Synopsis
Environmental enteropathy is a chronic condition of the small intestine that is associated with increased intestinal permeability, mucosal inflammation, malabsorption, and systemic inflammation. It is commonly accompanied by enteric infections and is misleadingly considered a subclinical disease. Potential effects of enteric infections and enteropathy on vaccine responses, child growth, cognitive development, and even later life obesity, diabetes, and metabolic syndrome are increasingly being recognized. Here, we review the evolving challenges to defining environmental enteropathy and enteric infections, current evidence for the magnitude and determinants of its burden, new assessment tools, and relevant interventions.
Keywords: Environmental enteropathy, Environmental enteric dysfunction, Enteric infections, Growth faltering
Introduction
As the cause of approximately 500,000 deaths in 20151 resulting from 1.7 billion episodes2 globally, early childhood diarrhea has been a major focus of global health efforts to improve child health in low-resource settings. However, as we have witnessed substantial reductions in diarrhea-related mortality and learned how to provide appropriate oral rehydration therapy, diarrhea has revealed itself to be only one, though particularly visible, consequence of exposure to enteropathogens in environments with poor sanitation and hygiene. A potentially more widespread and debilitating consequence for long-term development is environmental enteropathy (EE, also termed environmental enteric dysfunction, EED), a chronic condition of the small intestine that is commonly considered a “subclinical” problem and often involves enteric infections without overt symptoms.3 However, since it lacks definition as a “disease” and is challenging to diagnose, its magnitude and importance are only beginning to be appreciated.
Growing evidence for the diverse effects of EE on child development have revealed the “subclinical” designation to be a misnomer. There is a largely under-recognized range of potential impacts of common enteric infections and enteropathy that extend far beyond the typical assumption that they mainly cause diarrhea or other intestinal complaints like abdominal pain, nausea, or vomiting. Recognized examples of non-intestinal outcomes associated with enteric infections include associations of toxoplasmosis with birth defects and congenital brain damage (“TORCH” syndrome)4 and of Campylobacter infections with Guillain-Barré Syndrome.5 However, as research studies include detection of wider arrays of bacterial, viral, and parasitic pathogens and longer term follow-up, potential effects of enteric infections and enteropathy on vaccine responses,6,7 child growth,8,9 cognitive development,10,11 and even later life obesity, diabetes, and metabolic syndrome are increasingly being recognized.12–15 Here, we review the evolving challenges to defining EE and enteric infections, current evidence for the magnitude and determinants of its burden, new assessment tools, and relevant interventions.
Historical perspective
Environmental enteropathy has its initial appreciation and origins in the mid-20th century as tropical sprue, a symptomatic disease first identified among military personnel and Peace Corps volunteers stationed in low-resource settings. Tropical sprue was characterized by chronic diarrhea, steatorrhea, weight loss, malabsorption, and abnormalities in intestinal morphology. In severe cases, it included manifestations of nutritional deficiencies, including night blindness and neurologic symptoms.16 In 1966, a study found that 40% of volunteers stationed in Pakistan had signs of malabsorption and none of their jejunal biopsies showed normal finger-like villous architecture with varying degrees of abnormality.17 The condition was associated with residence in tropical countries, though the specific cause was unknown.
Further study of intestinal morphology by jejunal biopsy in asymptomatic individuals from Africa, Asia, and Latin America found common abnormalities of shorter and thickened villi, increased crypt depth, and inflammatory cellular infiltration, which did not necessarily result in overt symptoms.18–24 Histological abnormalities were often accompanied by excess fecal fat excretion and malabsorption of xylose and vitamin B12.21–23 Similar abnormalities were documented in malnourished children with Kwashiorkor and severe wasting.25–27 While the condition among expatriates reverted after returning to their home countries,28 populations in endemic settings experienced the condition chronically.
Current definitions
Environmental enteropathy
Environmental enteropathy is thought (by most authors) to be the result of chronic exposure to enteropathogens, though the potentially synergistic and disruptive role of poor nutrition is increasingly being recognized. In addition to the histopathological findings of villous blunting, the main components of EE are increased intestinal permeability (from impaired barrier function), mucosal inflammation, malabsorption, and systemic inflammation.29–32 The condition is considered distinct from tropical sprue and overt symptoms of diarrhea.29 Because it is dependent on exposure to unsanitary environments, it is common in both long-term residents and in travelers, and is reversible once the environment improves,28 EE is thought to be environmentally derived and likely widespread in low-resource settings.29
However, the determination of a clear consensus definition for EE remains an elusive challenge. Because EE is without overt acute symptoms (although it may manifest in subacute weight loss and impaired growth or development over longer periods of time), the traditional gold standard for diagnosis has been intestinal biopsy to identify abnormalities in intestinal histology. Such an invasive diagnostic is infeasible in most research and many clinical settings and is also limited by potentially inadequate sampling, as the biopsied sample may not be representative of the whole intestine. However, the diagnosis of EE in the absence of these invasive procedures has proved challenging.
In response to the inability to regularly perform biopsies in healthy, “asymptomatic” individuals, recent research on EE has aimed to identify biomarkers to characterize EE that can be measured in stool, blood, or urine. At least 40 different biomarkers or metabolites have been investigated as potential indicators for EE that is clinically significant enough to result in growth faltering (Table 1).6,29,33–39 These include markers of disrupted intestinal barrier or absorptive function (e.g. lactulose and mannitol, rhamnose, or D-xylose absorption and excretion in the urine, alpha-1-antitrypsin in stool, tight junction components in plasma or intestinal tissue staining); translocation of microbes or their products (e.g. LPS or anti-LPS antibody); intestinal inflammation (e.g. myeloperoxidase, lactoferrin, calprotectin, or lipocalin in the stool); and systemic inflammation (e.g. hsCRP or AGP; serum amyloid A and other acute phase proteins). Other indicators include metabolites such as citrulline or tryptophan that may signal a healthy intestinal mucosa.
Table 1.
