Abstract
The underlying hypothalamic neurocircuitry by which metabolism and feeding regulates reproductive function has been well-studied in the rodent; however, recent data demonstrated significant neuroanatomical differences in the human brain. The current study had three objectives, centered on arcuate nucleus neuropeptides regulating feeding and reproduction: 1) characterize coexpression patterns in the female nonhuman primate, 2) establish whether these neuronal populations make potential contacts with GnRH neurons and 3) determine whether these contacts differ between the low and high GnRH-releasing states of pre-puberty and adulthood, respectively. Female nonhuman primates have several coexpression patterns of hypothalamic neuropeptides that differ from those reported in rodents. Cocaine- and amphetamine-regulated transcript (CART) is not coexpressed with proopiomelanocortin (POMC) but instead with neuropeptide Y (NPY). CART is also expressed in a subpopulation of kisspeptin cells in the nonhuman primate, similar to observations in humans but diverging from findings in rodents. Very few GnRH-expressing neurons received close appositions from double-labeled kisspeptin/CART fibers; however, both single-labeled kisspeptin and CART fibers were in frequent apposition with GnRH neurons with no differences between prepubertal and adult animals. NPY/AgRP coexpressing fibers contacted significantly more GnRH neurons in prepubertal animals than adults, consistent with increased NPY and AgRP mRNA observed in prepubertal animals. The current findings detail significant differences in arcuate nucleus neuropeptide coexpression in the monkey compared to the rodent and are consistent with the hypothesis that arcuate nucleus NPY/AgRP neurons play an inhibitory role in controlling GnRH neuronal regulation in the prepubertal primate.
Keywords: arcuate nucleus, AgRP, neuroendocrine
INTRODUCTION
Metabolic status regulates reproductive function at the level of hypothalamic gonadotropin releasing-hormone (GnRH) output, which is critical for downstream activation of the pituitary and gonads. This interconnectivity offers the evolutionary advantage of halting fertility and conserving energy in times of food scarcity, when it is unlikely a pregnancy could successfully be brought to term [1, 2]. This connection also conveys signals of sufficient growth required for the onset of puberty, which is initiated by increases in hypothalamic GnRH release. Human conditions associated with undernutrition, such as anorexia nervosa and extreme exercise, result in delayed puberty and hypothalamic amenorrhea due to decreased pulsatile GnRH release [3–5]. Many hormonal and neuronal signals originally characterized for their roles in feeding and energy homeostasis are now believed to also provide critical regulatory feedback to GnRH neurons on the metabolic status of the organism.
Research conducted in rodent models has been invaluable for highlighting key metabolic signals participating in the regulation of GnRH release. Particularly, electrophysiological recordings from both rat and mouse brain slices reveal that hypothalamic neuropeptides regulating appetite also modulate GnRH neuronal firing both directly and indirectly. This includes stimulation of GnRH neuronal firing by the satiety signaling peptides proopiomelanocortin (POMC) [6, 7] and cocaine- and amphetamine-regulated transcript (CART) [8–10], as well as inhibition of GnRH neuronal firing by the appetitive signaling peptides neuropeptide Y (NPY) [6, 11–13] and agouti-related peptide (AgRP)[6]. Many of these neuropeptides also interact with kisspeptin/neurokinin B/dynorphin (KNDy) coexpressing neurons, which are themselves critical upstream regulators of GnRH release [10, 14, 15]. Arcuate nucleus KNDy neurons stimulate GnRH release and evidence from numerous models of negative energy balance indicate that inhibition of KNDy neurons likely contributes to metabolic driven reproductive dysfunction [16–20]. This regulatory circuitry is hypothesized to provide feedback cues to reproductive circuits on the level of food availability and energy homeostasis, which are needed to maintain reproductive function.
The arcuate nucleus has been an area of intense focus for both circuits regulating metabolism and reproduction due to its expression of numerous neuropeptides. Taken together with the fact that GnRH fibers terminate in the adjacent median eminence, it is hypothesized that the arcuate nucleus is a key site of integration between the metabolic and reproductive regulatory systems. Recent neuroanatomical studies have noted key differences between humans and rodents in the expression patterns of neuropeptides in the arcuate nucleus [21–23]. Colocalization of neuropeptides within many arcuate nucleus cell types is hypothesized to lend important physiological specialization. Colocalization of NPY and AgRP is thought to promote an extremely powerful appetite-inducing effect because NPY stimulates appetite through binding of NPY receptors, while AgRP is an endogenous antagonist for the appetite-suppressing melanocortin 4 receptor [24–26]. Similarly, kisspeptin release is hypothesized to be regulated by two neuropeptides that are coexpressed in the arcuate population, neurokinin B (NKB) and dynorphin, to create the “GnRH pulse generator” [27–32]. Differences in coexpression patterns between species raises the possibility that metabolic regulation of reproduction is controlled by fundamentally different neural circuitry in primates compared to rodents. The goal of the current study was to examine the neuroanatomy of circuits regulating feeding and reproduction in the female rhesus macaque. Specifically, we investigated colocalization patterns of arcuate nucleus neuropeptides previously implicated in GnRH regulation, including NPY, AgRP, POMC, CART, kisspeptin and dynorphin. We also gathered indirect evidence for whether these neuropeptides regulate GnRH in the primate by determining whether fibers made close appositions onto GnRH neurons and whether these connections fluctuated between animals with low GnRH release (prepubertal) compared to animals with high GnRH release (ovariectomized adults).
METHODS
Animals
Brains from female rhesus macaques were collected at the Oregon National Primate Research Center (ONPRC). All animal procedures were approved by the ONPRC Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guidelines on the ethical use of animals. Animals were deeply sedated with ketamine, then perfused transcardially with saline followed by 4% paraformaldehyde. The hypothalamus was blocked and post-fixed in paraformaldehyde overnight, then incubated in ascending concentrations of sucrose before flash freezing in 2-methylbutane. Frozen tissue was cut into 25 μm sections in a 1:24 series on a microtome with a freezing stage and stored in cryoprotectant at −20° C until further use. Brains were collected from intact prepubertal animals (n=5) between 1–2.5 years old, and ovariectomized (OVX) adult females (n=5) between 9–18 years old. Time between OVX and tissue collection was at least 2 months. Blood collected at the time of necropsy confirmed low or undetectable estrogen and progesterone levels for all animals.
