adjust for major outcome determinants in acute stroke, both including baseline NIHSS,34 time from symptom onset to recanalization,6 and age.34 LRM‐3 additionally corrects for successful recanalization and rates of complete (TICI‐3) recanalization.
Results
Patients
A set of 325 patients was included. Mean age was 71.9±14.4 years. Successful recanalization was achieved in 80.3% of patients (n=261). In 117 cases postinterventional DSA runs revealed complete reperfusion (TICI‐3, 36.0%, n=117). Patients presented with severe neurological deficits (median baseline NIHSS 14) and improved to a median NIHSS of 7 at the day of discharge. Mean time from symptom onset to first DSA runs, precisely determinable for 305 patients, was 219.2±77.5 minutes.
Thrombus Migration
In total, 97 (29.8%) patients with signs of TM were identified. Twenty‐six showed discrepancies of thrombus location between CTA and initial DSA runs (migration group A), 29 patients had a striatocapsular infarct despite the occlusion sparing all perforators, as revealed on a synopsis of CTA and first DSA runs (migration group B). Forty‐two patients had a striatocapsular territory infarction that clearly exceeded the regions that were suggested by the exact perforator group involvement (migration group C). There were no differences in age (70.5±16.8 vs 72.5±13.3, P=0.288), sex (female: 53.6% vs 53.7%, P=1.000), baseline NIHSS (15 vs 14, P=0.569), the frequency of bridging thrombolysis (63.9% vs 64.6%, P=0.899), and risk factor distribution between patients with TM compared to those without signs of TM (see Table). Rates of TM did not differ between patients receiving IV rtPA and those ineligible (13.5% vs 8.8%, P=0.386) also when analysis was confined to patients with sequential imaging available (n=221) and when only migration group A (n=26) is defined as TM. In this cohort the time window of TM is restricted to the interval after lysis (Table S2). Further, in 61 cases TM occurred without any association to IV rtPA administration. Either patients were ineligible for IV rtPA (n=35) or TM occurred prior to the administration of IV rtPA (n=26; see Data S1).
Table 1.
Baseline and Outcome Characteristics of Patients With and Without Signs of Thrombus Migration
| TM (n=97) | No TM (n=228) | P Value | |
|---|---|---|---|
| Age | 70.5±16.8 | 72.5±13.3 | 0.288 |
| Sex, female | 53.6% (52) | 53.7% (122) | 1.000 |
| Baseline NIHSS | 15 (11‐18) | 14 (11‐17) | 0.569 |
| Risk factors | |||
| Hypertension | 77.1% (74) | 74.8% (169) | 0.777 |
| Diabetes | 21.9% (21) | 15.9% (36) | 0.205 |
| AF | 54.2% (52) | 52.7% (119) | 0.809 |
| Prior stroke/TIA | 18.8% (18) | 18.1% (41) | 0.876 |
| Hemisphere, left | 45.4% (44) | 50.0% (114) | 0.469 |
| Wake‐up stroke | 6.2% (6) | 6.1% (14) | 1.000 |
| Drip and ship patients | 42.3% (41) | 43.0% (98) | 1.000 |
| Time to first DSA run, min | 212 (175‐268) | 206 (158‐258) | 0.258 |
| IV rtPA bridging | 63.9% (62) | 64.9% (148) | 0.899 |
| Time to IV rtPA, min | 100 (80‐135) | 110 (73‐135) | 0.981 |
| Successful recanalization | 75.3% (73) | 82.5% (188) | 0.170 |
| TICI‐3 recanalization | 20.6% (20) | 42.5% (97) | <0.001* |
| First‐pass recanalization | 22.7% (22) | 28.1% (64) | 0.339 |
| Time to recanalization, min | 276 (241‐330) | 264 (204‐320) | 0.085 |
| NIHSS at discharge | 9 (3‐13) | 7 (2‐12) | 0.069 |
| “Substantial neurologic improvement” | 34.7% (33) | 47.3% (105) | 0.048 † |
AF indicates atrial fibrillation; DSA, digital subtraction angiography; IV, intravenous; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue plasminogen activator; TIA, transient ischemic attack; TICI, Thrombolysis in Cerebral Infarction grade; TM, thrombus migration.
