Abstract
Objective
The rapid worldwide rise in incidence of human papillomavirus (HPV)-positive oropharyngeal squamous cell carcinoma (OPSCC) has generated studies confirming this disease as an entity distinct from traditional OPSCC. Based on pathology, surgical studies have revealed prognosticators specific to HPV-positive OPSCC. The current AJCC/UICC staging and pathologic nodal (pN)-classification do not differentiate for survival, demonstrating the need for new, HPV-specific OPSCC staging. The objective of this study was to define a pathologic staging system specific to HPV-positive OPSCC.
Methods
Data were assembled from a surgically-managed, p16-positive OPSCC cohort (any T, any N, M0) of 704 patients from five cancer centers. Analysis was performed for a) the AJCC/UICC pathologic staging, b) newly published clinical staging for non-surgically managed HPV-positive OPSCC and c), a novel, pathology-based, “HPVpath” staging system that combines features of the primary tumor and nodal metastases.
Results
A combination of AJCC/UICC pT-classification and pathology-confirmed metastatic node count (<4 versus ≥5) yielded three groups, stages I (pT1-T2, ≤ 4 nodes), II (pT1-T2, ≥ 5 nodes; pT3-T4, ≤ 4 nodes), and III (pT3-T4, ≥ 5 nodes), with incrementally worse prognosis (Kaplan-Meier overall survival of 90%, 84% and 48% respectively). Existing AJCC/UICC pathologic staging lacked prognostic definition. Newly published HPV-specific clinical stagings from non-surgically managed patients, although prognostic, showed lower precision for this surgically managed cohort.
Conclusions
Three loco-regional “HPVpath” stages are identifiable for HPV-positive OPSCC, based on a combination of AJCC/UICC primary tumor pT-classification and metastatic node count. A workable, pathologic staging system is feasible to guide prognosis and adjuvant therapy decisions in surgically-managed HPV-positive OPSCC.
INTRODUCTION
It is well-recognized that an increasing proportion of oropharyngeal squamous cell carcinoma (OPSCC) are associated with the human papillomavirus (HPV).[1] The population demographics, patient comorbidity, clinical presentation, histopathology, molecular biology and prognosis of HPV-positive OPSCC (identified by its surrogate immunohistochemical marker, p16-positivity or by a combination of p16 and HPV-specific test positivity) differ markedly from those of p16-negative, tobacco-related OPSCC.[2]
While surgery and (chemo)radiation-based treatment approaches to OPSCC are effective, no prospective clinical trials compare one approach with the other and both are endorsed by National Comprehensive Cancer Network (NCCN).[3] Single,[4–10] and multi-institutional[11,12] cohort studies support surgical approaches, including minimally invasive, transoral laser microsurgical (TLM) and more recently, transoral robotic surgical (TORS) pharyngectomy, combined with neck dissection for primary management of OPSCC, without or with adjuvant therapy. Transoral surgical studies demonstrate oncologic outcomes and functional recovery comparable to those of non-surgical approaches for OPSCC, and the need for radiation or chemotherapy is obviated for a proportion of patients.11 These studies also document the improved prognosis of HPV-positive, p16-overexpressing OPSCC compared to non-HPV-positive OPSCC.[5,13] Pathologic data on HPV-positive OPSCC from primary tumor and neck surgical specimens emerged from these studies. The American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) pathologic T-classification (pT) proved prognostic, but the pathologic N-classification (pN) was a poor discriminator.[14] The presence and size of nodal deposits, AJCC/UICC pN-classification and extranodal extension (ENE) had no prognostic impact in p16-positive OPSCC.[5,11,14–21] The prognostic association of AJCC/UICC pT-classification[14] is comparable to that of clinical T-classification in radiation-treated cohorts of HPV-positive OPSCC.[22] New clinical staging systems[23,24] have been proposed via modification of the existing AJCC/UICC T- and N-classifications based on HPV-positive OPSCC patients receiving radiation-based treatment. Additional risk-categorization that incorporates age and smoking has also been proposed.[25] The need to supplant the AJCC/UICC staging for HPV-positive OPSCC is also supported by national database studies.[26]
An important goal of staging and risk-stratification in surgically-managed HPV-positive OPSCC is the avoidance or minimization of potential toxicities from adjuvant therapy.[27] Disparity between the clinical AJCC/UICC TNM stage and survival in HPV-positive OPSCC[14,17] speaks to the need for a new staging paradigm. Moreover, there is significant discordance between clinical/radiological and pathologic nodal staging for head and neck carcinoma in general[28] and specifically for HPV-positive OPSCC.[14] Precise pathology-verified staging data from resection specimens are lacking. Disease staging should be prognostic, validated by internal and external cohorts, and readily accomplished from medical records. Prognostic pathologic parameters have been identified in surgical HPV-positive OPSCC cohorts.13–18, 23,27 To test these prognosticators, this study assembled a p16-positive OPSCC database from five centers. The study objectives were to assess:
the prognostic quality of the AJCC/UICC 7th edition pathologic staging system,[30]
the prognostic validity of proposed clinical staging systems[23] when applied to pathology data, and,
to define, if feasible, a pathologic staging system specific to HPV-positive OPSCC. It is essential that any proposed staging system adhere to the TNM approach endorsed by AJCC/UICC.
METHODS
Study design and data sources
The study group combined data from consecutive cases of surgically-managed, HPV-positive OPSCC from five cancer centers, four in the United States (Washington University School of Medicine (WUSM) in St. Louis 1996–2014, Mayo Clinic Rochester 1990–2015, University of Alabama 2004–2012, Memorial Sloan-Kettering Cancer Center 1985–2005) and one in United Kingdom (University of Liverpool 2006–2013). For inclusion in the study, pathology reports from primary tumor resection and neck dissection specimens were required. A de-identified database from each center was submitted to the study headquarters at WUSM, where Institutional Review Board-approved data management and investigation were performed.