Immunohistochemistry
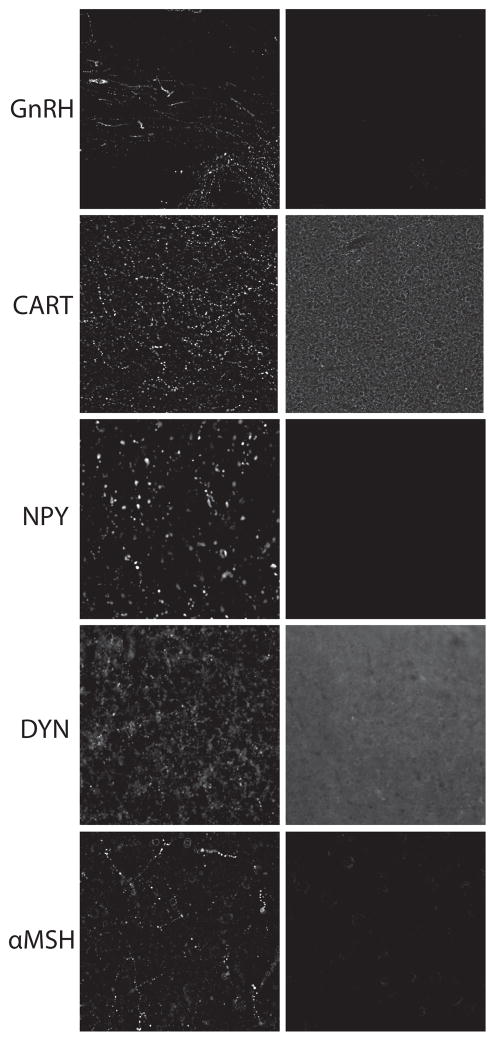
Free-floating immunohistochemistry was performed on a 1:12 hypothalamic series containing sections with the arcuate nucleus. Tissue was rinsed of cryoprotectant in potassium phosphate-buffered saline (KPBS), then blocked serially in 1% hydrogen peroxide, avidin and biotin blocking solutions, 3% bovine serum albumin and 2% normal donkey serum before incubation in primary antibody cocktail overnight at 4° C (Table 1). All antibodies except CART and dynorphin were detected with direct secondary labeling with Alexa fluorophores (1:1000). CART and dynorphin signals were amplified by incubation serially with biotin-conjugated secondary antibody (1:5000), avidin and biotin solution (Vectastain ABC kit PK-4000), biotinylated tyramide and fluorophore-conjugated streptavidin. Two antibodies raised in the same host species of rabbit were used in the same tissue as described previously [10]. Briefly, the rabbit anti-CART antibody was used first at very dilute concentrations that produced no signal with direct secondary detection. This signal was amplified with biotin/tyramide/streptavidin protocol as described above. Tissue was then washed and incubated with the second rabbit primary antibody, which was detected with direct secondary labeling. Tissue was mounted on gelatin-subbed slides and coverslipped with SlowFade medium. All antibodies had previously been characterized and validated by preadsorption experiments (Table 1) [33–39]. However, only the AgRP and kisspeptin antibodies had previously been validated in nonhuman primate tissue. To verify specificity within primate tissue for the other antibodies, preadsorption experiments were performed for GnRH (blocking peptide PEP-168, Thermo Fisher Scientific), CART (blocking peptide H-003-61, Phoenix Pharmaceuticals), NPY (blocking peptide H-049-03, Phoenix Pharmaceuticals), DYN (blocking peptide H-021-03, Phoenix Pharmaceuticals), and αMSH (blocking peptide H-043-01, Phoenix Pharmaceuticals) using at least 100-fold higher molar concentration of antigen to antibody. These experiments confirmed a lack of staining for all antibodies following preadsorption (Figure 1).
Table 1.
Antibody specifications for immunohistochemistry.
| Source | Host Species | Concentration | Validation | |
|---|---|---|---|---|
| α-MSH | Phoenix Pharmaceuticals H-043-01 | Rabbit | 1:15,000 | [36] |
| AgRP | Antibodies Australia | Guinea Pig | 1:5,000 | [39]⊥ |
| CART | Phoenix Pharmaceuticals H-003-62 | Rabbit | 1:1,000,000* | [37] |
| DYN | Phoenix Pharmaceuticals H-021-03 | Rabbit | 1:18,000* | [35] |
| GnRH | Sigma PA1-120 | Rabbit | 1:3,000 | [38] |
| Kisspeptin | Bloom Laboratory GQ2 | Sheep | 1:15,000 | [34]⊥ |
| NPY | Millipore AB1583 | Sheep | 1:3,000 | [33] |
Biotin/tyramide/streptavidin secondary amplication, see Methods.
Specificity determined in nonhuman primate tissue.
Figure 1.
Antibody validation in female rhesus macaque mediobasal hypothalamus. Antibody labeling (left panel) was validated for specificity by an absence of staining following preadsorption (right panels) with either GnRH, CART, NPY, DYN, or αMSH. Higher background staining was noted for CART and DYN likely due to the tyramide amplification of fluorescent labeling, but no specific staining was observed.
Confocal Imaging and ImageJ Analysis
Confocal analysis was performed on a Leica SP5 AOBS spectral confocal system. Fluorophores were imaged sequentially to avoid spectral overlap. For fiber colocalization analysis images were obtained using a 20x objective, while close apposition analysis was scanned using a 40x objective and a zoom factor of 2. For all images, 1 μm z-plane stacks were imaged through the tissue entirety. For all analyses, endogenous autofluorescence was detected through excitation with the 488 laser and detection of emission in the red spectrum. Spectral Unmixing in ImageJ was used to remove autofluorescence from 488 and 568 immunostaining.
Colocalized Cell Body Quantification
For analysis of the percentage of kisspeptin cell bodies coexpressing CART, all kisspeptin cells observed in 4 arcuate photomicrographs/animal were counted manually in ImageJ and then checked for CART-ir by flipping back and forth between color channels using the plugin Image5D. On average 96 kisspeptin cells were examined per animal in the dynorphin experiment and 88 kisspeptin cells/animal in the CART experiment. NPY immunoreactivity was not visible, consistent with previous reports [40]; therefore, quantification of the percentage of CART and AgRP cell coexpressing NPY was not possible. No αMSH-immunoreactivity was observed within CART cells.
Colocalized Axon Varicosity Quantification
Analysis of axon varicosities was adapted from Skraptis et al. [23]. Confocal photomicrographs were analyzed in ImageJ by placing grid overlays of 50 μm x 50 μm squares onto 3 arcuate photomicrographs and a total area of ≈135,000 μm2 was analyzed per animal (two 3x3 grids/section were analyzed for three sections/animal). Axon varicosities that crossed a grid line were examined for immunoreactivity and manually scored for single or double-immunoreactivity, again assisted by the Image5D plugin to flip back and forth between fluorophore channels. The number of double-labeled fibers was divided by the total number of the fibers of interest and expressed as a percentage. This percentage was then averaged across the grids for each animal.
Quantification of Axon Varicosity Density
For quantification of axon density, maximum intensity images were compiled across a 10 μm stack for four arcuate sections per animal. A region of interest was placed near the ventral border of the brain adjacent to third ventricle and consisted of approximately 122,500 μm2. For each fluorophore a threshold was set for all images and any cell body immunoreactivity or fluorescent artifacts from the region of interest were erased using the ImageJ Drawing Tool Eraser function. ImageJ measurements were made of “area fraction” which is the percentage of the region of interest with signal intensity over the set threshold. For quantification of double-label staining, a threshold was set for each fluorophore individually and then a mask was created for overlapping pixels at each 1 μm plane. A maximum intensity 10 μm stack from this colocalization mask was then stacked to 10 μm and quantified for area fraction as above.
Close Apposition Analysis
Analysis of close appositions between fibers and cell bodies was performed as described previously [10]. All GnRH neurons were analyzed across three sections/animal using a 63x objective. On average 20 GnRH neurons were analyzed per animal. Values are reported as the percentage of GnRH neurons with close appositions to normalize differences in total number of cells examined per animal. In addition, for GnRH cells with close appositions, the number of close appositions per cell was calculated. To determine close appositions onto GnRH neurons, 1 μm focal planes were manually examined to determine the presence or absence of close apposition of either kisspeptin, CART, kisspeptin/CART, NPY, AgRP or NPY/AgRP immunoreactive fibers. An apposition was defined as an abutment of an axon with GnRH cell body resulting either in overlap of immunoreactivities or an absence of black ir-negative pixels between fiber and cell body in all orthogonal views of the potential contact.
In situ hybridization
The same brains used for immunohistochemistry were also used for in situ hybridization (n=5/group). For each probe a 1:12 series of arcuate hypothalamic tissue was mounted onto slides in RNase free conditions for each animal. Separate in situ hybridizations for AgRP and NPY were performed as previously described [39, 41]. Briefly, brain sections were fixed in 4% paraformaldehyde (pH 7.4), treated with proteinase K at 37 °C to increase penetration, and then with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0). Sections were then rinsed in 2X SSC, dehydrated through graded series of alcohols, delipidated in chloroform, rehydrated through a second series of alcohols and air-dried. The sections were exposed to either human NPY or human AgRP cRNA probes labeled with 33P UTP overnight in a moist chamber at 55 °C. After incubation, the slides were washed in 4X SSC, in RNase A at 37 °C, and in 0.1X SSC at 60 °C. Slides were then dehydrated through a graded series of alcohols and dried. For visualization of the probes, labeled sections were exposed to film (Biomax MR, Kodak) overnight. For quantitative analysis of NPY and AgRP mRNA, arcuate nucleus film images were captured using a CoolSNAP charge-coupled camera (Photometrics) and analyzed using the MetaMorph Imaging system (Universal Imaging). Fifteen to twenty images containing half the arcuate nucleus were imaged per animal. The images were analyzed using a sampling box that encompassed the entire region of interest and measured as the integrated intensity. Background labeling, determined using the same sampling box over an adjacent region that contained no expression, was subtracted from this measurement. For each animal, adjusted integrated intensities were averaged across all sections and are reported as relative units.
