Abstract
Recently, two-dimensional paper networks have been developed to enable multistep assays to be performed in a lateral flow format. These devices have been used to perform simple enzyme linked immunoassays on paper. However, these devices have yet to incorporate more complex immunoassays, including the use of streptavidin-biotin detection strategies. Here we present a modified two-dimensional paper network capable of consecutively delivering six reagents. The device requires only a single user step and delivers (i) the sample, (ii) the biotinylated detection antibody, (iii) streptavidin horse-radish peroxidase (iv) a wash buffer (v) a colorimetric substrate and (vi) a final wash buffer. To demonstrate the utility of this approach we designed an assay to detect the malaria protein Pf HRP2. Using this platform, we were able to achieve a limit-of-detection equivalent to that of a traditional 96-well plate sandwich ELISA. In addition to improvements in the limit-of-detection, the inclusion of streptavidin-biotin simplifies the development of similar tests for other targets.
Graphical Abstract
INTRODUCTION
Microfluidic paper-based devices are a novel class of point-of-care rapid diagnostic test (RDT) capitalizing on paper’s low cost, ubiquity, and wicking properties1. A subset of microfluidic paper-based devices, lateral flow tests (LFTs), are a staple of clinical point-of-care (POC) diagnostics due to their simplicity, speed and low cost2. LFTs are typically simple sandwich immunoassays designed to detect proteins of clinical importance. They often have higher limits-of-detection than their conventional enzyme linked immunoassay (ELISA) counterparts, reducing their clinical utility3,4. Increased sensitivity requires the ability to implement more complicated, multistep assays, i.e. utilizing chemical amplification techniques. Recently, two dimensional paper networks (2DPN) have been developed to expand the utility of traditional LFTs4. These 2DPNs provide the ability to run multistep immunoassays with minimal user interaction and do not require additional instruments or significantly increase cost4. These devices allow for the inclusion of strategies which improve the limit-of-detection (LOD) relative to “one-dimensional” LFTS3–8, providing the means to develop highly sensitive POC assays for clinically relevant targets.
One potential application of highly sensitive RDTs is the diagnosis of malaria. The World Health Organization (WHO) reports that half the world’s population is at risk for malaria9. Malaria causes an estimated 627,000 deaths annually with 90% occurring in sub-Saharan Africa9. Most of these deaths result from infections with the Plasmodium falciparum (Pf) parasite, making its detection particularly important10. RDTs that diagnose Pf malaria often target the Pf histidine-rich protein 2 (Pf HRP2), an antigen released by blood stages of Pf parasites11. Using traditional ELISA techniques, researchers have been able to reliably measure Pf HRP2 levels as low as 0.11 ng/mL12, corresponding to approximately 0.7 parasites/uL, using the maximum circulating approximation developed by Marquart et al13. In contrast, traditional lateral flow RDTs for Pf HRP2 are able to achieve limits-of-detection as low as 6.94 ng/mL (8 parasites/μL)7,13. Fu et al. demonstrated that by implementing a 2DPN gold enhancement immunoassay, they could achieve a limit-of-detection of 2.9 ng/mL (4 parasites/μL)6,13. While a significant improvement, additional steps are necessary to achieve a LOD comparable to the best reported traditional ELISAs. Malaria elimination programs, which aim to detect subclinical malaria infections, require low LODs equivalent to standard HRP2 ELISAs14.