Eligibility criteria and Study variables
Eligibility criteria for inclusion of cases from each institutional database were: previously untreated, biopsy-proven OPSCC (any T, any N, M0), p16-positivity on immunohistochemistry (IHC),[5] curative treatment with primary resection and neck dissection ± adjuvant therapy, pathology data and survival status (alive/dead) at last follow-up. p16 IHC was considered positive at four study centers when >70% nuclear and cytoplasmic staining was observed. For one center (WUSM), a cutoff of >70% was also used, although two patients were included with p16 staining >50% because the literature does not definitively support the 70% cutoff over 50%.” For patients from all institutions who had not undergone p16-testing at the time of OPSCC diagnosis, IHC was performed on tissues retrieved from the surgical specimen at the time of primary resection.
Based on the published literature about surgically-treated, HPV-positive OPSCC,[14–19] the pathology data requirements for inclusion in the study were availability of pT-classification, pN-classification, and metastatic lymph node counts. The pathologic data was recorded from the pathology reports at each institution and then sent to the study headquarters at WUSM. Cumulative positive lymph node count was used for patients treated with bilateral dissections. With synchronous primaries, the greater pT was used as the sole index primary for that patient. T4-classification was not further subdivided into T4a or T4b, since no difference in outcomes between the two has been reported in surgical[31] and non-surgical[24,32] studies. Lymph node ratio was not used due to its previously reported non-predictive status.27 Other data sought were clinical T- and N-classification, recurrence, cause of death, ENE, adjuvant treatment, lymphovascular invasion (LVI) and perineural invasion (PNI).
Statistical methods
Overall survival (OS) was the primary outcome and disease-free survival (DFS), the secondary outcome. OS was defined as the probability of survival from the time of surgery to death from any cause and DFS was the probability of survival from surgery to the date of disease recurrence or death. Cases from the combined database were grouped and analyzed using the staging systems to address the objectives detailed above.
We first employed the AJCC/UICC 7th edition OPSCC pathologic stage system. Two newly proposed clinical staging systems, Huang et al (stage I:T1-3,N0-2b; II:T1-3N2c; III:T4 or N3)[23] and International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S, stage I:T1-T2,N0-N2b; II:T1-T2, N2c or T3,N0-N2c; III:T4,any N or any T,N3)[24] were then analyzed, but using pathologic data. Finally, we developed a pathology-derived staging approach, based on HPV-positive OPSCC-specific publications, which had demonstrated prognostication from pT-classification and metastatic node count, [14,17,29] using the conjunctive consolidation (CC) method.[33] CC combines information from significant prognostic variables and consolidates this information into fewer, similarly prognostic groups. As the outcome of interest was 5-year OS, only cases with follow-up of five years were included.
CC analysis resulted in the creation of three stage groupings based on combinations of pT-classification (early, pT1-T2 versus advanced, pT3-T4) and metastatic node count with a cut-off of 4 positive nodes (pN1 for ≤ 4 nodes versus pN2 ≥ 5 nodes). Analogous to the overall TNM stage, CC analysis derived three stage groups (termed as HPVpath stages):
HPVpath stage I: pT1 or T2 with ≤ 4 nodes
HPVpath stage II: pT1 or T2 with ≥ 5 nodes; pT3 or pT4 with ≤ 4 nodes
HPVpath stage III: pT3 or pT4 with ≥ 5 nodes
Kaplan-Meier analysis was performed to compute the OS with 95% confidence intervals (CI) for each of the staging systems under investigation. Multivariate Cox proportional hazard (PH) analysis was performed to control for confounders including other prognosticators, center effect and study period. In addition, c-index was determined to measure the discriminatory power across the (tested) staging systems, and between the prognostic variables of pT-classification and metastatic node count.[34] All tests were 2-tailed and were evaluated at alpha level of 0.05. Data were analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC), SPSS (Version 22.0. Armonk, NY) and Stata Statistical Software (Release 13. College Station, TX:StataCorp LP).
RESULTS
The five study centers (1–5) submitted data for 812 patients of which 704 fulfilled all eligibility criteria (Fig. 1). Table 1 shows pertinent demographic, treatment, and pathology data. Median follow-up period for the 704 patients was 43.9 (minimum=10, maximum=227) months. Majority (81%) were pT1-T2. A small proportion (6%) was pN0, with the modal AJCC/UICC pN-classification being N2b (48%). HPVpath stages I, II and III were recorded in 493 (70%), 172 (24%) and 39 (6%) patients respectively.
Figure 1. Flow diagram depicting study flow.
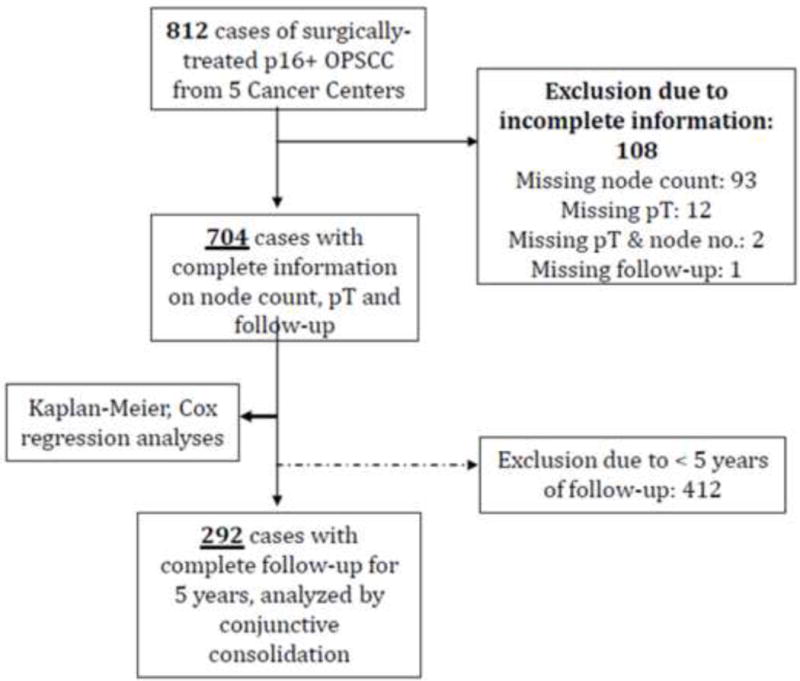
OPSCC: Oropharyngeal squamous cell carcinoma
TABLE 1.