*P<0.001; † P<0.05.
Confirming the Time Point of Infarction
To clarify whether infarction of the striatocapsular region in cases where the thrombus spared all perforators occurred preinterventionally (migration group B), a blinded multimodal imaging analysis was performed. In 93.1% (27/29) of cases imaging analysis revealed clear evidence of preinterventional infarction (see Table S3 for positive criteria and ratings of controls). In contrast, only 1 of the concomitantly rated 29 (3.4%) controls was rated false positive, ie, as showing signs of preinterventional infarction despite the patient not developing infarction in the striatocapsular region. This implies that infarction of the discrepant area, which was already reperfused on first DSA runs, occurred before thrombectomy and hence cannot be explained by either infarction during the thrombectomy procedure or by secondary tissue damage due to inflammation16, 17 or extrafocal degeneration.18, 19
Effect on Endovascular Success and Patients' Outcome
Patients with TM had lower rates of complete (TICI‐3) recanalizations (20.6% vs 42.5%, P<0.001, Table). In a multivariate logistic regression model, occurrence of TM was associated with lower rates of complete (TICI‐3) recanalization (adjusted OR 0.400, 95% CI 0.226‐0.707, P=0.002) after correcting for age, time to first DSA run, device choice, and administration of IV rtPA (LRM‐1). There was no significant difference either regarding this effect or other baseline characteristics whether TM was based on indirect or direct evidence (Table S4). Correspondingly, patients with TM tended to have higher NIHSS scores at discharge (median 9 vs 7, P=0.069) and had lower rates of “substantial neurologic improvement” (34.4% vs 45.9%, P=0.048, see Table). TM remains a statistically tangible factor associated with lower rates of “substantial neurologic improvement” when corrections were made for baseline parameters using the LRM‐2 (adjusted OR 0.541, 95% CI 0.309‐0.946, P=0.031). However, when additional corrections were made for rates of successful and complete (TICI‐3) recanalization (LRM‐3), TM was no longer found to be an independent factor influencing the incidence of “substantial neurologic improvement” (adjusted OR 0.697, 95% CI 0.386‐1.258, P=0.231).
Discussion
This study shows 4 major findings: (1) TM should be regarded as the first and foremost reason causing discrepancies between exact occlusion site (perforator involvement) and infarct size in the striatocapsular region supplied by the MCA; (2) TM is not infrequent, occurring in at least one‐third of MCA occlusions; (3) the occurrence of TM does not seem to be facilitated by the use of IV rtPA and often occurs spontaneously; (4) TM independently predicted lower rates of complete recanalization, presumably as a sign indicating increased thrombus fragility, and hence was associated with lower rates of neurologic improvement.
The results were derived from a large prospective cohort of patients with a baseline (stroke severity, symptom onset to DSA, etc) and technical setting (success rates, TICI‐3 rates, etc) highly comparable to those of the large randomized trials.1, 2, 3, 4, 5 The data presented are thus representative of current standards in endovascular treatment of large vessel occlusions.
Olsen et al were the first to observe discrepancies among infarct size, reactive hyperemia, and thrombus location and suggested a model of “embolic migration” in MCA occlusions.15 Since then, further cases with clear discrepancies between infarct size and thrombus location have been reported. Saito et al observed discrepancies between thrombus location as revealed by DSA and infarct extent in the striatocapsular region.14 They reported 5 cases in which the occlusion was confined to the distal M1 segment or M2 branches, but the infarct involved the basal ganglia. Further, in a previous large‐cohort study the correlation of infarct extent and exact thrombus location within the striatocapsular region was systemically investigated.11 Our results suggest that TM occurs frequently and causes preinterventional infarction of more proximal territories than expected by the thrombus location on preinterventional angiography imaging (CTA/DSA). The data presented also underscore that the literature on that topic may underestimate its incidence and especially its relevance for endovascular stroke therapy.
