Abstract
Background
Vasoplegic syndrome, defined by hypotension despite normal or increased cardiac output, is associated with high mortality after cardiopulmonary bypass. Methylene blue (MB) is reported to ameliorate vasoplegic syndrome through the nitric oxide pathway. We hypothesized that early administration of MB would improve outcomes in patients with vasoplegic syndrome after cardiopulmonary bypass.
Methods
All patients that underwent cardiopulmonary bypass at our institution (1/1/11–6/30/16) were identified through our Society of Thoracic Surgery database. Pharmacy records identified patients receiving MB within 72 hours of cardiopulmonary bypass. Multivariate logistic regression identified predictors of major adverse events among patients receiving MB.
Results
A total of 118 (3.3%) cardiopulmonary bypass patients received MB for vasoplegic syndrome. These patients had a higher incidence of comorbidities and cases were more commonly reoperative (76.1 vs 41.2%, p<0.0001) and complex (70.3 vs 31.8%, p<0.0001). The only difference in preoperative medications was that MB patients had a higher rate of amiodarone use (15.3 vs 2.2%, p<0.0001). MB patients had significantly higher rates of postoperative complications, except atrial fibrillation. Early (operating room, 40.7%) versus late (intensive care unit, 59.3%) administration of MB was associated with significantly reduced operative mortality (10.4 vs 28.6%, p=0.018) and risk-adjusted major adverse events (OR 0.35, p=0.037).
Conclusions
Operative mortality is high in patients receiving methylene blue for the treatment of vasoplegia after cardiopulmonary bypass. Early administration of methylene blue improves survival and reduces the risk-adjusted rate of major adverse events in these patients.
Classifications: Cardiac Surgery, Cardiopulmonary Bypass, Methylene Blue, Vasoplegia
Over the last 20 years, there has been an increase in the incidence of vasoplegic syndrome, which affects over 5% of patients undergoing cardiopulmonary bypass (CPB) and is associated with a mortality rate approaching 25% (1–3). Standard treatment options for severe refractory vasoplegic syndrome are extremely limited and include vasopressor support and systemic corticosteroids.(3–5) With the increasing incidence of vasoplegic syndrome, the use of alternative treatment options such as methylene blue (MB) has also increased. MB has demonstrated beneficial effects on the severe vasodilation associated with vasoplegic syndrome(6–8).
MB is a potent inhibitor of nitric oxide (NO) synthase in vascular endothelial cells; resulting in decreased NO release and increased systemic vascular resistance (SVR)(9). There have been a number of retrospective studies examining the use of this drug in the treatment of vasoplegic syndrome with mixed results regarding efficacy(6, 10, 11). Two prospective, randomized trials evaluated MB administration at the onset of cardiopulmonary bypass (CPB) in 30 and 100 patients with risk factors for vasoplegic syndrome and demonstrated safety in the CPB population(12, 13). Despite promising results, significant criticism has focused on the fact that neither trial selected for patients with the clinical diagnosis of vasoplegic syndrome. Additionally, several animal studies evaluated methylene blue’s mechanism of action and biochemical pathways for resolution of vasoplegia. These demonstrated a time dependent decrease in NO synthase production, improved microvascular function, and decreased transendothelial inflammatory cell migration(14–17).
Currently MB does not have any U.S. Food and Drug Administration (FDA) approved indications but is most commonly used for treatment of methemoglobinemia. The purpose of this study is to determine the incidence of CPB induced vasoplegic syndrome and describe contemporary practice patterns of MB use at an academic cardiac surgery center. We hypothesized that early administration of MB would reduce the risk of morbidity and mortality in patients with vasoplegic syndrome.
