Abstract
Recent work has provided new insights into how altered B cell-intrinsic signals — through the B cell receptor (BCR) and key co-receptors — function together to promote the pathogenesis of autoimmunity. These combined signals affect B cells at two distinct stages: first, in the selection of the naive repertoire; and second, during extrafollicular or germinal centre activation responses. Thus, dysregulated signalling can lead to both an altered naive BCR repertoire and the generation of autoantibody-producing B cells. Strikingly, high-affinity autoantibodies predate and predict disease in several autoimmune disorders, including type 1 diabetes and systemic lupus erythematosus. This Review summarizes how, rather than being a downstream consequence of autoreactive T cell activation, dysregulated B cell signalling can function as a primary driver of many human autoimmune diseases.
Despite the established importance of B cells in the pathogenesis of human autoimmunity, the immune mechanisms that underlie initial breaks in B cell tolerance have not been completely defined. In addition to clonally rearranged B cell receptors (BCRs), B cells express innate pattern recognition receptors (including Toll-like receptors (TLRs)), co-stimulatory molecules (including CD40, CD80 and CD86) and cytokine receptors. Both the establishment of the naive B cell repertoire and B cell activation during an immune response depend on the coordinated, synergistic activation of these receptor families.
Genome-wide association studies (GWAS) have identified hundreds of gene polymorphisms that are associated with an increased risk of developing auto-immunity1–5. Importantly, the vast majority of these genetic changes are predicted to affect immune function. Most are located in non-coding elements that probably have an effect on gene expression, whereas only a limited number result in altered protein structures. Despite this increasingly robust genetic dataset, there is only a limited amount of mechanistic data with respect to the cell lineage-specific and stage-specific effects of candidate risk variants. Notably, autoimmunity-associated variants identified by GWAS are highly enriched for signalling programmes that may affect B cell function, including in genes that encode receptors, signalling effectors and downstream transcriptional regulators of the BCR, CD40, TLRs or cytokine receptors6. Taken together, these data suggest that in an appropriate environmental setting, even modest alterations in B cell signalling may be sufficient to initiate, promote and/or sustain autoimmune disease, particularly diseases that are associated with humoral autoimmunity.
In this Review, we present a model in which dysregulated B cell signalling functions to initiate autoimmunity by modulating the naive BCR repertoire during immature and transitional B cell development, and by promoting the peripheral activation of auto-reactive B cell clones. First, we describe how altered B cell signalling affects the negative and positive selection of B cells during development, skewing the naive B cell repertoire towards self-reactivity or poly-reactivity. Next, we highlight the importance of T cell-independent and T cell-dependent extrafollicular B cell activation in the pathogenesis of humoral autoimmunity. Finally, we discuss how dysregulated B cell-intrinsic BCR, TLR and cytokine signalling can be sufficient to initiate spontaneous, autoimmune germinal centre (GC) responses, resulting in a loss of T cell tolerance, epitope spreading and GC-dependent systemic autoimmunity. In this context, we propose that GWAS-identified risk variants promote autoimmunity by affecting B cell signalling across a continuum of developmental selection and peripheral activation responses.
Receptor crosstalk shapes the naive repertoire
BCRs are generated by the random recombination of germline-encoded variable, diversity and joining gene segments. Although necessary for the generation of receptors that can recognize diverse pathogens, an inherent trade-off of this process is the creation of self-reactive receptors that have the potential to elicit an autoimmune response. Throughout development, immature B cells in the bone marrow (BM) and transitional type 1 (T1) and type 2 (T2) B cells in the periphery are subject to an interplay of positive and negative selection mechanisms to ensure the establishment of a diverse but ‘safe’ repertoire within the follicular mature or marginal zone (MZ) compartments7,8 (BOX 1). Importantly, although the strength of BCR ligation is the dominant driver of B cell tolerance, recent studies indicate that signalling through the B cell-activating factor receptor (BAFFR; also known as TNFRSF13C), TLRs and CD40 synergizes with BCR activation to define the mature B cell repertoire (FIG. 1). Although the effect of GWAS-identified autoimmunity-associated polymorphisms on this process has not been extensively studied, emerging data indicate that altered signalling downstream of these receptor families can modulate selection, thereby skewing the naive B cell repertoire towards autoreactive B cell specificities.
Box 1. Positive and negative selection of autoreactive B cells.
The majority of autoreactive B cells are removed or segregated from the developing repertoire through the processes of negative selection, which include deletion171, receptor editing172 and the induction of anergy173. In addition to these negative selection mechanisms, positive selection of distinct B cell receptor (BCR) specificities also contributes to the mature B cell repertoire. Provided that it does not surpass a presumed threshold for negative selection, BCR engagement with self-ligands promotes the survival advantage of a limited number of competing B cells during development174–176. Consistent with an effect of positive selection on B cell development, specific immunoglobulin variable-domain gene families are enriched in the mature B cell compartments177,178. In addition to BCR engagement, B cell selection is promoted by BAFF-mediated survival signals179, by engagement with Toll-like receptor ligands52 and/or by CD40 signalling53,55. Notably, although co-receptor signalling has primarily been viewed as restricted to the periphery, accumulating data also suggest earlier roles in the bone marrow38,46,55. Collectively, these studies support a model in which self-ligand binding to B cells that results in a signal that is below the threshold for negative selection, combined with adequate co-receptor signalling, results in a competitive advantage of B cell clones in entering the mature repertoire.
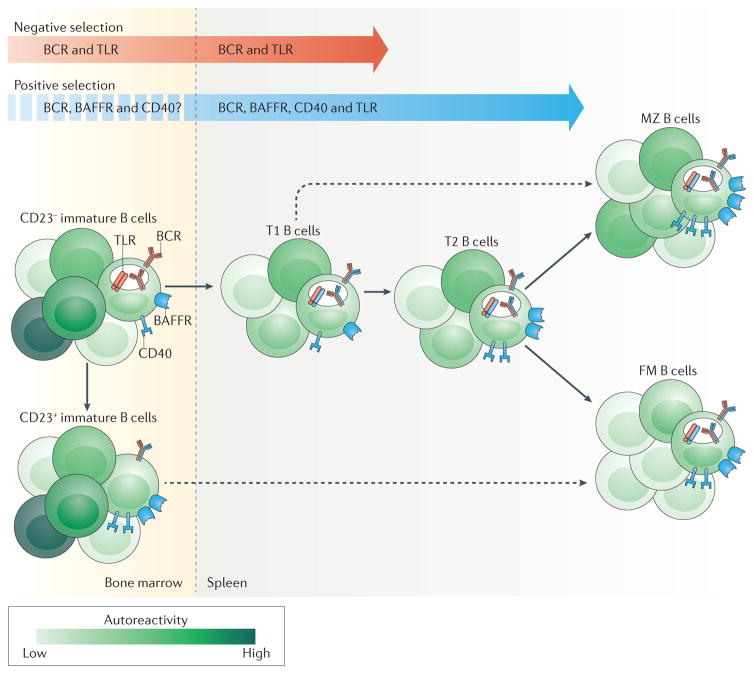
Figure 1. B cell receptor and co-receptor signalling govern B cell selection and maturation.
Self-reactive B cells are subject to positive or negative selection throughout their development in the bone marrow and periphery (spleen). The selective fate of an individual B cell clone depends on multiple factors, including the location and form of self-antigen encounter, the strength of the B cell receptor (BCR) signal and synergy with co-receptor pathways. Negative selection mechanisms (such as deletion, receptor editing and anergy) are mediated primarily by BCR signalling, with potential input from specific Toll-like receptors (TLRs). By contrast, positive selection through survival and/or clonal expansion occurs primarily in transitional B cells in the periphery, and is driven by a complex interplay between BCR signalling and co-receptor signalling mediated by B cell-activating factor receptor (BAFFR), CD40 and TLRs. As the developing repertoire is fine-tuned, transitional B cells mature and populate the follicular mature (FM) compartment or the marginal zone (MZ) compartment. Although the majority of autoreactive BCR specificities are purged by negative selection, a proportion of mature naive B cells exhibit self-reactivity and/or polyreactivity, particularly within the MZ compartment. The dashed arrows indicate ongoing research regarding nonlinear routes for B cell development. T1, transitional type 1; T2, transitional type 2.
BCR signalling
The BCR is a master regulator of negative and positive selection mechanisms
Sustained BCR signalling is required for the survival of both immature BM B cells and mature B cells in the periphery9 through the induction of nuclear factor-κB (NF-κB)-dependent and/or phosphatidylinositol 3-kinase (PI3K)-dependent pro-survival signalling10–12. Conversely, stronger BCR signalling can promote apoptosis in both immature13 and transitional14 B cells in vitro. Thus, an ‘intermediate’ level of tonic BCR signalling may be optimal for immature B cell survival. However, it is evident that the context of self-antigen encounter, rather than signal strength per se, helps to determine the outcome of these events. As detailed in BOX 1, additional factors — including the location of BCR engagement, the form of self-antigen and synergy with additional co-receptor signals — all affect the developmental fate of individual B cells (FIG. 1).
Recent studies from our laboratory and others have expanded our understanding of ligand-mediated positive selection in the periphery. For example, our group identified a subset of T2 B cells that enter the cell cycle in response to antigen engagement15. Using the M167 VH1 heavy chain transgenic model, in which B cells that express self-reactive phosphorylcholine-specific BCRs are detected by an idiotype-specific antibody, we demonstrated that M167-idiotype+ B cells are enriched within this cycling transitional subset and are further enriched in the MZ compartment, which is consistent with antigen-driven positive selection15,16. Importantly, the effect of self-ligand engagement on B cell selection is probably effective across a range of antigen specificities. Using the Nur77–GFP reporter strain, in which BCR signalling activates GFP expression under control of the region that regulates Nur77 (also known as Nr4a1), Zikherman et al.17 demonstrated that BCR signalling occurs as a continuum during development. Consistent with observations in the M167 model15 and other transgenic models18, GFP reporter expression initially increases in T2 B cells, implying that the selection of transitional B cells into follicular mature and MZ compartments is refined by antigen stimulation.
The BCR signalling variant PTPN22R620W promotes greater autoreactivity in mature cells
Although not yet studied in detail, autoimmunity-associated genetic risk variants that affect BCR signalling probably also modulate B cell selection during development. For example, protein tyrosine phosphatase nonreceptor 22 (PTPN22) negatively regulates signalling downstream of both BCR and T cell receptor (TCR) activation. A single-nucleotide polymorphism in PTPN22 (C1858T; R620W) is associated with increased susceptibility to several autoimmune diseases19,20, including systemic lupus erythematosus (SLE)21, type 1 diabetes (T1D)22 and rheumatoid arthritis (RA)23. Consistent with a role for this polymorphism in modulating B cell tolerance, healthy individuals who carry the autoimmunity-associated allele of PTPN22 exhibit increased autoreactivity in the naive B cell repertoire, together with an expansion of transitional and anergic IgD+IgM−CD27−B cell subsets, relative to individuals who do not carry the allele24,25.
An unresolved question is whether the PTPN22R620W polymorphism is a gain-of-function or loss-of-function variant20. Although initial human studies indicated that this polymorphism might increase the phosphatase activity of PTPN22 (resulting in reduced antigen-receptor signalling)24,26,27, independent mouse knock-in models demonstrated enhanced BCR and TCR signalling28,29. Although this discrepancy between human and animal studies remains unresolved, T cells from aged Ptpn22 knock-in mice exhibit blunted TCR signalling, which probably reflects chronic antigen stimulation (X. Dai and D.J.R., unpublished observations). On the basis of this observation, we hypothesize that chronic, enhanced antigen-receptor signalling in PTPN22R620W carriers might explain the observed decreases in BCR and TCR signalling in humans. Importantly, this dichotomy also raises the question as to whether the increase in B cell self-reactivity within the pre-immune repertoire of PTPN22R620W carriers reflects enhanced positive selection, rather than reduced negative selection, of B cells during development. In support of this concept, we have observed marked alterations in the naive B cell repertoire in mice that have a mutation orthologous to the human PTPN22R620W variant (G.M. and D.J.R., unpublished observations).
