Abstract
Background and aims
Lipoprotein(a) [Lp(a)] is a proatherogenic lipoprotein associated with coronary heart disease, ischemic stroke, and more recently aortic stenosis and heart failure (HF). We examined the association of Lp(a) levels with incident HF hospitalization in the Atherosclerosis Risk in Communities (ARIC) study. We also assessed the relationship between Lp(a) levels and arterial stiffness as a potential mechanism for development of HF.
Methods
Lp(a) was measured in 14,154 ARIC participants without prevalent HF at ARIC visit 1 (1987–1989). The association of Lp(a) quintiles with incident HF hospitalization was assessed using Cox proportional-hazards models. Arterial stiffness parameters were stratified based on Lp(a) quintiles, and p-trend was calculated across ordered groups.
Results
At median follow-up of 23.4 years, there were 2,605 incident HF hospitalizations. Lp(a) levels were directly associated with incident HF hospitalization in models adjusted for age, race, gender, systolic blood pressure, history of hypertension, diabetes, smoking status, body mass index, heart rate, and high-density lipoprotein cholesterol (quintile 5 vs. quintile 1: hazard ratio [HR] 1.24, 95% confidence interval [CI] 1.09–1.41; p-trend across increasing quintiles <0.01), but not after excluding prevalent and incident myocardial infarction cases (HR 1.07, 95% CI 0.91–1.27; p-trend = 0.70). When adjusted for age, gender, and race, Lp(a) quintiles were not significantly associated with arterial stiffness parameters.
Conclusions
Increased Lp(a) levels were associated with increased risk of incident HF hospitalization. After excluding prevalent and incident myocardial infarction, the association was no longer significant. Lp(a) levels were not associated with arterial stiffness parameters.
Keywords: lipoproteins, heart failure, risk factors, risk prediction
Introduction
Lipoprotein (a) [Lp(a)] is a proatherogenic lipoprotein composed of a low-density lipoprotein (LDL)–like moiety with a unique glycoprotein, apolipoprotein (a) [apo(a)], which is covalently linked to a single molecule of apolipoprotein B-100 (apoB-100) of the LDL moiety. Elevated plasma levels of Lp(a) are a significant risk factor for atherosclerotic cardiovascular disease.1–6 We have previously shown that high levels of Lp(a) are significantly associated with an increased risk for coronary heart disease (CHD) and stroke in the Atherosclerosis Risk in Communities (ARIC) cohort.1,7
Elevated levels of Lp(a) may be associated with higher risk for heart failure for several reasons. Given its atherothrombotic properties, Lp(a) may increase the risk for heart failure after ischemic myocardial injury. Additionally, Lp(a) has been implicated in the development of aortic valve stenosis,8–12 a phenomenon thought to be related to valvular calcification and stiffness, which also contribute to the development of heart failure.13 Furthermore, through enhanced atherosclerosis, Lp(a) may increase arterial stiffness, which could also augment heart failure risk.14
To our knowledge, only one study has assessed the relationship between Lp(a) levels and risk for heart failure.15 In addition, no published study has examined the relationships among Lp(a) levels, arterial stiffness, and heart failure. We hypothesized that higher levels of Lp(a) would be associated with greater risk for incident heart failure hospitalization. We also postulated that increased Lp(a) levels would be associated with increased arterial stiffness and subsequent incident heart failure hospitalization.
Therefore, the purpose of this study is to examine the association of Lp(a) levels with incident heart failure hospitalization and arterial stiffness in the ARIC study.
Participants and methods
Study participants
The ARIC study is a prospective study of cardiovascular disease incidence in 15,792 men and women between the ages of 45 and 64, who were recruited from four US communities (Minneapolis, Minnesota; Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi) in 1987–1989. Additional description of the ARIC study design has been published elsewhere.16 At ARIC visit 1 (1987–1989), 752 participants had a preexisting diagnosis of heart failure. We excluded those without data on Lp(a), incident heart failure hospitalization, or covariates (n = 1,535). We also excluded race other than African American or White (n = 48) and African Americans from Minnesota and Washington County field centers (n = 55), as these cohorts have numbers that are too small for adequate statistical adjustment. This resulted in a total of 14,154 participants who were included in our analysis of Lp(a) and incident heart failure hospitalization (Supplemental Fig. 1). Arterial stiffness parameters from ARIC visit 2 (1990–1992) were available in 9,523 participants, in whom we assessed associations of Lp(a) with arterial stiffness parameters and heart failure.