Patients and Methods
Patients
This study was approved the University of Virginia Institutional Review Board with waiver of informed consent (IRB Protocol # 19247). All patients receiving MB within 72 hours of CPB at our institution between January 2011 and March 2016 were identified. Our Society of Thoracic Surgeons (STS) database prospectively collects preoperative characteristics, intraoperative details, and postoperative outcomes through 30-days for all patients undergoing cardiac surgery at our institution. These records were linked with all episodes of MB administration recorded in our institutional pharmacy database. Additionally the Clinical Data Repository (CDR) was queried which includes laboratory values, cost data, and detailed hemodynamic data for all inpatient hospitalizations. Finally, long-term survival data was collected from the Social Security Death Master File.
Post CPB Vasoplegia University of Virginia MB Protocol
Since 2011, the Division of Thoracic and Cardiovascular Surgery at our institution has used a consensus approach to the management of post-CPB vasoplegic syndrome. Diagnosis includes vasodilation characterized by low SVR with elevated CI resulting in hypotension despite high doses of vasopressors on pump or postoperatively. Standard vasopressor support is used with a preference for vasopressin and norepinephrine to combat vasodilation with phenylephrine used as a second line therapy. Given the common hyperdynamic cardiac function during vasoplegic syndrome with cardiac indices typically above 4.0 L/min/m2, epinephrine is used sparingly. Additionally, after clinical diagnosis of vasoplegic syndrome, patients receive stress-dose steroids, diphenhydramine and famotidine to target the systemic inflammatory response. Our MB administration protocol for patients with refractory vasoplegic syndrome was a bolus dose of 2 mg/kg of intravenous MB followed by 12-hour infusion at 0.5mg/kg/hr. This protocol was in place for all patients during the study period.
Statistical Methods
The primary outcome was major adverse event (MAE) after administration of MB for vasoplegic syndrome. MAE included the STS major morbidities (permanent stroke, renal failure, reoperation, deep sternal wound infection, and prolonged ventilation) as well as operative mortality (in-hospital or 30-day). Standard STS definitions were utilized for all variables including new onset renal failure and prolonged ventilation (>24 hours). Additionally, preoperative and intraoperative variables associated with MB treatment for vasoplegic syndrome were assessed. Early administration was defined as operating room while patients not receiving MB until reaching the postoperative intensive care unit were classified as late administration. Univariate analysis was performed using Chi squared and Mann Whitney-U for categorical and continuous variables respectively. Multivariable logistic regression was used to identify risk-adjusted predictors of MAE in patients receiving MB for vasoplegic syndrome. SAS version 9.4 (SAS Institute, Cary, NC) statistical software was used for analysis with a statistical threshold for significance set at 0.05.
Results
Vasoplegic Population
A total of 3,608 patients underwent cardiopulmonary bypass during the study period, of whom 118 (3.3%) received MB for the treatment of vasoplegic syndrome. Patients receiving MB were more likely younger (63 vs. 67 years, p=0.031) and had higher rates of medical comorbidities, especially heart failure (74.6 vs. 49.4%, p<0.0001) (Table 1). Preoperative amiodarone use was higher (15.3 vs. 2.2%, p<0.0001), with no difference in ACE-inhibitor use within 48 hours of surgery (11.0 vs. 11.4%, p=0.896). Patients receiving MB for vasoplegic syndrome had significantly higher rates of all STS major morbidities as well as operative mortality (Table 2).
Table 1.