Description of Study population and Hazards ratio from Cox univariate analysis for Overall Survival
| n (%) | HR | p-value | |
|---|---|---|---|
|
| |||
| Center | |||
| 1 | 264 (37) | 1 | ref |
| 2 | 56 (8) | 2.31 (1.27–4.20) | 0.006 |
| 3 | 98 (14) | 0.64 (0.25–1.66) | 0.355 |
| 4 | 98 (14) | 1.48 (0.73–3.02) | 0.271 |
| 5 | 188 (27) | 1.53 (0.86–2.70) | 0.145 |
| Age (years) | |||
| Mean (Standard Deviation) | 57 (9.7) | 1.05 (1.03–1.07) | <0.001 |
| Sex | |||
| Male | 589 (84) | 1 | ref |
| Female | 115 (16) | 1.67 (0.88–2.44) | 0.142 |
| Smoking* | |||
| Never | 230 (45) | 1 | ref |
| Former | 171 (34) | 1.92 (1.08–3.41) | 0.026 |
| Current | 107 (21) | 2.49 (1.35–4.61) | 0.004 |
| Subsite | |||
| Tonsil | 414 (59) | 1 | Ref |
| Base of tongue | 276 (39) | 1.15 (0.75–1.76) | 0.528 |
| Other§ | 14 (2) | 1.75 (0.42–7.23) | 0.440 |
| Extranodal extension (pN> 0) | |||
| Negative | 233 (35) | 1 | Ref |
| Positive | 427 (65) | 1.61 (0.98–2.63) | 0.060 |
| AJCC pathological stage | |||
| 1 | 10 (1) | 1 | Ref |
| 2 | 24 (3) | 0.42 (0.03–6.64) | 0.534 |
| 3 | 95 (14) | 0.68 (0.08–5.44) | 0.714 |
| 4 | 575 (82) | 1.28 (0.18–9.22) | 0.806 |
| Pathological T-classification | |||
| 1 | 279 (40) | 1 | Ref |
| 2 | 290 (41) | 1.77 (1.03–3.05) | 0.039 |
| 3 | 92 (13) | 2.67 (1.38–5.16) | 0.004 |
| 4 | 43 (6) | 5.69 (2.67–11.31) | <0.001 |
| Pathological N-classification | |||
| 0 | 44 (6) | Ref | |
| 1 | 91 (13) | 0.89 (0.23–3.47) | 0.870 |
| 2a | 141 (20) | 1.24 (0.35–4.40) | 0.737 |
| 2b | 337 (48) | 1.89 (0.59–6.08) | 0.285 |
| 2c | 52 (7) | 3.56 (1.01–12.62) | 0.049 |
| 3 | 39 (6) | 2.70 (0.64–11.31) | 0.174 |
| Positive Lymph nodes | |||
| ≤4 | 589 (84) | 1 | Ref |
| ≥5 | 115 (16) | 2.93 (0.21–0.53) | <0.001 |
| Margins* | |||
| Negative | 543 (90) | 1 | Ref |
| Positive | 61 (10) | 1.55 (0.89–2.71) | 0.123 |
| Perineural Invasion* | |||
| Negative | 367 (86) | 1 | Ref |
| Positive | 60 (14) | 3.33 (1.92–5.81) | <0.001 |
| Lymphovascular Invasion* | |||
| Negative | 307 (74) | 1 | Ref |
| Positive | 109 (26) | 1.92 (1.14–3.23) | 0.014 |
| Treatment# | |||
| Surgery only | 147 (21) | 1 | Ref |
| Surgery + Radiation | 322 (46) | 0.54 (0.32–0.90) | 0.019 |
| Surgery + Chemoradiation | 233 (33) | 0.49 (0.27–0.87) | 0.014 |
Variables with incompletely recorded data for study cohort.
Other subsites included soft palate in 10, the oropharynx subsite was unlisted in 4 patients.
Adjuvant therapy unknown for two patients, both did not have any recurrence and were alive at last follow-up.
Adjuvant therapy was administered in 79% of cases, with radiation alone in 46% and chemoradiation in 33%. Recurrence occurred in 89 patients (13%), local in 25, regional in 34 and distant in 39 patients. Nine patients developed recurrences at multiple sites. The distant metastasis rate by treatment type was 3.4% (n=5/147) for surgery alone, 4.6% (n=15/322) for surgery plus radiation, and 8.2% (n=19/233) for surgery plus chemoradiation. The 5-year OS for the entire cohort of 704 cases was 86% (95% CI: 82 to 99%).
AJCC/UICC pT-classification
A progressive decline in OS was seen as the pT-classification increased from T1 to T4 (Supplemental Fig. A1). The 569 (81%) pT1 and pT2 cases exhibited a 5-year OS of 93% (95% CI: 85 to 92%) and the 135 (19%) pT3 and pT4 cases had an OS of 73% (95% CI: 64 to 82%).
AJCC/UICC pN-classification
In contrast to pT-classification, increasing AJCC/UICC pN-classification from pN0 to pN3 was not associated with progressive worsening of OS in this surgical cohort (Supplemental Fig. A2). pN0 cases demonstrated poorer survival than N1, and N2c survival was worse than that of N3. pN2c showed slightly poorer survival compared with the other pN-categories (non-significant). The N2c cohort was associated with a significantly higher proportions of pT3-T4 (39%) and ≥5 metastatic nodes (n=21, 40%) than was seen in the overall cohort.