Incorporating a streptavidin-biotin detection system can improve the limit-of-detection of immunoassays. Streptavidin-biotin detection strategies have been used for decades in ELISAs due to their many advantages15. Briefly, streptavidin has an extremely high affinity for biotin16 (Kd ~ 4 x 10−14), streptavidin binds to biotin with high specificity, and biotin is small (244 Da) allowing it to be readily covalently linked to molecules without altering biological activity15. Streptavidin is available with a variety of detection labels that produce additional amplification, including the enzyme horse-radish peroxidase15, enabling the detection of biotinylated molecules. Furthermore, streptavidin-biotin detection strategies offer an inherently amplified signal versus direct detection strategies because streptavidin and biotin both provide four binding sites for one another15. Additional sensitivity can be achieved by utilizing streptavidin conjugated to polymers of horse-radish peroxidase, providing a larger number of enzyme molecules per bound molecule. Here we demonstrate that by using a larger sample size than previously reported 2DPN RDTs and employing a streptavidin poly- horse-radish peroxidase detection system, 2DPNs can achieve limits-of-detection comparable to the lowest reported limit-of-detection by traditional ELISA.
MATERIALS AND METHODS
Two-Dimensional Paper Network Device
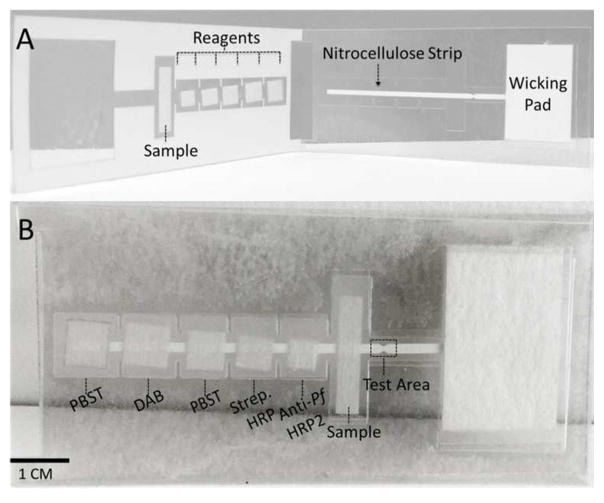
The two-dimensional paper network (2DPN) devices, depicted in Figure 1, are constructed on a plastic backbone consisting of 10 mil Dura-Lar (Blick Art Supplies, Galesburg, Illinois) and 5 mil adhesive-backed Dura-Lar (Blick Art Supplies, Galesburg, Illinois). The five reagent storage and release pads and the sample pad are cut from glass fiber pads (grade 8951, Ahlstrom, Helsinki, Finland). The wicking pad is made from cellulose (C083, Millipore, Billerica, MA). The primary lateral flow channel consists of 4 mil backed high flow nitrocellulose (HF135, Millipore, Billerica, MA). All materials were cut using a CO2 laser cutter (Universal Laser Systems, Scottsdale, Arizona).
Figure 1.
Device diagram. (A) Device prior to folding with key components labeled. (B) Device folded to initiate flow with reagents and test area
Sequential Fluid Flow Experiment
We performed experiments to evaluate the flow of reagents in two different 2DPNs designed to deliver six reagents sequentially. The first device was modeled after the device described by Fu et al6. Reagent pads were placed on “legs” extending at a ninety-degree angle off the main lateral flow body (Figure 2). This device is referred to as the “leg design” in the remainder of this manuscript. The second device consists of the same reagent pads placed directly on the main body of the device. Due to the fact that this device consists of a linear piece of nitrocellulose, it is referred to as the “linear device”.
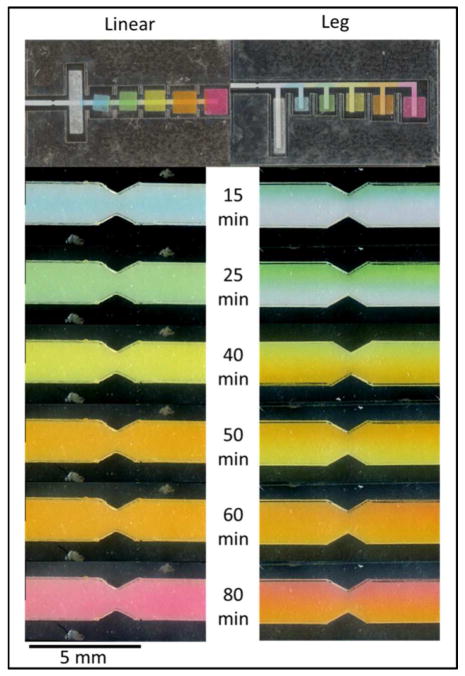
Figure 2.