| Function | Biomarker | Description | Sample type |
|---|---|---|---|
| Intestinal absorptive function | Lactulose | Disaccharide, indicator of gut barrier disruption | Administered orally and measured in urine |
| Mannitol | Monosaccharide, indicator of gut absorptive surface | Administered orally and measured in urine | |
| Lactulose:Mannitol ratio (%L and %M) | Indicator of barrier disruption per surface area | Administered orally and measured in urine | |
| Rhamnose | Monosaccharide, indicator of absorptive surface | Administered orally and measured in urine | |
| D-xylose | Monosaccharide, indicator of absorptive surface | Administered orally and measured in urine | |
| GMCSF antibody | Granulocyte macrophage colony stimulating factor autoantibody | Plasma | |
| Intestinal barrier function | alpha-1-antitrypsin | Plasma protease inhibitor, indicator of relatively severe gut barrier disruption | Stool |
| Claudin-2 | Tight junction peptide reflecting increased permeability | Intestinal tissue staining | |
| Claudin-15 | A marker of “healthy” gut absorptive and barrier function | Urine | |
| Translocation | LPS | Lipopolysaccharide | Plasma |
| IgA and IgG anti-LPS | Antibody produced against lipopolysaccharide | Plasma | |
| IgA and IgG anti-FliC | Antibody produced against bacterial FliC (flagellin) | Plasma | |
| Zonulin | Tight junction peptide regulator of gut permeability (haptoglobin) | Plasma | |
| TJP1 | Tight junction protein gene encoding for ZO-1 | Intestinal biopsy, tissue DNA | |
| Intestinal inflammation | MPO | Myeloperoxidase, a neutrophil granule component | Stool |
| Calprotectin | Neutrophil marker | Plasma; stool | |
| Neopterin | Monocyte/macrophage marker of immune activation (GTP metabolite) | Stool | |
| Lactoferrin | Neutrophil granule component | Stool | |
| Lipocalin | Protein in neutrophils and epithelial cells | Stool | |
| Reg 1A | Regenerating islet-derived protein-α | ||
| Reg1β | Marker of epithelial repair | Stool | |
| I-FABP | Intestinal fatty acid binding protein | Plasma | |
| Fecal S100A12 | Calcium (and zinc, copper)-binding protein regulator of inflammatory signaling | Stool | |
| Systemic inflammation | AGP | Acid glycoprotein, a hepatic “acute phase reaction” product | Plasma |
| IL-1β | Interleukin-1β, inflammatory cytokine produced by monocytes and macrophages | Plasma | |
| IL-4 | Th2 cytokine | Plasma | |
| IL-5 | Th2 and mast cell cytokine | Plasma | |
| IL-6 | Pro or anti-inflammatory T cell or macrophage cytokine or myokine | Plasma | |
| IL-7 | Lymphokine stimulator of stem cell differentiation into B, T or NK lymphoid cells | Plasma | |
| IL-10 | Anti-inflammatory STAT3 inducer cytokine | Plasma | |
| TNFα | Proinflammatory cytokine | Plasma | |
| MIP1β | Macrophage inflammatory protein 1β (CCL4) | Plasma | |
| Ferritin | Iron binding protein reflecting iron stores | Serum | |
| Hepcidin | Inflammation driven inhibitor of iron exporter ferroportin, thus blocker of iron uptake or release into the circulation; hence anemia of chronic inflammation | Plasma | |
| C-reactive Protein (CRP) | An acute phase reactant (APR) | Plasma | |
| hsCRP | High sensitivity C-reactive protein (APR) | Plasma | |
| sCD14 | Soluble CD14; shed by activated monocytes, binds LPS | Plasma | |
| EndoCAb | Antibody produced against bacterial lipopolysaccharide | Plasma | |
| Serum amyloid A | Acute phase reactant driver of inflammation | Plasma | |
| LBP | LPS binding protein | Plasma | |
| Metabolites/ Growth markers | Tryptophan | Essential amino acid for protein synthesis and growth as well as the neurotransmitter, serotonin | Plasma |
| Kynurenine | Tryptophan metabolite via IDO, potentially driven by inflammation | Plasma | |
| K:T ratio | Kynurenine:tryptophan ratio | Plasma | |
| Citrulline | Key amino acid for intestinal repair | Plasma | |
| IGFBP-3 | Insulin-like growth factor binding protein-3 | Plasma | |
| IGF-1 | Insulin-like growth factor 1 | Plasma | |
| Activin | Growth regulation factor | Plasma |
The biomarkers for the above processes have varying specificity for EE-induced growth faltering. Lactulose absorption and excretion, like fecal alpha-1-antitripsin and plasma LPS markers, all reflect disrupted intestinal barrier dysfunction, which is a proximal component of EE. Conversely, markers of systemic inflammation have diverse causes and cannot specifically identify EE. Of course, measures of growth impairment alone, such as height-for-age z-score (HAZ) or stunting, have been included as markers in some studies, but they have poor specificity for EE since they may be due to non-intestinal causes. Like later impairments in cognitive development (especially in higher executive function or semantic fluency40), these measures are indicators of outcomes of EE and may occur too late in the disease process to be of timely diagnostic use that could enable interventions. Tracking of growth trajectories may be useful to identify early decrements that could predict continuing growth deficits if EE conditions persist.
The identification of appropriate biomarkers for EE is challenged by their frequent comparison to non-specific outcomes like linear growth rather than a true gold standard diagnostic. As noted above, stunting is hypothesized to be an effect of EE and is highly multifactorial. The strongest predictor of stunting is birth size,41 which is necessarily unrelated to the development of EE in early life in the child (although likely relevant to EE in the mother). Therefore, single anthropometric measurements have poor sensitivity and specificity as a standard against which to validate biomarkers. However, growth trajectories, especially over the first two years of life (assessed as incremental changes in HAZ scores) may better reflect EE in early childhood. Validation against intestinal biopsy might be more specific, but is infeasible in many settings as discussed above.
In addition, the potential ubiquity of EE in low-resource settings makes it difficult to identify appropriate comparison groups. Because EE is acutely asymptomatic, it is unclear how to define “healthy” controls for a study in an environment conducive to EE and where the condition is expected to be common. Unlike the WHO growth standards,42 for example, there are no international reference standards for EE biomarkers based on a representative group of children from diverse areas. Distributions of EE biomarkers in healthy children in high-resource settings are also largely not available to be used as reference standards. Even if this information were available, external reference populations could be inappropriate since they would differ on many other characteristics that would confound comparisons. In multiple papers assessing EE among children in low-resource settings, the authors referenced comparison biomarker values based on small studies in healthy adults that were not assessing EE directly.36,37,43,44 Clearly these reference values were suboptimal.
Beyond the uncertainties with biomarkers, the scientific community has not come to a consensus on what type of definitions are relevant for understanding EE. If EE is to be considered a disease, it will require a specific pathologic definition. Conversely, as a syndrome, EE could encompass multiple disease states. Arbitrary assemblages of morphological or functional pathologies can have important acute or lasting consequences despite having multiple potential etiologies. Examples include pneumonia and diarrhea that are well recognized as “disease” entities, even though they are comprised of many component, more specific diagnoses such as pneumococcal pneumonia or shigellosis. EE could be considered similarly as a set of functional pathophysiologic alterations, with varying degrees of morphologic pathology caused by diverse environmental determinants. However, distinguishing EE from similar pathologies as HIV-associated enteropathy, for example, may be difficult. It is unclear whether and how similar presentations of enteropathy should be considered distinct when the conditions can be indistinguishable except for the underlying cause.
What needs to drive these definitions is the goal to identify EE as an entity for which its recognition and the development of effective interventions can improve long-term health outcomes that may range from growth to cognitive and even later life metabolic impairment. Both surveillance and clinical case definitions will be needed for future study of EE. A surveillance definition would be used to identify sentinel cases to inform population-level interventions, which would be relevant since EE appears to be widespread in low-resource settings. Because populations suffering from EE show a population shift in biomarker distributions, it may be difficult to make individual diagnoses, and population-level interventions may be most appropriate. On the other hand, a clinical case definition will be needed for targeted treatment and other individual-level interventions.
Geographic differences in EE manifestations may also pose challenges. Variations in environmental exposures across low-resource settings likely result in different pathologies. Perhaps it is more important to describe an EE spectrum, where milder cases may not include systemic inflammation, for example, and more severe cases may be associated with villous blunting and poor growth outcomes. It may be necessary to distinguish between consequential EE, with observable poor outcomes, and inconsequential EE, in which a child may show abnormal histology or biomarker levels, but no related outcomes such as poor growth. In this conception, EE constitutes a risk factor for health outcomes such as a significant drop in HAZ in the formative early years of childhood or an impairment of normal cognitive development, especially in higher executive function. EE describes a state that is probabilistically associated with poor development (e.g. growth, vaccine response, cognitive impairment), but is not a necessary or sufficient cause and may lead to no observable clinical outcomes in a significant proportion of cases.
As the field is quickly evolving, new biomarkers are being identified and refined to help enhance our understanding, if not the definition, of EE. In spite of the complex challenges in defining EE, improved clarity of this entity will help to develop appropriate surveillance and clinical case definitions against which to study risk factors, which will be vital to advancing our understanding and to testing, and ultimately investing in, potentially effective interventions.
Specific enteric infections
Overt diarrhea (defined as three or more unformed stools per day45), especially when prolonged or persistent, has predicted growth and even cognitive failure in many previous studies.11,46–51 However, reduced diarrhea rates observed in more recent studies have made overt diarrhea less useful in predicting growth and developmental outcomes. Despite these reductions, “subclinical” pathogen detection in stools continues to be common in many impoverished settings and has been associated with poor outcomes.8,9,52
These infections are hypothesized to be a key contributor to environmental enteropathy, and asymptomatic carriage of known enteric pathogens may cause or aggravate linear growth faltering, even in the absence of recognized episodes of diarrhea.8,9,53,54 For example, asymptomatic excretion of enteroaggregative E. coli (EAEC) has been associated with linear growth faltering.53 The mechanism for the growth effect may be through subclinical gut inflammation, which has been associated with EAEC detection in the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED), a birth cohort study performed at eight sites in South America, sub-Saharan Africa, and South Asia (Rogawski et.al., manuscript in preparation). Carriage of Campylobacter spp. has also been associated with both linear and ponderal growth faltering.8,55 This association may be mediated through environmental enteropathy; Campylobacter detection was associated with increased markers of permeability (alpha-1-antitrypsin), intestinal inflammation (myeloperoxidase), and systemic inflammation (AGP) in MAL-ED.8 Similarly, persistent Giardia detection in the first 6 months of life has been associated with malabsorption9 and reduced linear growth in two studies.9,56 Cryptosporidium parvum excretion has also been associated with growth faltering, although it is unclear if this association is driven by prolonged excretion after a symptomatic infection.57 These data suggest that a primary mechanism for the pathogenesis of EE may involve exposure to pathogens through poor hygiene practices and contaminated food and water resulting in enteric infections.