The apo(a) component of Lp(a) may contain a variable number of kringle IV type 2 repeats that can affect characteristics such as isoform size and plasma Lp(a) levels.17,18 ARIC investigators measured Lp(a) at ARIC visit 1 with a kringle IV type 2 repeat–sensitive assay19 for analysis of Lp(a) and incident heart failure hospitalization. We performed confirmatory analyses with Lp(a) values measured a decade later at visit 4 (1996–1998) using a kringle IV type 2 repeat–insensitive assay.20 Participants with prevalent heart failure at visit 4 were excluded.
Outcomes and covariates
Incident heart failure hospitalization was defined by International Statistical Classification of Diseases and Related Health Problems (ICD) codes of 428.x (9th Revision) or I50 (10th Revision) in any position on the hospital discharge list or on a death certificate with death from heart failure in any position.21 CHD events are defined as definite or probable MI, fatal CHD, or cardiac procedure.
Cigarette smoking and the use of antihypertensive and lipid-lowering medications were obtained from a standardized questionnaire. Hypertension was defined as systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mmHg, or the use of antihypertensive medications during the previous 2 weeks. Diabetes was defined as a fasting plasma glucose level ≥126 mg/dL, a nonfasting plasma glucose level ≥200 mg/dL, or a self-reported history of physician-diagnosed diabetes or treatment for diabetes.
Lipids and lipoproteins
Lipid measurements were performed on 12-hour fasting plasma samples that were stored at −70°C with ethylenediaminetetraacetic (EDTA) acid as the anticoagulant. Plasma total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides were measured using enzymatic methods.22 LDL cholesterol (LDL-C) was calculated using the Friedewald equation.23 At visit 1, Lp(a) was measured using a double-antibody enzyme-linked immunosorbent assay technique as previously described19 and at visit 4 using a commercially available automated immunoturbidimetric assay (Denka Seiken Co. Ltd., Tokyo, Japan).20 Lp(a) values at visit 1 were standardized using a conversion equation derived from a comparison between samples at visit 1 measured by both assays in 100 samples from an entire Lp(a) distribution with equal representation from both genders and ethnic groups, and there was excellent correlation (Pearson r=0.88) without evidence of systematic bias at high or low Lp(a) levels, as previously described.1
Ultrasound imaging and determination of arterial wall parameters
At ARIC visit 2, participants were asked to refrain from smoking, vigorous exercise, and caffeine the night prior to ultrasound imaging. Methods for the acquisition of electrocardiography-gated B-mode ultrasound images and for echo tracking of arterial diameter in the ARIC study have been described previously.16,24–26 Arterial wall characteristics were determined by measuring arterial diameter changes over the cardiac cycle for an average of 5.6 cardiac cycles. Arterial wall characteristics were derived from ultrasound measurements as well as from supine brachial blood pressure measured at ARIC visit 2. The arterial wall parameters used in the current study include pressure-strain modulus, carotid arterial strain, arterial distensibility, arterial compliance, and stiffness index,24–26 and equations used to calculate these indices are shown in Supplemental Table 1. Higher pressure-strain modulus and stiffness index and lower carotid arterial strain, arterial distensibility, and compliance suggest increased arterial stiffness.25
Statistical analysis
In the current analysis, we examined the association between Lp(a) levels from ARIC visit 1 and incident heart failure hospitalization. Characteristics were compared by Lp(a) levels at baseline (ARIC visit 1) using chi-squared test for categorical variables and Student’s t-test or Kruskal–Wallis rank test for continuous variables as appropriate. The p-trend tests a linear increase in log relative hazard with increasing quintiles. Participants were followed up for incident heart failure hospitalization or until loss to follow-up, death, or December 31, 2012 (whichever came first). We used multivariable Cox proportional-hazards regression models to investigate the association between visit 1 Lp(a) levels and incident heart failure hospitalization. Lp(a) level was treated as a categorical variable by quintiles. The lowest quintile was considered as the reference in the categorical analysis. The proportional hazard assumption was confirmed using time-dependent covariates and likelihood ratio tests. We adjusted for age, gender, and race in model 1. In model 2, we additionally adjusted for systolic blood pressure, history of hypertension, diabetes, current smoking status, body mass index, and heart rate (which have been shown to predict incident heart failure in ARIC).27 In model 3, we also added HDL-C. In model 4, we additionally adjusted for prevalent CHD at baseline to assess if the relationship would attenuate after accounting for prevalent CHD. Model 5 adjusts for LDL-C in addition to covariates used in models 1–4. Finally, model 6 additionally adjusts for Lp(a)- cholesterol corrected LDL-C that is not taken into account by the Friedewald equation. Lp(a)- cholesterol corrected LDL-C is derived using the formula Lp(a)-cholesterol = Lp(a) mass (mg/dL) × 0.3.28 Using models 1–6 as described, we also examined the relationship between Lp(a) levels and incident heart failure hospitalization after excluding both prevalent and incident myocardial infarction (MI) during the follow up period. Confirmatory analyses were done in visit 4 samples (1996–1998) using the Denka Seiken assay to measure Lp(a) levels.