Although the complex mechanisms that underlie the establishment of the mature B cell repertoire require further study, these combined observations suggest that self-antigen-induced BCR signalling shapes the mature naive B cell compartment by subtly altering transitional B cell survival and positive selection. By modulating this signalling — probably in concert with co-receptors, as described below — we propose that a subset of auto-immunity-associated variants skew the pre-immune repertoire towards increased autoreactivity, thus potentiating the risk of humoral autoimmunity.
BAFFR signalling
Coordinate regulation of BCR and BAFFR signalling in naive B cell development
BCR signalling is crucial for B cell survival and tolerance, but B cells also compete for access to limited survival signals during development. Among these signals, the cytokine B cell-activating factor (BAFF; also known as TNFSF13B) has prominent roles in B cell survival and homeostasis30. BAFF can bind to three distinct B cell surface receptors (namely, BAFFR, transmembrane activator and CAML interactor (TACI; also known as TNFRSF13B) and B cell maturation antigen (BCMA; also known as TNFRSF17)); however, the ligation of BAFFR seems to have a dominant role in peripheral B cell maturation.
The BCR pathway regulates BAFFR signalling through several mechanisms31,32. Strikingly, recent work demonstrates that BAFFR signalling can directly integrate with tonic BCR signalling to promote B cell survival. For example, BAFFR signalling promotes the phosphorylation of proximal BCR signalling components, including spleen tyrosine kinase (SYK) and Igα, and inducible Syk deletion results in reduced BAFF-dependent B cell survival despite intact alternative NF-κB signalling33. In addition, BAFF seems to co-opt signalling components of the BCR to promote CD19 phosphorylation, resulting in PI3K-dependent B cell survival34. Consistent with this, CD19 is required for the survival of SYK-deficient B cells35. In combination, these studies suggest that a complex interplay between the BCR and BAFFR signalling pathways promotes B cell survival, and that this crosstalk probably modulates transitional B cell selection and the establishment of the mature naive B cell repertoire (FIG. 2a).
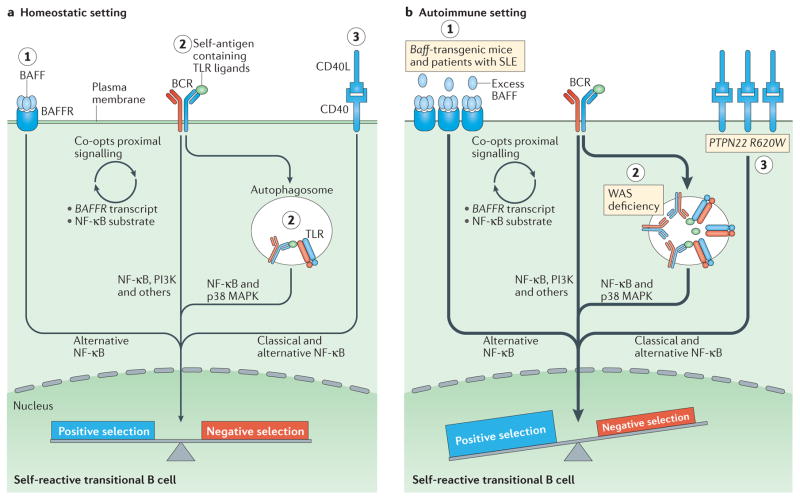
Figure 2. Altered B cell receptor and co receptor signalling promotes increased autoreactivity within the naive B cell repertoire.
a | Under homeostatic conditions, self-reactive B cells are subjected to both positive and negative selection mechanisms as they transit into the naive B cell pool and establish the naive repertoire. Whereas tonic B cell receptor (BCR) signalling and BCR engagement with self-antigen primarily regulate these events, synergy between the BCR and co-receptors fine-tunes the tolerance programme within a given B cell. Among these co-receptors, B cell-activating factor receptor (BAFFR) signalling (1) synergizes with BCR signalling during late bone marrow and transitional development through a series of complex events, including proximal biochemical crosstalk and the downstream transcriptional regulation of both receptor and substrate expression. Dual BCR and Toll-like receptor (TLR) signalling (2) is mediated by internalization and delivery of self-antigens that contain TLR ligands to autophagosomes, which contain endosome-resident TLRs. CD40 signalling (3), which is triggered by interaction with CD40 ligand (CD40L) on T cells and possibly other cell types, also integrates with the BCR signalling pathway. Although BCR signalling can modulate CD40 expression, other biochemical or transcriptional events that affect this crosstalk are less well understood. b | In genetic (or environmental) settings that promote an increased risk of developing autoimmunity, the homeostatic signalling thresholds are modulated, and self-reactive B cells exhibit greater positive selection and/or reduced negative selection, leading to a naive repertoire that is skewed towards autoreactivity. For example, excess amounts of B cell-activating factor (BAFF; 1) in the Baff-transgenic mouse model rescue low-affinity self-reactive B cells from negative selection. A similar mechanism has been proposed to exist in individuals with systemic lupus erythematosus (SLE). Similarly, in mouse and human settings of Wiskott–Aldrich syndrome (WAS) deficiency (2), hyper-responsive dual BCR and TLR signalling promotes the positive selection of transitional B cells with BCRs that use a limited subset of genes that encode self-reactive heavy-chain variable (VH) domains. Healthy individuals with the autoimmunity-associated variant PTPN22R620W (3) exhibit altered BCR and CD40 signalling, and have an enrichment of self-reactive BCR specificities within the naive B cell compartment. Although it has not yet been definitively demonstrated, it is likely that enhanced positive selection, rather than relaxed negative selection, predominantly mediates this change. The thickness of the arrows indicates the strength of pathway activation. MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; PI3K, phosphatidylinositol 3-kinase.
Given that immature IgM+ B cells seem to be normal in Baff−/− and Baffr−/− mice, BAFF was not thought to affect BM B cell development and selection. However, new studies challenge this idea and may support a model that is similar to that of BAFFR-driven peripheral selection. BAFFR is expressed by a CD23+ immature BM B cell subset, which also expresses B220 (also known as PTPRC), IgM and AA4.1 (also known as CD93). This subset further shares phenotypic and functional characteristics of splenic T2 B cells, including maturation from CD23− counterparts, dependence on both BAFFR and tonic BCR signalling, and proliferation following T cell help31,36,37. BAFFR-deficient ‘T2-like’ immature cells also exhibit a competitive disadvantage relative to their wild-type counterparts38. Collectively, these data support a revised model of BM B cell tolerance in which BAFFR signalling, in concert with tonic BCR signalling, promotes the differentiation and/or positive selection of certain immature B cells. Additional studies that assess the effects of antigen-mediated BCR signalling are required to better define the potential contribution of this cell population to the establishment of the naive repertoire and/or autoimmune responses.
Excess BAFF promotes the development of an altered repertoire of self-reactive naive B cells
In addition to its effect on B cell survival, BAFF has been implicated in the development of SLE and other autoimmune diseases. For example, BAFF overexpression in Baff-transgenic mice recapitulates several cardinal features of human SLE, including polyclonal B cell proliferation, antinuclear antibody production and the development of immune-complex-mediated glomerulonephritis39. In addition, BAFF levels are increased in a subset of patients with SLE40, and a BAFF-inhibiting therapeutic antibody, known as belimumab, demonstrated clinical efficacy in patients with SLE41, which emphasizes the importance of BAFF in the pathogenesis of the disease.
Although the mechanisms by which BAFF promotes autoimmunity have not been completely defined, excess BAFF probably contributes to disease development through effects on B cell selection during development. Specifically, increased BAFF levels rescue low-affinity self-reactive transitional cells, thereby allowing their maturation and entry into ‘forbidden’ splenic zones. In a model in which developing B cells compete for available BAFF, excess BAFF results in relaxed selection42–44. Thus, BAFF-dependent survival signals, presumably downstream of BAFFR, contribute to humoral autoimmunity in Baff-transgenic mice by increasing the proportion of autoreactive B cells within the mature naive repertoire (FIG. 2b). In addition, an essential role for B cell-intrinsic TACI signalling in BAFF-driven autoimmunity has recently been identified and is discussed in detail below.
TLR signalling
Dual BCR and TLR signalling orchestrates the selection of self-reactive transitional B cells
Dual BCR and TLR signalling, facilitated by the trafficking of self-antigen to endosomal TLRs, has an important role in the initial activation of mature naive autoreactive B cells45. Although biochemical studies of these pathways in transitional B cells have not yet been carried out, human and mouse studies nevertheless support a role for BCR and TLR crosstalk in regulating B cell tolerance, particularly tolerance towards nuclear self-antigens (FIG. 2a).
Interestingly, similarly to BCR signalling, TLR activation also seems to have a dichotomous role in promoting both the positive and negative selection of autoreactive B cells. On the one hand, Myd88−/− mice (which lack the gene that encodes myeloid differentiation primary response protein 88) have abnormal central tolerance, and patients who lack MYD88 or IRAK4 (which encodes IL-1 receptor-associated kinase 4) exhibit defects in central and peripheral tolerance, implicating TLR-dependent innate signalling in the removal of autoreactive clones during B cell development46–48. On the other hand, in a mouse model in which lupus is driven by an autoimmunity-associated Cd45 variant, strain-specific variance in TLR9 signal strength in C57BL/6 versus BALB/c mice either facilitated B cell negative selection and central tolerance, or promoted breaks in peripheral B cell tolerance (probably through enhanced positive selection)49. Additional evidence for TLR signalling promoting transitional B cell positive selection in both humans and mice comes from the primary immunodeficiency Wiskott–Aldrich syndrome (WAS; FIG. 2b). Mutations in the WAS protein influence actin polymerization and receptor signalling in nearly all haematopoietic cell lineages and, in B cells, WAS mutations result in modestly enhanced signalling downstream of both the BCR and TLRs50–52. In this context, although central tolerance is maintained in Was−/− mice, late T2 B cells exhibit enhanced proliferation52. High-throughput B cell repertoire sequencing of Was−/− mice and humans with WAS identified the preferential selection of specific heavy-chain variable (VH) gene families (VH10 in mice; VH4-34 in humans) as late transitional B cells become mature naive B cells52. Consistent with the BCR using these VH families to bind to antigenic complexes that also contain TLR ligands, both the increase in transitional B cell cycling and the altered naive repertoire in WAS were MYD88 dependent52. Thus, in addition to the known effects on the activation of mature autoreactive B cells, altered BCR and TLR signalling is coordinated to enhance the positive selection of self-reactive specificities into the naive B cell repertoire.
In summary, dual BCR and TLR signalling probably affects both negative and positive selection events during B cell development, with the effects depending predominantly on the developmental stage. TLR signalling in immature B cells primarily promotes negative selection, whereas dual signalling in late transitional B cells facilitates positive selection through clonal expansion. Additional studies that assess BCR crosstalk with specific TLRs during B cell development should improve our understanding for how these coordinated signals shape the naive repertoire.
CD40–CD40 ligand signalling
B cell-intrinsic CD40 signalling affects the naive B cell repertoire
In addition to its well-established role in T cell-dependent B cell responses, recent studies indicate that CD40 signalling can also directly modulate transitional B cell selection (FIG. 2a). CD40 signalling can both rescue transitional B cells from BCR-induced apoptosis in vitro14 and compensate for reduced BCR signalling during development, as shown by a markedly smaller peripheral B cell compartment in mice that lack both Bruton’s tyrosine kinase (BTK) and CD40 than is found in wild-type mice53. In addition, CD40 ligand (CD40L) on naive CD4+ T cells augments the survival of autoreactive B cells54. Consistent with this idea, our group demonstrated that CD40 signalling facilitates B cell positive selection, leading to an altered mature B cell repertoire, particularly within the MZ55. Thus, although Cd40−/− mice have normal B cell numbers56, CD40 signalling still affects the breadth of specificities in the mature compartment. It is also tempting to speculate about an earlier role for CD40 in central tolerance on the basis of its expression by immature BM cells (G.M. and D.J.R., unpublished observations)57 and the competitive disadvantage of Cd40−/− BM B cells observed by Schwartz et al.55. Future mechanistic studies that directly assess the intersecting roles of the CD40 and BCR pathways are likely to provide additional insights into immature and transitional B cell selection.