Because these infections are commonly diagnosed in stool by culture or molecular methods, it is challenging to distinguish between colonization and infection. Many microorganisms can play both the roles of commensal and pathogenic organism and mere detection does not illuminate the multifactorial impact of the organism.58 Some organisms, like C. difficile, Salmonella, and likely many other enteric bacterial pathogens, may change from commensal to pathogenic within an individual over time, and pathogenicity is highly dependent on presence of other microorganisms and a wide range of environmental and genetically-determined host factors.59 Further, the distinction between commensal and pathogenic is often made among organisms within the same species, as is the case for E. coli. Pathogenic E. coli are generally identified by an array of virulence factors, the significance of some of which have yet to be fully elucidated.60
The identification, quantification, and attribution of pathogenic versus non-pathogenic organisms is complicated in endemic settings by differences in host resistance, normal microbiota, and acquired immunity. Organisms that are known causes of diarrhea outbreaks, such as waterborne Giardia outbreaks, are often not associated with diarrhea among children in endemic settings. Giardia, for example, was more commonly identified in non-diarrheal stools than diarrheal stools in both major studies of diarrhea etiology, MAL-ED and GEMS.52,61 Even organisms that are statistically associated with diarrhea in these studies are commonly found in non-diarrheal stools and “healthy” controls. New methods to use quantitative PCR to incorporate quantity of pathogen have helped distinguish cases from controls, but they remain imperfect and quantities significantly associated with case status necessarily only apply at the population level. Detections above these quantities cannot conclusively identify etiology in individual cases. In contrast, detections of these pathogens are rare in high-resource settings, which makes it easier to confidently assign etiology.
Pathogenic organisms must be considered in the context of the gut microbiota, a highly complex “organ” of the body which impacts myriad processes from metabolism to cognition. However, microbiota-related studies rarely consider the presence of specific pathogenic organisms, and it is unknown what bilateral impact they may have. While the microbiota has been shown to be altered in many diseases states, it is unclear which compositions constitute beneficial microbiota and which represent dysbiosis. Diversity parameters for the microbiota (either alpha diversity within a sample or beta diversity between samples)62 can simplify the highly dimensional composition data, but high diversity is not uniformly positive. For example, while exclusive breastfeeding is recommended in early infancy, the predominant bacteria in the microbiota of breastfed infants are Bifidobacterium and Lactobacillus,63,64 while infants fed with formula milk have a more complex microbiota.63,65 Recently, measures of microbiota “maturity” have been developed to describe how malnourished children often have microbiota compositions that mirror the compositions of younger healthy children.66 However, the organisms identified as discriminating for immaturity differ across populations, limiting the generalizability of the index.66,67 These discriminating organisms are not those classically considered as enteric pathogens, and it is unclear if they are harmful themselves or simply indicators for generalized dysbiosis.
Animal models can help to separate and elucidate the roles of specific pathogen infections versus the microbiota by experimentally controlling infections and the microbiota in specific diet and host contexts. For example, protozoal, bacterial and viral infections in murine models have strikingly different effects in the context of specific nutrient deficiencies with normal microbiota. Protein deficiency greatly enhanced both the intensity of cryptosporidial infection as well as its impact on growth, documenting a bidirectional impact of worsened infection with protein malnutrition and conversely worsened growth with infection.68,69 Cryptosporidial infection in the setting of protein deficiency also caused impaired turnover of infected epithelial cells.70 Similar synergies with protein or zinc deficiency were seen with EAEC infections.71–73 Conversely, murine rotavirus infections were less severe in undernourished conditions,74 which may be because the intestinal villi were blunted and less efficiently provided the lactase needed to “uncoat” the virus as part of pathogenesis.
The microbiota also affect susceptibility to infection in mouse models. Early studies from the 1960s and 1970s showed the intestinal flora was antagonistic to Salmonella, Shigella, and Vibrio cholerae infection.75(pp313–316) Several other recent studies have found that a normal microbiota in mice successfully prevents colonization by Salmonella enterica serovar Typhimurium. Conversely, mice with altered microbiotas due to antibiotic administration are more susceptible to intestinal infection and disease due to Salmonella and other enterobacteria such as E. coli.76–78 One study showed a dose-response such that greater alterations to the microbiota led to higher colonization by S. enterica serovar Typhimurium, with more severe inflammation and intestinal pathology.77,79 Further, modification of the microbiota through the antibiotic treatment of mice increased susceptibility to infection by vancomycin-resistant Enterococcus and C. difficile.77
In terms of the microbiota and susceptibility to viral infections, there are examples both where intestinal bacteria promote and are antagonistic to viral infection.78,80,81 For example, Bacteroides thetaiotaomicron and Lactobacillus casei have been shown to prevent infection of the intestinal epithelial cells by rotavirus in vitro. Similarly, mice with depleted microbiotas through antibiotic treatment or development in germ-free conditions are more susceptible to influenza compared to normal mice.78,80 On the other hand, the GI microbiota has been shown to enhance replication and infection of other viruses.80,82,83 Antibiotic-treated mice were less susceptible to poliovirus compared to mice with normal microbiota, resulting in a mortality rate among normal mice twice that among antibiotic-treated mice.82 A similar study demonstrated that mouse mammary tumor virus, a retrovirus, was more efficiently transmitted in the presence of a rich microbiota, and correspondingly virus transmission to offspring was reduced in antibiotic-treated mice and germ-free mice.83
Clinical studies have supported laboratory based evidence of the importance of the microbiota. Prior antibiotic treatment has been associated with increased susceptibility to E. coli, Salmonella, Shigella and Campylobacter infections and with longer duration of infection compared to patients who did not receive antibiotics.78,84,85(p433) Antibiotic treatment also reduces the inoculum required to cause infection with Salmonella.85(p433) The clear association between the microbiota and susceptibility to infection has led some researchers to suggest that people with an altered microbiota are functionally immunocompromised and less resilient against new and opportunistic pathogens and recurrent infections.77 The complex interactions in the gut between enteropathogens and the microbiota likely play a key role in development of environmental enteropathy.
Magnitude of burden
Because a clear case definition with diagnostic cut-offs for EE biomarkers has not yet been developed to classify individuals with and without EE using non-invasive methods, no large-scale population-based surveillance has been completed to estimate global burden of EE. Symptomatic diarrhea remains the main indicator for pediatric enteric disease. Only symptomatic diarrhea was included in the most recent Global Burden of Disease study, and the longer-term consequences of even overt diarrhea remain controversial and poorly defined.1 The inadequacy of definitions of EE has limited the ability to include its consequences in enteric disease-associated DALY calculations, which would help garner the attention needed for their amelioration.86
Smaller-scale studies of histology are not necessarily globally representative, but provide evidence for the universality of EE in low-resource settings. In a study of 57 Indian children with chronic diarrhea, almost three-quarters showed abnormal histology of the jejunum and approximately two-thirds showed atrophy of villi.87 In a cohort study of two hundred adults in Zambia, all of the jejunal biopsies demonstrated some degree of enteropathy, with varying levels of abnormality in villous height and crypt depth.88 Among 414 children presenting to a hospital in London with chronic diarrhea, almost half had mild (25%), moderate (10%), or severe (9%) enteropathy by proximal small intestinal mucosal biopsy.89
EE biomarkers are increasingly being measured in studies of enteric disease. In MAL-ED, a large multisite birth cohort study from 8 countries, a panel of potential EE biomarkers was measured across the first two years of life.38 In a subset of children with biomarkers measured at 3–9 months of age, median alpha-1-antitrypsin, neopterin, and myeloperoxidase concentrations in stool were elevated compared to sparse but available data from healthy individuals in non-tropical countries.36 Specifically, in quarterly stools from 3–21 months of age from the complete MAL-ED cohort in the Bangladesh site, more than half of samples were above normal for alpha-1-antitrypsin and myeloperoxidase, and 95% were abnormal for neopterin.37 Similarly, in PROVIDE, a study of EE and oral polio and rotavirus vaccine response in Bangladesh, fecal EE biomarkers measured at 12 weeks of age were abnormal in the majority of infants (82% abnormal for myeloperoxidase and alpha-1-antitrypsin, and 94% abnormal for calprotectin).6 These results suggest the majority of infants may have had enteric inflammation even in the first few months of life. The entire distribution of EE biomarkers in study populations from low-resource settings is likely shifted compared to populations in high-resource settings.