We also assessed the relationship between Lp(a) and vascular arterial stiffness parameters. We examined the distribution of various arterial stiffness parameters across Lp(a) quintiles in unadjusted models and in models adjusted for age, gender and race, since the arterial stiffness parameters can vary by these demographic variables.29
The institutional review boards at all participating centers approved the ARIC study protocol, and all participants provided informed consent. We used SAS version 9.4 (SAS Institute Inc., Cary, NC) and Stata version 12 (StataCorp, College Station, TX) to carry out the above analyses. All tests were 2-tailed with a p-value <0.05 considered statistically significant.
Results
Baseline characteristics
Among the 14,154 ARIC participants included for the analysis of Lp(a) and incident heart failure hospitalization, mean age was 55 years; 55% were women, and 25% were African Americans. The mean follow-up period was 20±6.55 years (median follow-up [25th, 75th percentiles], 23.4 [16.8, 24.5] years). The 5th (highest) quintile of Lp(a) included more women (62% vs. 50% in quintile 1, p <0.001) and African Americans (46% vs. 6% in quintile 1, p <0.001) (Table 1). Participants in quintile 5 were also more likely to have a higher prevalence of cardiovascular risk factors and higher levels of total cholesterol and LDL-C than those in quintile 1. Individuals with Lp(a) levels in the highest quintile were also more likely to have incident MI and CHD after visit 1 than individuals in quintile 1.
Table 1.
Baseline characteristics in the overall cohort based on Lp(a) quintiles at ARIC visit 1.
| Lp(a) Quintiles | p trend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| n | 2943 | 2742 | 2815 | 2825 | 2829 | |
| Lp(a) range (mg/dL) | 0.02–2.41 | 2.54–5.59 | 5.73–11.29 | 11.43–22.96 | 23.10–108.23 | <0.001 |
| Age at baseline (years) | 54.0 ± 5.7 | 54.2 ± 5.7 | 54.1 ± 5.8 | 54.0 ± 5.8 | 54.1 ± 5.7 | 0.87 |
| Female (%) | 49.6 | 51.3 | 54.8 | 55.1 | 61.6 | <0.001 |
| African American (%) | 5.7 | 10.1 | 25.5 | 40.0 | 45.6 | <0.001 |
| Hypertension (%) | 28.3 | 26.6 | 30.8 | 37.1 | 39.3 | <0.001 |
| Diabetes (%) | 10.5 | 8.5 | 10.3 | 12.6 | 12.6 | <0.001 |
| Total cholesterol (mg/dL) | 207.6 ± 40.7 | 210.6 ± 39.8 | 212.9 ± 40.7 | 215.3 ± 41.5 | 228.1 ± 43.1 | <0.001 |
| LDL-C (mg/dL) | 129.2 ± 37.8 | 133.6 ± 36.8 | 136.7 ± 38.4 | 138.7 ± 39.2 | 150.2 ± 40.5 | <0.001 |
| HDL-C (mg/dL) | 50.4 ± 18.0 | 50.7 ± 16.7 | 51.7 ± 16.4 | 52.1 ± 16.6 | 54.1 ± 17.4 | <0.001 |
| Triglycerides (mg/dL) | 119 (82, 175) |
113 (80, 159) |
106 (77, 150) |
104 (76, 150) |
104 (76, 143) |
<0.001 |
| BMI (kg/m2) | 27.1 ± 4.8 | 26.9 ± 4.8 | 27.7 ± 5.2 | 27.9 ± 5.5 | 28.0 ± 5.7 | <0.001 |
| Current smoking (%) | 25.5 | 25.7 | 26.0 | 25.7 | 26.3 | 0.53 |
| Prevalent CHD (%) | 3.9 | 4.1 | 3.3 | 3.8 | 5.5 | 0.02 |
| Statin user (%) | 0.5 | 0.4 | 0.6 | 0.4 | 0.9 | 0.09 |
| Incident CHD (%) | 19.6 | 19.7 | 18.5 | 21.2 | 23.3 | <0.001 |
| Prevalent MI (%) | 3.2 | 3.3 | 3.0 | 3.2 | 4.7 | 0.01 |
| Incident MI (%) | 9.0 | 9.2 | 10.2 | 11.2 | 12.1 | <0.001 |
Data were expressed as mean ± SD or median (25th, 75th percentiles) for continuous variables and percentage for categorical variables.
BMI, body mass index; CHD, coronary heart disease; HDL-C, high-density lipoprotein cholesterol; Lp(a), lipoprotein(a); LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction.