Characteristics of Patients Receiving methylene blue (MB) for vasoplegic syndrome
| Variable | MB | No MB | p-value |
|---|---|---|---|
| Number of patients | 118 (3.3%)1 | 3490 (96.7%) | - |
| Isolated Coronary Artery Bypass and/or Valve | 35 (29.6%) | 2381 (68.2) | <0.0001 |
| Ventricular Assist Device | 40 (33.9%) | 130 (3.7%%) | <0.0001 |
| Prior Ventricular Assist Device | 32 (27.1%) | 28 (0.1%) | <0.0001 |
| Age | 63 [58, 71]2 | 67 [57, 75] | 0.031 |
| Male Sex | 81 (69.2%) | 2334 (67.7%) | 0.720 |
| Tobacco Use | 46 (39.3%) | 785 (22.8%) | <0.0001 |
| Heart Failure Diagnosis | 88 (74.6%) | 1740 (49.9%) | <0.0001 |
| Dialysis Dependent Renal Failure | 7 (6.9%) | 94 (2.6%) | 0.036 |
| Diabetes Mellitus | 46 (39.0%) | 3488 (36.5%) | 0.586 |
| Severe Chronic Lung Disease | 10 (8.6%) | 143 (4.1%) | 0.008 |
| Hypertension | 77 (65.8%) | 2646 (76.7%) | 0.007 |
| Ejection Fraction | 42 [20, 58] | 57 [45, 63] | <0.0001 |
| Preoperative Amiodarone Therapy | 18 (15.3%) | 78 (2.2%) | <0.0001 |
| ACE-Inhibitor within 48 hours | 13 (11.0%) | 398 (11.4%) | 0.896 |
| Prior Cardiac Surgery | 89 (76.1%) | 1435 (41.2%) | <0.0001 |
| Cross Clamp Time | 100 [79, 126] | 75 [58, 96] | <0.0001 |
n (%), all such values
median [interquartile range], all such values
Table 2.
Outcomes for Patients Receiving methylene blue (MB) for vasoplegic syndrome
| Variable | MB | No MB | p-value |
|---|---|---|---|
| Postoperative Atrial Fibrillation | 27 (22.9%) | 725 (20.8%) | 0.579 |
| Stroke | 6 (5.1%) | 71 (2.0%) | 0.025 |
| Renal Failure | 25 (21.2%) | 151 (4.3%) | <0.0001 |
| Deep Sternal Wound Infection | 1 (0.9%) | 2 (0.1%) | 0.003 |
| Reoperation | 31 (26.3%) | 222 (6.4%) | <0.0001 |
| Prolonged Ventilation | 27 (22.9%) | 232 (6.7%) | <0.0001 |
| Operative Mortality | 25 (21.2%) | 112 (3.2%) | <0.0001 |
| Major Adverse Event Composite | 55 (46.6%) | 516 (14.8%) | <0.0001 |
n (%), all such values
median [interquartile range], all such values
Methylene Blue Outcomes
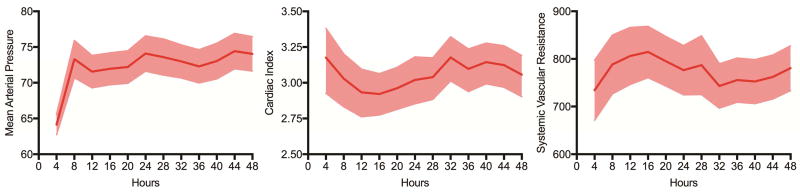
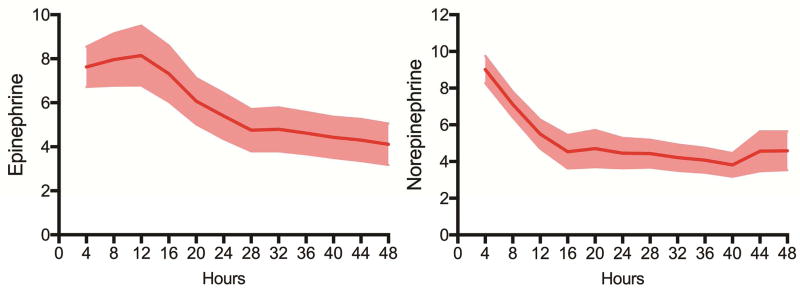
After MB administration, patients with vasoplegic syndrome demonstrated improvement in mean arterial pressure (MAP) in addition to gradual increases in systemic vascular resistance (SVR) and decreases in cardiac index (CI) (Figure 1). Additionally, patients demonstrated a gradual reduction in vasopressor requirements (Figure 2). When stratified by early (operating room) versus late (intensive care unit) administration of MB, patients demonstrated reduced renal failure (10.4 vs 28.6%, p=0.018) and reduced operative mortality (10.4 vs 28.6%, p=0.018) with early administration (Table 3, Table 4).