Non-diarrheal enteric infections are more straightforward to measure through detection of the enteropathogens in stool samples when diarrhea symptoms are not reported. The prevalence of such infections is high in low-resource settings, which has made it critical to assess presence of pathogens in both diarrheal and non-diarrheal stools when attributing population-level diarrhea etiology. In GEMS, a large study of moderate-to-severe diarrhea in seven sites in Africa and Asia, the prevalences of Shigella, Cryptosporidium, and LT-ETEC by qPCR were 27%, 21%, and 29% in enrolled controls without diarrhea in the second year of life.90 In MAL-ED, the prevalences of Campylobacter, Giardia, and EAEC using conventional methods91 in quarterly non-diarrheal stools from the same age period were 36%, 29%, and 26%, respectively.8,9 Because the prevalences of Giardia and EAEC were similar in diarrheal and non-diarrheal stools, neither pathogen was statistically associated with diarrhea for any age group or at any site in MAL-ED.52 Giardia carriage measured by repeated detections in non-diarrheal stools was common; 63% of the Giardia detections in diarrheal stools were preceded by Giardia detections in non-diarrheal stools in the prior two months, suggesting the Giardia detected during diarrhea may have been from previous infection.9
Even if the burden of EE may be difficult to define, the prevalence of risk factors for EE is high and widespread. The World Health Organization estimates that approximately 900 million people around the world do not have access to an improved drinking water source, including more than 40% of people in sub-Saharan Africa.92 Even those with access to an improved water source may have poor quality water due to recontamination and unsafe storage. Similarly, sanitation coverage and use is poor. Prevalence of open defecation globally was 18% in 2006 and is widely practiced in South Asia (48%) and sub-Saharan Africa (28%). Improved sanitation facilities that ensure hygienic separation between humans and their excreta are available to less than a third of people in Sub-Saharan Africa.92
Many challenges also remain to providing optimal nutrition both in utero and during early childhood. Globally each year, approximately 13 million infants are born with intrauterine growth restriction and about 20 million with low birth weight.93 Breastfeeding in the first 6 months of life is not exclusive as recommended for 16% of infants, and 14% of children discontinue breastfeeding before 2 years of age.94 Micronutrient deficiencies are also common; a quarter of the global population has Vitamin A deficiency,94 including 190 million (33.3%) children under 5 years of age.95 Overall, 16% of the global population has zinc deficiency, and 17% of women have iron deficiency,94 with anemia affecting almost half of the preschool age population.93 These risk factors are likely major contributors to EE. A global study of risk factors for stunting, an outcome associated with EE, identified fetal growth restriction and preterm birth as the leading risk factors for stunting prevalence, followed by environmental factors (unimproved water, unimproved sanitation, and biomass fuel use) and maternal and child nutrition. This analysis estimates that 22% of stunting cases were attributable to environmental factors and 14% were attributable to child nutrition.41
Challenges to assessing causality
The complex interplay between many of these factors, which are often associated, but related nonlinearly, make it challenging to assign causality in population based studies. For example, the highly cited vicious cycle between malnutrition and illness posits that malnutrition can both be a cause and effect of enteric infections.12 Even with longitudinal data to track children over time, it is difficult to model the complex system and determine temporality. Risk factors can confound the causal effect of other risk factors if the confounding factor is associated with both the risk factor of interest and the EE outcome. However, they could also be mediators, on the causal pathway between more distal risk factors and EE, or modifiers, which change the magnitude of association between risk factors and EE. We diagram the relationship between these different types of variables in Figure 1A, and provide an example in the context of EE in Figure 1B. These diagrams draw on the concepts of directed acyclic graphs,96 which are useful to develop causal models, but are extended here to include modifiers and allow multiple variables at one node.
Figure 1.
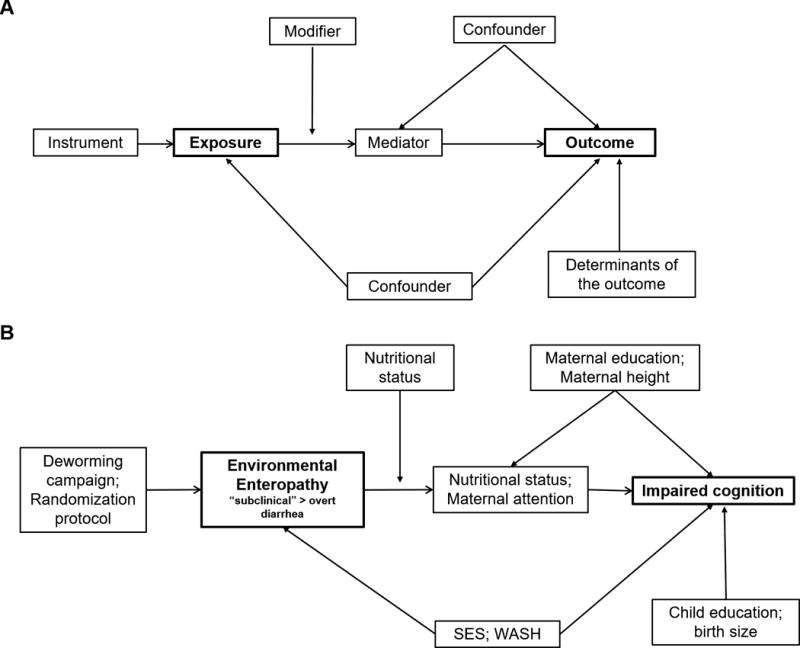
Modified directed acyclic graphs to diagram the complex interplay between factors associated with environmental enteropathy and its effects: 2A—a diagram of the different types of variables that could affect exposure-outcome relationships; 2B—an example showing relationships between relevant variables in the context of environmental enteropathy.
In observational studies, it may be difficult to disentangle highly correlated risk factors such as socioeconomic status and education. The distribution of risk factors associated with nutrition, WaSH, and social factors vary across and often within countries, as was shown in the multisite MAL-ED study, where there were major variations in breastfeeding practices, water, sanitation, handwashing, animal ownership, and antibiotic use across sites.8,9,97 These differences may limit the interpretation of cross-site comparisons. Alternatively, risk factors may be ubiquitous in some settings, such that it is impossible to compare outcomes between exposed and unexposed. For example, all participants had access to an improved water source (as defined by the WHO92) in five of the eight research sites in the MAL-ED study.8
Many of these risk factors are difficult to measure. Caregiver report, especially for behavioral factors such as handwashing behavior, is notoriously unreliable.98,99 Improved measurement of risk factors alongside better models of their complex interactions will all be needed to identify appropriate targets and develop interventions for EE.
New assessment tools create new perspectives and recognition of impact
Detection of enteric pathogens
The relatively recent focus on enteropathy and subclinical enteric infections has been spurred by technological advances that have made closer study of EE feasible. A widening array of protozoan, bacterial, and viral enteric pathogen assays are now available to assess pathogen burden in stool samples, including those for Cryptosporidium, Campylobacter, multiple types of diarrheagenic E. coli based on increasingly appreciated virulence traits such as heat labile or stable enterotoxins (LTEC or STEC), attaching and effacing traits (EPEC), invasiveness (EIEC), shigatoxin (STxEC), aggregative adherence (EAEC), and viral agents.100,91
While traditional culture and microscopy methods are still commonly used to detect these pathogens, new molecular methods have been developed with superior sensitivity and specificity in many cases.90,101 For example, culture assays for Shigella are known to have low sensitivity. In a reanalysis of stool samples from the GEMS case-control study, the use of quantitative PCR diagnostics increased Shigella attributable incidence approximately two and a half fold in the second year of life from 2.7 cases per 100 child-years to 7.0 cases per 100 child-years.90
Molecular tools not only provide greater sensitivity than most traditional methods, they also enable quantification of the pathogen load indicating the intensity of each pathogen infection.90,101 The quantification cycle (Cq) in quantitative PCR, the cycle number at which fluorescence from target amplification exceeds the background fluorescence, can be used as an inverse metric of nucleic acid quantity. For many of the diarrhea-associated pathogens, higher pathogen quantities, as indicated by lower Cq values, are more strongly associated with stools from diarrhea cases compared to non-diarrheal stools from controls. For example, the association between diarrhea and Rotavirus detected at a Cq of 34 was near the null, while the magnitude of the odds ratio for diarrhea with Rotavirus detected at a Cq of 20 was greater than 10 in GEMS.90 The difficulty of assigning diarrhea etiology has long been recognized given the frequent presence of enteropathogens in non-diarrheal stools. Quantification allows greater resolution to better discriminate causative organisms from potentially unrelated infections.