Metastatic node count
High numbers of pathologically-confirmed metastatic nodes correlated significantly with decreased OS; a significant decline was observed for ≥5 nodes. The 5-year OS was 89% for HPVpath pN1 (n=589, 84%) versus 71% for HPVpath pN2 (n=115, 16%). There was no overlap of the 95% CI for OS between HPVpath pN1 and HPVpath pN2. N0 patients demonstrated the same survival as the group with 1–4 nodes, supporting inclusion of cases with 0–4 nodes in a single group (Supplement Fig. A3).
Analyses of the staging systems
(a) AJCC/UICC Staging
Extant AJCC/UICC 7th Edition stage groupings did not demonstrate significantly different OS (Fig. 2).
Figure 2.
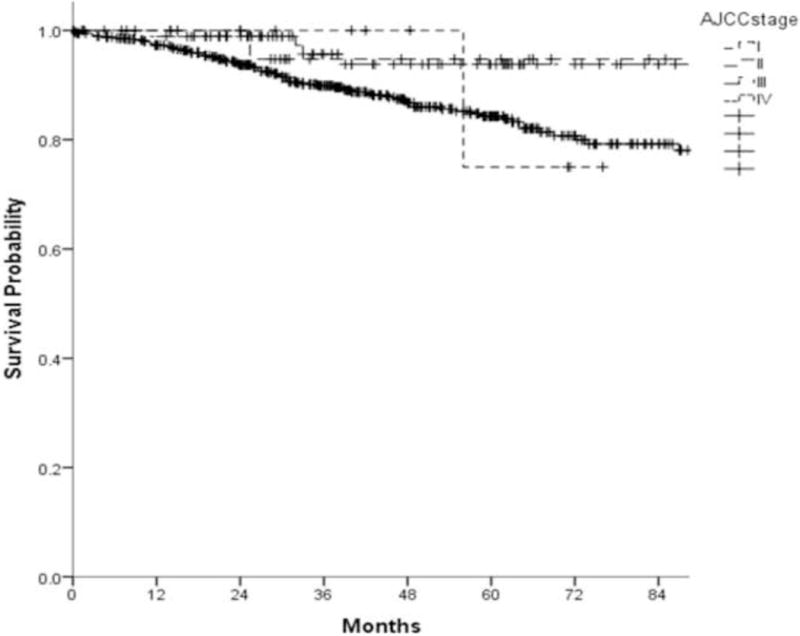
Kaplan-Meier overall survival by AJCC/UICC stage grouping for oropharynx (log rank, p-value=0.230)
(b) Proposed clinical Staging systems[23,24]
Re-arranged clinical AJCC/UICC T- and N-categories developed by Huang et al[23] and applied to our pathologic data demonstrated separation between stage I, with a 5-year OS of 88% (95% CI:84 to 92%) and stages II and III, with respective 5-year OS of 70% (95% CI:45 to 94%) and 71% (95% CI:59 to 83%) (Supplement Fig. A4). Sub-total overlap of the 95% CI for OS estimates was observed for stage II and stage III. This differs from published results in a radiation-treated cohort, using clinical data.[23] Overlap of the 95% CI of OS was also observed with the ICON-S staging[24] (stage I: 90%, 95% CI:87 to 93%; II: 79%, 95% CI:71 to 87%; III: 70%, 95% CI:57 to 83%, Supplement Fig. A5).
(c)“HPVpath” staging
Overall survival
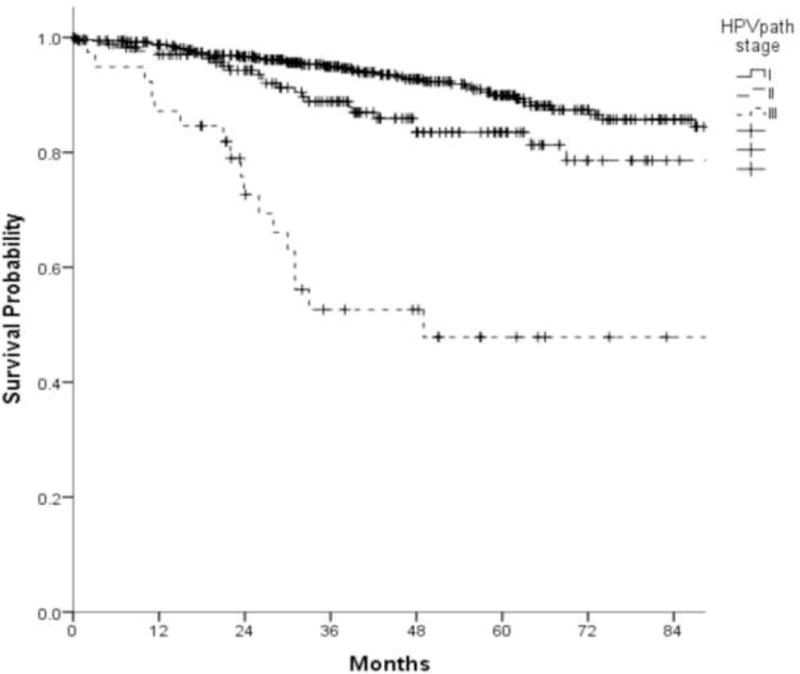
For the CC analysis, 292 cases met criteria of minimum, absolute 5-year follow-up, or earlier death. When categories of AJCC/UICC pT-classification and metastatic node count were cross tabulated (Table 2), three HPVpath stages I, II and III were identified, with 5-year OS estimates of 84% (95% CI: 79 to 89%), 68% (95% CI: 57 to 79%) and 26% (95% CI: 8% to 44%) respectively (Fig. 3A). HPVpath stage III had significantly worse OS than stages I and II, with no overlap of the 95% CI. Stage II showed minimal overlap of the 95% CI with stage I. The distribution of pertinent variables such as age, smoking, pT- and pN-classification, ENE or treatment for the 292 cases within the CC analysis cohort did not differ significantly from the remaining 412 cases.
TABLE 2.
Five-Year Survival as a Function of AJCC pathological T-classification and Number of Nodes Categories (‘HPVpath pN1 ≤ 4 nodes’ and ‘HPVpath pN2 ≥ 5 nodes’).