Figure 1.
Hemodynamic response (mean arterial pressure, cardiac index, and systemic vascular resistance [SVR) after MB administration. Graphs shown are means and 95% confidence intervals.
Figure 2.
Vasopressor requirements (epinephrine and norepinephrine) after MB administration. Graphs shown are means and 95% confidence intervals.
Table 3.
Characteristics of Early vs Late Administration of MB for vasoplegic syndrome
| Variable | Early | Late | p-value |
|---|---|---|---|
| Number of patients | 48 (40.7%)1 | 70 (59.3%) | - |
| Isolated Coronary Artery Bypass and/or Valve | 20 (28.6%) | 15 (31.3%) | 0.754 |
| Ventricular Assist Device | 23 (32.9%) | 17 (35.4%) | 0.773 |
| Age | 64 [60, 73]2 | 63 [56, 70] | 0.305 |
| Male Sex | 34 (70.8%) | 47 (68.1%) | 0.754 |
| Tobacco Use | 16 (33.3%) | 30 (43.5%) | 0.269 |
| Heart Failure | 34 (70.8%) | 54 (77.1%) | 0.439 |
| End Stage Renal Disease | 4 (8.3%) | 3 (4.3%) | 0.361 |
| Diabetes Mellitus | 20 (17.0%) | 26 (37.1%) | 0.621 |
| Severe Chronic Lung Disease | 2 (4.2%) | 8 (11.6%) | 0.304 |
| Hypertension | 36 (75.0%) | 41 (59.4%) | 0.081 |
| Ejection Fraction | 41 [18, 58] | 42 [23, 59] | 0.787 |
| Preoperative Amiodarone Therapy | 4 (8.3%) | 14 (20.0%) | 0.083 |
| ACE-Inhibitor within 48 hours | 3 (6.3%) | 10 (14.3%) | 0.171 |
| Prior Cardiac Surgery | 41 (85.4%) | 48 (69.6%) | 0.048 |
| Cross Clamp Time (minutes) | 100 [86, 118] | 102 [75, 134] | 0.848 |
n (%), all such values
median (interquartile range), all such values
Table 4.
Outcomes of Early vs Late Administration of MB for vasoplegic syndrome
| Variable | Early | Late | p-value |
|---|---|---|---|
| Postoperative Adverse Event | 34 (70.8%) | 53 (75.7%) | 0.554 |
| Blood Transfusion | 44 (91.7%) | 58 (82.9%) | 0.170 |
| Length Of Stay (days) | 12 [10, 18.5] | 12 [6, 21] | 0.447 |
| Postoperative Atrial Fibrillation | 14 (29.2%) | 13 (18.6%) | 0.178 |
| Postoperative Cardiac Arrest | 5 (10.4%) | 8 (11.4%) | 0.863 |
| Stroke | 2 (4.2%) | 4 (5.7%) | 0.707 |
| Renal Failure | 5 (10.4%) | 20 (28.6%) | 0.018 |
| Reoperation | 10 (20.8%) | 21 (30.0%) | 0.266 |
| Deep Sternal Wound Infection | 1 (2.2%) | 0 (0.00%) | 0.219 |
| Prolonged Ventilation | 11 (22.9%) | 16 (22.9%) | 0.994 |
| STS Major Morbidity | 17 (35.4%) | 37 (52.9%) | 0.062 |
| 30-day Mortality | 5 (10.4%) | 20 (28.6%) | 0.018 |
n (%), all such values
median (interquartile range), all such values
Major Adverse Events
A total of 46.6% (55/118) of patients receiving MB for vasoplegic syndrome experienced a MAE. The MAE group had lower rates of tobacco use (29.6 vs. 47.6%, p=0.047), isolated coronary artery bypass graft (CABG) and/or valve surgery (14.6 vs. 42.9%, p=0.001) and early administration of MB (35.4 vs. 64.6%, p=0.044), but a higher rate of prior cardiac surgery (81.5 vs. 71.4%, p=0.02) (Table 5). Additionally, preoperative diastolic blood pressure (58 vs 68 mmHg, p=0.024) was lower and cross clamp time (167 vs. 147 minutes, p=0.021) was longer in the MAE group. Multivariate logistic regression demonstrated early administration of MB independently reduces the risk of MAE in patients with vasoplegic syndrome (OR 0.35, p=0.037, c-statistic= 0.798) (Table 6).