Lp(a) levels and incident heart failure hospitalization
The median Lp(a) levels at visit 1 were 8 mg/dL for participants without heart failure and 10 mg/dL for participants with incident heart failure hospitalization. There were 2,605 incident heart failure hospitalizations after visit 1. Higher Lp(a) quintiles at visit 1 were significantly associated with greater risk for incident heart failure hospitalization in unadjusted models (log-rank p <0.001; Fig. 1) as well as adjusted models (Table 2). The risk was apparent in higher Lp(a) quintiles (i.e., quintiles 4–5) only, not in lower quintiles. Sequential adjustments in the models described earlier did not meaningfully alter the associations of Lp(a) and incident heart failure hospitalization within each quintile (Table 2). When adjusted for age, gender, race, systolic blood pressure, hypertension, diabetes, current smoking, body mass index, heart rate, and HDL-C (i.e., model 3), higher Lp(a) quintiles were significantly associated with an increased risk for incident heart failure hospitalization (quintile 5 vs. quintile 1: hazard ratio [HR] 1.24, 95% confidence interval [CI] 1.09–1.41; p-trend across increasing quintiles <0.01). Upon exclusion of participants with prevalent and incident MI (n = 490 and 1,411, respectively), the association between Lp(a) levels and incident heart failure hospitalization was no longer significant (quintile 5 vs. quintile 1: HR 1.07, 95% CI 0.91–1.27; p-trend across increasing quintiles = 0.70; Table 3). There was no significant interaction by race, gender, or diabetes (p for interaction: 0.65, 0.22, and 0.31, respectively). There was a significant association between the top 10% of Lp(a) values (Lp(a) range 33–108 mg/dL) and incident heart failure hospitalization (vs lowest quintile: HR 1.34, 95% CI 1.12–1.60; p = 0.001), but this association no longer remained significant after MI was excluded (HR 1.16, 95% CI 0.91–1.47). Results based on Lp(a) levels from visit 4, measured approximately a decade later using an automated assay, were qualitatively similar to those from visit 1 (p-trend across quintiles = 0.02; Supplemental Table 2). The results at visit 4 were also similar for the top 10% versus the lowest quintile: there was a significant association with incident heart failure hospitalization (HR 1.25, 95% CI 1.00–1.55; p = 0.046), but this association no longer remained significant after MI was excluded (HR 1.18, 95% CI 0.90–1.55; p = 0.230).
Fig. 1.
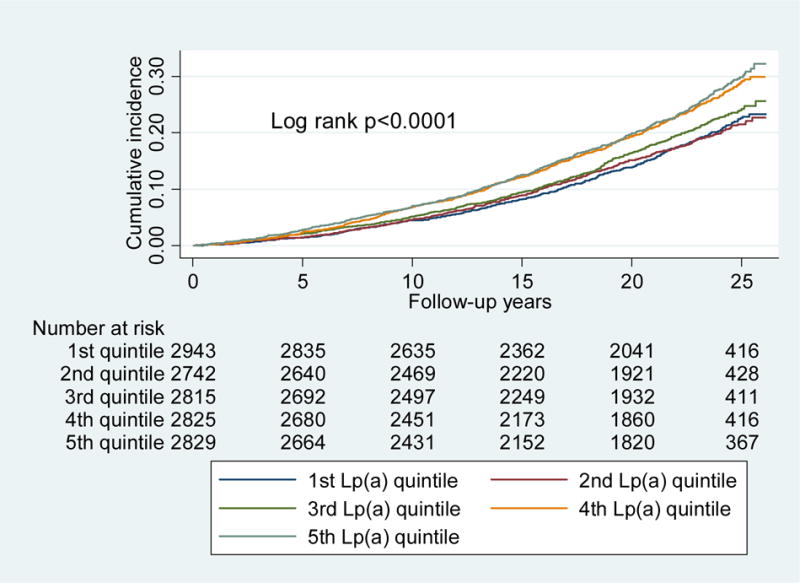
Cumulative incidence of heart failure hospitalization by Lp(a) quintiles at ARIC visit 1.
Higher Lp(a) quintiles at visit 1 were significantly associated with greater risk for incident heart failure hospitalization in unadjusted models. p values were calculated by log-rank trend tests of survival functions across Lp(a) quintiles.
Table 2.