Flow profile comparison between devices. Food coloring was used to compare the flow between two device designs. The linear device showed consistently sequential reagent delivery, while the leg device showed parallel reagent flow at the 25, 50 and 80-minute time points
In order to visualize the flow of reagents, we used food dye (McCormick, Sparks, Maryland) diluted in Phosphate Buffered Saline (PBS) with .05% Tween® 20 (PBST). Glass fiber pads with capacities of 50 μL, 15 μL, 20 μL, 25 μL, 30 μL and 35 μL were placed in their respective positions on the device. Different colors of food coloring, diluted in PBST, were dispensed with the appropriate volume on each pad, as shown in Figure 1. The device was folded in half to initiate fluid flow. Images were acquired with a flatbed scanner at 600 dots-per-inch (DPI) at 15, 25, 40, 50, 60 and 80 minutes after flow initiation.
PF HRP2 2DPN Assay Preparation
Prior to device assembly, we pipetted 0.4 μL of the capture antibody, a murine antibody to Pf HRP2 (Immunology Consultants Laboratory, Portland, OR), directly onto the nitrocellulose strip at the test location. The strip was allowed to dry for ten minutes before applying another 0.4 uL of antibody to the same spot. It was then dried for 90 minutes at 37 °C.
To prevent nonspecific binding, the nitrocellulose strip was blocked in a similar fashion to that described by Fu et al.6. The device was completely submerged for twenty minutes in PBS containing 5% sucrose, 2% bovine serum albumin (BSA), 0.25% PVP (40KD mW, Sigma Aldrich, St. Louis, Missouri), and .05% Tween 20. The nitrocellulose was dried for 60 minutes at 37°C before completely assembling the device. Finally, the device was assembled as shown in Figure 1.
The unconjugated murine detection antibody (Immunology Consultants Laboratory, Portland, OR) was conjugated to biotin using EZ-link biotinylation kit (Thermo Fisher Scientific, Waltham, Massachusetts). The biotin conjugated detection antibody was desalted using Zeba columns (Thermo Fisher Scientific, Waltham, Massachusetts) to remove any unbound biotin. A streptavidin- horse-radish peroxidase polymer consisting of 400 horse-radish peroxidase monomer molecules (poly-HRP80, Fitzgerald, Acton, Massachusetts) was used as the secondary detection mechanism. Diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, Missouri) served as the colorimetric substrate.
Running the 2DPN Assays
The biotinylated detection antibody was diluted to 40 ug/mL in PBST. The streptavidin-horse-radish peroxidase polymer was diluted to 8 ug/mL in PBST. DAB was dissolved in Millipore water at a concentration of 0.5 mg/mL. Samples were created by spiking recombinant Pf HRP2 (CTK Biotech, San Diego, CA) in fetal bovine serum (FBS). We tested concentrations of 0, 0.1 ng/mL, 0.25 ng/mL, 0.5 ng/mL, 1 ng/mL Pf HRP2 in triplicate on both the linear and leg 2DPNs. Immediately prior to running the assay, sodium percarbonate (Sigma-Aldrich, St. Louis, Missouri) was added to the DAB solution to a concentration of 5.7 mg/mL. Sodium percarbonate provides the necessary hydrogen peroxide for the DAB colorimetric reaction to occur.
To run the assay, 50 μL of sample was pipetted onto the first pad. Next, 15 uL detection antibody was applied to the second pad, followed by 20 uL streptavidin-horse-radish peroxidase, 25 uL PBST, 30 uL DAB-sodium percarbonate solution, and 35 uL PBST. The device was then folded in half to initiate fluid flow. Each device was scanned in black and white at 600 DPI using a flatbed scanner at 60 minutes and 90 minutes after assay initiation.