Statistics
Changes in mRNA and protein levels, as well as differences in close appositions to GnRH cells between prepubertal and OVX adult animals, were determined by unpaired Student’s t-tests in GraphPad Prism statistics software.
RESULTS
Coexpression patterns in the arcuate nucleus
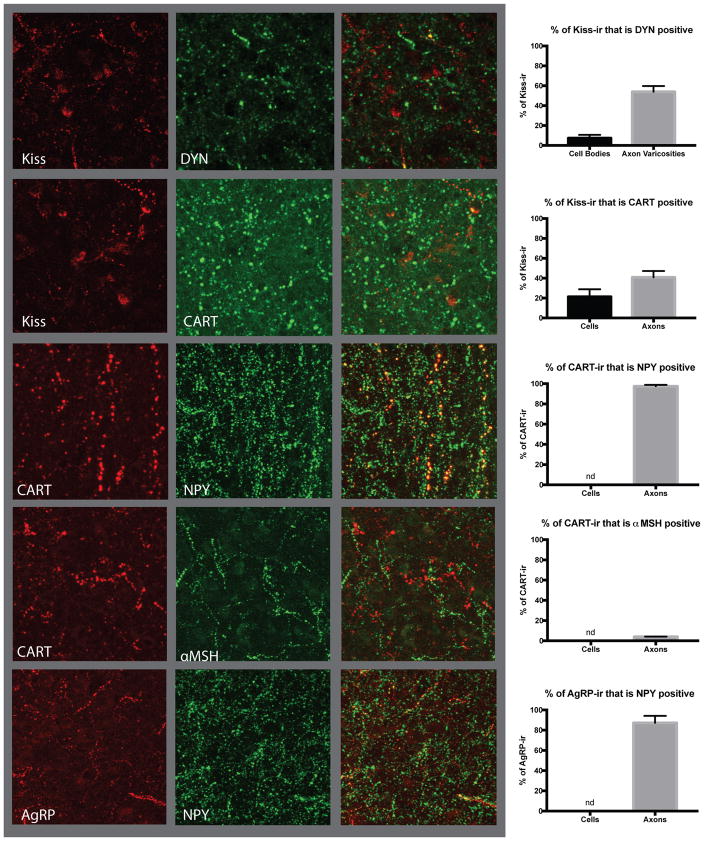
Immunohistochemistry was used to examine coexpression patterns of arcuate nucleus neuropeptides kisspeptin/DYN, kisspeptin/CART, NPY/CART, POMC/CART and NPY/AgRP, in the OVX adult female rhesus macaque (n=5). Only 7.3±3.3% of kisspeptin cell bodies showed coexpression with dynorphin in the primate arcuate nucleus, consistent with recent reports from humans (Figure 2, [21, 42]. However, axon varicosities showed significantly higher colocalization with 54±5.7% of kisspeptin fibers demonstrating dynorphin-ir. A similar pattern of colocalization was observed between kisspeptin and the anorexigenic neuropeptide CART, with 21.37±7.5% of kisspeptin cell bodies and 40.89±6.3% of kisspeptin axon varicosities being positive for CART (Figure 2).
Figure 2.
Neuropeptide coexpression patterns in the arcuate nucleus of ovariectomized (OVX) adult female rhesus macaques. Quantification of coexpression in cell bodies, when visible, and axon varicosities by immunohistochemistry, was performed for arcuate neuropeptides regulating reproduction and appetite. Kisspeptin showed only a 7% coexpression with dynorphin (top panel) in cell bodies but 54% colocalization in axon varicosities. CART-ir was present in 21% of kisspeptin-ir positive cell bodies and 41% of axon varicosities (second panel). CART axon varicosities showed a high level of colocalization of 97% with NPY (third panel), although no NPY-ir was observed in cell bodies; therefore, coexpression in cell bodies was not quantified. CART showed extremely low colocalization of only 4% of axon varicosities with α-MSH (fourth panel) while no cell bodies showed immunoreactivity for both neuropeptides. NPY-ir was also present in 87% of AgRP-ir axon varicosities (fifth panel). Images are approximately 150 x 150 μm.
CART was highly coexpressed with the orexigenic neuropeptide NPY in the arcuate nucleus of the nonhuman primate. NPY-ir is known to be absent from cell bodies so only axon varicosities were examined and 97.43±1.4% of CART axon varicosities are positive for NPY-ir (Figure 2). Only 4.00±0.19% of CART-ir axon varicosities are also positive for the POMC cleavage product α-melanin stimulating hormone (α-MSH)-ir and no colocalized cell bodies were observed. Examination of AgRP and NPY revealed that 87.2±6.9% of AgRP-ir axon varicosities are also NPY-ir positive, consistent with previous works in both rodents and non-human primates (Figure 2) [24, 43, 44].
Potential contacts with GnRH neurons across puberty
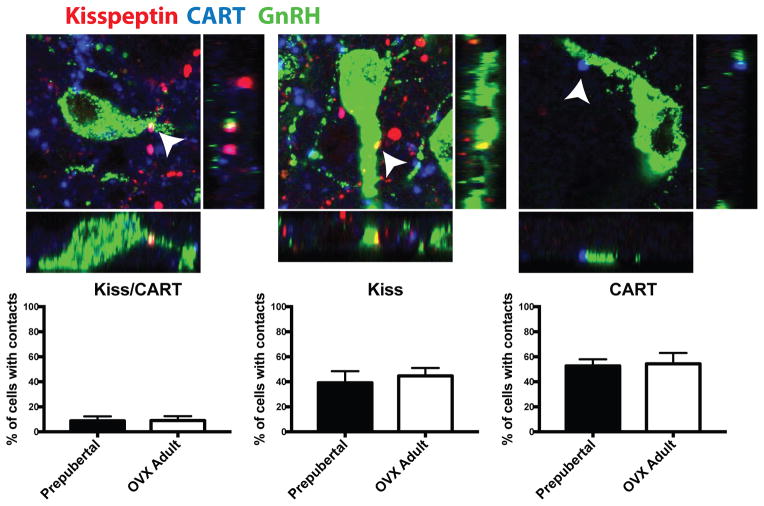
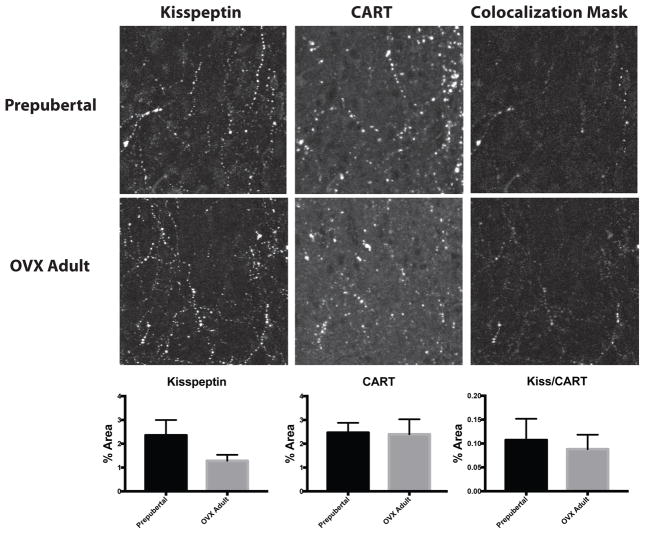
To better understand what role these neuropeptides might play in regulating GnRH release, the current study examined whether arcuate nucleus neuropeptides have fiber projections in close proximity with GnRH neurons and whether these close appositions differed between prepubertal animals, when GnRH release is low, and OVX adult animals, when GnRH release is high. Fibers coexpressing kisspepti- and CART-ir made infrequent close appositions onto GnRH neurons, contacting roughly 8% of GnRH cells examined with no difference between prepubertal (8.76±3.6%) and OVX adult animals (9.08±3.4%; Figure 3). For GnRH cells that did receive Kisspeptin/CART-ir close appositions, the number of appositions per GnRH cell also did not differ between prepubertal (1.42±0.25 contacts/cell) and OVX adult animals (1.29±0.24 contacts/cell). Single-labeled CART-ir and kisspeptin-ir fibers were in more frequent close apposition with GnRH neurons. Single-labeled kisspeptin-ir fibers were in close contact with approximately 42% of GnRH cells examined and there was no difference between prepubertal (39.20±9.3%) and OVX adults (44.74±6.3%; Figure 3). Of the cells receiving kisspeptin-ir close appositions, the number of close appositions per GnRH cell did not differ between prepubertal (2.90±0.5 contacts/cell) and OVX adult animals (1.89±0.2 contacts/cell). Single-labeled CART-ir fibers made close appositions to approximately 54% of GnRH cells examined, with no difference between prepubertal (52.67±5.3%) and OVX adult animals (54.37±8.7%). Of the cells receiving CART-ir close appositions, the number of CART-ir close appositions per GnRH cell did not differ between prepubertal (1.68±0.1 contacts/cell) and OVX adult animals (1.55±0.2 contacts/cell; Figure 3). In addition, no changes in kisspeptin-ir, CART-ir or colocalized kisspeptin/CART-ir fiber density were observed in the arcuate nucleus between prepubertal animals and OVX adults (Figure 4).