ARIC visit 1 Lp(a) quintiles hazard ratios (95% CI) for incident heart failure hospitalization (prevalent MI included).
| Lp(a) [HF/at risk] | Hazard ratio (95% confidence interval) | p trend | ||||
|---|---|---|---|---|---|---|
| Quintile 1 (0.02–2.41) [479/2943 (16%)] |
Quintile 2 (2.54–5.59) [447/2742 (16%)] |
Quintile 3 (5.73–11.29) [502/2815 (18%)] |
Quintile 4 (11.43–22.96) [583/2825 (21%)] |
Quintile 5 (23.10–108.23) [594/2829 (21%)] |
||
| Model 1 | reference | 0.96 (0.84–1.09) |
1.01 (0.89–1.14) |
1.14 (1.00–1.29) |
1.14 (1.00–1.29) |
0.02 |
| Model 2 | reference | 1.03 (0.90–1.17) |
1.09 (0.95–1.23) |
1.21 (1.06–1.37) |
1.23 (1.08–1.40) |
0.004 |
| Model 3 | reference | 1.02 (0.90–1.17) |
1.09 (0.96–1.24) |
1.21 (1.07–1.38) |
1.24 (1.09–1.41) |
0.002 |
| Model 4 | reference | 1.04 (0.91–1.19) |
1.10 (0.96–1.25) |
1.22 (1.07–1.38) |
1.19 (1.05–1.36) |
0.01 |
| Model 5 | reference | 1.05 (0.92, 1.20) |
1.09 (0.96, 1.25) |
1.21 (1.06, 1.37) |
1.16 (1.01, 1.33) |
0.049 |
| Model 6 | reference | 1.05 (0.92, 1.20) |
1.09 (0.96, 1.25) |
1.21 (1.06, 1.38) |
1.16 (1.02, 1.34) |
0.037 |
Follow-up to December 31, 2012; mean follow-up 20.0±6.55 years, median follow-up 23.4 (16.8, 24.5) years. Lp(a) presented as mg/dL. P trend tests a linear increase in log relative hazard with increasing quintiles.
Model 1: adjusted by age, gender, and race.
Model 2: model 1 plus systolic blood pressure, hypertension, diabetes, current smoking, BMI, and heart rate.
Model 3: model 2 plus HDL-C.
Model 4: model 3 plus prevalent CHD.
Model 5: model 4 plus LDL-C
Model 6: model 4 plus Lp(a)- cholesterol corrected LDL-C
HF, incident heart failure hospitalization.
Table 3.
ARIC visit 1 Lp(a) quintiles hazard ratios (95% CI) for incident heart failure hospitalization (prevalent and incident MI excluded).
| Lp(a) [HF/at risk] | Hazard ratio (95% confidence interval) | p trend | ||||
|---|---|---|---|---|---|---|
| Quintile 1 (0.02–2.28) [295/2456 (12%)] |
Quintile 2 (2.41–5.46) [294/2482 (12%)] |
Quintile 3 (5.59–11.03) [329/2436 (14%)] |
Quintile 4 (11.16–22.43) [356/2439 (15%)] |
Quintile 5 (22.57–107.60) [356/2440 (15%)] |
||
| Model 1 | reference | 0.95 (0.81–1.12) |
1.00 (0.85–1.17) |
1.04 (0.88–1.22) |
0.99 (0.84–1.17) |
0.89 |
| Model 2 | reference | 1.02 (0.87–1.20) |
1.07 (0.91–1.26) |
1.11 (0.94–1.31) |
1.07 (0.90–1.26) |
0.74 |
| Model 3 | reference | 1.02 0.86–1.20) |
1.08 (0.92–1.27) |
1.12 (0.95–1.31) |
1.07 (0.91–1.27) |
0.70 |
Follow-up to December 31, 2012; mean follow-up 20.0±6.55 years, median follow-up 23.4 (16.8, 24.5) years. Lp(a) presented as mg/dL. p trend tests a linear increase in log relative hazard with increasing quintiles.
Models 1–3 are as described for Table 2.
Lp(a) and arterial stiffness
Although higher Lp(a) quintiles were associated with greater pressure-strain modulus and stiffness index and lower arterial distensibility and compliance in the unadjusted model (p-trend across Lp(a) quintiles <0.01 for each; Table 4), after adjustment for age, race, and gender, no significant association was observed.
Table 4.
Arterial stiffness parameters by Lp(a) quintiles.