Running Standard ELISA Kit
We also tested all spiked FBS samples using the Malaria Ag CELISA (Cellabs, Sydney, Australia). We additionally tested 0.05 ng/mL of HRP2 spiked in FBS with the CELISA. The CELISA is the primary commercialized HRP2 ELISA14 and has reported sensitivity and specificity equivalent to quantitative polymerase chain reaction (qPCR) in symptomatic patients17.
The assay was run in accordance with the provided protocol. Briefly, 100 μL spiked FBS sample was added into each well and incubated for one hour at room temperature in a humid chamber. The wells were manually washed five times with 300 μL PBST. Next, 100 μL of detection antibody was added to each well, and the plate was incubated for 1 hour at room temperature in a humid chamber. After incubation, the wells were washed another five times with 300 μL PBST. Finally, 100 μL of substrate was added to each well, and the plate was incubated in the dark at room temperature for fifteen minutes. 50 uL of stop solution was added to each well, and the plate was imaged at 450 nm using a plate reader (Tecan, Zürich, Switzerland). A two-sided t-test was used to test for significant differences in mean absorbance between different HRP2 concentrations. P-values of less than 0.05 were considered significant.
Analysis of 2DPN Assay Signal
A fixed size rectangular region of interest (ROI) was placed at the capture region of each image. An additional background ROI of identical size was selected automatically halfway between the signal ROI and the wicking pad. To determine the signal, the complement of the image was first computed such that higher pixel intensity corresponded to a higher signal. The test signal was defined by calculating the mean of the maximum pixel intensity for each row of the test ROI. The background signal was calculated identically using the background ROI. Finally, the test ROI signal was divided by the background ROI signal to determine a signal-to-background ratio. A two-sided t-test assuming unequal variances was used to test whether differences in the signal-to-background ratio for different antigen concentrations were statistically significant; p-values of less than 0.05 were considered significant.
RESULTS AND DISCUSSION
Sequential Flow Comparison
The flow comparison between the leg and linear is shown in Figure 2. An image of each device is shown immediately after folding. Additional images of the test zone of each device are shown at various time points after folding to assess flow. In both devices, the final reagent reached the test zone in 80 minutes. However, the leg device resulted in parallel flow of consecutive reagents as opposed to sequential reagent delivery. This is particularly apparent at the 25, 50 and 80-minute time points. Conversely, the linear device shows consistent sequential reagent delivery at all time points.
Results suggest that the nitrocellulose inlets of the leg device, which are perpendicular to fluid flow, disrupt the desired sequential laminar flow. This effect is exacerbated relative to previously published 2DPNs by having a larger sample pad. In previously published devices, each sequential reagent pad is designed to hold a larger volume than the previous pad3–8. By placing the largest pad first, we introduced a strong force perpendicular to the desired flow immediately prior to the test area. Furthermore, we extended the total number of reagent pads. This increases the number of inlets perpendicular to the device, further exacerbating the effect. The linear device solves this challenge by placing reagent pads symmetrically centered on the device, resulting in no net force perpendicular to the primary nitrocellulose channel.