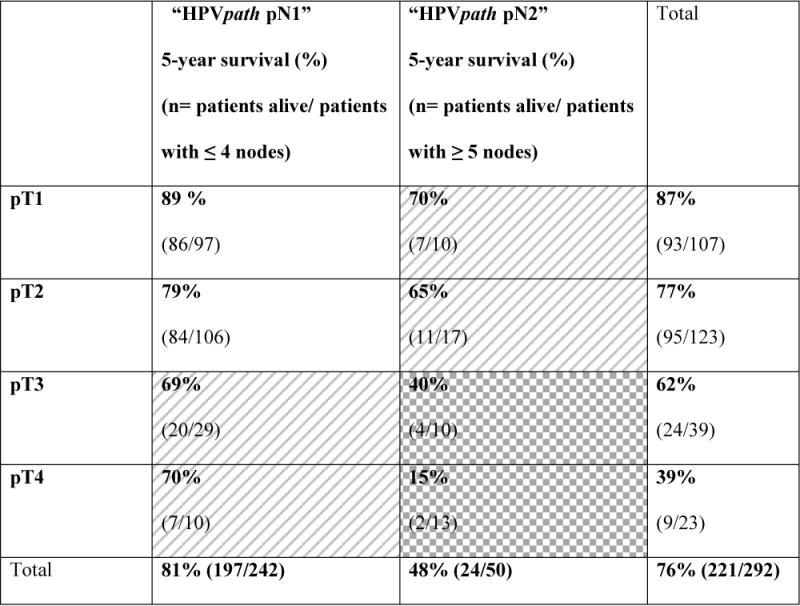
|
White=HPVpath stage I, Diagonal pattern= HPVpath stage II, Grid pattern= HPVpath stage III.
Figure 3.
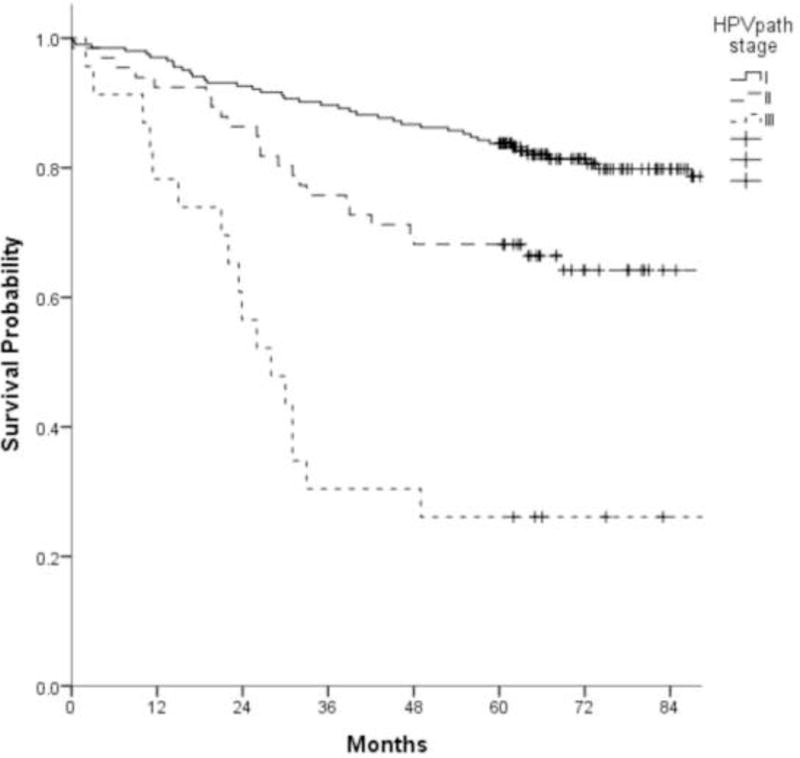
A. Kaplan-Meier overall survival curves of 292 patients used for conjunctive consolidation as a function of HPVpath stages I, II, and III (log rank, p-value<0.001)
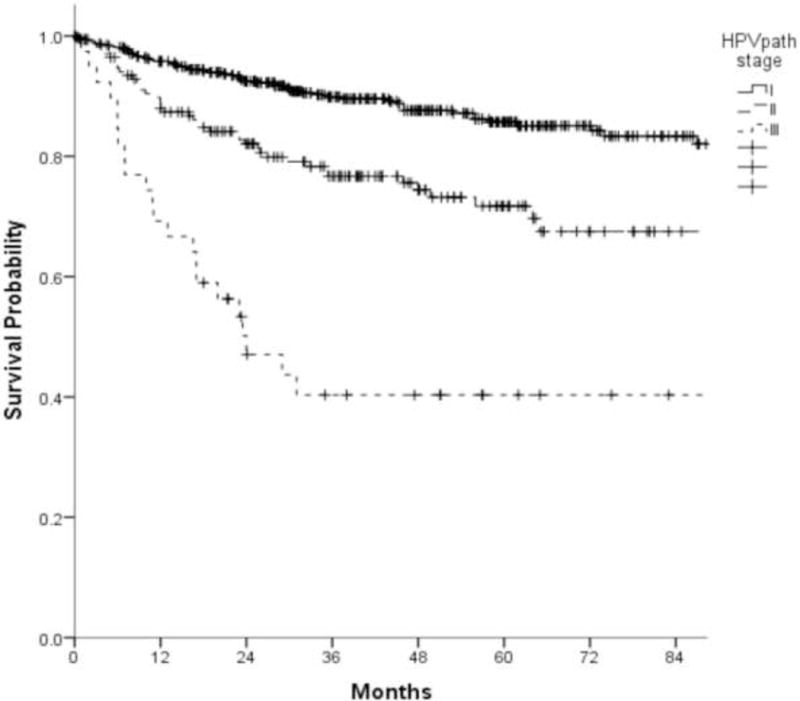
B. Kaplan-Meier overall survival curves of 704 patients in the overall study cohort as a function of HPVpath stages I, II, and III (log rank, p-value<0.001)
C. Kaplan-Meier disease free survival curves of 704 patients as a function of HPVpath I, II, and III (log rank, p-value<0.001)
When Kaplan-Meier analysis was performed for the entire cohort of 704 cases, the prognostic gradient was maintained for the three groups of HPVpath staging. Within this group (n=704), the 5-year OS estimates were 90% (95% CI:87 to 93%), 84% (95% CI:77 to 90%), and 48% (95% CI:30 to 66%) for HPVpath stages I, II and III, respectively (Fig. 3B).