However, with the increased sensitivity of molecular tools, poor specificity may be a problem if quantity of pathogen is not taken into account. To limit false positives, a Cq cut-off (e.g. at 35 cycles) is regularly set to classify amplifications beyond that cycle number as negative in the analysis. However, beyond the strength of statistical associations between pathogen quantity and diarrhea compared to non-diarrheal stools, the necessary or sufficient pathogen burdens to cause illness are unknown and likely vary across individuals and settings. Therefore, while these methods are especially useful for assigning etiology at the population level, they are less useful for determining causes of individual cases.
There are further limitations of these methods in standardization and comparability. Comprehensive platforms such as the TaqMan Array Card can detect 40+ pathogens simultaneously and are standardized to allow comparison of Cq values across assays.101 However, PCR Cq values are difficult to compare when measured by single reactions without a standardized platform. Cq values may differ by PCR machine as well as across experiments on the same machine, making cross-site comparisons of pathogen quantity unreliable. In addition, in many PCR programs, Cq values must be calculated or at least reviewed by a technician by eye, which can further increase variability. Assaying pathogen standards of known quantity to create quantification curves can improve comparability across platforms.102
Microbiome and innovative biomarker assays
Because the majority of bacteria in the gastrointestinal microbiota cannot be cultured, early studies of the microbiota that relied on bacterial culture provided a skewed representation of microbiota composition. Newer molecular techniques, which most commonly amplify and characterize nucleic acids from the 16S rRNA conserved gene through high-throughput sequencing technologies, have allowed higher resolution for the complex and diverse communities of the microbiota.103 These techniques are also rapidly becoming less expensive, which allows for larger sample sizes needed for group comparisons.
Similarly, as assays for current biomarkers are being refined, transcriptomics and metabolomics offer innovative methods to identify new types of biomarkers. In a recent study of Malawian children from 12–61 months of age, lactulose permeability as an indicator of EE was correlated with fecal messenger RNA copy number to identify transcripts with differential expression in EE. Twelve transcripts associated with EE and mapped to pathways related to cell adhesion and immune responses to viral, bacterial, and parasitic organisms. Transcripts associated with the maintenance of the mucous layer were under-expressed in children with EE.104 These transcripts may prove to be useful targets as relevant EE biomarkers.
Metabolomics also has the potential to identify new biomarkers associated with EE. In a study of urinary metabolites in children enrolled in the case-control study component of MAL-ED in Northeast Brazil, an 1H nuclear magnetic resonance (NMR) spectroscopy-based metabolic profiling approach was used to identify metabolites associated with stunting (HAZ < −2) and catch-up growth (change in HAZ between baseline and 2–5 months follow-up). Undernutrition was associated with altered choline and tryptophan metabolism and increased proteolytic activity of the gut microbiome. The authors suggest that urinary N-methylnicotinamide and β-aminoisobutyric acid may be promising biomarkers for identifying children at risk for further growth shortfalls.105
Interventions
As our understanding of the mechanisms of EE pathogenesis increases, we can begin to appreciate the optimal markers to help define and track potentially effective interventions. This understanding will likely best derive from linking field clinical studies with targeted animal model studies that dissect metabolic pathways that are seen in both affected children and in the models. Examples include disrupted choline and tryptophan metabolism in both children and murine models. Furthermore, field and lab model studies can identify hypotheses to be tested in other study designs and raise potential interventions worthy of testing. For example, the impairment of cell turnover with protein deficiency that greatly increases the intensity and severity of cryptosporidial infection noted above70 raises the possibility that intestinal repair nutrients like glutamine or citrulline might reduce the impaired growth and development seen with this infection in clinical studies.
Multiple key interventions will likely be needed in combination to realize reductions in EE and its potentially devastating intermediate and long-term consequences. For example, behavioral or structural interventions that reduce exposure to enteric pathogens, such as interventions targeted to improve water, sanitation, and hygiene, will likely synergize with nutritional interventions, vaccines, and innovative interventions that directly target EE, such as those to repair intestinal injury. Because many of the available enteric vaccines have shown reduced efficacy in low-resource settings where EE is common,106 reducing quantity of pathogen ingested from contaminated food and water may improve vaccine impact. Likewise, key nutrients likely enhance protective host immune (and other) responses to pathogen disruption of intestinal function. Such protection could range from improved innate or acquired immunity to epithelial cell turnover, an important component of host defense.70 Equally, very limited, carefully targeted, single-dose antimicrobials might enable catch-up growth responses to nutrition therapy to set a child’s growth onto a new trajectory. Finally, an alert and healthy child will engage more parental and caregiver interactions to support the critical impact of cognitive stimulation on child development.
Furthermore, the potential to identify subtypes of EE may help identify targeted interventions which will be most effective. For example, high levels of fecal MPO suggesting an invasive pathogen like Shigella, enteroinvasive E. coli (EIEC) or Campylobacter infections might be promptly ameliorated by single dose antimicrobial (such as azithromycin) treatment. Alternatively, disrupted intestinal absorptive or barrier function might suggest that interventions targeting injury repair, such as certain amino acids in an Oral Rehydration and Repair Therapy (ORRT), could be helpful. Vaccines are available for only a few enteropathogens, including rotavirus, cholera, and polio, and are limited by suboptimal effectiveness in low-resource settings. Development of new vaccines, especially for Shigella, may be warranted given the recently appreciated burden of both moderate-to-severe watery diarrhea and dysentery associated with Shigella.90
Challenges remain with regional and cultural differences in the uptake, effectiveness, and adherence to selected interventions. Water, sanitation, and hygiene interventions have been particularly challenged by suboptimal coverage, even in the setting of randomized trials with intense community interaction and follow-up.107,108 Appropriate water treatment processes, latrine designs, and sanitation and hygiene behaviors must be tailored to individual communities to maximize acceptability.109 Intervention planning should also account for existing practices in the targeted community. For example, interventions involving targeted antimicrobial use may be severely limited by current widespread use even early in life110 and concerns about antimicrobial resistance.
Conclusions
The complexities inherent in defining and studying EE and subclinical infections have made it difficult to characterize burden as well as test potential interventions. Calls to update diarrhea DALYs86 have largely been unanswered because of the difficulty in quantifying non-diarrheal impact of enteric pathogen exposure and EE. A significant drop in HAZ in the formative early years of childhood or an impairment of normal cognitive development remain to be counted, largely because we still desperately need better data on their causes and burden. The years lost to disability (YLD) due to early childhood EE in the form of growth and cognitive developmental deficits, as well as long-term metabolic effects, may exceed the rapidly decreasing years of life lost (YLL) due to diarrhea. Including these components into the DALYs will appropriately highlight the importance and value of diagnosis and effective interventions needed to improve these outcomes for impoverished children around the world.
Similar pathways and effects may also have relevance among the elderly whose quality of life is also impaired by poor sanitation and physical and cognitive impairment. Although these are not addressed in this overview, they likely involve similar, largely unrecognized effects of EE, as shown by the surprising frequencies of evidence for intestinal inflammation and of C. difficile infections that may be seen in more than 30–40% of “asymptomatic” nursing home residents.111–113 Further expanding the potential DALY impact of the often clinically unrecognized problem of EE in this population is also likely warranted.
Numerous research and translational gaps remain in the appreciation of EE and its causes, biomarkers, and consequences. Because EE can develop in the first few months of life, methods for early recognition are vitally needed to intervene before long-term consequences manifest. The appropriate targets of these interventions and mode of delivery for biggest impact are still unknown. Some of the most important long-term outcomes for growth or cognitive development require much longer follow-up than most current projects or funding mechanisms address. Focus on shorter-term outcomes may miss critical aspects of EE or of its impact, and inappropriately discount the effectiveness of interventions. Innovative enteropathogen detection, biomarker, microbiota, and metabolomics methods noted above have improved our ability to tackle questions about which pathogens have greatest relevance, which host and microbial pathways are involved, and which biomarkers will be most relevant for diagnosis, health outcomes, and interventions. It is clear that we can no longer consider EE to be “subclinical.” It does a disservice to the field by understating the potential long-term and multifaceted impact of EE among children in low-resource settings.