Enhanced CD40 signalling in the presence of the autoimmunity-associated variant PTPN22R620W
Whether these CD40-dependent effects on the pre-immune repertoire also contribute to the risk of autoimmunity has not been addressed. However, individuals who carry the autoimmunity-associated R620W variant of PTPN22 exhibit both altered B cell selection and increased CD40 expression in mature naive cells25. These findings raise the possibility that dysregulated BCR signalling in PTPN22R620W carriers functions, in part, to facilitate CD40 signalling, culminating in events that may promote the increased survival of autoreactive cells (FIG. 2b). Consistent with this idea, although the increased frequency of autoreactive B cells observed in the periphery of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX syndrome) has been attributed to a lack of regulatory T cell-dependent regulation of B cell tolerance48,58, an alternative interpretation is that the increased levels of CD40L found on CD4+ T cells in these patients instead directly promote the survival and positive selection of autoreactive B cells.
In summary, the studies described above support a paradigm in which BCR signalling coordinates with BAFF family receptors, TLRs and CD40 to shape the mature naive B cell repertoire. Although these signalling pathways are frequently considered separately, crosstalk between them seems to be the norm rather than the exception. Depending on the developmental stage and context, this ongoing signal integration can function to modulate either negative or positive selection. Importantly, we predict that autoimmunity-associated genetic risk variants alter this process by coordinately affecting these B cell-intrinsic pathways, resulting in increased self-reactivity and polyreactivity within the pre-immune repertoire.
Receptor crosstalk shapes B cell activation
Although carriers of GWAS-identified autoimmunity-associated variants frequently exhibit increased auto-reactivity within the naive B cell repertoire, this increase in the proportion of autoreactive naive B cell clones is insufficient for the development of systemic autoimmunity. Indeed, up to 20% of mature B cells in healthy individuals exhibit antinuclear reactivity in the absence of clinical autoimmunity59. Therefore, additional immune signals are required to initiate the activation of autoreactive B cell clones and thereby precipitate humoral autoimmunity. During a humoral immune response, antibody-secreting cells (ASCs) are generated through two distinct pathways: namely, the extrafollicular and GC B cell pathways. Importantly, although these pathways are often considered separately, they probably occur in parallel to protect the host from infectious challenge and during autoimmunity. The specific B cell activation pathway that is responsible for the majority of autoantibodies remains undefined, largely because it is difficult to determine whether an ASC is derived from an extrafollicular response or whether it developed within a GC.
Extrafollicular B cell responses
Extrafollicular B cell activation contributes to the production of class-switched autoantibodies
The pathophysiology of SLE is particularly complex, as both human clinical studies and animal models indicate that serum antinuclear autoantibodies can be generated through both extrafollicular and GC B cell activation pathways. This issue is not only of academic interest, as short-lived plasma cells (predominantly derived from extrafollicular responses) are sensitive to anti-proliferative immunosuppression and B cell depletion by CD20-specific antibody, whereas GC-derived long-lived plasma cells are largely resistant to such treatment60–62.
The importance of extrafollicular B cell activation in autoantibody generation has been most extensively studied in the MRL.Faslpr mouse lupus model. Using MRL.Faslpr mice that express a transgene that encodes rheumatoid factor (RF), William et al.63 demonstrated that the spontaneous activation of RF+ B cells occurs through an extrafollicular pathway, independently of GCs. Microdissection and BCR cloning revealed extensive somatic hypermutation (SHM) in RF+ B cells at the T cell–red pulp border, confirming that antigen-driven SHM can occur outside the GC63. The prominent roles of extrafollicular B cell responses in lupus pathogenesis are not unique to the MRL.Faslpr model. For example, humoral autoimmunity in transgenic mice that over-express BAFF occurs independently of CD4+ T cells64. In addition, up to 60% of autoreactive ASCs in NZB/W mice are short-lived plasmablasts, which are sensitive to cyclophosphamide treatment60.
Continuous extrafollicular B cell activation is probably also relevant to the pathogenesis of human SLE. Plasmablast-like cells are expanded in the peripheral blood of patients with SLE, and their numbers correlate with titres of double-stranded DNA (dsDNA)-specific antibodies and disease flares65. In addition, a peripheral blood plasmablast gene signature identifies a subset of patients with increased disease activity66. Moreover, a recent study used deep sequencing and single-cell analysis of peripheral blood ASCs in patients with SLE to characterize the phenotype of autoantibody-producing cells67. By studying activated B cells that express the intrinsically self-reactive VH4-34 heavy chain (see BOX 2), this study demonstrated that a considerable proportion of VH4-34+ SLE plasmablasts were derived from newly activated B cells that exhibited clonality that persisted for months. In addition, a subset of these autoreactive ASCs lacked BCR mutations, which is most consistent with a non-GC origin.
Box 2. VH4 34+ B cells.
VH4-34 is a human heavy chain family that has germline-encoded polyreactivity towards multiple self-antigens, including double-stranded DNA, apoptotic cells and red blood cell carbohydrate antigens180–183. VH4-34+ B cells can be identified in the peripheral blood using the anti-idiotype monoclonal antibody 9G4. In healthy individuals, 5–10% of naive B cells are 9G4+, but 9G4+ B cells are largely excluded from the germinal centre, memory cell and plasma cell compartments, which suggests the existence of a negative selection checkpoint that prevents autoreactive B cell activation. By contrast, 9G4+ B cells are enriched in memory B cell subsets and plasma cells in patients with systemic lupus erythematosus, which is consistent with the observation that the levels of VH4-34+ autoantibodies are increased in this disease184–188.
B cell signals required for extrafollicular responses
Continuously generated, short-lived plasmablasts could be an important therapeutic target in humoral autoimmune diseases, and thus we need to identify the key signals that control their generation. B cells show a unique propensity for activation by integrated signals downstream of the BCR and TLRs45,68. Although this arrangement has probably evolved to resist viral infection69, it carries the risk of autoimmunity as the endosomal TLRs can also respond to RNA-containing and DNA-containing epitopes within apoptotic particles. Consistent with this idea, MYD88-deficient MRL.Faslpr mice lack antinuclear antibodies and are protected from systemic autoimmunity70. After activation by integrated BCR and TLR signalling, activated B cells migrate to the border of the T cell zone and the follicle, where they interact with CD4+ T cells. Although certain auto-antibody specificities can develop in MRL.Faslpr mice in a T cell-independent manner, CD4+ T cells facilitate extra-follicular B cell responses through interactions between CD40 and CD40L, interactions between inducible T cell co-stimulator (ICOS) and ICOS ligand (ICOSL), and the provision of interleukin-21 (IL-21)71 (FIG. 3).
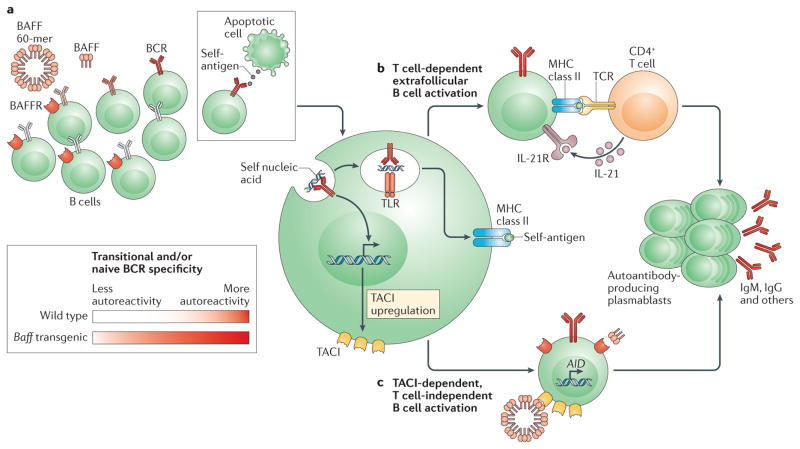
Figure 3. T cell-dependent and T cell-independent extrafollicular activation pathways in autoimmunity.
Excess amounts of B cell-activating factor (BAFF) promote the survival of autoreactive B cells (part a), there by increasing the proportion of self-reactive B cells within the transitional and mature naive B cell compartments. Following engagement by self-antigen, autoreactive B cells undergo activation in response to dual B cell receptor (BCR)-dependent and Toll-like receptor (TLR)-dependent signalling. Subsequently, activated B cells differentiate into extrafollicular autoantibody-producing plasmablasts through either T cell-dependent (part b) or T cell-independent (part c) pathways. Activated B cells that engage cognate CD4+ T cells at the T cell–B cell border undergo class-switch recombination and differentiation into extrafollicular plasmablasts in response to co-stimulatory signals provided by T cells (part b), including CD40 ligand (CD40L; not shown), inducible T cell co-stimulator (ICOS; not shown) and interleukin-21 (IL-21). Following engagement by self-antigen, autoreactive B cells upregulate their surface expression of transmembrane activator and CAML interactor (TACI) in response to BCR-dependent signalling. TACI engagement by multimeric BAFF 60-mers (part c) promotes the expression of activation-induced cytidine deaminase (AID), resulting in somatic hypermutation, class-switch recombination and the subsequent generation of autoantibody-producing plasmablasts. Although additional B cell subsets may contribute to TACI-dependent autoantibody production in high BAFF settings, transitional B cells make a prominent contribution to the pool of IgG-producing plasmablasts in Baff-transgenic mice. BAFFR, BAFF receptor; TCR, T cell receptor.
Of the known MYD88-dependent receptors, the endosomal receptors TLR7 and TLR9 have prominent effects on the pathogenesis of autoimmunity. As predicted by in vitro studies68,72, TLR7-deficient and TLR9-deficient MRL.Faslpr mice lack RNA-specific and dsDNA-specific autoantibodies, respectively73,74. However, Tlr7 and Tlr9 deletions result in unexpected opposing effects on disease severity. Specifically, Tlr7 deletion is protective in several mouse lupus models, whereas Tlr9 deletion surprisingly exacerbates disease74. Notably, TLR signalling might affect disease severity by facilitating dual BCR-mediated and TLR-mediated activation of autoreactive B cells, or by enhancing type I interferon (IFN) production by plasmacytoid dendritic cells73,75. However, consistent with a dominant role for B cells, B cell-intrinsic Myd88 deletion abrogates the generation of antinuclear antibodies and nephritis in MRL.Faslpr mice76.
TACI signalling is required for T cell-independent, BAFF-driven autoimmunity
Baff-transgenic mice develop extrafollicular plasmablasts, high-titre class-switched autoantibodies and systemic autoimmunity in the absence of CD4+ T cells64. Although BAFF-driven autoimmunity requires B cell-intrinsic MYD88 function64, the receptor that binds to BAFF and promotes autoantibody production had, until recently, not been identified. As discussed above, excess BAFF promotes B cell hyperplasia and the survival of autoreactive B cells in the naive repertoire, whereas BAFFR deficiency results in a loss of mature B cells42,43,77, thereby implicating BAFFR in BAFF-driven autoimmunity. However, TACI signals through MYD88 (REF. 78) and promotes T cell-independent antibody responses79, suggesting a role for TACI in this process. Consistent with this idea, two independent studies demonstrated the surprising finding that BAFF-driven autoimmunity is abrogated by Taci deletion80,81.