Figure 3.
Apposition of kisspeptin-ir, CART-ir and kisspeptin/CART-ir fibers onto GnRH neurons of the mediobasal hypothalamus in prepubertal and OVX adult female rhesus macaques. No differences were observed between prepubertal and OVX animals in the percentage of GnRH neurons (green) with close appositions from coexpressing kisspeptin/CART-ir fibers (magenta; left panel), single-labeled kisspeptin-ir fibers (red; middle panel) or single-labeled CART-ir fibers (blue; right panel). Close apposition colocalization with GnRH neurons is indicated by arrows and shown with orthogonal views (below and to the right). Photomicrographs are of z-stack of 3 1-μm planes and approximately 40 x 40 μm in size, N=5.
Figure 4.
Arcuate kisspeptin and CART fiber density by immunohistochemistry in prepubertal and OVX adult female rhesus macaques. Quantification of kisspeptin-ir (left panels), CART-ir (middle panels) and colocalized kisspeptin/CART-ir (right panels) showed similar levels of protein in prepubertal (top panels) and OVX adult animals (bottom panel). N=5, image size is approximately 150 x 150 μm.
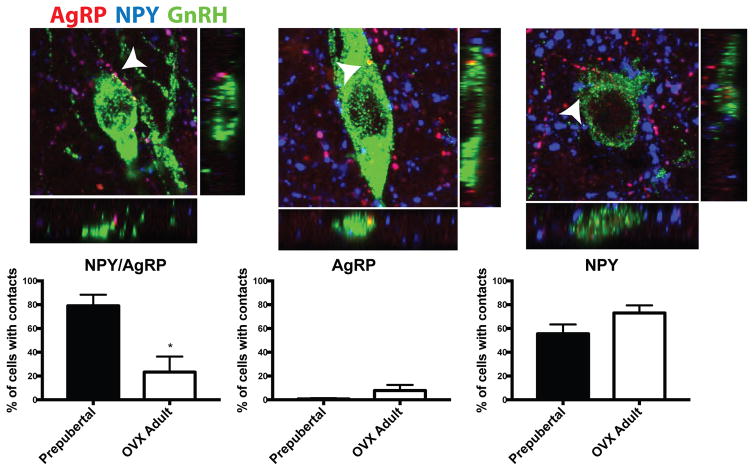
Fibers coexpressing NPY- and AgRP-ir were also observed in close apposition to GnRH neurons (Figure 5). Interestingly, 79.0±9.4% of GnRH neurons examined received close appositions from fibers coexpressing AgRP/NPY-ir in prepubertal animals, while only 23.3±13.1% of GnRH neurons examined in OVX adult animals had close appositions (p<0.01). The number of NPY/AgRP close appositions per contacted GnRH neuron also trended higher in prepubertal animals (3.06±0.3 contacts/cell) compared to OVX adults (1.93±0.4 contacts/cell; p=0.07). Single-labeled AgRP-ir close appositions to GnRH neurons were rare and there was no significant difference in the percentage of GnRH neurons with these appositions between prepubertal animals (0.67±0.7%) and OVX adults (7.75±4.8%; Figure 5). The number of animals demonstrating AgRP-ir close appositions to GnRH cells was too low to statistically compare the number of appositions/contacted cell, but more than one close appositions per GnRH cell, in either prepubertal animals or OVX adults, was rarely observed. The percentage of GnRH neurons with potential contacts from single-labeled AgRP fibers is considerably lower than that observed for AgRP/NPY coexpressing fibers, indicating the majority of AgRP input to GnRH neurons comes from NPY-coexpressing cells (Figure 5). This finding is consistent with widespread expression of NPY in the brain [45]. In contrast to the relatively few number of GnRH neurons receiving single-labeled AgRP-ir close appositions, roughly 64% of GnRH neurons received close appositions from single-labeled NPY-ir fibers (Figure 5). The percent of GnRH neurons with NPY-ir close appositions trended higher in OVX adults compared to prepubertal animals, but this was not significant (OVX adults: 73.1 ± 6.4%; prepubertal animals: 55.6 ± 7.9 %; p=0.12). However, of the neurons receiving close appositions, OVX adults received significantly more NPY-ir close appositions per GnRH cell than prepubertal animals (OVX adults: 2.89 ± 0.4; prepubertal animals: 1.62 ± 0.1; p<0.05).
Figure 5.
Close apposition of AgRP-ir, NPY-ir, and AgRP/NPY-ir fibers onto GnRH neurons of the mediobasal hypothalamus in prepubertal and OVX adult female rhesus macaques. Coexpressing AgRP/NPY-ir fibers (magenta; left panel) were in close apposition with significantly more GnRH neurons (green) in the prepubertal state (75%) compared to the OVX adult state (27%, left panel). Prepubertal and OVX adults showed a similarly low percentage of GnRH neurons receiving close appositions from single-labled AgRP-ir fibers (red; middle panel). There was no difference in the percentage of GnRH neurons with close appositions from single-labeled NPY-ir fibers (blue; right panel) in OVX adults compared to prepubertal animals. Close apposition colocalization with GnRH neurons is indicated by arrows and shown with orthogonal views (below and to the right). Photomicrographs are of z-stack of 3 1-μm planes and approximately 40 x 40 μm in size, N=5.