Table 5.
Baseline Characteristics for MB Patients Experiencing a Major Adverse Event (MAE)
| Variable | MAE | No MAE | p-value |
|---|---|---|---|
| Cases | 25 (21.2%)1 | 93 (78.8%) | - |
| Male sex | 37 (68.5%) | 44 (69.8%) | 0.877 |
| Age (years) | 61 [56, 70]2 | 64 [59, 73] | 0.280 |
| Heart Failure | 45 (81.8%) | 43 (68.3%) | 0.291 |
| Hypertension | 36 (66.7%) | 41 (65.1%) | 0.857 |
| Dialysis | 3 (5.45%) | 4 (6.4%) | 0.837 |
| Diabetes | 21 (38.2%) | 25 (39.7%) | 0.868 |
| Severe Lung Disease | 4 (7.4%) | 6 (9.5%) | 0.912 |
| Prior Cardiac Surgery | 44 (81.5%) | 45 (71.4%) | 0.204 |
| Tobacco Use | 16 (29.6%) | 30 (47.6%) | 0.047 |
| Preoperative Amiodarone Therapy | 7 (12.7%) | 11 (17.5%) | 0.476 |
| ACE-Inhibitor within 48 hours | 7 (12.7%) | 6 (9.5%) | 0.579 |
| Preoperative Diastolic Blood Pressure | 58 [43, 68] | 68 [52, 84] | 0.024 |
| Initial Postoperative White Blood Cell Count | 17.5 [13.5, 26.0] | 16.2 [13.0, 21.8] | 0.582 |
| Isolated CABG/Valve | 8 (14.6%) | 27 (42.9%) | 0.001 |
| Cross Clamp Time (minutes) | 167 [125, 234] | 147 [118, 190] | 0.021 |
| Early MB Administration | 17 (35.4%) | 31 (64.6%) | 0.044 |
n (%), all such values
median (interquartile range), all such values
Table 6.
Predictors of Major Adverse Events
| Variable | Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Tobacco Use | 0.26 | 0.087 | 0.771 | 0.015 |
| Early MB Administration | 0.35 | 0.127 | 0.936 | 0.037 |
| Isolated CABG/Valve | 0.29 | 0.095 | 0.855 | 0.025 |
| Cross Clamp Time | 1.01 | 0.999 | 1.022 | 0.077 |
| Preoperative Diastolic Blood Pressure | 1.00 | 0.981 | 1.022 | 0.904 |
| Prior Cardiac Surgery | 2.89 | 0.968 | 8.635 | 0.057 |
C-statistic = 0.798
Comment
Vasoplegic syndrome following CPB is a growing problem as cardiac surgery case complexity increases and continuous flow mechanical circulatory support devices are used more frequently. In the present study, 3.3% of patients at a quaternary care academic medical center received MB for the treatment of severe refractory vasoplegia. These patients had significantly worse outcomes including higher rates of STS major morbidities and operative mortality. Specific predictors for development of vasoplegic syndrome are limited and related to case complexity and comorbidities(1, 3, 18, 19). A standardized approach to the treatment of severe vasoplegic syndrome resulted in an operative mortality rate of 21.2% compared to rates as high as 44% previously reported(10, 20, 21). This study demonstrated that MB administration for treatment of vasoplegic syndrome results in an improvement in MAP, with a compensatory increase in SVR and decrease in CI, coinciding with a reduction in vasopressor requirements. In addition to confirming MB improves vasoplegia, we validate that early administration of MB in the operating room at first signs of vasoplegic syndrome provide better outcomes and improved survival.