In the multivariable Cox PH analysis, HPVpath stage remained significant after controlling for center, age and adjuvant treatment (Table 3). The c-indices for each of the staging systems in multivariate model risk-adjusted for age, center, and treatment were 0.73 for HPVpath, 0.72 each for Huang[23] and ICON-S[24] staging and 0.68 for AJCC/UICC. The c-indices for each of the three staging systems in multivariate model risk-adjusted for age, center, treatment, smoking, and PNI were 0.78 for HPVpath, 0.75 each for Huang[23] and ICON-S[24] staging, and 0.72 for AJCC/UICC.
TABLE 3.
Multivariate Cox Regression Analysis for Overall Survival with data from all five Centers using HPVpath stages.
| aHR (adjusted hazard ratio) | 95% CI for aHR | p-value | |
|---|---|---|---|
|
| |||
| Center | |||
| 1 | 1 | Ref | |
| 2 | 1.71 | 0.90–3.25 | 0.102 |
| 3 | 0.71 | 0.27–1.76 | 0.483 |
| 4 | 1.73 | 0.83–3.61 | 0.147 |
| 5 | 1.84 | 1.01–3.33 | 0.045 |
|
| |||
| Age | 1.04 | 1.01–1.06 | 0.001 |
|
| |||
| Treatment | |||
| Surgery Only | 1 | Ref | |
| Surgery + Radiation | 0.55 | 0.31–0.95 | 0.033 |
| Surgery + Chemoradiation | 0.46 | 0.24–0.87 | 0.017 |
|
| |||
| HPVpath stage | |||
| I | 1 | Ref | |
| II | 1.59 | 0.95–2.64 | 0.077 |
| III | 6.83 | 3.77–12.38 | <0.001 |
Disease-free survival
When using DFS as the outcome of interest, separation of curves for the three HPVpath stages was similar to that for OS (Fig. 3C), without any overlap of the 95% CI for 5-year DFS estimates (stage I:86%, 95% CI:82 to 90%; II:72%, 95% CI:64 to 79%; III:40%, 95% CI:24 to 56%).
Analysis of “other” prognostic features
Center, age, current smoking and adjuvant therapy were significantly associated with OS at the univariate level. There was no significant difference between adjuvant chemoradiation and adjuvant radiation alone. Of the pathological variables, advanced T-classification, high metastatic node count (≥5), LVI, PNI, and HPVpath staging were prognostic for OS.
AJCC/UICC pN-classification, AJCC/UICC pTNM staging and ENE were not significant. HPVpath staging remained prognostic in multivariate analysis, adjusted for center, adjuvant treatment and age.
DISCUSSION
Analysis of HPV-positive OPSCC cases from five centers, with pathology data derived from primary tumor resection and neck dissection, generated pathologic stage groups unique to HPV-positive OPSCC. By cross-tabulation of AJCC/UICC pT-classification groups (T1-T2 versus T3-T4) with pathology-based, node count groups (pN1 with ≤4 and pN2 with ≥5 metastatic nodes), we observed three groups, with progressive decline in OS using either the CC method or Kaplan-Meier analysis. Significant separation for the three HPVpath groupings was also seen using DFS as the outcome. The HPVpath system identifies a small group (pT3 or T4 combined with ≥ 5 metastatic nodes) that has a particularly poor prognosis, which comprise only 8% of the CC cohort (n=292) and 6% of the entire cohort (n=704).
By contrast, AJCC/UICC 7th Edition pathological stage groups and pN-classification did not predict survival, confirming previous reports.[14,23] The prognostic significance of the AJCC/UICC pT-classification was preserved, with a significant, absolute decline of 33% in the 5-year OS estimate from 93% for pT1 to 60% for pT4-classification cases. AJCC/UICC 7th edition pN-classification showed no difference in OS, with substantial overlap of 5-year OS and 95% CI estimates between various pN-categories, and slightly poorer estimate for the pN2c group. Neither adverse nodal features of AJCC/UICC pN-classification nor ENE have been associated with a poor prognosis in HPV-positive OPSCC in single-institution surgical series.[14–19] This report, using a multi-institutional experience confirms their failure to confer a worse prognosis.
Guidelines, such as NCCN, rely upon pathology-derived risk stratification and staging to direct post-operative adjuvant therapy for OPSCC.[3] AJCC/UICC pN-classification and TNM stages I–IV did not prove prognostic, justifying the application of a new pathologic staging system (and risk-stratification) for HPV-positive OPSCC. Of nodal parameters evaluated, the presence of five or more pathologically-confirmed metastatic nodes was associated with significantly worse prognosis, supporting previous work.[14,17,29] Incorporation of node number in pathologic staging is consistent with the approach at other disease sites traditionally managed by surgery (such as gastric and colorectal cancer), where metastatic node count forms the basis of AJCC/UICC pN-staging.[30]
It was important to analyze the performance of the recently published clinical staging system by Huang et al in our surgical cohort using pathologic data.[23] Huang et al also found poor performance of the existing AJCC/UICC 7th edition in (chemo)radiation-treated patients, and proposed a new clinical classification into three groups from an HPV-positive OPSCC cohort (n=573).[23] This approach was validated in an ICON-S study in which 98% of patients received primary radiation-based treatment.[24] The application of these staging systems[23,24] to surgically-managed patients using pathology data, yielded similar OS estimates for groups I and II but higher survival for group III. In addition, there was significant overlap of 95% CI for groups II and III. The 5-year survivals of 53%[24] and 54%[23] for the Group III, (chemo)radiation-treated cohort versus 70% in the surgically-treated cohort might be explained by better survival when HPV-positive OPSCC pN3 (> 6cm nodal metastasis) patients are managed with primary surgical resection.[35] Reliable estimation of node count for the purpose of clinical staging classification[24] is limited given the lack of concordance between clinical/radiological and pathologic data.[14,28] Accurate enumeration of pathologically-confirmed node metastasis is important for staging of surgically-managed carcinoma as also suggested by O’Sullivan et al.[24] Determination of discrimination power (c-index) amongst the three staging systems yielded the highest value for HPVpath stages followed by the recently proposed clinical stages,[23,24] and the AJCC/UICC Stage,[30] in our cohort with complete pathologic data. This held up in risk-adjusted multivariate models.