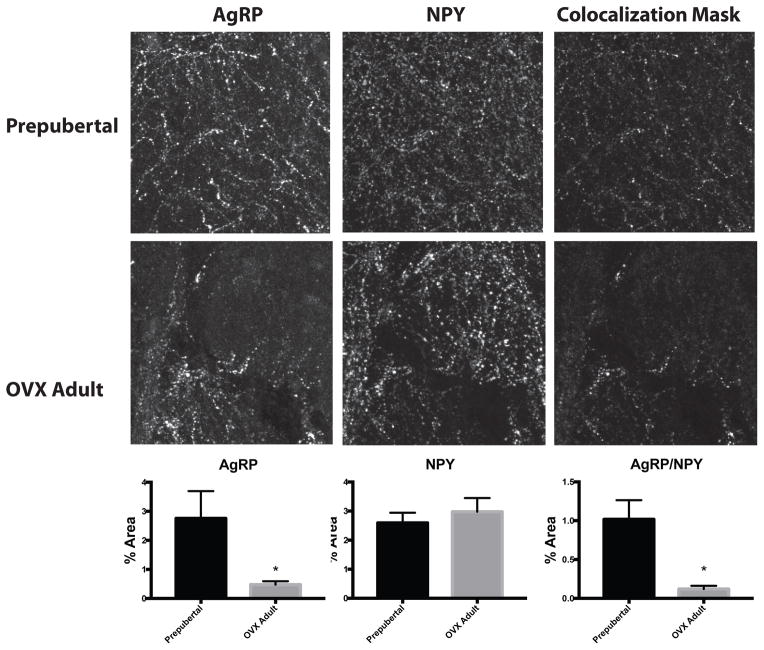
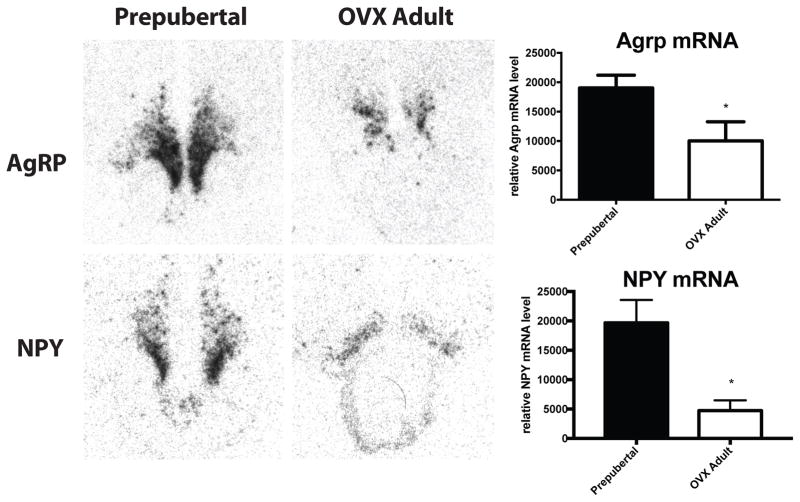
Quantification of arcuate nucleus AgRP-ir fiber density by IHC revealed significantly higher fiber density in prepubertal animals (2.76±0.94 % of area) compared to OVX adults (0.48±0.12% of area, p=0.04; Figure 6). Arcuate nucleus NPY-ir fiber density was not changed across groups (Figure 6). Colocalized AgRP/NPY-ir fiber density was significantly higher in prepubertal animals (1.02±0.25% of area) compared to OVX adults (0.12±0.04% of area, p<0.01; Figure 6). To investigate whether differences in protein density were supported by differences in mRNA, ISH was performed on the same brains for AgRP and NPY. Quantification of arcuate nucleus AgRP mRNA expression by ISH revealed a similar increase in mRNA expression in prepubertal animals (19038±2163 relative units) compared to OVX adults (10040±3234 relative units, unpaired t test p=0.0495; Figure 7). Despite no difference in protein levels by IHC, NPY mRNA levels were significantly elevated in prepubertal animals (19672±3911 relative units) compared to OVX adults (4748±1736 relative units, unpaired t test p=0.0149; Figure 7).
Figure 6.
Arcuate AgRP and NPY fiber density by immunohistochemisty in prepubertal and OVX adult female rhesus macaques. Quantification of AgRP-ir (left panels), NPY-ir (middle panels) and colocalized AgRP/NPY-ir (right panels) showed significantly lower levels of AgRP-ir and AgRP/NPY-ir in OVX adult animals (bottom panels) compared to prepubertal animals (top panels). N=5, * p<0.05 by t-test and images are approximately 150 x 150 μm.
Figure 7.
Arcuate NPY and AgRP mRNA expression as measured by in situ hybridization in prepubertal and OVX adult female rhesus macaques. Analysis of radiographic films following labeling with a P33 NPY (top) or AgRP (bottom) anti-sense cRNA probe revealed significantly higher expression of NPY and AgRP mRNA in prepubertal animals compared to OVX adults. N=5, * p<0.05 by t-test.
DISCUSSION
Nonhuman primates show distinct neuroanatomical differences from rodents in the neurocircuitry regulating both feeding and reproduction. Like the human, rhesus macaques show a lack of colocalization between the anorexigenic neuropeptides CART and POMC as observed in rodents [46], but instead CART is coexpressed with the orexigenic neuropeptide NPY and a subpopulation of kisspeptin expressing neurons [22, 23]. These key differences in coexpression patterns from the rodent suggest that the mechanisms of metabolic regulation of reproduction may differ between species. Importantly, arcuate nucleus NPY and AgRP expression decreases from puberty to adulthood as do close appositions from fibers coexpressing NPY and AgRP onto GnRH neurons indicating this population, traditionally implicated in the regulation of feeding and metabolism, may act to decrease GnRH and function as a prepubertal brake.
Several neuropeptides that regulate feeding and metabolism also provide metabolic feedback to GnRH neurons. The anorexigenic neuropeptide CART directly stimulates GnRH neurons, as well as upstream kisspeptin neurons in the arcuate nucleus of rodents, illustrating two potential paths for reproductive regulation [9, 10, 14]. Consistent with this observation in rodents, the current study found that almost half of GnRH neurons receive potential contacts from CART-ir fibers. Although the number of cells receiving close appositions did not change between prepubertal and adult animals, this high percentage of GnRH cells receiving input from CART fibers suggests that they could be an important regulatory signal for GnRH cells in the nonhuman primate. The current study only examined GnRH neurons in the infundibular nucleus and there are scattered GnRH neurons in other hypothalamic nuclei [47]; therefore, it is possible that neuropeptide input onto GnRH neurons across puberty and adulthood may differ depending on the neuroanatomical location. CART is known to inhibit feeding and increase metabolism and, consistent with these functions, CART expression is suppressed under conditions of negative energy balance in rodents, sheep and primates [10, 48–53]. Given the stimulatory role of CART on kisspeptin and GnRH neuronal firing we hypothesize that decreases in this signal during negative energy balance may contribute to the metabolic inhibition of reproductive function.
The orexigenic neuropeptide NPY is also implicated in regulating GnRH release [54, 55]. NPY directly inhibits GnRH neuronal firing via the Y1 and Y5 receptor in the rodent [6, 11, 14]. Previous work in rodents and primates has demonstrated that NPY levels are elevated in prepubertal animals and decrease after the initiation of puberty, indicating that NPY could be a brake on prepubertal GnRH release [56, 57]. The current study found a similar decrease in arcuate nucleus NPY mRNA in female adults compared to prepubertal monkeys as well as a decrease in NPY/AgRP-ir colabeled fibers in the arcuate nucleus and a specific decrease in NPY/AgRP-ir fibers in close apposition with GnRH neurons. High expression and contact of NPY fibers onto GnRH neurons in prepubertal animals confirms this signal likely inhibits GnRH release and that attenuation of this inhibition is a trigger for puberty.
However, while NPY/AgRP-ir close appositions with GnRH neurons were decreased in adults, close appositions from single-labeled NPY-ir fibers were modestly increased compared to prepubertal animals. In addition, the number of NPY-ir close appositions per GnRH cell was significantly increased in OVX adults compared to prepubertal animals. These findings could raise the possibility that AgRP colocalization with NPY in the arcuate nucleus decreases after puberty. However, arcuate nucleus NPY mRNA is decreased in OVX animals, indicating potentially less NPY-ir is originating from the arcuate nucleus in these animals. We hypothesize that these single-labeled fibers likely originate outside of the arcuate nucleus. Other potential sources of NPY could include the dorsomedial hypothalamus and brainstem populations; however, there is limited information on the projection patterns of these specific NPY populations in the primate. Furthermore, the increase in the number of NPY-ir close appositions per GnRH neuron in OVX adults, when GnRH release is high, could indicate a stimulatory role for this alternative population of NPY neurons. Consistent with this hypothesis is data from rodents indicating both a stimulatory and inhibitory role for NPY in the regulation of GnRH neurons, depending on steroid hormone milieu [58]. Differences in steroid hormone levels are unlikely to account for the apparent discrepancy in the current study, since the same OVX adult animals had increased single-labeled NPY close appositions per GnRH neuron and decreased appositions from fibers coexpressing NPY and AgRP compared to prepubertal animals. It is possible that changes in expression of NPY receptors contribute to the dual role of NPY in GnRH regulation, given there are multiple NPY receptors with different intracellular signaling cascades. There is limited data on which NPY receptors are expressed on GnRH neurons in monkeys, and further work is needed to determine whether developmental changes in receptor profiles might underlie these confounding observations.