Surprisingly, the previously reported link between ACE-inhibitors and vasoplegic syndrome was not identified in this study. These findings can likely be attributed to the low rate of ACE-inhibitor use within 48-hours of surgery (11%), due to increased vigilance ensuring patients hold this medication as it is a known risk factor(5, 13, 22, 23). However, preoperative amiodarone therapy was significantly higher in the MB group than the standard population. Given the retrospective nature of this study, it is impossible to ascertain if this is an association or causation, as patients requiring preoperative amiodarone are likely higher surgical risk. In light of the increasing use of amiodarone and the growing prevalence of vasoplegic syndrome, further investigation is warranted in a prospective manner. Prior heart failure and ejection fraction were also important patient level factors associated with higher rates of MB treatment for vasoplegic syndrome, which have been previously described(3, 19).
The composite MAE was used to identify risk factors for poor outcomes after MB administration for vasoplegic syndrome. Univariate analysis demonstrated six variables associated with MAE including markers of case complexity, such as prior cardiac surgery, cross clamp time and procedure performed, as well as patient specific factors like tobacco use and preoperative diastolic blood pressure. The timing of MB administration was also significant, with patients receiving the medication in the operating room having a reduced rate of MAE compared to receiving it in the intensive care unit despite no major baseline differences. On multivariate regression, tobacco use was independently associated with reduced rates of MAE suggesting a potential reversible cause of vasoplegic syndrome through the NO pathway, which is impacted by MB treatment. Tobacco use may cause endothelial cell activation, which predisposes patients to vasoplegic syndrome that is attenuated though the MB pathway(24).
Additionally, isolated CABG/valve operations reduced risk-adjusted odds of MAE highlighting case complexity as a predictor of poor outcomes. This was particularly pronounced in the population undergoing ventricular assist device (VAD) placement, especially in patients with prior continuous flow VADs, or heart transplants, which has been well described in the literature(25, 26).
Early administration of MB resulted in reduced incidence of postoperative renal failure and operative mortality. This benefit is likely due to early attenuation of the NO induced vasodilation cascade, which reduces the severe systemic inflammatory response(15, 20). Multivariate regression confirmed early MB administration as an independent predictor of reduced risk-adjusted MAE. These results highlight the importance of early recognition and treatment of vasoplegic syndrome. A standardized treatment protocol as described above is a critical step in improving outcomes in these patients with a high risk of morbidity and mortality.
This study is limited by the retrospective single center nature of the data available for analysis. However, this design allowed for increased granularity of patient specific data, including hourly hemodynamic and vasopressor dosages. Additionally, vasoplegic syndrome is an often mentioned but poorly described disease process in the literature, characterized by low SVR and high CI with no widely accepted cutoffs. As such we used administration of MB as a marker of severe refractory vasoplegic syndrome, which limits the availability of a true control group. We were unable to identify any patients at our institution having vasoplegic syndrome who did not receive MB. However, given practice patterns and current vasoplegic syndrome protocols, all patients in the past 5 years with severe vasoplegic syndrome received MB and were captured for analysis. For this reason, we did not seek to identify predictors of vasoplegic syndrome after CBP as others have done. Instead, we focused on predictors of favorable response to MB in order to identify patients most likely to benefit from receiving this non-FDA approved drug. Finally, timing of MB administration was at the discretion of the attending surgeon and patients receiving the therapy in the operating room represent a more aggressive treatment.
In conclusion, early administration of MB improves survival and reduces the risk of MAE in patients with CPB-induced vasoplegic syndrome. These patients have a high mortality rate that may be improved with standardized protocols that encourage MB initiation at the first signs of vasoplegic syndrome. Currently, MB is not FDA approved and is administered off-label to treat this life-threatening condition. These results support the need for a prospective study and further investigation into the use of MB for vasoplegic syndrome.