| n | Lp(a) Quintiles (mg/dL) | p trend | ||||
|---|---|---|---|---|---|---|
| 1 2,025 |
2 1,899 |
3 1,876 |
4 1,832 |
5 1,891 |
||
| Pressure-strain modulus (kPa) | 137.4 (74.4, 200.4) |
134.6 (74.8, 194.4) |
139.9 (77.4, 202.4) |
142.8 (79.1,206.5) |
144.8 (75.9, 213.7) |
<0.001 |
| Adjusteda |
141.7 (139.0, 144.3) |
137.5 (134.7, 140.2) |
139.8 (137.1, 142.5) |
139.9 (137.2, 142.7) |
140.2 (137.4, 142.9) |
0.28 |
| Carotid arterial strain (%) | 5.37 (3.56, 7.18) |
5.36 (3.59, 7.13) |
5.33 (3.53, 7.13) |
5.32 (3.60, 7.04) |
5.32 (3.56, 7.08) |
0.54 |
| Adjusteda |
5.35 (5.28, 5.43) |
5.36 (5.28, 5.44) |
5.33 (5.25, 5.41) |
5.33 (5.25, 5.41) |
5.33 (5.25, 5.41) |
0.98 |
| Arterial distensibility (%/kPa) | 1.77 (1.05, 2.50) |
1.79 (1.07,2.51) |
1.74 (1.01,2.47) |
1.69 (1.01,2.37) |
1.69 (0.98, 2.40) |
<0.001 |
| Adjusteda |
1.72 (1.69, 1.75) |
1.76 (1.73, 1.79) |
1.74 (1.71, 1.77) |
1.73 (1.69, 1.76) |
1.74 (1.71, 1.77) |
0.44 |
| Arterial compliance (mm3/kPa) | 8.01 (4.88, 11.14) |
8.13 (4.89,11.37) |
7.82 (4.66, 10.98) |
7.76 (4.65, 10.87) |
7.54 (4.49, 10.59) |
<0.001 |
| Adjusteda |
7.79 (7.65, 7.92) |
7.97 (7.84, 8.11) |
7.82 (7.69, 7.95) |
7.87 (7.73, 8.00) |
7.84 (7.70, 7.98) |
0.35 |
| Stiffness index | 0.11 (0.07, 0.15) |
0.11 (0.07, 0.15) |
0.11 (0.07, 0.15) |
0.11 (0.07, 0.15) |
0.11 (0.07, 0.16) |
0.001 |
| Adjusteda |
0.11 (0.11, 0.11) |
0.11 (0.11, 0.11) |
0.11 (0.11, 0.11) |
0.11 (0.11, 0.11) |
0.11 (0.11, 0.11) |
0.52 |
Data expressed as median (25th, 75th percentiles).
p-trend was calculated by trend test across ordered groups.
Lp(a) levels were measured at ARIC visit 1 (1987–1989). Arterial stiffness parameters were measured at ARIC visit 2 (1990–1992).
Adjusted for age, race, and gender.
Discussion
In this study, we demonstrated that higher levels of Lp(a) are significantly associated with an increased risk for incident heart failure hospitalization. After the exclusion of prevalent and incident MI, the association was no longer statistically significant.
A recent study by Kamstrup et al. in the Copenhagen City Heart Study and Copenhagen General Population Study showed that elevated Lp(a) levels were associated with an increased risk for heart failure.15 We confirmed this association between Lp(a) and heart failure in the ARIC study. After the exclusion of MI and aortic stenosis, Kamstrup et al. found an attenuated, but significant, association between Lp(a) and heart failure. Their analyses suggested that 63% of the increased risk for heart failure was mediated by MI and aortic stenosis, and they hypothesized that arterial stiffness and vascular noncompliance may be a potential mechanism to explain the relationship of Lp(a) and heart failure.15
In our study, we examined the association of Lp(a) measured at visit 1 with parameters of arterial stiffness assessed at visit 2. Although higher Lp(a) levels were associated with increased pressure-strain modulus and stiffness and reduced arterial compliance and distensibility in a simple model, the associations were no longer significant after adjustment for age, race, and gender. These findings do not support a direct effect of Lp(a) on arterial stiffness as a likely mechanism to explain the increased risk for heart failure with increased Lp(a) levels.
In the current study, the association between Lp(a) quintiles and heart failure hospitalization was no longer significant after excluding prevalent and incident MI. We did not find evidence in the ARIC study that the association between Lp(a) and heart failure is due to a mechanism independent of CHD.
Strengths of the current study include a large, well-characterized, randomly sampled population with lengthy follow-up in a prospective study designed to examine cardiovascular disease incidence and risk factors. Lp(a) was measured in the entire cohort at two separate time points with two different assays. The findings based on Lp(a) levels measured at visit 4, approximately a decade after visit 1, were quantitatively similar to those based on Lp(a) at visit 1 and suggest that higher levels of Lp(a) continue to be associated with heart failure when measured later in life.
One limitation of this study is that the endpoint was determined based on ICD codes and that echocardiographic data were not available to characterize heart failure as with preserved or reduced ejection fraction. However, validation of hospitalizations for heart failure indicated that the positive predictive value of discharge and death certificate codes was 93% for acute decompensated heart failure and 97% for chronic heart failure.21
Kamstrup et al. also demonstrated an association between high-risk genetic variants in the LPA gene (rs3798220 and rs10455872) and heart failure in the Copenhagen studies.15 We confirmed a strong association between these variants and Lp(a) levels (data not shown). Although we did not find a significant association with incident heart failure hospitalization, the high-risk variants were associated with numerically greater HRs (data not shown). Because of the smaller size of the ARIC study, the mixed racial cohort in ARIC, and the low allele frequency of these variants, our sample size had insufficient statistical power to confirm the findings of the Copenhagen studies, which included a much larger and more homogeneous cohort.