Mechanistically, whereas TACI expression is usually limited to mature B cells, we noted a marked upregulation of TACI expression by CD21lowCD24hi transitional B cells in Baff-transgenic mice81. TACI expression identified a subset of activated, proliferating cells that were enriched for autoreactive specificities and that coordinately exhibited the expression of activation-induced cytidine deaminase (AID) and the production of class-switched, somatically mutated autoantibodies81. On the basis of these findings, we propose a model in which activated transitional B cells and/or naive mature B cells generate a pool of ASCs that produce pathogenic IgG autoantibodies in Baff-transgenic mice (FIG. 3). BAFF levels are increased in many patients with autoimmune disease (including those with SLE, RA and systemic sclerosis), which suggests that BAFF-driven TACI signalling may promote pathogenic plasmablast formation40, although this possibility has not yet been examined in humans. In addition, dysregulated transitional B cell activation is likely to be relevant in other clinical scenarios that are characterized by high BAFF levels, including autoimmune disease relapse after B cell-depletion therapy82,83 and de novo humoral autoimmunity following stem cell transplantation84. Notably, as systemic infectious challenges frequently promote local BAFF generation85, TACI-dependent activation of transitional B cells may contribute to rapid, T cell-independent antibody responses to blood-borne pathogens.
Autoimmune GC B cell responses
GC B cell responses during autoimmunity
In addition to the extrafollicular B cell responses described above, several lines of evidence indicate that spontaneous GCs are an important site of autoreactive B cell activation in autoimmune diseases6,86. For example, autoantibody-producing B cells cloned from patients with SLE are frequently class-switched and exhibit extensive SHM87. Although SHM can occur within extrafollicular foci in autoimmunity, these findings implicate autoantigen-driven GC selection in the generation of high-affinity, autoantibody-producing B cells. Consistent with this idea, spontaneous GCs are observed in several mouse lupus models, and the B cell-intrinsic signals required for autoantibody production are also necessary for spontaneous GC formation50,76,88–90. In addition, tertiary lymphoid structures and ectopic GCs develop within inflamed tissues in human autoimmunity, including kidneys in lupus nephritis and arthritic joints in rheumatoid arthritis86,91. Finally, serum autoantibody titres increase years before disease onset and persist despite aggressive immunosuppression, implicating GC-derived long-lived plasma cells in the pathogenesis of autoimmune disease.
Most importantly, in addition to GCs being a site of autoreactive plasma cell generation, emerging evidence demonstrates that autoreactive B cells can direct the formation of spontaneous GCs by initiating the expansion of cognate T follicular helper (TFH) cells. In this model, rather than being a downstream consequence of self-reactive T cells, dysregulated B cell signalling functions as the primary driver of disease initiation (FIG. 4). On the basis of this paradigm, we review the specific B cell immune mechanisms that underlie the formation of spontaneous autoimmune GCs. Where possible, we focus on the specific signalling pathways that have been shown to promote autoimmunity in a B cell-intrinsic manner.
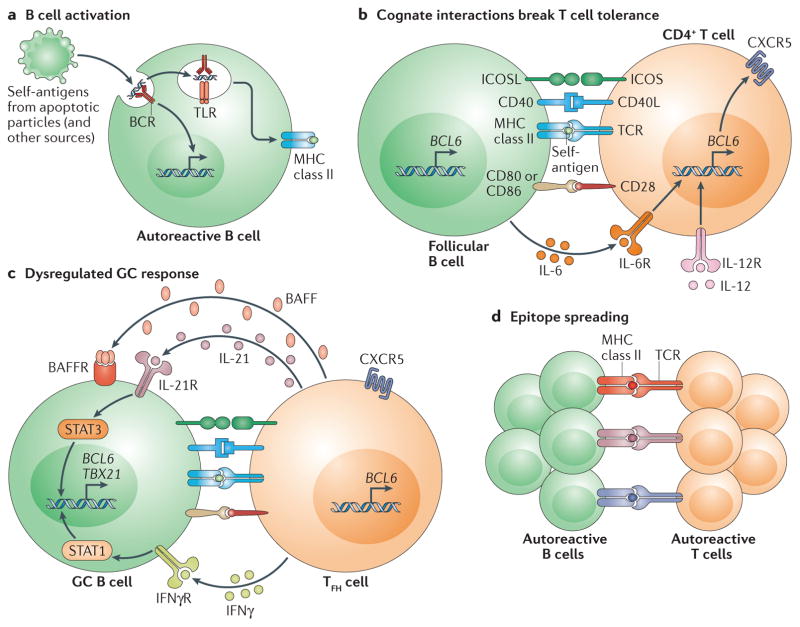
Figure 4. Self reactive B cells initiate autoimmune germinal centre formation by facilitating breaks in T cell tolerance.
a | After binding to self-antigen (either soluble or bound to antigen-presenting cells) derived from apoptotic particles or other disease-specific targets, autoreactive B cell receptors (BCRs) traffic nuclear antigens to the endosomal receptors Toll-like receptor 7 (TLR7) and TLR9, resulting in initial B cell activation in response to integrated BCR-dependent and TLR-dependent signalling. In parallel, endolysosomal enzymes also process internalized self-antigens (including a broad range of nucleic acid-associated proteins) into peptides for loading onto MHC class II. b | B cells function as antigen-presenting cells to present MHC class II-bound peptides to cognate self-reactive CD4+ T cells at the T cell–B cell border of lymphoid follicles. Together with co-stimulatory signals provided by CD80 and/or CD86 and inducible T cell co-stimulator ligand (ICOSL), self-reactive B cells initiate breaks in CD4+ T cell tolerance. Activated CD4+ T cells subsequently express CXC-chemokine receptor 5 (CXCR5) and B cell lymphoma 6 (BCL-6), resulting in their migration to the B cell follicle as early T follicular helper (TFH) cells (not shown). Activated B cells also produce interleukin-6 (IL-6), which may facilitate TFH cell differentiation by inducing BCL-6 expression, although this has not yet been directly tested. c | TFH cells promote germinal centre (GC) formation through the production of IL-21, which sustains B cell BCL-6 expression and promotes B cell activation, class-switch recombination and plasma cell differentiation. In autoimmune settings, interferon-γ (IFNγ; probably derived from activated CD4+ T cells) drives GC formation in a B cell-intrinsic, signal transducer and activator of transcription 1 (STAT1)-dependent manner, in part by enhancing BCL-6 expression. IFNγ also promotes B cell-intrinsic expression of the transcription factor T-bet (encoded by TBX21), which is required for class-switch recombination to pathogenic IgG2a and IgG2c isotypes, but is redundant for IFNγ-driven GC formation. Although not yet directly tested in autoimmune models, this dysregulated GC response is probably affected by additional cytokines, including B cell-activating factor (BAFF), which promotes the selection of high-affinity GC B cell clones, and IL-12, which facilitates T cell IFNγ production and TFH cell differentiation. d | Iterative interactions between GCB cells and cognate TFH cells within ongoing autoimmune GCs probably result in epitope spreading and the recruitment of additional autoreactive T cell and B cell clones. BAFFR, BAFF receptor; CD40L, CD40 ligand; ICOS, inducible T cell co-stimulator; IFNγR, IFNγ receptor; IL-6R, IL-6 receptor; TCR, T cell receptor.
Dysregulated BCR signalling promotes GC-dependent systemic autoimmunity
Studies in several independent animal models have clearly shown that dysregulated BCR-dependent signalling is sufficient to promote autoimmunity in a B cell-intrinsic manner. For example, patients with WAS exhibit high rates of humoral autoimmunity92,93. To examine the cell-intrinsic mechanism that underlies autoimmunity in WAS, we generated BM chimaeras in which B cells, but not other haematopoietic lineages, lacked expression of the WAS protein50. In this setting, Was−/− B cells initiated spontaneous humoral autoimmunity characterized by high-titre class-switched antinuclear antibodies, TFH cell expansion, spontaneous GC formation and the development of progressive immune-complex-mediated glomerulonephritis. Subsequently, Recher et al.94 demonstrated that conditional deletion of Was in B cells also resulted in the formation of spontaneous GCs and the production of class-switched autoantibodies. Mechanistically, Was−/− B cells are modestly hyper-responsive to both BCR and TLR activation, which suggests that dysregulated dual BCR and TLR signalling may be sufficient to promote autoantibody production.
In support of this idea, B cell-intrinsic deletion of the SRC family tyrosine kinase-encoding gene Lyn also promotes spontaneous autoimmunity95. Notably, Lyn−/− B cells exhibit enhanced BCR-mediated calcium flux96, which is probably explained by the role of LYN in driving immunoreceptor tyrosine-based inhibition motif (ITIM)-mediated inhibitory signalling by CD22 or Fcγ receptor IIB (FcγRIIB). These observations are relevant beyond mouse models, as polymorphisms in LYN and the related kinase BLK are associated with SLE97–99. In addition, rare loss-of-function mutations in SIAE (which encodes sialic acid acetyl esterase) — an enzyme that is required for post-translational modifications that facilitate CD22 activity — increase the risk of an individual developing SLE, T1D and RA100.
Similarly, the autoimmunity-associated PTPN22R620W variant also modulates BCR signalling21–23. To elucidate the mechanisms that drive autoimmune disease, two groups independently generated mouse models in which the gene that encodes the orthologous risk variant in mouse phosphatase (Pep) was knocked in28,29. Both knock-in strains exhibited enhanced BCR and TCR signalling, spontaneous GC formation and an age-related expansion of effector memory T cells. However, systemic autoimmunity was only observed in mice that had a mixed C57BL/6J×129/Sv background, which potentially reflects the influence of signalling lymphocyte activation molecule (SLAM) family polymorphisms derived from the 129 background on autoimmune GC responses101,102.
As the autoimmunity-associated PTPN22R620W variant might promote autoimmunity through T cell-dependent or B cell-dependent mechanisms, we generated a separate strain to evaluate B cell-intrinsic expression of this risk-associated variant. Remarkably, aged animals developed spontaneous GCs and produced high-titre autoantibodies, which indicates that B cell-specific expression of the risk-associated variant was sufficient to promote a break in B cell tolerance28. Notably, consistent with the ability of modest alterations in BCR signal strength to promote an autoimmune GC response, transgenic overexpression of the TEC family kinase BTK, which is essential for BCR-triggered phospholipase Cγ2 (PLCγ 2) activation, was sufficient to trigger analogous events and lead to T cell-dependent autoantibody production103.
Together, these studies demonstrate the crucial importance of dysregulated BCR signalling in driving humoral autoimmunity, both by modulating the pre-immune B cell repertoire and by facilitating the activation of autoreactive B cells in the periphery.
B cell antigen presentation in autoimmunity
B cell-targeted therapies are approved by the US Food and Drug Administration for the treatment of RA104, anti-neutrophil cytoplasmic autoantibody-associated vascu-litis105 and SLE41, and the therapeutic benefits of these treatments have also been observed in a diverse range of human autoimmune diseases, including T1D106, primary membranous nephropathy107, pemphigus vulgaris108 and multiple sclerosis109. However, although clinical improvement correlates with the decrease in autoantibody titres in certain diseases108,110, B cell depletion frequently results in clinical responses despite persistent serum autoantibody titres111,112. These observations suggest that additional B cell functions, including MHC class II-dependent antigen presentation or cytokine production, have important roles during the pathogenesis of autoimmune disease.
Initial evidence for antibody-independent B cell effector functions in SLE came from seminal studies using the MRL.Faslpr model. Specifically, B cell-deficient MRL.Faslpr mice lacked T cell infiltrates in kidneys, whereas MRL.Faslpr mice in which B cells express surface but not secreted IgM developed prominent nephritis in the absence of serum autoantibodies113,114. Although these studies suggested important roles for B cell antigen presentation in the pathogenesis of SLE, direct evidence of B cell antigen-presenting cell (APC) activity promoting autoimmunity was limited to a few models. For example, in the non-obese diabetic mouse (NOD mouse) model of T1D, B cell-intrinsic loss of MHC class II prevented CD4+ T cell activation and the development of diabetes; findings that are consistent with studies showing the antibody-independent development of diabetes in secretory IgM-deficient NOD mice115,116. Similarly, in the experimental autoimmune encephalitis model of multiple sclerosis, B cell-intrinsic MHC class II-dependent antigen presentation, rather than myelin oligodendrocyte glycoprotein-specific antibody production, was required for progressive neurological disease117.