PF HRP2 Assay
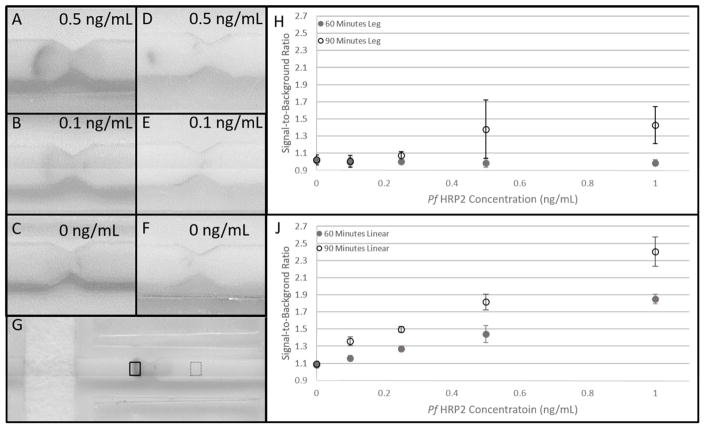
Example images for 0.5 ng/mL, 0.1 ng/mL and 0 ng/mL HRP2 concentrations are provided for both the linear and leg devices in Figure 3. In Figure 3G, the signal ROI is outlined in a solid black box and the background ROI in the dotted black box. Figure 3 also shows a scatter plot of the calculated signal-to-background ratios for concentrations ranging from 0 to 1 ng/mL with their standard deviations for both linear and leg devices. For the linear 2DPN, at 60 minutes, the mean SBR between 0 ng/mL and 0.1 ng/mL were not statistically different. The SBR between 0.5 ng/mL and 0.25 ng/mL were also not statistically different. The mean SBR for all other concentrations were significantly different at 60 minutes for the linear 2DPN. At 90 minutes, the mean SBR for all antigen concentrations were significantly different from one another for the linear 2DPN. For the leg 2DPN, there were no significant differences in mean SBR between HRP2 concentrations at 60 or 90 minutes. Although signal was present for 0.5 ng/mL and 1.0 ng/mL after 90 minutes, the large variability in SBR prevented differences from reaching statistical significance. We believe the inferior performance for the leg device is due to the parallel reagent delivery illustrated with the food coloring experiments. When signal is present for the leg device, it is only present in the middle of the test zone as shown in Figure 3D. This is likely the only location where all reagents are delivered in sufficient quantity.
Figure 3.
Results of Pf HRP2 2DPN assays. Representative scanned image for the linear device of (A) 0.5 ng/mL Pf HRP2. (B) 0.1 ng/mL and (C) 0 ng/mL after 90 minutes. Representative scanned images for the leg device are shown in (D), (E) and (F) for the same concentrations. (G) Signal and background regions-of-interest. The solid black box corresponds to the signal region-of-interest and the dotted black box to the background region-of-interest. (H) A plot of the signal-to-background ratio for the leg 2DPN for concentrations ranging from 0 to 1 ng/mL Pf HRP2 at 60 and 90 minutes after assay initiation. Each data point corresponds to the average of three separate tests. There is no significant difference in mean SBR between any antigen concentration at 60 or 90 minutes. (J) Presents the same plot for the linear 2DPN. At 60 minutes, there is no significant difference between 0.1 ng/mL and 0 ng/mL or between 0.5 ng/mL and 0.25 ng/mL. The mean SBR is significantly different between all other antigen concentrations. At 90 minutes, the mean SBR is significantly different between all antigen concentrations.
For the CELISA kit, there was no significant difference between the mean absorbance for 0.05 ng/mL and 0 ng/mL. Mean absorbance were significantly different between all other concentrations. The LOD for the linear device at 90 minutes is 0.1 ng/mL, comparable to the lowest reported ELISA LOD of 0.11 ng/mL12, corresponding to approximately 0.7 parasites/uL13. The CELISA HRP2 ELISA kit used here also produced a LOD of 0.1 ng/mL. Results using this kit are shown in supplemental figure 1.
The improvement in limit-of-detection is due to both the use of polymerized horse-radish peroxidase conjugated streptavidin in tandem with a biotinylated detection antibody and the 50 μL sample size. Ramachandran et al. reported a 2DPN achieving a LOD of 6.5 ng/mL using 10 μL of spiked FBS sample employing the same capture and detection antibody and the same colorimetric substrate reported here8. Increasing the sample size would have maximally improved the LOD to 1.3 ng/mL. The additional improvement in limit-of-detection is attributable to the streptavidin-biotin detection system. A 50 μL plasma sample size is obtainable from a finger prick using a high-flow lancet, e.g. the BD Microtainer Contact-Activated Blue Lancet which provides up to 500 μL of blood per single puncture18.