Abbreviations
- FDA
Food and Drug Administration
- MB
Methylene blue
- NO
Nitric oxide
- SVR
Systemic Vascular Resistance
- CPB
Cardiopulmonary Bypass
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Byrne JG, Leacche M, Paul S, Mihaljevic T, Rawn JD, Shernan SK, et al. Risk factors and outcomes for 'vasoplegia syndrome' following cardiac transplantation. Eur J Cardiothorac Surg. 2004;25(3):327–32. doi: 10.1016/j.ejcts.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 2.Cremer J, Martin M, Redl H, Bahrami S, Abraham C, Graeter T, et al. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg. 1996;61(6):1714–20. doi: 10.1016/0003-4975(96)00055-0. [DOI] [PubMed] [Google Scholar]
- 3.Fischer GW, Levin MA. Vasoplegia during cardiac surgery: current concepts and management. Semin Thorac Cardiovasc Surg. 2010;22(2):140–4. doi: 10.1053/j.semtcvs.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Levin MA, Lin HM, Castillo JG, Adams DH, Reich DL, Fischer GW. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation. 2009;120(17):1664–71. doi: 10.1161/CIRCULATIONAHA.108.814533. [DOI] [PubMed] [Google Scholar]
- 5.Mekontso-Dessap A, Houel R, Soustelle C, Kirsch M, Thebert D, Loisance DY. Risk factors for post-cardiopulmonary bypass vasoplegia in patients with preserved left ventricular function. Ann Thorac Surg. 2001;71(5):1428–32. doi: 10.1016/s0003-4975(01)02486-9. [DOI] [PubMed] [Google Scholar]
- 6.Evora PR, Jose Rodrigues A, Celotto AC. "Methylene blue should be relegated to rescue use and not as first-line therapy" cannot become a paradigm. J Cardiothorac Vasc Anesth. 2014;28(2):e11–2. doi: 10.1053/j.jvca.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Evora PR, Levin RL. Methylene blue as drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2004;127(3):895–6. doi: 10.1016/j.jtcvs.2003.09.046. author reply 6. [DOI] [PubMed] [Google Scholar]
- 8.Evora PR, Ribeiro PJ, Vicente WV, Reis CL, Rodrigues AJ, Menardi AC, et al. Methylene blue for vasoplegic syndrome treatment in heart surgery: fifteen years of questions, answers, doubts and certainties. Rev Bras Cir Cardiovasc. 2009;24(3):279–88. doi: 10.1590/s0102-76382009000400005. [DOI] [PubMed] [Google Scholar]
- 9.Omar S, Zedan A, Nugent K. Cardiac vasoplegia syndrome: pathophysiology, risk factors and treatment. Am J Med Sci. 2015;349(1):80–8. doi: 10.1097/MAJ.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 10.Weiner MM, Lin HM, Danforth D, Rao S, Hosseinian L, Fischer GW. Methylene blue is associated with poor outcomes in vasoplegic shock. J Cardiothorac Vasc Anesth. 2013;27(6):1233–8. doi: 10.1053/j.jvca.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Riha H, Augoustides JG. Pro: methylene blue as a rescue therapy for vasoplegia after cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25(4):736–8. doi: 10.1053/j.jvca.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Ozal E, Kuralay E, Yildirim V, Kilic S, Bolcal C, Kücükarslan N, et al. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg. 2005;79(5):1615–9. doi: 10.1016/j.athoracsur.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Maslow AD, Stearns G, Butala P, Schwartz CS, Gough J, Singh AK. The hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary bypass. Anesth Analg. 2006;103(1):2–8. doi: 10.1213/01.ane.0000221261.25310.fe. table of contents. [DOI] [PubMed] [Google Scholar]