Future studies may consider distinguishing between heart failure with preserved and with reduced ejection fraction when assessing the relationship between Lp(a) and heart failure. Since echocardiography data were not collected at earlier ARIC visits, we could not examine the associations of Lp(a) levels, aortic stenosis, and heart failure in the ARIC study. Although we assessed the associations of Lp(a), arterial stiffness, and heart failure in 9,523 individuals, our study could still be underpowered if the association of Lp(a) with arterial stiffness is only modest. In that case, a much larger study population would help confirm our findings. Another limitation of this investigation is that we assessed only local arterial stiffness as opposed to regional arterial stiffness. Finally, despite comprehensive adjustment, residual confounding is still possible in an observational study.
In summary, individuals with higher levels of Lp(a) had an increased risk for heart failure hospitalization even after adjustment for other risk factors in ARIC. We have previously shown that high levels of Lp(a) were significantly associated with an increased risk for CHD and stroke in both European Americans and African Americans. These combined data suggest that novel therapies such as PCSK9 inhibitors, which lower both LDL-C and Lp(a), and antisense oligonucleotides targeting apo(a),30 which selectively lower Lp(a), may reduce risk for heart failure hospitalization as well as CHD and stroke in high-risk individuals with high levels of Lp(a).
Supplementary Material
Highlights.
Lipoprotein(a) level was associated with heart failure risk in the ARIC study
The association persisted after adjustment for other risk factors
The relation was not significant after excluding prevalent and incident MI
Lipoprotein(a) level was not associated with arterial stiffness parameters
Acknowledgments
The authors thank the ARIC participants and staff for their important contributions.
Financial support
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Y. Pokharel is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL110837.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
A. Agarwala: none
Y. Pokharel: supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL110837.
A. Saeed: none
W. Sun: none
S. S. Virani: none
V. Nambi: provisional patent along with Roche, Baylor College of Medicine, University of North Carolina on use of biomarkers in prediction of heart failure, regional advisory board Sanofi Regeneron, event adjudicator for a study sponsored by Siemens, site PI for study sponsored by Merck
C. Ndumele: none,
E. Shahar: none
G. Heiss: none
E. Boerwinkle: officer, director, trustee, or other fiduciary role for Codified Genomics
S. Konety: none
R. C. Hoogeveen: research grants from Denka Seiken, Ltd., and Roche.
C. M. Ballantyne: consultant fees/honoraria from Abbott Diagnostic, Amarin, Amgen, AstraZeneca, Eli Lilly, Esperion, Genzyme, Ionis, Matinas BioPharma Inc, Merck & Company, Novartis, Pfizer, Regeneron, Roche Diagnostic, Sanofi-Synthelabo; research/research grants from Abbott Diagnostic, Amarin, Amgen, Eli Lilly, Esperion, Novartis, Otsuka, Pfizer, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, Takeda; a provisional patent (patent #61721475) entitled ”Biomarkers to Improve Prediction of Heart Failure Risk” was filed by Drs. Ballantyne, Hoogeveen, and Nambi, Baylor College of Medicine and Roche.
Author contributions
A. Agarwala: conceived and designed the research; drafted the manuscript; made critical revision of the manuscript for important intellectual content.
Y. Pokharel: drafted the manuscript; made critical revision of the manuscript for important intellectual content.
A. Saeed: made critical revision of the manuscript for important intellectual content.
W. Sun: performed statistical analysis; made critical revision of the manuscript for important intellectual content.
S. S. Virani, V. Nambi, C. Ndumele, E. Shahar, G. Heiss, E. Boerwinkle, S. Konety: made critical revision of the manuscript for important intellectual content.