To test whether B cell-intrinsic antigen presentation was similarly required to initiate CD4+ T cell activation and spontaneous GC formation in SLE, we generated WAS chimaeras in which all B cells lacked MHC class II90. Remarkably, the loss of B cell APC function abrogated the expansion of activated effector memory T cells and the development of spontaneous GCs. These findings were corroborated by an independent study in which B cell-intrinsic Cre-mediated deletion of MHC class II in MRL.Faslpr mice prevented CD4+ T cell activation and the production of class-switched autoanti-bodies118. Together, these findings confirm that B cells that function as APCs directly initiate CD4+ T cell activation in mouse models of SLE, and correspondingly, that a loss of B cell APC activity may explain the antibody-independent benefits of B cell depletion therapy. Consistent with this idea and its relevance beyond SLE, spontaneous anti-insulin GCs are generated in mice that have anti-insulin transgenic T cells and B cells through a process that requires GC B cells to present a repertoire of insulin epitopes derived from circulating insulin119.
B cell-intrinsic TLR signalling in autoimmune GCs
In addition to its role in extrafollicular autoantibody responses, dysregulated TLR signalling is implicated in GC-dependent autoimmunity. For example, B cell-intrinsic MYD88 signalling is crucial for spontaneous GC formation and antinuclear antibody production in several mouse lupus models50,89,120. Of the MYD88-dependent TLRs, B cell TLR7 signalling is most prominently linked with GC-driven autoimmunity. For example, TLR7 overexpression in lupus-prone mouse strains that carry the Yaa translocation or in Tlr7-transgenic mice results in systemic autoimmunity characterized by spontaneous GC formation121,122. Moreover, polymorphisms in TLR7 have been linked to the development of SLE in human candidate gene studies123–125. These effects on autoimmune GCs are most likely explained by B cell-intrinsic TLR7 over-expression, as Tlr7-transgenic B cells are preferentially expanded in the GC relative to wild-type B cells in competitive chimaeras122.
However, although B cell-intrinsic TLR9 expression is required for the formation of antinucleosome antibodies126, the relative effect of TLR7 signalling versus TLR9 signalling in autoimmune GC responses had not been assessed. For this reason, we generated WAS chimaeras with B cells that were deficient in either TLR7 or TLR9 (REF. 88). As predicted, B cell-intrinsic Tlr7 and Tlr9 deletion prevented the production of RNA-associated and DNA-associated autoantibodies, respectively. However, whereas B cell-intrinsic TLR7 deficiency prevented spontaneous GC formation and systemic autoimmunity, disease severity was notably exacerbated by B cell-intrinsic Tlr9 deletion. Interestingly, spontaneous GCs also develop in non-autoimmune C57BL/6 mice with increasing age in a manner that is dependent on B cell-intrinsic TLR7 signalling but is restrained by TLR9 activation121. Although the mechanisms by which TLR9 activation restrains autoimmunity remain elusive, these studies demonstrate that the protective roles of TLR9 rely on B cells and not myeloid subsets.
Of relevance to human disease pathogenesis, genetic variants that influence TLR signalling pathways — including TNFAIP3 (which encodes tumour necrosis factor-induced protein 3), TNIP1 (which encodes TNFAIP3-interacting protein 1), ATG5 (which encodes autophagy-related gene 5), IRF5 (which encodes IFN-regulatory factor 5), IRF7 and SLC15A4 (which encodes solute carrier family 15 member 4) — are highly associated with the risk of developing SLE127,128. Although the effects of individual polymorphisms on downstream signalling and the key cell lineages involved remain to be determined, these findings from GWAS emphasize the crucial importance of dysregulated TLR responses in the pathogenesis of systemic autoimmunity.
IL-6, IL-21 and BAFF in autoimmune GC responses
In addition to cognate interactions between GC B cells and TFH cells, cytokine signalling markedly influences GC biology, both during infectious responses and in autoimmunity. The pleiotropic cytokine IL-21 facilitates GC formation by promoting the cell-intrinsic activation and differentiation of TFH cells and GC B cells129–131. Consistent with important roles for IL-21 in the pathogenesis of autoimmune disease, serum IL-21 levels are increased in mouse and human lupus132–135. In addition, Il21r deletion or therapeutic IL-21 blockade limits autoimmunity in the MRL.Faslpr mice and BXSB-Yaa mice132,136,137 in a manner that is dependent on B cell-intrinsic IL-21R activation138.
In cooperation with IL-21, the pro-inflammatory cytokine IL-6 promotes the formation of GC B cells and TFH cells. Although an absolute requirement for IL-6 in TFH cell generation is controversial131,139–141, IL-6 is known to promote early TFH cell differentiation by transiently inducing B cell lymphoma 6 (BCL-6) expression in CD4+ T cells binding cognate antigen142. In addition, Il6 deletion reduces disease severity in the BXSB-Yaa mouse lupus model, and increased serum IL-6 levels have been observed in human SLE, which suggests potential roles for this cytokine in disease pathogenesis143,144. Consistent with this idea, IL-6 receptor blockade with tocilizumab decreased the levels of dsDNA-specific antibodies and circulating plasmablasts in early-stage clinical trials in patients with SLE145. Interestingly, in addition to dendritic cells and other myeloid subsets, activated B cells produce IL-6, which suggests that B cell-derived IL-6 might initiate TFH cell development and spontaneous GC formation in SLE146,147, although this hypothesis has not yet been directly tested.
Finally, although systemic autoimmunity in Baff-transgenic mice occurs independently of T cells, TFH cells are an important source of local BAFF production within GCs148,149. Although TFH cell-derived BAFF is redundant for GC formation, T cell-derived BAFF promotes the selection of high-affinity GC B cell clones, thereby implicating BAFF in the pathogenesis of GC-dependent autoimmunity.
IFN signalling in autoimmune GC responses
In addition to important functions during antiviral immune responses, the type I IFN cytokine family (comprising IFNα, IFNβ, IFNε and IFNω) has been implicated in the pathogenesis of systemic autoimmunity. For example, a prominent subset of patients with SLE exhibit increased levels of type I IFN-induced transcripts in peripheral blood mononuclear cells, a phenomenon termed the ‘interferon signature’ (REFS 150–152). Mechanistically, TLR-dependent activation of plasmacytoid dendritic cells by nucleic acid-containing immune complexes has been proposed to propagate SLE through increased type I IFN production. Consistent with this idea, human genetic variants that affect type I IFN production or signalling — including variants in IRF5, STAT4 (which encodes signal transducer and activator of transcription 4) and TREX1 (which encodes 3′-repair exonuclease 1) — are associated with an increased risk of developing SLE, whereas type I IFN receptor deficiency ameliorates disease in some mouse lupus models153,154. Importantly, in addition to type I IFNs, type II IFN (IFNγ) signalling has also been implicated in the pathogenesis of SLE in animal models and human gene expression studies150,155–157.
Recently, human and animal studies have begun to reconcile these overlapping roles for type I and type II IFNs in the pathogenesis of SLE. Whereas B cell-intrinsic type I IFN signalling is redundant for humoral autoimmunity in WAS chimaeras, IFNγ promotes GC-dependent autoantibody production in the Roquinsan/san, B6.Sle1b and WAS chimaera lupus models90,157,158. Spontaneous GC formation required B cell-intrinsic and T cell-intrinsic IFNγ receptor (IFNγR) signalling for the generation of GC B cells and TFH cells, respectively. Mechanistically, although IFNγ-driven upregulation of the T-box transcription factor T-bet was required for the production of pathogenic IgG2c autoantibodies, neither complete nor B cell-intrinsic T-bet deficiency affected spontaneous GC formation90. Instead, IFNγ synergized with co-stimulatory signals to promote cell-intrinsic expression of the GC master regulator BCL-6 by both CD4+ T cells and B cells90,157. In contrast to these crucial effects of IFNγ on the pathogenesis of mouse lupus, B cell-intrinsic type I IFN signalling accelerates, but is not required for, the production of class-switched autoantibodies and spontaneous GC formation in the WAS model90.
Remarkably, these findings precisely model recent longitudinal studies that showed that initial SLE-associated autoantibodies develop years before clinical symptoms and that their appearance correlates with increased serum IFNγ levels159. By contrast, the type I IFN signature typically develops shortly before the diagnosis of SLE, years after the first detection of antinuclear antibodies159,160. Thus, increased levels of type I IFNs probably propagate autoimmunity through feed-forward mechanisms before the clinical diagnosis of SLE150–152, whereas B cell-intrinsic IFNγ signalling may be crucial for initial breaks in B cell tolerance that result in spontaneous GC formation, autoantibody production and a loss of T cell tolerance.
Importantly, the paradigm in which the serial accumulation of disease-associated autoantibodies precedes an increase in pro-inflammatory cytokines and disease onset is not unique to SLE. Rather, antibodies that are specific for islet cell antigens (including 65 kDa glutamic acid decarboxylase (GAD65; also known as glutamate decarboxylase 2), insulinoma-associated protein 2 (IA-2) and insulin) and cyclic citrullinated peptides develop years before clinical symptoms in T1D and RA, respectively161,162. Although the effect of specific cytokines on disease progression in T1D is less well understood (probably because such data would require local tissue sampling in the setting of organ-specific autoimmunity), an increase in the serum levels of pro-inflammatory cytokines predicts the imminent development of active RA163. In this context, there is increasing interest in examining whether immunomodulatory treatments given to at-risk individuals with latent autoimmunity can prevent progression to symptomatic disease. On the basis of the hypothesis that hydroxychloroquine might inhibit endolysosomal TLR signalling or processing of autoantigens for MHC class II-dependent presentation, clinical trials are in progress, or in development, to examine whether hydroxychloroquine treatment of asymptomatic, autoantibody-positive individuals can prevent or delay the development of RA (the StopRA trial164; ClinicalTrials.gov: NCT02603146) and T1D (run by the T1D TrialNet consortium; C. Greenbaum, personal communication), respectively. Thus, future studies that address the specific immune mechanisms and cytokine signalling pathways that are responsible for the initiation versus the progression of humoral autoimmunity may ultimately hold the promise of establishing treatments to prevent autoimmune disease in at-risk individuals.
Conclusions and future perspectives
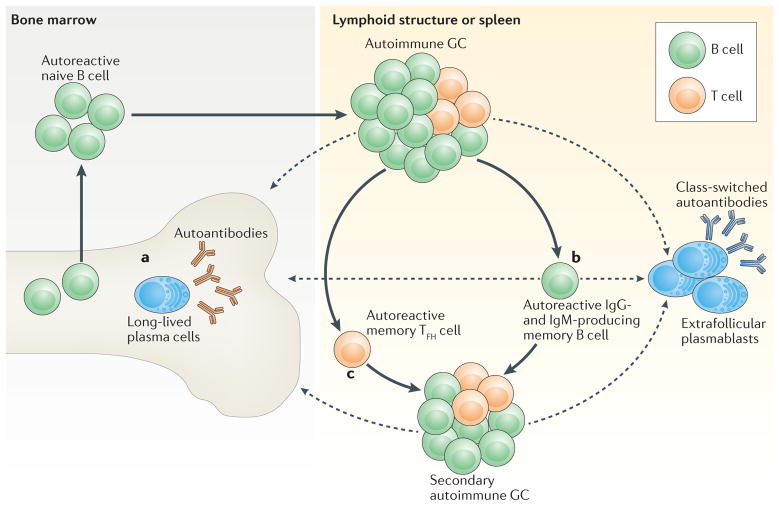
Emerging data from animal and human studies demonstrate that even modest alterations in B cell-intrinsic signalling programmes can be sufficient to promote breaks in T cell tolerance and initiate systemic autoimmunity. On the basis of these data, we propose a model in which GWAS-identified autoimmunity-associated variants and/or other genetic alterations in B cell gene products promote humoral autoimmunity through several distinct mechanisms. First, altered B cell signalling programmes increase the risk of autoimmunity by modulating positive and negative selection during B cell development, culminating in an increase in the proportion of mature B cells that exhibit autoreactivity. However, although contributing to disease risk, it is unlikely that increased autoreactivity alone is sufficient for disease development. As a crucial second step, autoantibody-producing B cells are generated from mature or naive B cells through parallel programmes that lead to extrafollicular and GC-dependent B cell activation, respectively. As described here, dual BCR and TLR signalling is crucial for the generation of short-lived, extrafollicular plasmablasts in a process that is markedly facilitated by BAFF-driven TACI signalling. In parallel, this BCR-driven and TLR-driven activation programme can also promote interactions between cognate T cells and B cells, which result in TFH cell differentiation and the formation of autoimmune GCs, leading to a break in T cell tolerance. As part of this iterative process, rounds of B cell antigen presentation allow for the display of a broad range of self-antigen-derived epitopes, and this probably promotes epitope spreading and the activation of additional autoreactive T cell and B cell clones (FIG. 4d). Finally, autoimmune GCs generate long-lived cell populations that are crucial for the maintenance and propagation of systemic autoimmunity (FIG. 5). Long-lived plasma cells derived from autoimmune GCs secrete pathogenic class-switched autoantibodies. In addition, autoreactive GC B cells can differentiate into IgM+ or class-switched memory B cells that can participate in the formation of new autoimmune GCs and/or self-reactive extrafollicular B cell responses. Finally, autoimmune GCs probably promote the generation of self-reactive memory TFH cells that can initiate the formation of new autoimmune GCs and/or instigate T cell-dependent extrafollicular responses165,166, events that probably no longer require triggering by self-reactive B cells.