In addition to NPY, AgRP has been implicated in both the stimulation and inhibition of GnRH release. In the rodent, AgRP directly inhibits neuronal firing in some GnRH neurons while stimulating GnRH firing in others [6]. Ablation of AgRP neurons is sufficient to restore reproductive function in leptin-deficient mice [59], indicating a strong and potentially overriding inhibitory role for this neuronal population in reproductive function. This inhibitory role is consistent with the current observation that AgRP mRNA and protein levels are higher in prepubertal animals, when GnRH release is low, compared to OVX adults, when GnRH release is high. It is likely that changes in NPY and AgRP expression are driven in part by changing metabolic demands between prepubertal and adult animals; therefore, we cannot rule out that changes in expression of these neuropeptides are independent of the differential GnRH release rates between these two conditions. However, the reduction of close appositions of fibers coexpressing NPY and AgRP onto GnRH neurons in OVX adult animals indicates a specific change in how these neuropeptides signal to the reproductive axis and suggest that these changes likely have effects beyond adapting to changing metabolic demands. Recent evidence indicates that AgRP also directly inhibits kisspeptin neurons in the mouse, and optogenetic activation of AgRP neurons is sufficient to inhibit reproductive cycling [60]. Future studies could explore whether this connection is apparent in primates and differentially regulated across puberty.
Kisspeptin is another key neuropeptide regulating GnRH release and low levels of kisspeptin are associated with GnRH inhibition during negative energy balance conditions [16–19, 61–63]. The current study did not detect changes in either kisspeptin-ir cell bodies or fiber density in the arcuate nucleus or close appositions onto GnRH neurons between prepubertal and OVX adult animals. This finding is at odds with previous literature showing a developmental increase in kisspeptin mRNA expression at the time of puberty in rodents and primates [64, 65]. In addition, the removal of steroid hormones further increases kisspeptin expression and fiber density due to the removal of negative feedback from estradiol in adult animals [66]. However, discrepancies between kisspeptin mRNA and protein were previously noted [16], indicating changes in peptide secretion may not always be simplistically correlated with changes in mRNA and protein levels. In addition, previous studies compared kisspeptin expression from prepubertal animals to animals immediately at the pubertal transition, in contrast to much older adults studied here (ages 9–18 years). It is possible kisspeptin levels are higher at the initiation of puberty to reawaken GnRH release and the HPG axis, and then decrease modestly once the axis has been established in adulthood.
Coexpression of other neuropeptides with kisspeptin in arcuate neurons is hypothesized to play an important role in the regulation of this critical reproductive output signal [27, 30, 67–70]. The observed level of roughly 20% CART colocalization within kisspeptin neurons and 40% in axons reported here in monkeys is similar to that reported in humans (17% in axons and 48% in cells) [23]. Both studies also noted a similarly low level of fibers coexpression kisspeptin and CART in close apposition to GnRH neurons indicating a potential limited role for this population in GnRH regulation in the primate. Although the current findings are largely consistent with reports in humans, one discrepancy was in the colocalization of kisspeptin and dynorphin. The current study observed significant colocalization between kisspeptin and dynorphin in axon varicosities in monkeys, while colocalization between kisspetin and dynorphin in human axons is reportedly very rare [21, 42]. It is possible that this is a species difference, which is supported by the observation that substance P colocalizes in kisspeptin cells of humans but not monkeys [71, 72]. Dynorphin neurons were rarely observed in the current study, although colabeling within kisspeptin neurons was observed occasionally, making it possible to distinguish rare double-labeled cell bodies. A lack of dynorphin cell bodies was also noted in human males and females, indicating that there may be technical limitation to studying dynorphin perikarya in primates by immunohistochemistry. Lack of steroid hormones may also contribute to low dynorphin detection since OVX decreases dynorphin expression in sheep [73]. Future studies should examine whether dynorphin levels are modulated by sex steroids in primates as described in sheep and determine whether this contributes to the low detection and colocalization with kisspeptin reported in both OVX nonhuman primates and postmenopausal women.
The current study has on one hand confirmed potential contacts between arcuate neuropeptides and GnRH neurons as previously observed in rodents, but also found important differences in the coexpression patterns of these same neuropeptides in both cell bodies and axon varicosities. This raises a significant question: what is the physiological importance of coexpression of neuropeptides? Surprisingly little information is known about how coexpressed neuropeptides might act in coordination to regulate downstream neurons. In most cases, it is unknown whether coexpressed neuropeptides have the same downstream targets or whether they are released simultaneously and in proportional amounts. Examination of colocalization within both cell bodies and axon varicosities in the current study revealed inherent differences in shuttling and accumulation within the cell bodies of these neuropeptides. Differences in subcellular localization of peptides also makes it difficult to quantify colocalized axons and the current study likely reports an underestimate of total colocalization, particularly where masks of overlapping pixels are used for quantification. All four neuropeptides examined in the current study (NPY, AgRP, CART and kisspeptin) are capable of regulating GnRH neuronal firing in the rodent [6, 10, 74], but similar experiments are difficult and costly to conduct in nonhuman primates. Several reports, including a recent study in humans, have coadministered KNDy agonists and antagonists peripherally to begin to understand the interactions of these neuropeptide systems [75]; however, these neuropeptides and their corresponding receptors have wide expression throughout the brain. Thus, it remains unclear, for any species, how these neuropeptides specifically act in coordination at the level of GnRH neurons. Subtle changes in the ratio of co-released neuropeptides at GnRH neurons might underlie changes in firing and pulse generation in different nutritional and developmental states. Advancements in opto- and pharmaco-genetic approaches should help elucidate how activation of these populations in vivo changes GnRH release in primates and rodents, and whether release of each neuropeptide in a coexpressed pair is required for this regulation.
Of note, there are limitations in the interpretation of the current results. Close appositions detected by confocal microscopy do not prove synaptic contact, which requires electron microscopy (EM). However, previous work in the monkey hypothalamus found that close appositions by light microscopy were frequently associated with synaptic contacts as determined by EM [76]. In fact, this analysis found that contacts by light microscopy often underestimated the number of synaptic contacts observed by EM. Consistent with this finding, it is likely that the number of close appositions per cell in the current report underestimates synaptic contact per cell, and this could likely be further confounded by tyramide amplification techniques which amplify the IHC signal and can obscure instances of multiple fine close appositions. An additional limitation, is the reliance on IHC for the determination of colocalization, especially for neuropeptides like NPY which do not accumulate in cell bodies. Quantification of colocalization within fibers was used to circumvent a lack of immunolabeled NPY soma; however, this does not necessarily represent the level of colocalization present at cell bodies, since differential transport of neuropeptides along the axon is possible. RNAscope offers an advantageous new system to begin to explore colocalization at the levels of cell bodies that relies on mRNA instead of protein. The reported increase in sensitivity of this assay will aid in detecting even low levels of colocalization and could allow for quantification of more subtle regulation of coexpressed neuropeptides within the same cells.