Interestingly, TM was found to occur with the same frequency in patients receiving IV rtPA as in those ineligible for IV treatment. Although TM has been reported after the use of IV rtPA,20, 23, 25 the evidence was clearly insufficient to conclude that IV rtPA favors TM. In contrast, our data militate against those assumptions, at least in the setting of timely subsequent ET. No significant association between IV rtPA and thrombus migration could be observed in group A cases, and in 61 out of 97 cases, TM occurred without administration of IV rtPA. Hence, our data suggest that TM is a phenomenon that is not predominantly caused by medical therapy; rather, it indicates that other factors (ie, thrombus histology and vessel anatomy) may be major predisposing factors.
Discrepancies between thrombus locations as revealed by CTA and subsequent DSA may also be caused by proximal thrombolysis, leaving more distal parts of the thrombus as the “newly formed” proximal end of the thrombus. However, first, this would imply that proximal thrombolysis accounts for discrepancies of roughly more than 10 to 20 mm, and second, as mentioned above, the administration of IV rtPA was not associated with the occurrence of these discrepancies. Another study has suggested that the use of IV rtPA affects thrombus stability and technical success of subsequent ET in distal MCA occlusions.33 However, the effects on complete reperfusion of TM seem to be independent of prior use of IV rtPA, as we corrected for this potential confounding effect of IV rtPA regarding this issue in multiple logistic regression.
The poorer outcome of patients with TM was shown to be mediated mainly through lower rates of complete recanalizations (see impact of adding rates of TICI‐3 recanalizations to the logistic regression model). Logically, lower rates of TICI‐3 recanalizations have been associated with less favorable neurologic outcome8, 35; however, its association with TM has never been studied so far. Incomplete recanalization in the setting of preinterventional TM may be caused by either preprocedural thrombus fragmentation during the course of migration or by increased rates of periprocedural thrombus fragmentation because migrated thrombi may generally be more fragile during the thrombectomy maneuver. We propose that preinterventional thrombus fragmentation might be the less prevalent event causing incomplete recanalization, as most intracranial thrombi have been shown to be unilocular.36 However, if pretherapeutic fragmentation of a thrombus is present, it is associated with poorer treatment effects of IV rtPA and ET.36 In contrast to the presumably low incidence of fragmented thrombi prior to endovascular treatment, periprocedural thrombus fragmentation during thrombectomy has been described as a common risk in endovascular stroke therapy. Several factors influencing its incidence have been suggested, including proximal flow modulation,37 device characteristics,38, 39 and thrombus architecture.40 This study also implies that if a sign of TM is observable on first DSA runs, it is desirable to pull out all the stops to reduce the—in this case a priori increased—risk of periinterventional thrombus fragmentation and adjust the technical approach and safety considerations accordingly, eg, by using aspiration techniques or additional proximal flow modulation.
This study has several limitations beyond the common limitations of an observational study. The results are likely to underestimate the true incidence of TM for several reasons. First, the discrepancies observable between CTA and DSA represent a short time period, and TM may occur before (false negative direct evidence for TM). Second, TM may occur in the distal M1 segment without reliable indirect imaging signs because this occlusion pattern lacks end‐artery involvement (false negative indirect evidence for TM). Third, TM may occlude perforators for such a short time that irreversible ischemia does not occur (false negative indirect evidence for TM). Fourth, in 27 patients the time interval of thrombus migration cannot be narrowed down (Data S1). Correlations in these 27 patients are thus prone to biases. If patients receive IV rtPA but thrombus migration had occurred already, these cases may suggest a false positive “pseudocorrelation” between use of IV rtPA and the occurrence of thrombus migration. However, that bias should be primarily kept in mind if any statistical association between IV rtPA and thrombus migration would have been observed. This was not the case, and, relatively speaking, it strengthens our conclusion that IV rtPA does not seem to facilitate thrombus migration. Fifth, our outcome analysis is based on discharge NIHSS scores and not on 3‐month modified Rankin Scale scores, the most commonly used outcome measure in current stroke research. The NIHSS, however, is a dedicated scoring system more directly reflecting the multifaceted and complex neurologic damage caused by ischemic lesions.30, 41 Further, short‐term NIHSS scores have been shown to be a sensitive outcome measure in detecting small treatment effects30 and are less prone to confounding effects such as age and comborbidities.42 This particularly holds true because our institution did not apply an age limit, and thus, a considerable portion of octo‐ and nonagenarians were included (n=103). The mentioned reasons make this outcome measure particularly suitable for the purpose of this study, which is detecting small, TM‐associated shifts in outcomes due to decreased rates of complete reperfusion.8 Last, we have ensured preinterventional infarction only in migration group B (striatocapsular infarct despite initial DSA runs showing no perforator involvement) and not in migration group C (more proximal territory affection in the striatocapsular region than would be expected from the exact thrombus location), which theoretically, unlikely as it is, could mean that migration group C discrepancies could have occurred for other reasons. We have confined our analysis to migration group B based on observations that preinterventional infarct signs on multimodal imaging are generally subtle, and it is relatively difficult to ensure infarction of, eg, a small more proximally located territory on a native preinterventional CT or detect a blush or AV shunt affecting only the more proximal perforator group but sparing the medial ones. Of note, however, if the analysis had been redone and limited to migration group A and B cases as “confirmed” thrombus migration, the results would have remained basically the same.