Surgical management permits definitive, “gold standard” assessment of the pathologic extent of malignant disease. We therefore propose that the three HPVpath stage groupings be employed for pathologic staging of loco-regional, HPV-positive OPSCC. This approach can be derived from standard pathology reporting, and was validated internally. To complete such staging, HPVpath stage IV can be assigned to patients presenting with distant metastatic disease,[36]concordant with the recently proposed clinical staging systems. Our findings may also be useful for stratification in clinical trials of adjuvant treatment for HPV-positive OPSCC.[13] HPVpath stage III cases represent logical candidates for testing novel adjuvant therapy approaches. Standard adjuvant chemoradiation was not associated with significant benefit over radiation alone in this monograph, supporting previous reports on HPV-positive OPSCC.[14,15,17,20,29]
The chief limitation of this work is the inevitable case selection for surgical resection. The absence of external validation, as a limitation, can be addressed through analyzing the results of other institutions according to the HPVpath stages, to assess their applicability beyond the five centers included in this study.
CONCLUSION
This study describes a pathologic staging system for HPV-positive OPSCC that associates increasing extent of loco-regional disease with significant decline in prognosis. The system is based on the existing AJCC/UICC pT-classification, cross-tabulated with cohorts who have either ≤4 or ≥5 pathologically-confirmed, metastatic neck nodes. The system employs pathology-based categorization resulting from primary surgical management and is complementary to recently published clinical staging systems[23,24] for HPV-positive OPSCC. The three HPVpath stages described here can be abstracted from standard pathology reports and are recommended for use in future studies, clinical trial stratification and consideration by cancer staging organizations such as AJCC/UICC.
Supplementary Material
Figure 4.
HIGHLIGHTS.
Multi-institutional surgical cohort of 704, p16-positive oropharynx cancer cases
Existing AJCC/UICC pathologic staging lacked prognostic definition
3 “HPVpath” stages identified using pT-classification and node count (≤ 4 vs ≥ 5)
“HPVpath” Stages were I (T1-T2, ≤ 4), II (T1-T2,≥ 5; T3-T4,≤ 4), and III (T3-T4,≥5)
Acknowledgments
Funding: No funding received.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest Statement
None of the co-authors had any conflicts of interest which could have affected the current study.
References
- 1.Chaturvedi AK, Engels Ea, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744–54. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ, et al. Head and Neck Cancers, Version 1. 2015. J Natl Compr Canc Netw. 2015;13:847–55. doi: 10.6004/jnccn.2015.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant DG, Salassa JR, Hinni ML, Pearson BW, Perry WC. Carcinoma of the tongue base treated by transoral laser microsurgery, part one: Untreated tumors, a prospective analysis of oncologic and functional outcomes. Laryngoscope. 2006;116:2150–5. doi: 10.1097/01.mlg.0000244159.64179.f0. [DOI] [PubMed] [Google Scholar]
- 5.Rich JT, Milov S, Lewis JS, Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) +/− adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119:1709–19. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panje WR, Scher N, Karnell M. Transoral carbon dioxide laser ablation for cancer, tumors, and other diseases. Arch Otolaryngol Head Neck Surg. 1989;115:681–8. doi: 10.1001/archotol.1989.01860300035012. [DOI] [PubMed] [Google Scholar]
- 7.Steiner W, Fierek O, Ambrosch P, Hommerich CP, Kron M. Transoral laser microsurgery for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg. 2003;129:36–43. doi: 10.1001/archotol.129.1.36. [DOI] [PubMed] [Google Scholar]
- 8.Williams CE, Kinshuck AJ, Derbyshire SG, Upile N, Tandon S, Roland NJ, et al. Transoral laser resection versus lip-split mandibulotomy in the management of oropharyngeal squamous cell carcinoma (OPSCC): a case match study. Eur Arch Otorhinolaryngol. 2014;271:367–72. doi: 10.1007/s00405-013-2501-5. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MA, Weinstein GS, O’Malley BW, Feldman M, Quon H. Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck. 2011;33:573–80. doi: 10.1002/hed.21500. [DOI] [PubMed] [Google Scholar]
- 10.Moore EJ, Olsen SM, Laborde RR, García JJ, Walsh FJ, Price DL, et al. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin Proc. 2012;87:219–25. doi: 10.1016/j.mayocp.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haughey BH, Hinni ML, Salassa JR, Hayden RE, Grant DG, Rich JT, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck. 2011;33:1683–94. doi: 10.1002/hed.21669. [DOI] [PubMed] [Google Scholar]
- 12.De Almeida JR, Li R, Magnuson JS, Smith RV, Moore E, Lawson G, et al. Oncologic Outcomes After Transoral Robotic Surgery : A Multi-institutional Study. JAMA Otolaryngol Head Neck Surg. 2015;141:1043–51. doi: 10.1001/jamaoto.2015.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adelstein DJ, Ridge JA, Brizel DM, Holsinger FC, Haughey BH, O’Sullivan B, et al. Transoral resection of pharyngeal cancer: Summary of a National Cancer Institute Head and Neck Cancer Steering Committee Clinical Trials Planning Meeting, November 6–7, 2011, Arlington, Virginia. Head Neck. 2012;34:1681–1703. doi: 10.1002/hed.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. 2012;122(Suppl):S13–33. doi: 10.1002/lary.23493. [DOI] [PubMed] [Google Scholar]