R. C. Hoogeveen, C. M. Ballantyne: conceived and designed the research; handled funding and supervision; made critical revision of the manuscript for important intellectual content
References
- 1.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, Folsom AR, Boerwinkle E, Ballantyne CM. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–249. doi: 10.1161/CIRCULATIONAHA.111.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, Rumley A, Lowe GD, Danesh J, Gudnason V. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008;168:598–608. doi: 10.1001/archinte.168.6.598. [DOI] [PubMed] [Google Scholar]
- 3.Johannsen TH, Kamstrup PR, Andersen RV, Jensen GB, Sillesen H, Tybjaerg-Hansen A, Nordestgaard BG. Hepatic lipase, genetically elevated high-density lipoprotein, and risk of ischemic cardiovascular disease. J Clin Endocrinol Metab. 2009;94:1264–1273. doi: 10.1210/jc.2008-1342. [DOI] [PubMed] [Google Scholar]
- 4.Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296:1363–1370. doi: 10.1001/jama.296.11.1363. [DOI] [PubMed] [Google Scholar]
- 5.Kardys I, Oemrawsingh RM, Kay IP, Jones GT, McCormick SP, Daemen J, Van Geuns RJ, Boersma E, Van Domburg RT, Serruys PW. Lipoprotein(a), interleukin-10, C-reactive protein, and 8-year outcome after percutaneous coronary intervention. Clin Cardiol. 2012;35:482–489. doi: 10.1002/clc.21988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Momiyama Y, Ohmori R, Fayad ZA, Tanaka N, Kato R, Taniguchi H, Nagata M, Ohsuzu F. Associations between serum lipoprotein(a) levels and the severity of coronary and aortic atherosclerosis. Atherosclerosis. 2012;222:241–244. doi: 10.1016/j.atherosclerosis.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, Folsom AR. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006;37:1407–1412. doi: 10.1161/01.STR.0000222666.21482.b6. [DOI] [PubMed] [Google Scholar]
- 8.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Arsenault BJ, Boekholdt SM, Dube MP, Rheaume E, Wareham NJ, Khaw KT, Sandhu MS, Tardif JC. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7:304–310. doi: 10.1161/CIRCGENETICS.113.000400. [DOI] [PubMed] [Google Scholar]
- 10.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 11.Grainger DJ, Kirschenlohr HL, Metcalfe JC, Weissberg PL, Wade DP, Lawn RM. Proliferation of human smooth muscle cells promoted by lipoprotein(a) Science. 1993;260:1655–1658. doi: 10.1126/science.8503012. [DOI] [PubMed] [Google Scholar]
- 12.Cote C, Pibarot P, Despres JP, Mohty D, Cartier A, Arsenault BJ, Couture C, Mathieu P. Association between circulating oxidised low-density lipoprotein and fibrocalcific remodelling of the aortic valve in aortic stenosis. Heart. 2008;94:1175–1180. doi: 10.1136/hrt.2007.125740. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa K, Aguero J, Oh JG, Hammoudi N, Fish LA, Leonardson L, Picatoste B, Santos-Gallego CG, Fish KM, Hajjar RJ. Increased stiffness is the major early abnormality in a pig model of severe aortic stenosis and predisposes to congestive heart failure in the absence of systolic dysfunction. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 15.Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail. 2016;4:78–87. doi: 10.1016/j.jchf.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 16.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Lackner C, Cohen JC, Hobbs HH. Molecular definition of the extreme size polymorphism in apolipoprotein(a) Hum Mol Genet. 1993;2:933–940. doi: 10.1093/hmg/2.7.933. [DOI] [PubMed] [Google Scholar]
- 18.Marcovina SM, Hobbs HH, Albers JJ. Relation between number of apolipoprotein(a) kringle 4 repeats and mobility of isoforms in agarose gel: basis for a standardized isoform nomenclature. Clin Chem. 1996;42:436–439. [PubMed] [Google Scholar]
- 19.Gaubatz JW, Heideman C, Gotto AM, Jr, et al. Human plasma lipoprotein [a]: structural properties. J Biol Chem. 1983;258:4582–4589. [PubMed] [Google Scholar]
- 20.Marcovina SM, Albers JJ, Scanu AM, Kennedy H, Giaculli F, Berg K, Couderc R, Dati F, Rifai N, Sakurabayashi I, Tate JR, Steinmetz A. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a) Clin Chem. 2000;46:1956–1967. [PubMed] [Google Scholar]
- 21.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1994;14:1098–1104. doi: 10.1161/01.atv.14.7.1098. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW. Variation of common carotid artery elasticity with intimal-medial thickness: the ARIC Study. Atherosclerosis Risk in Communities. Ultrasound Med Biol. 1997;23:157–164. doi: 10.1016/s0301-5629(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 25.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 26.Arnett DK, Boland LL, Evans GW, Riley W, Barnes R, Tyroler HA, Heiss G. Hypertension and arterial stiffness: the Atherosclerosis Risk in Communities Study. ARIC Investigators. Am J Hypertens. 2000;13:317–323. doi: 10.1016/s0895-7061(99)00281-2. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seman LJ, Breckenridge WC. Isolation and partial characterization of apolipoprotein (a) from human lipoprotein (a) Biochem Cell Biol. 1986;64:999–1009. doi: 10.1139/o86-133. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 30.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, Crooke ST, Witztum JL, Stroes ES, Tsimikas S. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