Conclusion
TM occurs frequently, at least in about one‐third of MCA occlusions. TM does not seem to be facilitated by the administration of IV rtPA and often occurs spontaneously. However, TM was associated with lower rates of complete reperfusion in subsequent ET, suggesting increased clot fragility. Occurrence of TM thereby has substantial impact on the outcome of endovascularly treated stroke patients.
Sources of Funding
This work was supported by the German Research Foundation and the Technical University of Munich in the framework of the Open Access Publishing Program.
Disclosures
None.
Supporting information
Data S1. Information on Device Choice
Data S2. Time Intervals of Thrombus Migration
Table S1. Logistic Regression Models Used in the Study
Table S2. Thrombus Migration Confined to Patients With Available Sequential Imaging and Migration Group A
Table S3. Ratings of Signs of Preinterventional Infarction
Table S4. Comparison of Baseline Characteristics of Patients With Direct and Indirect Evidence of Thrombus Migration and Those Without Signs of Thrombus Migration
(J Am Heart Assoc. 2017;6:e005149. DOI: 10.1161/JAHA.116.005149.)
References
- 1. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Roman L, Serena J, Abilleira S, Ribo M, Millan M, Urra X, Cardona P, Lopez‐Cancio E, Tomasello A, Castano C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez‐Perez M, Goyal M, Demchuk AM, von Kummer R, Gallofre M, Davalos A; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 2. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators . Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. N Engl J Med. 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 3. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND‐IA Investigators . Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med. 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 4. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 5. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama a Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg‐Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 6. Sheth SA, Jahan R, Gralla J, Pereira VM, Nogueira RG, Levy EI, Zaidat OO, Saver JL; Trialists S‐S . Time to endovascular reperfusion and degree of disability in acute stroke. Ann Neurol. 2015;78:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoo AJ, Simonsen CZ, Prabhakaran S, Chaudhry ZA, Issa MA, Fugate JE, Linfante I, Liebeskind DS, Khatri P, Jovin TG, Kallmes DF, Dabus G, Zaidat OO; Cerebral Angiographic Revascularization Grading Collaborators . Refining angiographic biomarkers of revascularization: improving outcome prediction after intra‐arterial therapy. Stroke. 2013;44:2509–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kleine JF, Wunderlich S, Zimmer C, Kaesmacher J. Time to redefine success? TICI 3 versus TICI 2b recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerv Surg. 2017;9:117–121. [DOI] [PubMed] [Google Scholar]
- 9. Bozzao L, Fantozzi LM, Bastianello S, Bozzao A, Fieschi C. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke. 1989;20:735–740. [DOI] [PubMed] [Google Scholar]
- 10. Decavel P, Vuillier F, Moulin T. Lenticulostriate infarction. Front Neurol Neurosci. 2012;30:115–119. [DOI] [PubMed] [Google Scholar]
- 11. Kleine JF, Beller E, Zimmer C, Kaesmacher J. Lenticulostriate infarctions after successful mechanical thrombectomy in middle cerebral artery occlusion. J Neurointerv Surg. 2016. Available at: http://jnis.bmj.com/content/early/2016/03/03/neurintsurg-2015-012243. Accessed February 9, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Feekes JA, Hsu SW, Chaloupka JC, Cassell MD. Tertiary microvascular territories define lacunar infarcts in the basal ganglia. Ann Neurol. 2005;58:18–30. [DOI] [PubMed] [Google Scholar]