Key points.
Despite commonly being considered a subclinical condition, environmental enteropathy in early childhood is associated with important long-term health impacts including impaired vaccine responses, child growth faltering, cognitive impairment, and later life obesity, diabetes, and metabolic syndrome.
Insufficient diagnostic biomarkers, the inability to identify appropriate control groups, and geographic differences in manifestations make it challenging to define environmental enteropathy, which will be necessary for further research, prevention, and treatment.
Enteric infections, even in the absence of overt diarrhea, are highly frequent in low-resource settings, contribute to environmental enteropathy, and may cause or aggravate growth faltering.
New tools for detecting enteric pathogens, characterizing the microbiome, and assessing the transcriptome and metabolome may help understand environmental enteropathy and identify potential interventions.
Acknowledgments
The authors thank Dr. Sean Moore and Dr. James Platts-Mills for their helpful inputs.
This work was supported by the Fogarty International Center, National Institutes of Health (D43-TW009359 to ETR).
Footnotes
Disclosures: We declare no conflicts of interest.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keusch GT, Denno DM, Black RE, et al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis. 2014;59(Suppl 4):S207–S212. doi: 10.1093/cid/ciu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neu N, Duchon J, Zachariah P. TORCH infections. Clin Perinatol. 2015;42(1):77–103. viii. doi: 10.1016/j.clp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Loshaj-Shala A, Regazzoni L, Daci A, et al. Guillain Barré syndrome (GBS): new insights in the molecular mimicry between C. jejuni and human peripheral nerve (HPN) proteins. Journal of Neuroimmunology. 2015;289:168–176. doi: 10.1016/j.jneuroim.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Naylor C, Lu M, Haque R, et al. Environmental Enteropathy, Oral Vaccine Failure and Growth Faltering in Infants in Bangladesh. EBioMedicine. 2015;2(11):1759–1766. doi: 10.1016/j.ebiom.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmartin AA, Petri WA. Exploring the role of environmental enteropathy in malnutrition, infant development and oral vaccine response. Philos Trans R Soc Lond, B, Biol Sci. 2015;370(1671) doi: 10.1098/rstb.2014.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amour C, Gratz J, Mduma E, et al. Epidemiology and impact of Campylobacter infection in children in eight low-resource settings: results from the MAL-ED study. Clin Infect Dis. 2016 Aug; doi: 10.1093/cid/ciw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogawski ET, Bartelt LA, Platts-Mills JA, et al. Determinants and impact of Giardia infection in the first two years of life in the MAL-ED birth cohort. Journal of the Pediatric Infectious Diseases Society. 2016 doi: 10.1093/jpids/piw082. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eppig C, Fincher CL, Thornhill R. Parasite prevalence and the worldwide distribution of cognitive ability. Proc Biol Sci. 2010;277(1701):3801–3808. doi: 10.1098/rspb.2010.0973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Pinkerton R, Oriá RB, Lima AAM, et al. Early Childhood Diarrhea Predicts Cognitive Delays in Later Childhood Independently of Malnutrition. Am J Trop Med Hyg. 2016 Sep; doi: 10.4269/ajtmh.16-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AAM. The impoverished gut--a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10(4):220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharf RJ, Deboer MD, Guerrant RL. Recent advances in understanding the long-term sequelae of childhood infectious diarrhea. Curr Infect Dis Rep. 2014;16(6):408. doi: 10.1007/s11908-014-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBoer MD, Lima AAM, Oría RB, et al. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr Rev. 2012;70(11):642–653. doi: 10.1111/j.1753-4887.2012.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBoer MD, Chen D, Burt DR, et al. Early childhood diarrhea and cardiometabolic risk factors in adulthood: the Institute of Nutrition of Central America and Panama Nutritional Supplementation Longitudinal Study. Ann Epidemiol. 2013;23(6):314–320. doi: 10.1016/j.annepidem.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batheja MJ, Leighton J, Azueta A, Heigh R. The Face of Tropical Sprue in 2010. Case Rep Gastroenterol. 2010;4(2):168–172. doi: 10.1159/000314231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenbaum J, Kent TH, Sprinz H. Malabsorption and jejunitis in American Peace Corps volunteers in Pakistan. Ann Intern Med. 1966;65(6):1201–1209. doi: 10.7326/0003-4819-65-6-1201. [DOI] [PubMed] [Google Scholar]
- 18.Cook GC, Kajubi SK, Lee FD. Jejunal morphology of the African in Uganda. J Pathol. 1969;98(3):157–169. doi: 10.1002/path.1710980302. [DOI] [PubMed] [Google Scholar]
- 19.Sprinz H, Sribhibhadh R, Gangarosa EJ, Benyajati C, Kundel D, Halstead S. Biopsy of small bowel of Thai people. With special reference to recovery from Asiatic cholera and to an intestinal malabsorption syndrome. Am J Clin Pathol. 1962;38:43–51. doi: 10.1093/ajcp/38.1.43. [DOI] [PubMed] [Google Scholar]
- 20.Colwell EJ, Welsh JD, Legters LJ, Proctor RF. Jejunal morphological characteristics in South Vietnamese residents. JAMA. 1968;206(10):2273–2276. [PubMed] [Google Scholar]
- 21.Lindenbaum J, Alam AK, Kent TH. Subclinical small-intestinal disease in East Pakistan. Br Med J. 1966;2(5530):1616–1619. doi: 10.1136/bmj.2.5530.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García S. Malabsorption and malnutrition in Mexico. Am J Clin Nutr. 1968;21(9):1066–1076. doi: 10.1093/ajcn/21.9.1066. [DOI] [PubMed] [Google Scholar]
- 23.England NW, O’Brien W. Appearances of the jejunal mucosa in acute tropical sprue in Singapore. Gut. 1966;7(2):128–139. doi: 10.1136/gut.7.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathan VI, Baker SJ. An epidemic of tropical sprue in southern India. I. Clinical features. Ann Trop Med Parasitol. 1970;64(4):439–451. doi: 10.1080/00034983.1970.11686715. [DOI] [PubMed] [Google Scholar]
- 25.Amin K, Walia BN, Ghai OP. Small bowel functions and structure in malnourished children. Indian Pediatr. 1969;6(2):67–72. [PubMed] [Google Scholar]
- 26.Cook GC, Lee FD. The jejunum after kwashiorkor. Lancet. 1966;2(7476):1263–1267. doi: 10.1016/s0140-6736(66)91686-2. [DOI] [PubMed] [Google Scholar]
- 27.Stanfield JP, Hutt MS, Tunnicliffe R. Intestinal biopsy in kwashiorkor. Lancet. 1965;2(7411):519–523. doi: 10.1016/s0140-6736(65)91474-1. [DOI] [PubMed] [Google Scholar]
- 28.Lindenbaum J, Gerson CD, Kent TH. Recovery of small-intestinal structure and function after residence in the tropics. I. Studies in Peace Corps volunteers. Ann Intern Med. 1971;74(2):218–222. doi: 10.7326/0003-4819-74-2-218. [DOI] [PubMed] [Google Scholar]
- 29.Korpe PS, Petri WA., Jr Environmental enteropathy: critical implications of a poorly understood condition. Trends in Molecular Medicine. 2012;18(6):328–336. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K, Petri WA. Environmental Enteropathy: Elusive but Significant Subclinical Abnormalities in Developing Countries. EBioMedicine. 2016;10:25–32. doi: 10.1016/j.ebiom.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syed S, Ali A, Duggan C. Environmental Enteric Dysfunction in Children. J Pediatr Gastroenterol Nutr. 2016;63(1):6–14. doi: 10.1097/MPG.0000000000001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keusch GT, Rosenberg IH, Denno DM, et al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr Bull. 2013;34(3):357–364. doi: 10.1177/156482651303400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerrant RL, Leite AM, Pinkerton R, et al. Biomarkers of Environmental Enteropathy, Inflammation, Stunting, and Impaired Growth in Children in Northeast Brazil. PLoS ONE. 2016;11(9):e0158772. doi: 10.1371/journal.pone.0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prata M de MG, Havt A, Bolick DT, Pinkerton R, Lima A, Guerrant RL. Comparisons between myeloperoxidase, lactoferrin, calprotectin and lipocalin-2, as fecal biomarkers of intestinal inflammation in malnourished children. J Transl Sci. 2016;2(2):134–139. doi: 10.15761/JTS.1000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosselin KB, Aboud S, McDonald CM, et al. Etiology of Diarrhea, Nutritional Outcomes and Novel Intestinal Biomarkers in Tanzanian Infants: A Preliminary Study. J Pediatr Gastroenterol Nutr. 2016 Jun; doi: 10.1097/MPG.0000000000001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosek M, Haque R, Lima A, et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88(2):390–396. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arndt MB, Richardson BA, Ahmed T, et al. Fecal Markers of Environmental Enteropathy and Subsequent Growth in Bangladeshi Children. Am J Trop Med Hyg. 2016;95(3):694–701. doi: 10.4269/ajtmh.16-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosek M, Guerrant RL, Kang G, et al. Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clin Infect Dis. 2014;59(Suppl 4):S239–S247. doi: 10.1093/cid/ciu457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uddin MI, Islam S, Nishat NS, et al. Biomarkers of Environmental Enteropathy are Positively Associated with Immune Responses to an Oral Cholera Vaccine in Bangladeshi Children. PLoS Negl Trop Dis. 2016;10(11):e0005039. doi: 10.1371/journal.pntd.0005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oriá RB, Costa CMC, Lima AAM, Patrick PD, Guerrant RL. Semantic fluency: a sensitive marker for cognitive impairment in children with heavy diarrhea burdens? Med Hypotheses. 2009;73(5):682–686. doi: 10.1016/j.mehy.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danaei G, Andrews KG, Sudfeld CR, et al. Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels. PLoS Med. 2016;13(11):e1002164. doi: 10.1371/journal.pmed.1002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. [Accessed May 9, 2016];WHO Child Growth Standards: Length/height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age, Methods and Development. 2006 http://www.who.int/childgrowth/standards/Technical_report.pdf?ua=1.