Species differences in the neuroanatomy of the reproductive neuroendocrine systems observed in this study are consistent with well-documented discrepancies in regulation of the hypothalamic-pituitary-gonadal axis between rodent and primates [77]. For example, rodents display a circadian entrained timing of the luteinizing hormone surge, while humans do not [78, 79]. An upstream surge of GnRH appears to trigger the pituitary LH surge in rodents, but the role of GnRH in this process in the primate remains controversial [80, 81]. Understanding differences between primates and rodents at the most basic neuroanatomical level provides important clues to the underlying physiology of these complex systems. While laboratory rodent models continue to provide critical and fast-paced insights into the underlying regulatory signals for neuroendocrine function, primate species offer the best models to study how human reproductive neuroendocrine diseases occur and can potentially be treated. Furthermore, by understanding species differences the field will be better equipped to anticipate which rodent findings might be directly translatable to humans and hold the most therapeutic advantage.
Acknowledgments
The authors would like to acknowledge Dr. Richard Stouffer for his comments and suggestions on the manuscript. Authors would also like to thank the Oregon National Primate Research Center Endocrine Technology Support Core for estradiol and progesterone assays. This work was supported by the Eunice Shriver Kennedy National Institute of Child Health and Human Development National Center for Translational Research in Reproduction and Infertility NIH P50 HD071836. This work was also funded in part by NIH awards R01 HD014643 and R01 DK079194.
Funding sources: This work was supported by the Eunice Shriver Kennedy National Institute of Child Health and Human Development NCTRI NIH P50 HD071836, R01 HD014643 and National Institute of Diabetes and Digestive and Kidney Diseases R01 DK079194.
Footnotes
Disclosure: DT, CM, AA and SS have nothing to declare. CT, MK, SRL PK and KG participate in research funded by Novo Nordisk Partnership grant. KG is employed by Novo Nordisk. PK is a consultant with Pfizer and GLWL Research.
References
- 1.Evans JJ, Anderson GM. Balancing ovulation and anovulation: integration of the reproductive and energy balance axes by neuropeptides. Hum Reprod Update. 2012;18:313–332. doi: 10.1093/humupd/dms004. [DOI] [PubMed] [Google Scholar]
- 2.Scaramuzzi RJ, Campbell BK, Downing JA, Kendall NR, Khalid M, Munoz-Gutierrez M, Somchit A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod Nutr Dev. 2006;46:339–354. doi: 10.1051/rnd:2006016. [DOI] [PubMed] [Google Scholar]
- 3.Boyar RM, Katz J, Finkelstein JW, Kapen S, Weiner H, Weitzman ED, Hellman L. Anorexia nervosa. Immaturity of the 24-hour luteinizing hormone secretory pattern. N Engl J Med. 1974;291:861–865. doi: 10.1056/NEJM197410242911701. [DOI] [PubMed] [Google Scholar]
- 4.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 5.Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab. 1985;61:851–858. doi: 10.1210/jcem-61-5-851. [DOI] [PubMed] [Google Scholar]
- 6.Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012;153:5587–5599. doi: 10.1210/en.2012-1470. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leslie RA, Sanders SJ, Anderson SI, Schuhler S, Horan TL, Ebling FJ. Appositions between cocaine and amphetamine-related transcript- and gonadotropin releasing hormone-immunoreactive neurons in the hypothalamus of the Siberian hamster. Neurosci Lett. 2001;314:111–114. doi: 10.1016/s0304-3940(01)02291-1. [DOI] [PubMed] [Google Scholar]
- 9.Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience. 2004;125:735–748. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 10.True C, Verma S, Grove KL, Smith MS. Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology. 2013;154:2821–2832. doi: 10.1210/en.2013-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Kirigiti MA, Cowley MA, Grove KL, Smith MS. Suppression of basal spontaneous gonadotropin-releasing hormone neuronal activity during lactation: role of inhibitory effects of neuropeptide Y. Endocrinology. 2009;150:333–340. doi: 10.1210/en.2008-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catzeflis C, Pierroz DD, Rohner-Jeanrenaud F, Rivier JE, Sizonenko PC, Aubert ML. Neuropeptide Y administered chronically into the lateral ventricle profoundly inhibits both the gonadotropic and the somatotropic axis in intact adult female rats. Endocrinology. 1993;132:224–234. doi: 10.1210/endo.132.1.8380374. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Chen P, Smith MS. Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin-releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology. 1999;140:5382–5390. doi: 10.1210/endo.140.11.7093. [DOI] [PubMed] [Google Scholar]
- 14.Verma S, Kirigiti MA, Millar RP, Grove KL, Smith MS. Endogenous kisspeptin tone is a critical excitatory component of spontaneous GnRH activity and the GnRH response to NPY and CART. Neuroendocrinology. 2014;99:190–203. doi: 10.1159/000365419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backholer K, Smith J, Clarke IJ. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology. 2009;150:5488–5497. doi: 10.1210/en.2009-0604. [DOI] [PubMed] [Google Scholar]
- 16.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23:52–64. doi: 10.1111/j.1365-2826.2010.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.True C, Kirigiti MA, Kievit P, Grove KL, Smith MS. Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance. J Neuroendocrinol. 2011;23:1099–1112. doi: 10.1111/j.1365-2826.2011.02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 19.Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- 20.Navarro VM, Ruiz-Pino F, Sanchez-Garrido MA, Garcia-Galiano D, Hobbs SJ, Manfredi-Lozano M, Leon S, Sangiao-Alvarellos S, Castellano JM, Clifton DK, Pinilla L, Steiner RA, Tena-Sempere M. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32:2388–2397. doi: 10.1523/JNEUROSCI.4288-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hrabovszky E, Sipos MT, Molnar CS, Ciofi P, Borsay BA, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153:4978–4989. doi: 10.1210/en.2012-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menyhert J, Wittmann G, Lechan RM, Keller E, Liposits Z, Fekete C. Cocaine- and amphetamine-regulated transcript (CART) is colocalized with the orexigenic neuropeptide Y and agouti-related protein and absent from the anorexigenic alpha-melanocyte-stimulating hormone neurons in the infundibular nucleus of the human hypothalamus. Endocrinology. 2007;148:4276–4281. doi: 10.1210/en.2007-0390. [DOI] [PubMed] [Google Scholar]
- 23.Skrapits K, Borsay BA, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Hrabovszky E. Colocalization of cocaine- and amphetamine-regulated transcript with kisspeptin and neurokinin B in the human infundibular region. PLoS One. 2014;9:e103977. doi: 10.1371/journal.pone.0103977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 25.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 26.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 27.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–210. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 31.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrington AM, Hutson JM, Southwell BR. Immunohistochemical localisation of cholinergic muscarinic receptor subtype 1 (M1r) in the guinea pig and human enteric nervous system. J Chem Neuroanat. 2007;33:193–201. doi: 10.1016/j.jchemneu.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada K, Park H, Sato S, Onozuka M, Kubo K, Yamamoto T. Dynorphin-A immunoreactive terminals on the neuronal somata of rat mesencephalic trigeminal nucleus. Neurosci Lett. 2008;438:150–154. doi: 10.1016/j.neulet.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Helwig M, Khorooshi RM, Tups A, Barrett P, Archer ZA, Exner C, Rozman J, Braulke LJ, Mercer JG, Klingenspor M. PC1/3 and PC2 gene expression and post-translational endoproteolytic pro-opiomelanocortin processing is regulated by photoperiod in the seasonal Siberian hamster (Phodopus sungorus) J Neuroendocrinol. 2006;18:413–425. doi: 10.1111/j.1365-2826.2006.01431.x. [DOI] [PubMed] [Google Scholar]