Figure 5. A sequential model of the progression of systemic autoimmunity.
After initial breaks in B cell and T cell tolerance, long-lived effector and memory populations are generated within spontaneous, autoimmune germinal centres (GCs) and contribute to the maintenance and progression of systemic autoimmunity. After iterative rounds of B cell proliferation and selection, B cells that express high-affinity B cell receptors (BCRs) exit the GC as either plasma cells or memory B cells. a | In response to CXC-chemokine receptor 4 (CXCR4)-dependent signalling, autoantibody-secreting plasma cells traffic to the bone marrow, where they exhibit long-term survival within bone marrow stromal niches. b | Alternatively, GC B cells can differentiate into autoreactive memory B cells. Long-lived memory B cells probably contribute to the progression of autoimmunity and to disease relapse following treatment owing to an increased propensity for either differentiation into short-lived, autoantibody-secreting, extrafollicular plasmablasts, or recruitment to and activation within secondary autoimmune GCs. c | In addition to memory B cells, emerging evidence suggests that a subset of T follicular helper (TFH) cells differentiate into memory TFH cells that can seed secondary GCs and promote the progression of autoimmunity. Importantly, after these initial breaks in T cell tolerance and the establishment of autoimmune T cell memory, additional naive B cell clones that exhibit self-reactivity may be recruited into established GCs in the absence of initial priming signals, thereby broadening the range of high-affinity autoantibodies that are generated. Dashed arrows indicate the movement of long-lived and other plasma cell populations to bone marrow or other lymphoid compartments, respectively.
Key open questions that are relevant to these models include the issue of how specific GWAS variants influence these events and lead to altered tolerance. Rather than a series of linear events, it is likely that variants with greater effect may function across multiple signalling cascades, leading to alterations in the repertoire as well as in cell activation, and some variants may exert this effect across multiple immune cell lineages. It will be important to design studies that specifically define B cell-intrinsic and alternative lineage-intrinsic effects. Moreover, multiple autoimmunity-associated polymorphisms show evidence of selection during human evolution, which may reflect a trade-off between an enhanced immune response to infection and an increased risk of autoimmunity167,168. For example, the PTPN22R620W polymorphism may provide increased protection against tuberculosis169, and SLE-associated polymorphisms in FCGR2B (which encodes FcγRIIB) protect against severe Plasmodium falciparum infection170. We hypothesize that, by increasing the breadth of the naive B cell repertoire and by lowering B cell activation thresholds, genetic variants associated with autoimmunity also facilitate rapid antibody responses to pathogens and protect against infectious challenge. Gaining a better understanding of the basis of positive selection will provide insights into the autoimmune events that are driven by such ‘friendly fire’.
Importantly, for the vast majority of disease-associated genetic variants the mechanisms that influence immune responses remain undefined. In addition, two or more autoimmunity-associated variants may function in an additive and/or synergistic manner. In this regard, recent advances in gene editing techniques will probably facilitate mechanistic studies by, for example, allowing the introduction of multiple human-relevant risk alleles into embryonic stem cells or zygotes from mouse disease models or the direct editing of primary human haematopoietic cells. Finally, although the BCR and co-receptor signalling pathways are frequently considered individually, as highlighted here, there is extensive crosstalk between these pathways, and these coordinate signals are integral to B cell activation. Therefore, individual genetic polymorphisms will probably affect multiple signalling cascades, potentially across a range of immune cell types. New technologies, including single-cell transcriptome analysis, phospho-mass-spectroscopy and improved bioinformatic tools, have begun to address some of this complexity. In addition to mechanistic insights, these next-generation approaches may facilitate the identification of specific pathways that drive disease in individual patients. Ultimately, this more precise understanding of disease mechanisms may inform both individualized treatment decisions and the development of novel targeted therapies for multiple autoimmune and other disorders.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (NIH) under award numbers R01HL075453 (to D.J.R), R01A1084457 (to D.J.R.), R01A1071163 (to D.J.R.), DP3DK097672 (to D.J.R.), DP3DK111802 (to D.J.R.), DP3DK097672-01S1 (to G.M.), T32AI106677 (to M.W.-D.) and K08AI112993 (to S.W.J.). The content of this Review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional support of the work was provided by the Benaroya Family Gift Fund (to D.J.R.); a Howard Hughes Medical Institute–NIH Molecular Medicine Training Grant (to G.M.); the American College of Rheumatology Research and Education Foundation Rheumatology Scientist Development Award and Career Development K Supplement (to S.W.J.); an Arthritis National Research Foundation grant (to S.W.J.); a Novel Research Grant from the Lupus Research Alliance (to S.W.J.); and the Arnold Lee Smith Endowed Professorship for Research Faculty Development (to S.W.J.).
Glossary
- CD40
A tumour necrosis factor receptor superfamily member expressed by antigen-presenting cells that transmits activation signals in response to CD40 ligand (CD40L). Binding of CD40 on transitional B cells to CD40L on naive CD4+ T cells activates both the classical and alternative nuclear factor-κB pathways to promote cell survival
- Humoral autoimmunity
Causes a broad range of autoimmune diseases that are characterized by the production of pathogenic antibodies. These disorders frequently share risk alleles. For example, the R620W polymorphism in PTPN22 (which encodes protein tyrosine phosphatase nonreceptor 22) is associated with an increased risk of developing several diseases, including rheumatoid arthritis, type 1 diabetes, systemic lupus erythematosus, Hashimoto thyroiditis, Addison disease and others
- Extrafollicular B cell activation
In response to infectious challenge, B cell receptor signalling induces the rapid division and differentiation of B cells into short-lived, antibody-secreting plasmablasts at the T cell zone–red pulp border. This extrafollicular B cell response can occur in a T cell-independent or T cell-dependent manner, and is the predominant source of the protective antibodies that are generated early during an infection
- Germinal centre
(GC). A specialized lymphoid structure in which activated B cells cycle between anatomically distinct dark zones and light zones as they undergo iterative rounds of affinity selection, which ultimately leads to the generation of both antigen-specific memory B cells and high-affinity, long-lived plasma cells
- B cell-activating factor receptor
(BAFFR). A tumour necrosis factor receptor superfamily member that promotes the survival and maturation of B cells. Following ligation by B cell-activating factor (BAFF), BAFFR mainly induces the alternative nuclear factor-κB pathway to promote pro-survival signals. Mice that are deficient in BAFF or BAFFR have a block in B cell development at the transitional type 1 stage
- Systemic lupus erythematosus
(SLE). A chronic, multisystem inflammatory disease that is characterized by high-titre, class-switched autoantibodies against nuclear antigens; these autoantibodies are invariably present before disease onset and ultimately lead to the formation of immune complexes that precipitate systemic and/or organ-targeted injury
- Type 1 diabetes
(T1D). An autoimmune disease that is characterized by the destruction of insulin-producing pancreatic islet cells. Although T cells are crucial in this process, high-affinity, somatically mutated autoantibodies that target pancreatic islet antigens are present before disease onset, which emphasizes the importance of B cells in the pathogenesis of T1D
- Rheumatoid arthritis
(RA). A chronic, autoimmune disease that is characterized by symmetrical polyarticular arthritis and the production of rheumatoid factor and autoantibodies against cyclic citrullinated peptides; the appearance of rheumatoid factor and autoantibodies precedes clinical disease, implicating altered B cell responses early in the disease process
- Transmembrane activator and CAML interactor
(TACI). A tumour necrosis factor receptor family member that activates the classical nuclear factor-κB (NF-κB) pathway in response to ligation by multimeric B cell-activating factor (BAFF) or a proliferation-inducing ligand (APRIL; also known as TNFSF13) on developing and mature B cells. TACI signalling promotes T cell-independent antibody responses, and a subset of patients with common variable immunodeficiency have TACI mutations. TACI-deficient animals develop B cell hyperplasia, probably owing to increased serum BAFF levels
- Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome
(IPEX syndrome). A syndrome caused by a lack of functional regulatory T cells that is due to mutations in FOXP3 (which encodes forkhead box P3). It is characterized by severe autoimmunity driven by both activated effector T cells and multiple autoantibodies
- MRL.Faslpr
A strain of mouse generated by breeding multiple mouse strains to select those that exhibit features of lupus-like disease, including lethal autoimmunity and high-titre antinuclear antibodies. The lpr mutation is an autosomal-recessive mutation in Fas, which results in lymphoproliferation and accelerated autoimmunity
- NZB/W mice
F1 mice that are hybrids of NZB (New Zealand black) and NZW (New Zealand white) strains. They develop class-switched antinuclear antibodies, systemic inflammation and immune-complex-mediated glomerulonephritis. Autoimmune susceptibility loci in this strain include polymorphisms in Fcgr2b (which encodes Fcγ receptor IIB) and in signalling lymphocyte activation molecule (SLAM) family genes
- Non-obese diabetic mouse
(NOD mouse). A polygenic animal model of type 1 diabetes characterized by spontaneous leukocyte infiltration within the pancreatic islets and by insulin-dependent diabetes, with a bias towards accelerated disease in female mice. Disease in this model depends on both B cell and T cell activation
- Tlr7-transgenic mice
A mouse model of lupus generated by transgenic overexpression of the gene that encodes Toll-like receptor 7 (TLR7), which results in systemic autoimmunity and high titres of RNA-specific autoantibodies. The phenotype of Tlr7-transgenic mice partially mimics that of lupus-prone strains that express duplicated Tlr7 through the Y-chromosome-linked autoimmune accelerator (Yaa) mutation
- BXSB-Yaa
Lupus-prone male mice that develop accelerated disease due to Y-chromosome-linked autoimmune accelerator (Yaa), a translocation of the telomeric end of the X chromosome to the Y chromosome, which results in the duplication of several genes, including Tlr7 (which encodes Toll-like receptor 7)
- Roquinsan/san
A strain of mice with an N-ethyl-N-nitrosourea (ENU)-driven ‘san’ mutation in Roquin (which encodes a RING-type ubiquitin ligase; also known as Rc3h1), resulting in a lupus-like disease that is characterized by spontaneous germinal centre formation, high-affinity double-stranded DNA-specific antibodies, autoimmune thrombocytopenia and immune-complex-mediated glomerulonephritis
- B6.Sle1b
A strain of C57BL/6 mice that has the Sle1b sublocus derived from lupus-prone NZW mice. The Sle1b sublocus contains signalling lymphocyte activation molecule (SLAM) family genes with polymorphisms that are involved in antinuclear antibody production, B cell and T cell activation, and spontaneous germinal centre formation.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Deng Y, Tsao BP. Advances in lupus genetics and epigenetics. Curr Opin Rheumatol. 2014;26:482–492. doi: 10.1097/BOR.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niewold TB. Advances in lupus genetics. Curr Opin Rheumatol. 2015;27:440–447. doi: 10.1097/BOR.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarwood A, Huizinga TW, Worthington J. The genetics of rheumatoid arthritis: risk and protection in different stages of the evolution of RA. Rheumatology (Oxford) 2016;55:199–209. doi: 10.1093/rheumatology/keu323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkes M, Cortes A, van Heel DA, Brown MA. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat Rev Genet. 2013;14:661–673. doi: 10.1038/nrg3502. [DOI] [PubMed] [Google Scholar]
- 5.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–1654. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 6.Jackson SW, Kolhatkar NS, Rawlings DJ. B cells take the front seat: dysregulated B cell signals orchestrate loss of tolerance and autoantibody production. Curr Opin Immunol. 2015;33:70–77. doi: 10.1016/j.coi.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 8.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otipoby KL, et al. The B-cell antigen receptor integrates adaptive and innate immune signals. Proc Natl Acad Sci USA. 2015;112:12145–12150. doi: 10.1073/pnas.1516428112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JL, Chiles TC, Sen RJ, Rothstein TL. Inducible nuclear expression of NF-κB in primary B cells stimulated through the surface Ig receptor. J Immunol. 1991;146:1685–1691. [PubMed] [Google Scholar]
- 13.Norvell A, Mandik L, Monroe JG. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J Immunol. 1995;154:4404–4413. [PubMed] [Google Scholar]