- 15.Sinha P, Lewis JS, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012;118:3519–30. doi: 10.1002/cncr.26671. [DOI] [PubMed] [Google Scholar]
- 16.Klozar J, Koslabova E, Kratochvil V, Salakova M, Tachezy R. Nodal status is not a prognostic factor in patients with HPV-positive oral/oropharyngeal tumors. J Surg Oncol. 2013;107:625–33. doi: 10.1002/jso.23292. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell JH, Ferris RL, Gooding W, Cunningham D, Mehta V, Kim S, et al. Extracapsular spread in head and neck carcinoma: Impact of site and human papillomavirus status. Cancer. 2013:1–7. doi: 10.1002/cncr.28169. [DOI] [PubMed] [Google Scholar]
- 18.Kaczmar JM, Tan KS, Heitjan DF, Lin A, Ahn PH, Newman JG, et al. HPV-related oropharyngeal cancer: risk factors for treatment failure in patients managed with primary surgery (TORS) Head Neck. 2016;38:59–65. doi: 10.1002/hed.23850. [DOI] [PubMed] [Google Scholar]
- 19.Iyer NG, Dogan S, Palmer F, Rahmati R, Nixon IJ, Lee N, et al. Detailed Analysis of Clinicopathologic Factors Demonstrate Distinct Difference in Outcome and Prognostic Factors Between Surgically Treated HPV-Positive and Negative Oropharyngeal Cancer. Ann Surg Oncol. 2015;22:4411–21. doi: 10.1245/s10434-015-4525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar B, Cipolla MJ, Old MO, Brown NV, Kang SY, Dziegielewski PT, et al. Surgical management of oropharyngeal squamous cell carcinoma: Survival and functional outcomes. Head Neck. 2016;38(Suppl 1):E1794–1802. doi: 10.1002/hed.24319. [DOI] [PubMed] [Google Scholar]
- 21.Wilkie MD, Upile NS, Lau AS, Williams SP, Sheard J, Helliwell TR, et al. Transoral laser microsurgery for oropharyngeal squamous cell carcinoma: A paradigm shift in therapeutic approach. Head Neck. 2016 doi: 10.1002/hed.24432. [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, et al. Deintensification Candidate Subgroups in Human Papillomavirus-Related Oropharyngeal Cancer According to Minimal Risk of Distant Metastasis. J Clin Oncol. 2013;31:543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 23.Huang SH, Xu W, Waldron J, Siu L, Shen X, Tong L, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol. 2015;33:836–45. doi: 10.1200/JCO.2014.58.6412. doi:10. [DOI] [PubMed] [Google Scholar]
- 24.O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 25.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane FK, Chen Y-H, Neville BA, Tishler RB, Schoenfeld JD, Catalano PJ, et al. Changing prognostic significance of tumor stage and nodal stage in patients with squamous cell carcinoma of the oropharynx in the human papillomavirus era. Cancer. 2015;121:2594–602. doi: 10.1002/cncr.29402. [DOI] [PubMed] [Google Scholar]
- 27.Dziegielewski PT, Teknos TN, Durmus K, Old M, Agrawal A, Kakarala K, et al. Transoral Robotic Surgery for Oropharyngeal Cancer: Long-term Quality of Life and Functional Outcomes. JAMA Otolaryngol Head Neck Surg. 2013;139:1099–1108. doi: 10.1001/jamaoto.2013.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch WM, Ridge JA, Forastiere A, Manola J. Comparison of clinical and pathological staging in head and neck squamous cell carcinoma: results from Intergroup Study ECOG 4393/RTOG 9614. Arch Otolaryngol Head Neck Surg. 2009;135:851–8. doi: 10.1001/archoto.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha P, Kallogjeri D, Gay H, Thorstad WL, Lewis JS, Chernock R, et al. High metastatic node number, not extracapsular spread or N-classification is a node-related prognosticator in transorally-resected, neck-dissected p16-positive oropharynx cancer. Oral Oncol. 2015;51:514–20. doi: 10.1016/j.oraloncology.2015.02.098. [DOI] [PubMed] [Google Scholar]
- 30.American Joint Committee on Cancer (AJCC) Staging Manual. 7. New York, NY: Springer; 2009. [Google Scholar]
- 31.Zenga J, Wilson M, Adkins DR, Gay HA, Haughey BH, Kallogjeri D, et al. Treatment Outcomes for T4 Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141:1118–27. doi: 10.1001/jamaoto.2015.0764. [DOI] [PubMed] [Google Scholar]
- 32.Garden AS, Kies MS, Morrison WH, Weber RS, Frank SJ, Glisson BS, et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol. 2013;8:21. doi: 10.1186/1748-717X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccirillo JF, Wells CK, Sasaki CT, Feinstein AR. New clinical severity staging system for cancer of the larynx. Five-year survival rates. Ann Otol Rhinol Laryngol. 1994;103:83–92. doi: 10.1177/000348949410300201. [DOI] [PubMed] [Google Scholar]
- 34.Harrel FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer-Verlag; 2001. (Series in Statistics). [Google Scholar]
- 35.Zenga P, Haughey BH, Jackson RS, et al. Treatment outcomes for N3 disease from HPV-related Oropharyngeal Squamous Cell Carcinoma; American Head and Neck Society 9th International Conference on Head and Neck Cancer, Abstract ID 75445; Seattle, WA. Jul 16–20, 2016. [Google Scholar]
- 36.Sinha P, Thorstad WT, Nussenbaum B, Haughey BH, Adkins DR, Kallogjeri D, et al. Distant metastasis in p16-positive oropharyngeal squamous cell carcinoma: a critical analysis of patterns and outcomes. Oral Oncol. 2014;50:45–51. doi: 10.1016/j.oraloncology.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