- 13. Behme D, Kowoll A, Weber W, Mpotsaris A. M1 is not M1 in ischemic stroke: the disability‐free survival after mechanical thrombectomy differs significantly between proximal and distal occlusions of the middle cerebral artery M1 segment. J Neurointerv Surg. 2015;7:559–563. [DOI] [PubMed] [Google Scholar]
- 14. Saito I, Segawa H, Shiokawa Y, Taniguchi M, Tsutsumi K. Middle cerebral artery occlusion: correlation of computed tomography and angiography with clinical outcome. Stroke. 1987;18:863–868. [DOI] [PubMed] [Google Scholar]
- 15. Olsen TS, Lassen NA. A dynamic concept of middle cerebral artery occlusion and cerebral infarction in the acute state based on interpreting severe hyperemia as a sign of embolic migration. Stroke. 1984;15:458–468. [DOI] [PubMed] [Google Scholar]
- 16. Gauberti M, De Lizarrondo SM, Vivien D. The “inflammatory penumbra” in ischemic stroke: from clinical data to experimental evidence. Eur Stroke J. 2016;1:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in stroke brain damage. Stroke. 2016;47:1165–1172. [DOI] [PubMed] [Google Scholar]
- 18. Winter B, Brunecker P, Fiebach JB, Jungehulsing GJ, Kronenberg G, Endres M. Striatal infarction elicits secondary extrafocal MRI changes in ipsilateral substantia nigra. PLoS One. 2015;10:e0136483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakane M, Teraoka A, Asato R, Tamura A. Degeneration of the ipsilateral substantia nigra following cerebral infarction in the striatum. Stroke. 1992;23:328–332. [DOI] [PubMed] [Google Scholar]
- 20. Alexandrov AV, Burgin WS, Demchuk AM, El‐Mitwalli A, Grotta JC. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short‐term improvement. Circulation. 2001;103:2897–2902. [DOI] [PubMed] [Google Scholar]
- 21. Akopov S, Whitman GT. Hemodynamic studies in early ischemic stroke: serial transcranial Doppler and magnetic resonance angiography evaluation. Stroke. 2002;33:1274–1279. [DOI] [PubMed] [Google Scholar]
- 22. Watanabe M, Mori T, Imai K, Izumoto H, Hirano T, Uchino M. Distal migration of a floating carotid thrombus in a patient using oral contraceptives: a case report. J Med Case Rep. 2009;3:8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoon JH, Shin YS, Lim YC, Kim HS, Nam HS, Heo JH. Distal migration of thrombus during intra‐arterial thrombolysis. Eur Neurol. 2010;63:62–63. [DOI] [PubMed] [Google Scholar]
- 24. Siu KL, Lee DG, Shim JH, Suh DC, Lee DH. Mechanical thrombectomy using the Solitaire FR system for occlusion of the top of the basilar artery: intentional detachment of the device after partial retrieval. Neurointervention. 2014;9:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. López‐Cuevas R, Lago A, Tembl JI. Downstream migration and fragmentation of a spontaneous calcific embolus after thrombolysis in a patient with ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:e165–e166. [DOI] [PubMed] [Google Scholar]
- 26. Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D; Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology, Technology Assessment Committee of the Society of Interventional Radiology . Trial design and reporting standards for intra‐arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. [DOI] [PubMed] [Google Scholar]
- 27. Shahi V, Fugate JE, Kallmes DF, Rabinstein AA. Early basal ganglia hyperperfusion on CT perfusion in acute ischemic stroke: a marker of irreversible damage? AJNR Am J Neuroradiol. 2014;35:1688–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fritzsch D, Reiss‐Zimmermann M, Lobsien D, Quaschling U, Hoffmann KT. Arteriovenous shunts and capillary blush as an early sign of basal ganglia infarction after successful mechanical intra‐arterial thrombectomy in ischaemic stroke. Eur Radiol. 2015;25:3060–3065. [DOI] [PubMed] [Google Scholar]
- 29. Dorn F, Kuntze‐Soderqvist A, Popp S, Lockau H, Haller B, Zimmer C, Andersson T, Liebig T. Early venous drainage after successful endovascular recanalization in ischemic stroke—a predictor for final infarct volume? Neuroradiology. 2012;54:745–751. [DOI] [PubMed] [Google Scholar]