- 14.Sater RA, Sandel PC, Monroe JG. B cell receptor-induced apoptosis in primary transitional murine B cells: signaling requirements and modulation by T cell help. Int Immunol. 1998;10:1673–1682. doi: 10.1093/intimm/10.11.1673. [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny JJ, O’Connell C, Sieckmann DG, Fischer RT, Longo DL. Selection of antigen-specific, idiotype-positive B cells in transgenic mice expressing a rearranged M167-μ heavy chain gene. J Exp Med. 1991;174:1189–1201. doi: 10.1084/jem.174.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–164. doi: 10.1038/nature11311. Using an in vivo reporter of BCR signalling, this paper describes how BCR engagement with self-ligand occurs across a continuum during transitional B cell development, thereby shaping peripheral B cell tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 19.Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP. Why is PTPN22 a good candidate susceptibility gene for autoimmune disease? FEBS Lett. 2011;585:3689–3698. doi: 10.1016/j.febslet.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Rawlings DJ, Dai X, Buckner JH. The role of PTPN22 risk variant in the development of autoimmunity: finding common ground between mouse and human. J Immunol. 2015;194:2977–2984. doi: 10.4049/jimmunol.1403034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyogoku C, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75:504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottini N, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 23.Begovich AB, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habib T, et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol. 2012;188:487–496. doi: 10.4049/jimmunol.1102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menard L, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rieck M, et al. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007;179:4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 27.Vang T, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 28.Dai X, et al. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest. 2013;123:2024–2036. doi: 10.1172/JCI66963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43:902–907. doi: 10.1038/ng.904. Reference 25 describes how the expression of a common autoimmunity-associated PTPN22 variant interferes with human B cell selection checkpoints, whereas references 28 and 29 report the phenotype of independently generated knock-in models of the homologous mouse gene. In combination, these studies demonstrate that the R620W polymorphism in PTPN22 promotes autoimmunity, in part through effects on B cell developmental selection and peripheral activation responses. [DOI] [PubMed] [Google Scholar]
- 30.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 31.Stadanlick JE, et al. Tonic B cell antigen receptor signals supply an NF-κB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SH, Cancro MP. Cutting edge: B cell receptor signals regulate BLyS receptor levels in mature B cells and their immediate progenitors. J Immunol. 2003;170:5820–5823. doi: 10.4049/jimmunol.170.12.5820. [DOI] [PubMed] [Google Scholar]
- 33.Schweighoffer E, et al. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity. 2013;38:475–488. doi: 10.1016/j.immuni.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jellusova J, et al. Context-specific BAFF-R signaling by the NF-κB and PI3K pathways. Cell Rep. 2013;5:1022–1035. doi: 10.1016/j.celrep.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hobeika E, et al. CD19 and BAFF-R can signal to promote B-cell survival in the absence of Syk. EMBO J. 2015;34:925–939. doi: 10.15252/embj.201489732. References 31, 33, 34 and 35 dissect the complex biochemical crosstalk between the BCR and BAFFR pathways that regulates context-dependent pro-survival programmes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 37.Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 2007;109:2339–2345. doi: 10.1182/blood-2006-05-021089. [DOI] [PubMed] [Google Scholar]
- 38.Rowland SL, Leahy KF, Halverson R, Torres RM, Pelanda R. BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J Immunol. 2010;185:4570–4581. doi: 10.4049/jimmunol.1001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackay F, et al. Mice transgenic for Baff develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stohl W, et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003;48:3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- 41.Navarra SV, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 42.Thien M, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Lesley R, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. References 42 and 43 describe how increased serum BAFF levels can rescue low-affinity self-reactive B cells from negative selection during peripheral B cell development. [DOI] [PubMed] [Google Scholar]
- 44.Hondowicz BD, et al. The role of BLyS/BLyS receptors in anti-chromatin B cell regulation. Int Immunol. 2007;19:465–475. doi: 10.1093/intimm/dxm011. [DOI] [PubMed] [Google Scholar]
- 45.Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isnardi I, et al. IRAK-4-and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuraoka M, et al. BCR and endosomal TLR signals synergize to increase AID expression and establish central B cell tolerance. Cell Rep. 2017;18:1627–1635. doi: 10.1016/j.celrep.2017.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann NY Acad Sci. 2011;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mills RE, et al. Unbiased modifier screen reveals that signal strength determines the regulatory role murine TLR9 plays in autoantibody production. J Immunol. 2015;194:3675–3686. doi: 10.4049/jimmunol.1500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker-Herman S, et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J Exp Med. 2011;208:2033–2042. doi: 10.1084/jem.20110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pala F, et al. Lentiviral-mediated gene therapy restores B cell tolerance in Wiskott–Aldrich syndrome patients. J Clin Invest. 2015;125:3941–3951. doi: 10.1172/JCI82249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolhatkar NS, et al. Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott–Aldrich syndrome. J Exp Med. 2015;212:1663–1677. doi: 10.1084/jem.20150585. Using WAS deficiency as a model of humoral autoimmunity, references 50 and 52 reveal how modestly enhanced BCR and TLR signalling can both promote the positive selection of self-reactive transitional B cells into the mature repertoire and trigger autoimmune GC responses, effects that result in B cell-driven systemic autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan WN, et al. Impaired B cell maturation in mice lacking Bruton’s tyrosine kinase (Btk) and CD40. Int Immunol. 1997;9:395–405. doi: 10.1093/intimm/9.3.395. [DOI] [PubMed] [Google Scholar]
- 54.Lesley R, Kelly LM, Xu Y, Cyster JG. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proc Natl Acad Sci USA. 2006;103:10717–10722. doi: 10.1073/pnas.0601539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz MA, Kolhatkar NS, Thouvenel C, Khim S, Rawlings DJ. CD4+ T cells and CD40 participate in selection and homeostasis of peripheral B cells. J Immunol. 2014;193:3492–3502. doi: 10.4049/jimmunol.1400798. Reference 54 demonstrates that naive CD4+ T cells augment the survival of developing B cells through CD40L–CD40 interactions, and reference 55 expands on this idea to show how CD40 signalling broadens the naive BCR repertoire. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castigli E, et al. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci USA. 1994;91:12135–12139. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castigli E, Young F, Carossino AM, Alt FW, Geha RS. CD40 expression and function in murine B cell ontogeny. Int Immunol. 1996;8:405–411. doi: 10.1093/intimm/8.3.405. [DOI] [PubMed] [Google Scholar]
- 58.Kinnunen T, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121:1595–1603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. This seminal paper demonstrates that >50% of immature B cells in humans exhibit self-reactivity and that these autoreactive specificities are partially removed from the mature naive repertoire at two discrete checkpoints during B cell development. [DOI] [PubMed] [Google Scholar]
- 60.Hoyer BF, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci USA. 2010;107:4658–4663. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahevas M, Michel M, Weill JC, Reynaud CA. Long-lived plasma cells in autoimmunity: lessons from B-cell depleting therapy. Front Immunol. 2013;4:494. doi: 10.3389/fimmu.2013.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. This study provides the first demonstration that SHM and class-switch recombination of autoreactive B cells can occur outside of GCs within extrafollicular foci in the spleen. [DOI] [PubMed] [Google Scholar]
- 64.Groom JR, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobi AM, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1332–1342. doi: 10.1002/art.10949. [DOI] [PubMed] [Google Scholar]
- 66.Banchereau R, et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell. 2016;165:551–565. doi: 10.1016/j.cell.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tipton CM, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16:755–765. doi: 10.1038/ni.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leadbetter EA, et al. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 69.Hou B, et al. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011;34:375–384. doi: 10.1016/j.immuni.2011.01.011. This study demonstrates that the physical context of antigen and adjuvant influences the ability of TLR ligands to enhance antibody formation and shows that B cell-intrinsic MYD88 signalling is crucial for antiviral responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nickerson KM, et al. TLR9 regulates TLR7-and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christensen SR, et al. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Yasuda K, et al. Murine dendritic cell type I IFN production induced by human IgG–RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J Immunol. 2007;178:6876–6885. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- 76.Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity. 2013;38:528–540. doi: 10.1016/j.immuni.2012.11.017. References 64, 68, 74 and 76 highlight the crucial importance of integrated BCR and TLR signalling in promoting B cell activation and antinuclear antibody production in SLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson JS, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 78.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 80.Figgett WA, et al. Deleting the BAFF receptor TACI protects against systemic lupus erythematosus without extensive reduction of B cell numbers. J Autoimmun. 2015;61:9–16. doi: 10.1016/j.jaut.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 81.Jacobs HM, et al. Cutting edge: BAFF promotes autoantibody production via TACI-dependent activation of transitional B cells. J Immunol. 2016;196:3525–3531. doi: 10.4049/jimmunol.1600017. References 80 and 81 highlight the crucial importance of B cell-intrinsic TACI signalling in the generation of class-switched autoantibodies in mice that overexpress BAFF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carter LM, Isenberg DA, Ehrenstein MR. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum. 2013;65:2672–2679. doi: 10.1002/art.38074. [DOI] [PubMed] [Google Scholar]
- 83.Cambridge G, et al. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum. 2006;54:723–732. doi: 10.1002/art.21650. [DOI] [PubMed] [Google Scholar]
- 84.Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20:349–360. doi: 10.1016/j.beha.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 85.MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 86.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 87.Wellmann U, et al. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci USA. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jackson SW, et al. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J Immunol. 2014;192:4525–4532. doi: 10.4049/jimmunol.1400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hua Z, et al. Requirement for MyD88 signaling in B cells and dendritic cells for germinal center antinuclear antibody production in Lyn-deficient mice. J Immunol. 2014;192:875–885. doi: 10.4049/jimmunol.1300683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jackson SW, et al. B cell IFN-γ receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med. 2016;213:733–750. doi: 10.1084/jem.20151724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 92.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott–Aldrich syndrome. J Pediatr. 1994;125:876–885. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 93.Crestani E, et al. Broad spectrum of autoantibodies in patients with Wiskott–Aldrich syndrome and X-linked thrombocytopenia. J Allergy Clin Immunol. 2015;136:1401–1404.e3. doi: 10.1016/j.jaci.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Recher M, et al. B cell-intrinsic deficiency of the Wiskott–Aldrich syndrome protein (WASp) causes severe abnormalities of the peripheral B-cell compartment in mice. Blood. 2012;119:2819–2828. doi: 10.1182/blood-2011-09-379412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lamagna C, Hu Y, DeFranco AL, Lowell CA. B cell-specific loss of Lyn kinase leads to autoimmunity. J Immunol. 2014;192:919–928. doi: 10.4049/jimmunol.1301979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 97.Harley JB, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hom G, et al. Association of systemic lupus erythematosus with C8orf13–BLK and ITGAM–ITGAX. N Engl J Med. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- 99.Lu R, et al. Genetic associations of LYN with systemic lupus erythematosus. Genes Immun. 2009;10:397–403. doi: 10.1038/gene.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Surolia I, et al. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 2010;466:243–247. doi: 10.1038/nature09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soni C, et al. Distinct and synergistic roles of FcγRIIB deficiency and 129 strain-derived SLAM family proteins in the development of spontaneous germinal centers and autoimmunity. J Autoimmun. 2015;63:31–46. doi: 10.1016/j.jaut.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bygrave AE, et al. Spontaneous autoimmunity in 129 and C57BL/6 mice—implications for autoimmunity described in gene-targeted mice. PLoS Biol. 2004;2:E243. doi: 10.1371/journal.pbio.0020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corneth OB, et al. Enhanced expression of Bruton’s tyrosine kinase in B cells drives systemic autoimmunity by disrupting T cell homeostasis. J Immunol. 2016;197:58–67. doi: 10.4049/jimmunol.1600208. [DOI] [PubMed] [Google Scholar]