CONCLUSIONS
We have demonstrated an extended 2DPN capable of delivering the sample and five reagents sequentially with a limit-of-detection equivalent to the Cellabs CELISA Malaria Antigen Kit. Improvements for this device could be made by incorporating dry reagent storage7,8 and a plasma separating membrane, allowing for whole blood analysis. Because of the ease of biotinylation, the device can be readily modified for the detection of other antigens that require a highly sensitive point-of-care test. The improvement LOD comes at the cost of increased assay time. However, for assays in which clinically necessary LOD cannot be achieved by conventional lateral flow tests, this method offers a novel point-of-care strategy.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA186132. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1450681
This material is based upon work supported by the Howard Hughes Medical Institute Summer Fellows Medical Program.
References
- 1.Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Anal Chem. 2010;82(1):3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 2.Ngom B, Guo Y, Wang X, Bi D. Analytical and Bioanalytical Chemistry. 2010:1113–1135. doi: 10.1007/s00216-010-3661-4. [DOI] [PubMed] [Google Scholar]
- 3.Fu E, Lutz B, Kauffman P, Yager P. Lab Chip. 2010;10(7):918–920. doi: 10.1039/b919614e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu E, Kauffman P, Lutz B, Yager P. Sensors Actuators B Chem. 2011;149(1):325–328. doi: 10.1016/j.snb.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu E, Liang T, Houghtaling J, Ramachandran S, Ramsey Sa, Lutz B, Yager P. Anal Chem. 2011;83(20):7941–7946. doi: 10.1021/ac201950g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu E, Liang T, Spicar-Mihalic P, Houghtaling J, Ramachandran S, Yager P. Anal Chem. 2012;84(10):4574–4579. doi: 10.1021/ac300689s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridley GE, Le H, Yager P. Anal Chem. 2014;86(13):6447–6453. doi: 10.1021/ac500872j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandran S, Fu E, Lutz B, Yager P. Analyst. 2014:1456–1462. doi: 10.1039/c3an02296j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heseltine E, editor. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: Round 5 (2013) WHO Press; 2014. [Google Scholar]
- 10.World malaria report 2014. WHO Press; 2014. [Google Scholar]
- 11.Beadle C, Long GW, Weiss WR, McElroy PD, Maret SM, Oloo aJ, Hoffman SL. Lancet. 1994;343(8897):564–568. doi: 10.1016/s0140-6736(94)91520-2. [DOI] [PubMed] [Google Scholar]
- 12.Butterworth AS, Robertson AJ, Ho M-F, Gatton ML, McCarthy JS, Trenholme KR. Malar J. 2011;10:95. doi: 10.1186/1475-2875-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marquart L, Butterworth A, McCarthy JS, Gatton ML. Malar J. 2012;11(1):74. doi: 10.1186/1475-2875-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PATH. [accessed Jan 15, 2016];Malaria Diagnostics Technology Landscape: Enzyme-Linked Immunosorbent Assays (ELISA) for Histidine-Rich Protein 2. http://sites.path.org/dx/files/2012/11/HRP-2-landscape_FINAL_forwebsite.pdf.
- 15.Diamandis EP, Christopoulos TK. Clin Chem. 1991;37(5):625–636. [PubMed] [Google Scholar]
- 16.Green M. Methods Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- 17.Noedl H, Yingyuen K, Laoboonchai A, Fukuda M, Sirichaisinthop J, Miller RS. Am J Trop Med Hyg. 2006;75(6):1205–1208. [PubMed] [Google Scholar]
- 18. [accessed Jul 10, 2015];BD Microtainer Contact-Activated Lancet. http://www.bd.com/vacutainer/pdfs/vs7573.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.