- 43.Saiki T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. Kurume Med J. 1998;45(1):69–73. doi: 10.2739/kurumemedj.45.69. [DOI] [PubMed] [Google Scholar]
- 44.Ledjeff E, Artner-Dworzak E, Witasek A, Fuchs D, Hausen A. Neopterin Concentrations in Colon Dialysate. Pteridines. 2013;12(4):155–160. [Google Scholar]
- 45.World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers. 2005 http://www.who.int/maternal_child_adolescent/documents/9241593180/en/index.html.
- 46.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61(5):707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 47.Lima AA, Moore SR, Barboza MS, Jr, et al. Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J Infect Dis. 2000;181(5):1643–1651. doi: 10.1086/315423. [DOI] [PubMed] [Google Scholar]
- 48.Niehaus MD, Moore SR, Patrick PD, et al. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg. 2002;66(5):590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 49.Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of Acute Diarrhea on Linear Growth in Peruvian Children. Am J Epidemiol. 2003;157(2):166–175. doi: 10.1093/aje/kwf179. [DOI] [PubMed] [Google Scholar]
- 50.Moore SR, Lima NL, Soares AM, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139(4):1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Assis AMO, Barreto ML, Santos LMP, Fiaccone R, da Silva Gomes GS. Growth faltering in childhood related to diarrhea: a longitudinal community based study. Eur J Clin Nutr. 2005;59(11):1317–1323. doi: 10.1038/sj.ejcn.1602245. [DOI] [PubMed] [Google Scholar]
- 52.Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Glob Health. 2015;3(9):e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steiner TS, Lima AA, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177(1):88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 54.Acosta GJ, Vigo NI, Durand D, et al. Diarrheagenic Escherichia coli: Prevalence and Pathotype Distribution in Children from Peruvian Rural Communities. Am J Trop Med Hyg. 2016;95(3):574–579. doi: 10.4269/ajtmh.16-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee G, Pan W, Peñataro Yori P, et al. Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis. 2013;7(1):e2036. doi: 10.1371/journal.pntd.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donowitz JR, Alam M, Kabir M, et al. A Prospective Longitudinal Cohort to Investigate the Effects of Early Life Giardiasis on Growth and All Cause Diarrhea. Clin Infect Dis. 2016;63(6):792–797. doi: 10.1093/cid/ciw391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum Infection in Peruvian Children: Growth Faltering and Subsequent Catch-up Growth. Am J Epidemiol. 1998;148(5):497–506. doi: 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- 58.Galdys AL, Nelson JS, Shutt KA, et al. Prevalence and Duration of Asymptomatic Clostridium difficile Carriage among Healthy Subjects in Pittsburgh, Pennsylvania. J Clin Microbiol. 2014;52(7):2406–2409. doi: 10.1128/JCM.00222-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine MM, Robins-Browne RM. Factors That Explain Excretion of Enteric Pathogens by Persons Without Diarrhea. Clin Infect Dis. 2012;55(Suppl 4):S303–S311. doi: 10.1093/cid/cis789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 61.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 62.Morgan XC, Huttenhower C. Chapter 12: Human Microbiome Analysis. PLoS Comput Biol. 2012;8(12):e1002808. doi: 10.1371/journal.pcbi.1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hopkins MJ, Sharp R, Macfarlane GT. Variation in human intestinal microbiota with age. Dig Liver Dis. 2002;34(Suppl 2):S12–S18. doi: 10.1016/s1590-8658(02)80157-8. [DOI] [PubMed] [Google Scholar]
- 64.Di Mauro A, Neu J, Riezzo G, et al. Gastrointestinal function development and microbiota. Ital J Pediatr. 2013;39:15. doi: 10.1186/1824-7288-39-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guarino A, Wudy A, Basile F, Ruberto E, Buccigrossi V. Composition and roles of intestinal microbiota in children. J Matern Fetal Neonatal Med. 2012;25(Suppl 1):63–66. doi: 10.3109/14767058.2012.663231. [DOI] [PubMed] [Google Scholar]
- 66.Subramanian S, Huq S, Yatsunenko T, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014 doi: 10.1038/nature13421. advance online publication. doi:10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blanton LV, Charbonneau MR, Salih T, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351(6275) doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coutinho BP, Oriá RB, Vieira CMG, et al. Cryptosporidium Infection Causes Undernutrition and, Conversely, Weanling Undernutrition Intensifies Infection. Journal of Parasitology. 2008;94(6):1225–1232. doi: 10.1645/GE-1411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa LB, Noronha FJ, Roche JK, et al. Novel in vitro and in vivo models and potential new therapeutics to break the vicious cycle of Cryptosporidium infection and malnutrition. J Infect Dis. 2012;205(9):1464–1471. doi: 10.1093/infdis/jis216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J, Bolick DT, Kolling GL, Fu Z, Guerrant RL. Protein malnutrition impairs intestinal epithelial turnover: a potential mechanism of increased cryptosporidiosis in a murine model. Infect Immun. 2016 Oct; doi: 10.1128/IAI.00705-16. IAI.00705-00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roche JK, Cabel A, Sevilleja J, Nataro J, Guerrant RL. Enteroaggregative Escherichia coli (EAEC) Impairs Growth while Malnutrition Worsens EAEC Infection: A Novel Murine Model of the Infection Malnutrition Cycle. J Infect Dis. 2010;202(4):506–514. doi: 10.1086/654894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bolick DT, Roche JK, Hontecillas R, Bassaganya-Riera J, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli strain in a novel weaned mouse model: exacerbation by malnutrition, biofilm as a virulence factor and treatment by nitazoxanide. J Med Microbiol. 2013;62(6):896–905. doi: 10.1099/jmm.0.046300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bolick DT, Kolling GL, Moore JH, et al. Zinc deficiency alters host response and pathogen virulence in a mouse model of enteroaggregative Escherichia coli-induced diarrhea. Gut Microbes. 2014;5(5):618–627. doi: 10.4161/19490976.2014.969642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Preidis GA, Saulnier DM, Blutt SE, et al. Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. J Pediatr Gastroenterol Nutr. 2012;55(3):299–307. doi: 10.1097/MPG.0b013e31824d2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hentges DJ. Role of the intestinal microflora in host defense against infection. In: Hentges DJ, editor. Human Intestinal Microflora in Health and Disease. New York: Academic Press, Inc; 1983. pp. 311–331. [Google Scholar]
- 76.Wardwell LH, Huttenhower C, Garrett WS. Current concepts of the intestinal microbiota and the pathogenesis of infection. Curr Infect Dis Rep. 2011;13(1):28–34. doi: 10.1007/s11908-010-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sekirov I, Finlay BB. The role of the intestinal microbiota in enteric infection. J Physiol (Lond) 2009;587(Pt 17):4159–4167. doi: 10.1113/jphysiol.2009.172742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Looft T, Allen HK. Collateral effects of antibiotics on mammalian gut microbiomes. Gut Microbes. 2012;3(5):463–467. doi: 10.4161/gmic.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sekirov I, Tam NM, Jogova M, et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun. 2008;76(10):4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moon C, Stappenbeck TS. Viral interactions with the host and microbiota in the intestine. Curr Opin Immunol. 2012;24(4):405–410. doi: 10.1016/j.coi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grzybowski MM, Długońska H. Natural microbiota in viral and helminth infections. Addendum to: Personalized vaccination. II. The role of natural microbiota in a vaccine-induced immunity. Ann Parasitol. 2012;58(3):157–160. [PubMed] [Google Scholar]