- 37.Dun NJ, Dun SL, Kwok EH, Yang J, Chang J. Cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat sympatho-adrenal axis. Neurosci Lett. 2000;283:97–100. doi: 10.1016/s0304-3940(00)00935-6. [DOI] [PubMed] [Google Scholar]
- 38.Chung WC, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. Detection and localization of an estrogen receptor beta splice variant protein (ERbeta2) in the adult female rat forebrain and midbrain regions. J Comp Neurol. 2007;505:249–267. doi: 10.1002/cne.21490. [DOI] [PubMed] [Google Scholar]
- 39.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010;151:1622–1632. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everitt BJ, Hokfelt T, Terenius L, Tatemoto K, Mutt V, Goldstein M. Differential co-existence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience. 1984;11:443–462. doi: 10.1016/0306-4522(84)90036-8. [DOI] [PubMed] [Google Scholar]
- 41.Grove KL, Sekhon HS, Brogan RS, Keller JA, Smith MS, Spindel ER. Chronic maternal nicotine exposure alters neuronal systems in the arcuate nucleus that regulate feeding behavior in the newborn rhesus macaque. J Clin Endocrinol Metab. 2001;86:5420–5426. doi: 10.1210/jcem.86.11.8033. [DOI] [PubMed] [Google Scholar]
- 42.Skrapits K, Borsay BA, Herczeg L, Ciofi P, Liposits Z, Hrabovszky E. Neuropeptide co-expression in hypothalamic kisspeptin neurons of laboratory animals and the human. Front Neurosci. 2015;9:29. doi: 10.3389/fnins.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience. 2006;143:975–986. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 44.Mihaly E, Fekete C, Tatro JB, Liposits Z, Stopa EG, Lechan RM. Hypophysiotropic thyrotropin-releasing hormone-synthesizing neurons in the human hypothalamus are innervated by neuropeptide Y, agouti-related protein, and alpha-melanocyte-stimulating hormone. J Clin Endocrinol Metab. 2000;85:2596–2603. doi: 10.1210/jcem.85.7.6662. [DOI] [PubMed] [Google Scholar]
- 45.de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system--II. Immunohistochemical analysis. Neuroscience. 1986;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 46.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 47.Witkin JW, Ferin M, Popilskis SJ, Silverman AJ. Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology. 1991;129:1083–1092. doi: 10.1210/endo-129-2-1083. [DOI] [PubMed] [Google Scholar]
- 48.Robson AJ, Rousseau K, Loudon AS, Ebling FJ. Cocaine and amphetamine-regulated transcript mRNA regulation in the hypothalamus in lean and obese rodents. J Neuroendocrinol. 2002;14:697–709. doi: 10.1046/j.1365-2826.2002.00830.x. [DOI] [PubMed] [Google Scholar]
- 49.Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1862–1867. doi: 10.1152/ajpregu.2001.281.6.R1862. [DOI] [PubMed] [Google Scholar]
- 50.Adam CL, Archer ZA, Findlay PA, Thomas L, Marie M. Hypothalamic gene expression in sheep for cocaine- and amphetamine-regulated transcript, pro-opiomelanocortin, neuropeptide Y, agouti-related peptide and leptin receptor and responses to negative energy balance. Neuroendocrinology. 2002;75:250–256. doi: 10.1159/000054716. [DOI] [PubMed] [Google Scholar]
- 51.Henry BA, Rao A, Ikenasio BA, Mountjoy KG, Tilbrook AJ, Clarke IJ. Differential expression of cocaine- and amphetamine-regulated transcript and agouti related-protein in chronically food-restricted sheep. Brain Res. 2001;918:40–50. doi: 10.1016/s0006-8993(01)02918-3. [DOI] [PubMed] [Google Scholar]
- 52.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 53.Van Vugt DA, Lujan ME, Froats M, Krzemien A, Couceyro PR, Reid RL. Effect of fasting on cocaine-amphetamine-regulated transcript, neuropeptide Y, and leptin receptor expression in the non-human primate hypothalamus. Neuroendocrinology. 2006;84:83–93. doi: 10.1159/000097494. [DOI] [PubMed] [Google Scholar]
- 54.McShane TM, May T, Miner JL, Keisler DH. Central actions of neuropeptide-Y may provide a neuromodulatory link between nutrition and reproduction. Biol Reprod. 1992;46:1151–1157. doi: 10.1095/biolreprod46.6.1151. [DOI] [PubMed] [Google Scholar]
- 55.McDonald JK, Lumpkin MD, DePaolo LV. Neuropeptide-Y suppresses pulsatile secretion of luteinizing hormone in ovariectomized rats: possible site of action. Endocrinology. 1989;125:186–191. doi: 10.1210/endo-125-1-186. [DOI] [PubMed] [Google Scholar]
- 56.El Majdoubi M, Sahu A, Ramaswamy S, Plant TM. Neuropeptide Y: A hypothalamic brake restraining the onset of puberty in primates. Proc Natl Acad Sci U S A. 2000;97:6179–6184. doi: 10.1073/pnas.090099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molnar CS, Sarvari M, Vastagh C, Maurnyi C, Fekete C, Liposits Z, Hrabovszky E. Altered Gene Expression Profiles of the Hypothalamic Arcuate Nucleus of Male Mice Suggest Profound Developmental Changes in Peptidergic Signaling. Neuroendocrinology. 2016;103:369–382. doi: 10.1159/000439430. [DOI] [PubMed] [Google Scholar]
- 58.Kalra SP, Crowley WR. Norepinephrine-like effects of neuropeptide Y on LH release in the rat. Life Sci. 1984;35:1173–1176. doi: 10.1016/0024-3205(84)90187-5. [DOI] [PubMed] [Google Scholar]
- 59.Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci U S A. 2012;109:3155–3160. doi: 10.1073/pnas.1120501109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, Ronnekleiv OK, Kelly MJ, Palmiter RD. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A. 2017;114:2413–2418. doi: 10.1073/pnas.1621065114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 62.Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol. 2008;20:1089–1097. doi: 10.1111/j.1365-2826.2008.01757.x. [DOI] [PubMed] [Google Scholar]
- 63.Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151:2233–2243. doi: 10.1210/en.2009-1190. [DOI] [PubMed] [Google Scholar]
- 64.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 66.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 67.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 68.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B Stimulates GnRH Release in the Male Monkey (Macaca mulatta) and Is Colocalized with Kisspeptin in the Arcuate Nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O’Byrne KT. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153:307–315. doi: 10.1210/en.2011-1641. [DOI] [PubMed] [Google Scholar]
- 70.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalil B, Ramaswamy S, Plant TM. The Distribution of Substance P and Kisspeptin in the Mediobasal Hypothalamus of the Male Rhesus Monkey and a Comparison of Intravenous Administration of These Peptides to Release GnRH as Reflected by LH Secretion. Neuroendocrinology. 2016;103:711–723. doi: 10.1159/000442420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hrabovszky E, Borsay BA, Racz K, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Substance P immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PLoS One. 2013;8:e72369. doi: 10.1371/journal.pone.0072369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146:1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- 74.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Narayanaswamy S, Prague JK, Jayasena CN, Papadopoulou DA, Mizamtsidi M, Shah AJ, Bassett P, Comninos AN, Abbara A, Bloom SR, Veldhuis JD, Dhillo WS. Investigating the KNDy hypothesis in humans by co-administration of kisspeptin, neurokinin B and naltrexone in men. J Clin Endocrinol Metab. doi: 10.1210/jc.2016-1911(2016)jc20161911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19:1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plant TM. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol. 2012;33:160–168. doi: 10.1016/j.yfrne.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 78.Chappell PE. Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol. 2005;17:119–130. doi: 10.1111/j.1365-2826.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- 79.Terasawa E, Yeoman RR, Schultz NJ. Factors influencing the progesterone-induced luteinizing hormone surge in rhesus monkeys: diurnal influence and time interval after estrogen. Biol Reprod. 1984;31:732–741. doi: 10.1095/biolreprod31.4.732. [DOI] [PubMed] [Google Scholar]
- 80.Pau KY, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology. 1993;133:1650–1656. doi: 10.1210/endo.133.4.8404606. [DOI] [PubMed] [Google Scholar]
- 81.Adams JM, Taylor AE, Schoenfeld DA, Crowley WF, Jr, Hall JE. The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J Clin Endocrinol Metab. 1994;79:858–864. doi: 10.1210/jcem.79.3.7521353. [DOI] [PubMed] [Google Scholar]