- 14.Nantais J, Dumbarton TC, Farah N, Maxan A, Zhou J, Minor S, et al. Impact of methylene blue in addition to norepinephrine on the intestinal microcirculation in experimental septic shock. Clin Hemorheol Microcirc. 2014;58(1):97–105. doi: 10.3233/CH-141874. [DOI] [PubMed] [Google Scholar]
- 15.Werner I, Guo F, Bogert NV, Stock UA, Meybohm P, Moritz A, et al. Methylene blue modulates transendothelial migration of peripheral blood cells. PLoS One. 2013;8(12):e82214. doi: 10.1371/journal.pone.0082214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menardi AC, Viaro F, Vicente WV, Rodrigues AJ, Evora PR. Hemodynamic and vascular endothelium function studies in healthy pigs after intravenous bolus infusion of methylene blue. Arq Bras Cardiol. 2006;87(4):525–32. doi: 10.1590/s0066-782x2006001700019. [DOI] [PubMed] [Google Scholar]
- 17.Siriwardena DK, Tagori H, Thiemermann C. Nitric oxide synthase inhibitors. Methods Mol Med. 2000;36:115–31. doi: 10.1385/1-59259-216-3:115. [DOI] [PubMed] [Google Scholar]
- 18.Alfirevic A, Xu M, Johnston D, Figueroa P, Koch CG. Transfusion increases the risk for vasoplegia after cardiac operations. Ann Thorac Surg. 2011;92(3):812–9. doi: 10.1016/j.athoracsur.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Kortekaas KA, Lindeman JH, Versteegh MI, Stijnen T, Dion RA, Klautz RJ. Preexisting heart failure is an underestimated risk factor in cardiac surgery. Neth Heart J. 2012;20(5):202–7. doi: 10.1007/s12471-012-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dagenais F, Mathieu P. Rescue therapy with methylene blue in systemic inflammatory response syndrome after cardiac surgery. Can J Cardiol. 2003;19(2):167–9. [PubMed] [Google Scholar]
- 21.Leite EG, Ronald A, Rodrigues AJ, Evora PRB. Is methylene blue of benefit in treating adult patients who develop catecholamine-resistant vasoplegic syndrome during cardiac surgery? Interactive CardioVascular and Thoracic Surgery. 2006;5(1):774–8. doi: 10.1510/icvts.2006.134726. [DOI] [PubMed] [Google Scholar]
- 22.Colson PH, Bernard C, Struck J, Morgenthaler NG, Albat B, Guillon G. Post cardiac surgery vasoplegia is associated with high preoperative copeptin plasma concentration. Crit Care. 2011;15(5):R255. doi: 10.1186/cc10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raja SG, Fida N. Should angiotensin converting enzyme inhibitors/angiotensin II receptor antagonists be omitted before cardiac surgery to avoid postoperative vasodilation? Interact Cardiovasc Thorac Surg. 2008;7(3):470–5. doi: 10.1510/icvts.2007.174698. [DOI] [PubMed] [Google Scholar]
- 24.Kortekaas KA, Lindeman JH, Reinders ME, Palmen M, Klautz RJ, de Groot PG, et al. Pre-existing endothelial cell activation predicts vasoplegia after mitral valve surgery. Interact Cardiovasc Thorac Surg. 2013;17(3):523–30. doi: 10.1093/icvts/ivt243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kofidis T, Struber M, Wilhelmi M, Anssar M, Simon A, Harringer W, et al. Reversal of severe vasoplegia with single-dose methylene blue after heart transplantation. J Thorac Cardiovasc Surg. 2001;122(4):823–4. doi: 10.1067/mtc.2001.115153. [DOI] [PubMed] [Google Scholar]
- 26.Michel S, Weis F, Sodian R, Beiras-Fernandez A, Bigdeli AK, Kaczmarek I, et al. Use of methylene blue in the treatment of refractory vasodilatory shock after cardiac assist device implantation: report of four consecutive cases. J Clin Med Res. 2012;4(3):212–5. doi: 10.4021/jocmr804w. [DOI] [PMC free article] [PubMed] [Google Scholar]