- 82.Kuss SK, Best GT, Etheredge CA, et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334(6053):249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kane M, Case LK, Kopaskie K, et al. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334(6053):245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol. 2011;8(9):523–531. doi: 10.1038/nrgastro.2011.133. [DOI] [PubMed] [Google Scholar]
- 85.Finegold SM, Mathisen GE, George WL, Hentges DL. Human Intestinal Microflora in Health and Disease. New York: Academic Press, Inc; 1983. Changes in human intestinal flora related to the administration of antimicrobial agents; pp. 355–446. [Google Scholar]
- 86.Guerrant RL, Kosek M, Lima AAM, Lorntz B, Guyatt HL. Updating the DALYs for diarrhoeal disease. Trends Parasitol. 2002;18(5):191–193. doi: 10.1016/s1471-4922(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 87.Mishra OP, Dhawan T, Singla PN, Dixit VK, Arya NC, Nath G. Endoscopic and histopathological evaluation of preschool children with chronic diarrhoea. J Trop Pediatr. 2001;47(2):77–80. doi: 10.1093/tropej/47.2.77. [DOI] [PubMed] [Google Scholar]
- 88.Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg. 2004;70(4):412–419. [PubMed] [Google Scholar]
- 89.Thomas AG, Phillips AD, Walker-Smith JA. The value of proximal small intestinal biopsy in the differential diagnosis of chronic diarrhoea. Arch Dis Child. 1992;67(6):741–743. doi: 10.1136/adc.67.6.741. discussion 743–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu J, Platts-Mills JA, Juma J, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Houpt E, Gratz J, Kosek M, et al. Microbiologic methods utilized in the MAL-ED cohort study. Clin Infect Dis. 2014;59(Suppl 4):S225–S232. doi: 10.1093/cid/ciu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Progress on Drinking Water and Sanitation: Special Focus on Sanitation. UNICEF, New York and WHO; Geneva: 2008. [Accessed February 5, 2016]. World Health Organization and United Nations Children’s Fund Joint Monitoring Programme for Water Supply and Sanitation, (JMP) http://www.wssinfo.org/fileadmin/user_upload/resources/1251794333-JMP_08_en.pdf. [Google Scholar]
- 93.World Health Organization. [Accessed November 29, 2016];Comprehensive Implementation Plan on Maternal, Infant and Young Child Nutrition. 2014 doi: 10.3945/an.114.007781. http://www.who.int/nutrition/publications/CIP_document/en/ [DOI] [PMC free article] [PubMed]
- 94.Forouzanfar MH, Afshin A, Alexander LT, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.World Health Organization. [Accessed November 29, 2016];Essential Nutrition Actions: Improving Maternal, Newborn, Infant and Young Child Health and Nutrition. 2013 http://www.who.int/nutrition/publications/infantfeeding/essential_nutrition_actions/en/ [PubMed]
- 96.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 97.Lang D MAL-ED Network Investigators. Opportunities to assess factors contributing to the development of the intestinal microbiota in infants living in developing countries. Microb Ecol Health Dis. 2015;26:28316. doi: 10.3402/mehd.v26.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Curtis V, Cousens S, Mertens T, Traore E, Kanki B, Diallo I. Structured observations of hygiene behaviours in Burkina Faso: validity, variability, and utility. Bull World Health Organ. 1993;71(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- 99.Biran A, Rabie T, Schmidt W, Juvekar S, Hirve S, Curtis V. Comparing the performance of indicators of hand-washing practices in rural Indian households. Trop Med Int Health. 2008;13(2):278–285. doi: 10.1111/j.1365-3156.2007.02001.x. [DOI] [PubMed] [Google Scholar]
- 100.Panchalingam S, Antonio M, Hossain A, et al. Diagnostic Microbiologic Methods in the GEMS-1 Case/Control Study. Clin Infect Dis. 2012;55(Suppl 4):S294–S302. doi: 10.1093/cid/cis754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J, Gratz J, Amour C, et al. A Laboratory-Developed TaqMan Array Card for Simultaneous Detection of 19 Enteropathogens. J Clin Microbiol. 2013;51(2):472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rutledge RG, Côté C. Mathematics of quantitative kinetic PCR and the application of standard curves. Nucleic Acids Res. 2003;31(16):e93. doi: 10.1093/nar/gng093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carroll IM, Threadgill DW, Threadgill DS. The gastrointestinal microbiome: a malleable, third genome of mammals. Mamm Genome. 2009;20(7):395–403. doi: 10.1007/s00335-009-9204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu J, Ordiz MI, Stauber J, et al. Environmental Enteric Dysfunction Includes a Broad Spectrum of Inflammatory Responses and Epithelial Repair Processes. Cell Mol Gastroenterol Hepatol. 2016;2(2):158–174. e1. doi: 10.1016/j.jcmgh.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mayneris-Perxachs J, Lima AAM, Guerrant RL, et al. Urinary N-methylnicotinamide and β-aminoisobutyric acid predict catch-up growth in undernourished Brazilian children. Sci Rep. 2016;6:19780. doi: 10.1038/srep19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Serazin AC, Shackelton LA, Wilson C, Bhan MK. Improving the performance of enteric vaccines in the developing world. Nat Immunol. 2010;11(9):769–773. doi: 10.1038/ni0910-769. [DOI] [PubMed] [Google Scholar]
- 107.Clasen T, Boisson S, Routray P, et al. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. The Lancet Global Health. 2014;2(11):e645–e653. doi: 10.1016/S2214-109X(14)70307-9. [DOI] [PubMed] [Google Scholar]
- 108.Patil SR, Arnold BF, Salvatore AL, et al. The Effect of India’s Total Sanitation Campaign on Defecation Behaviors and Child Health in Rural Madhya Pradesh: A Cluster Randomized Controlled Trial. PLOS Medicine. 2014;11(8):e1001709. doi: 10.1371/journal.pmed.1001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luby S. Is targeting access to sanitation enough? Lancet Glob Health. 2014;2(11):e619–e620. doi: 10.1016/S2214-109X(14)70326-2. [DOI] [PubMed] [Google Scholar]
- 110.Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bulletin of the World Health Organization. 2017;95:49–61. doi: 10.2471/BLT.16.176123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Archbald-Pannone L, Sevilleja JE, Guerrant R. Diarrhea, clostridium difficile, and intestinal inflammation in residents of a long-term care facility. J Am Med Dir Assoc. 2010;11(4):263–267. doi: 10.1016/j.jamda.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bentley DW. Clostridium difficile-associated disease in long-term care facilities. Infect Control Hosp Epidemiol. 1990;11(8):434–438. doi: 10.1086/646204. [DOI] [PubMed] [Google Scholar]
- 113.Rybolt AH, Bennett RG, Laughon BE, Thomas DR, Greenough WB, Bartlett JG. Protein-losing enteropathy associated with Clostridium difficile infection. Lancet. 1989;1(8651):1353–1355. doi: 10.1016/s0140-6736(89)92803-1. [DOI] [PubMed] [Google Scholar]