- 30. Kerr DM, Fulton RL, Lees KR; VISTA Collaborators . Seven‐day NIHSS is a sensitive outcome measure for exploratory clinical trials in acute stroke: evidence from the Virtual International Stroke Trials Archive. Stroke. 2012;43:1401–1403. [DOI] [PubMed] [Google Scholar]
- 31. Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol. 2015;42:86–92. [DOI] [PubMed] [Google Scholar]
- 32. Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain‐Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, Montalescot G, Weisel JW. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaesmacher J, Kleine JF. Bridging therapy with i. v. rTPA in MCA occlusion prior to endovascular thrombectomy: a double‐edged sword? Clin Neuroradiol. 2016. Available at: http://rd.springer.com/article/10.1007%2Fs00062-016-0533-0. Accessed February 9, 2017. [DOI] [PubMed] [Google Scholar]
- 34. Krishnan P, Saposnik G, Ovbiagele B, Zhang L, Symons S, Aviv R. Contribution and additional impact of imaging to the SPAN‐100 score. AJNR Am J Neuroradiol. 2015;36:646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rangaraju S, Aghaebrahim A, Streib C, Jadhav AP, Jovin TG. Abstract t MP7: TICI 2B vs. TICI 3: differences in infarct volumes and clinical outcomes in proximal intracranial large vessel occlusions treated with endovascular therapy. Stroke. 2014;45:ATMP7. [Google Scholar]
- 36. Gratz PP, Schroth G, Gralla J, Mattle HP, Fischer U, Jung S, Mordasini P, Hsieh K, Verma RK, Weisstanner C, El‐Koussy M. Whole‐brain susceptibility‐weighted thrombus imaging in stroke: fragmented thrombi predict worse outcome. AJNR Am J Neuroradiol. 2015;36:1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Velasco A, Buerke B, Stracke CP, Berkemeyer S, Mosimann PJ, Schwindt W, Alcázar P, Cnyrim C, Niederstadt T, Chapot R, Heindel W. Comparison of a balloon guide catheter and a non–balloon guide catheter for mechanical thrombectomy. Radiology. 2016;280:169–176. [DOI] [PubMed] [Google Scholar]
- 38. Mokin M, Setlur Nagesh SV, Ionita CN, Mocco J, Siddiqui AH. Stent retriever thrombectomy with the cover accessory device versus proximal protection with a balloon guide catheter: in vitro stroke model comparison. J Neurointerv Surg. 2016;8:413–417. [DOI] [PubMed] [Google Scholar]
- 39. Chueh J‐Y, Puri AS, Gounis MJ. An in vitro evaluation of distal emboli following Lazarus cover‐assisted stent retriever thrombectomy. J Neurointerv Surg. 2017;9:183–187. [DOI] [PubMed] [Google Scholar]
- 40. Mokin M, Morr S, Natarajan SK, Lin N, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI. Thrombus density predicts successful recanalization with solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2015;7:104–107. [DOI] [PubMed] [Google Scholar]
- 41. Young FB, Weir CJ, Lees KR; GAIN International Trial Steering Committee and Investigators . Comparison of the National Institutes of Health Stroke Scale with disability outcome measures in acute stroke trials. Stroke. 2005;36:2187–2192. [DOI] [PubMed] [Google Scholar]
- 42. Kleine JF, Boeckh‐Behrens T, Prothmann S, Zimmer C, Liebig T. Discrepancy between early neurological course and mid‐term outcome in older stroke patients after mechanical thrombectomy. J Neurointerv Surg. 2016;8:671–676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Information on Device Choice
Data S2. Time Intervals of Thrombus Migration
Table S1. Logistic Regression Models Used in the Study
Table S2. Thrombus Migration Confined to Patients With Available Sequential Imaging and Migration Group A
Table S3. Ratings of Signs of Preinterventional Infarction
Table S4. Comparison of Baseline Characteristics of Patients With Direct and Indirect Evidence of Thrombus Migration and Those Without Signs of Thrombus Migration