- 104.Edwards JC, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 105.Stone JH, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pescovitz MD, et al. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dahan K, et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2016;28:348–358. doi: 10.1681/ASN.2016040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355:1772–1779. doi: 10.1056/NEJMoa062930. [DOI] [PubMed] [Google Scholar]
- 109.Hauser SL, et al. B-Cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 110.Beck LH, Jr, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pendergraft WF, et al. Long-term maintenance therapy using rituximab-induced continuous B-cell depletion in patients with ANCA vasculitis. Clin J Am Soc Nephrol. 2014;9:736–744. doi: 10.2215/CJN.07340713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cornec D, Avouac J, Youinou P, Saraux A. Critical analysis of rituximab-induced serological changes in connective tissue diseases. Autoimmun Rev. 2009;8:515–519. doi: 10.1016/j.autrev.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- 115.Wong FS, et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes. 2004;53:2581–2587. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- 116.Noorchashm H, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet β cells of nonobese diabetic mice. J Immunol. 1999;163:743–750. [PubMed] [Google Scholar]
- 117.Molnarfi N, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Giles JR, Kashgarian M, Koni PA, Shlomchik MJ. B cell-specific MHC class II deletion reveals multiple nonredundant roles for B cell antigen presentation in murine lupus. J Immunol. 2015;195:2571–2579. doi: 10.4049/jimmunol.1500792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wan X, Thomas JW, Unanue ER. Class-switched anti-insulin antibodies originate from unconventional antigen presentation in multiple lymphoid sites. J Exp Med. 2016;213:967–978. doi: 10.1084/jem.20151869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soni C, et al. B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J Immunol. 2014;193:4400–4414. doi: 10.4049/jimmunol.1401720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walsh ER, et al. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc Natl Acad Sci USA. 2012;109:16276–16281. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shen N, et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci USA. 2010;107:15838–15843. doi: 10.1073/pnas.1001337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garcia-Ortiz H, et al. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann Rheum Dis. 2010;69:1861–1865. doi: 10.1136/ard.2009.124313. [DOI] [PubMed] [Google Scholar]
- 125.Kawasaki A, et al. TLR7 single-nucleotide polymorphisms in the 3′ untranslated region and intron 2 independently contribute to systemic lupus erythematosus in Japanese women: a case-control association study. Arthritis Res Ther. 2011;13:R41. doi: 10.1186/ar3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nickerson KM, et al. TLR9 promotes tolerance by restricting survival of anergic anti-DNA B cells, yet is also required for their activation. J Immunol. 2013;190:1447–1456. doi: 10.4049/jimmunol.1202115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Han JW, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41:1234–1237. doi: 10.1038/ng.472. [DOI] [PubMed] [Google Scholar]
- 128.Gateva V, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 130.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. References 129–131 demonstrate the crucial role of IL-21 in promoting GC formation by facilitating the differentiation of TFH cells and GC B cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bubier JA, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci USA. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ozaki K, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 134.Vinuesa CG, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 135.Dolff S, et al. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R157. doi: 10.1186/ar3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rankin AL, et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Faslpr/lpr/J mice. J Immunol. 2012;188:1656–1667. doi: 10.4049/jimmunol.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bubier JA, et al. Treatment of BXSB-Yaa mice with IL-21R–Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann NY Acad Sci. 2007;1110:590–601. doi: 10.1196/annals.1423.063. [DOI] [PubMed] [Google Scholar]
- 138.McPhee CG, et al. IL-21 is a double-edged sword in the systemic lupus erythematosus-like disease of BXSB.Yaa mice. J Immunol. 2013;191:4581–4588. doi: 10.4049/jimmunol.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med. 1998;188:1895–1906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Karnowski A, et al. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med. 2012;209:2049–2064. doi: 10.1084/jem.20111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Poholek AC, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Linker-Israeli M, et al. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- 144.Jain S, et al. Interleukin 6 accelerates mortality by promoting the progression of the systemic lupus erythematosus-like disease of BXSB.Yaa mice. PLoS ONE. 2016;11:e0153059. doi: 10.1371/journal.pone.0153059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Illei GG, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vanden Bush TJ, Bishop GA. TLR7 and CD40 cooperate in IL-6 production via enhanced JNK and AP-1 activation. Eur J Immunol. 2008;38:400–409. doi: 10.1002/eji.200737602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.de Valle E, et al. NFκB1 is essential to prevent the development of multiorgan autoimmunity by limiting IL-6 production in follicular B cells. J Exp Med. 2016;213:621–641. doi: 10.1084/jem.20151182. Reference 140 demonstrates that combined deletion of Il6 and Il21 prevents antiviral GC responses, which are partially restored by B cell-derived IL-6. Consistent with roles for B cell-derived IL-6 production in autoimmunity, reference 147 shows that spontaneous GC formation in the setting of B cell-intrinsic Nfkb1 deletion correlates with IL-6 production by activated B cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goenka R, et al. Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med. 2014;211:45–56. doi: 10.1084/jem.20130505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wensveen FM, Slinger E, van Attekum MH, Brink R, Eldering E. Antigen-affinity controls pregerminal center B cell selection by promoting Mcl-1 induction through BAFF receptor signaling. Sci Rep. 2016;6:35673. doi: 10.1038/srep35673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baechler EC, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kirou KA, et al. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 153.Deng Y, Tsao BP. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat Rev Rheumatol. 2010;6:683–692. doi: 10.1038/nrrheum.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kiefer K, Oropallo MA, Cancro MP, Marshak-Rothstein A. Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol. 2012;90:498–504. doi: 10.1038/icb.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Haas C, Ryffel B, Le Hir M. IFN-γ is essential for the development of autoimmune glomerulonephritis in MRL/Ipr mice. J Immunol. 1997;158:5484–5491. [PubMed] [Google Scholar]
- 156.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-γ receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Faslpr mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- 157.Lee SK, et al. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 158.Domeier PP, et al. IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J Exp Med. 2016;213:715–732. doi: 10.1084/jem.20151722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Munroe ME, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis. 2016;75:2014–2021. doi: 10.1136/annrheumdis-2015-208140. References 90, 157 and 158 demonstrate the crucial importance of IFNγ signalling in promoting spontaneous GC formation in mouse lupus and show that it acts through the cell-intrinsic induction of the differentiation of TFH cells and GC B cells. Reference 159 corroborated the importance of this pro-inflammatory cytokine in human SLE by demonstrating that increased serum IFNγ levels correlate with the first appearance of serum autoantibodies years prior to a clinical diagnosis of SLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arbuckle MR, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 161.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl 2):S52–S61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 162.Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am. 2010;36:213–241. doi: 10.1016/j.rdc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sokolove J, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS ONE. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.US National Library of Medicine. ClinicalTrialsgov. 2017 https://clinicaltrials.gov/ct2/show/NCT02603146.
- 165.Qi H. T follicular helper cells in space-time. Nat Rev Immunol. 2016;16:612–625. doi: 10.1038/nri.2016.94. [DOI] [PubMed] [Google Scholar]
- 166.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Raj T, et al. Common risk alleles for inflammatory diseases are targets of recent positive selection. Am J Hum Genet. 2013;92:517–529. doi: 10.1016/j.ajhg.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ramos PS, Shedlock AM, Langefeld CD. Genetics of autoimmune diseases: insights from population genetics. J Hum Genet. 2015;60:657–664. doi: 10.1038/jhg.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gomez LM, Anaya JM, Martin J. Genetic influence of PTPN22 R620W polymorphism in tuberculosis. Hum Immunol. 2005;66:1242–1247. doi: 10.1016/j.humimm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 170.Clatworthy MR, et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcγRIIb reduce susceptibility to malaria. Proc Natl Acad Sci USA. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hartley SB, et al. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 172.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 173.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-Cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gaudin E, et al. Positive selection of B cells expressing low densities of self-reactive BCRs. J Exp Med. 2004;199:843–853. doi: 10.1084/jem.20030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cyster JG, et al. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996;381:325–328. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 176.Julien S, Soulas P, Garaud JC, Martin T, Pasquali JL. B cell positive selection by soluble self-antigen. J Immunol. 2002;169:4198–4204. doi: 10.4049/jimmunol.169.8.4198. [DOI] [PubMed] [Google Scholar]
- 177.Gu H, Tarlinton D, Muller W, Rajewsky K, Forster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Levine MH, et al. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci USA. 2000;97:2743–2748. doi: 10.1073/pnas.050552997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 180.Richardson C, et al. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J Immunol. 2013;191:4926–4939. doi: 10.4049/jimmunol.1202263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jenks SA, et al. 9G4+ autoantibodies are an important source of apoptotic cell reactivity associated with high levels of disease activity in systemic lupus erythematosus. Arthritis Rheum. 2013;65:3165–3175. doi: 10.1002/art.38138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pascual V, et al. Nucleotide sequence analysis of the V regions of two IgM cold agglutinins. Evidence that the VH4-21 gene segment is responsible for the major cross-reactive idiotype. J Immunol. 1991;146:4385–4391. [PubMed] [Google Scholar]
- 183.Thompson KM, et al. Human monoclonal antibodies against blood group antigens preferentially express a VH4-21 variable region gene-associated epitope. Scand J Immunol. 1991;34:509–518. doi: 10.1111/j.1365-3083.1991.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 184.Pugh-Bernard AE, et al. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cappione A, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. References 67, 180 and 183–185 describe important roles of B cells that express the intrinsically autoreactive VH4-34 heavy chain in the pathogenesis in SLE. Reference 67 also demonstrates that a substantial proportion of VH4-34+ antibody-secreting cells are derived from recently-activated, clonally-expanded B cells, which implicates ongoing B cell activation outside the GC in the pathogenesis of SLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Isenberg D, Spellerberg M, Williams W, Griffiths M, Stevenson F. Identification of the 9G4 idiotope in systemic lupus erythematosus. Br J Rheumatol. 1993;32:876–882. doi: 10.1093/rheumatology/32.10.876. [DOI] [PubMed] [Google Scholar]
- 187.Stevenson FK, et al. Utilization of the VH4-21 gene segment by anti-DNA antibodies from patients with systemic lupus erythematosus. J Autoimmun. 1993;6:809–825. doi: 10.1006/jaut.1993.1066. [DOI] [PubMed] [Google Scholar]
- 188.van Vollenhoven RF, et al. VH4-34 encoded antibodies in systemic lupus erythematosus: a specific diagnostic marker that correlates with clinical disease characteristics. J Rheumatol. 1999;26:1727–1733. [PubMed] [Google Scholar]