Abstract
BACKGROUND
The interactions between biochemical and chemical agents in the body are important in many clinical processes. Affinity chromatography and high-performance affinity chromatography (HPAC), in which a column contains an immobilized biologically-related binding agent, are two methods that can be used to study these interactions.
CONTENT
This review looks at various approaches that can be used in affinity chromatography and HPAC to characterize the strength or rate of a biological interaction, the number and types of sites that are involved in this process, and the interactions between multiple solutes for the same binding agent. A number of applications for these methods are examined, with an emphasis on recent developments and high-performance affinity methods. These applications include the use of these techniques for fundamental studies of biological interactions, high-throughput screening of drugs, work with modified proteins, tools for personalized medicine, and studies of drug-drug competition for a common binding agent.
SUMMARY
The wide range of formats and detection methods that can be used with affinity chromatography and HPAC for examining biological interactions makes these tools attractive for various clinical and pharmaceutical applications. Future directions in the development of small-scale columns and the coupling of these methods with other techniques, such as mass spectrometry or other separation methods, should continue to increase the flexibility and ease with which these approaches can be used in work involving clinical or pharmaceutical samples.
Introduction
The interactions between biochemicals and chemicals in the body are important in many clinical processes. Examples include the binding of antibodies with antigens, the interactions of hormones with their receptors, and the binding of drugs with their biological targets or carrier agents (1,2). These interactions are usually reversible and range from having a weak to high binding strength, or “affinity”. These systems may also be highly selective in their binding (e.g., an antibody-antigen interaction) or more general in nature (e.g., the binding of drugs with a serum transport protein) (1–4). A variety of methods have been employed to study these and other types of biological interactions. These techniques have ranged from equilibrium dialysis and ultrafiltration to X-ray crystallography, absorption or fluorescence spectroscopy, surface plasmon resonance spectroscopy, and nuclear magnetic resonance spectroscopy (3–5). This review will discuss an alternative group of techniques that are based on affinity chromatography.
Affinity chromatography is a type of liquid chromatography in which the stationary phase is an immobilized form of a biologically-related binding agent. This binding agent, or “affinity ligand”, is used to retain specific compounds from applied samples (6). The presence of such an agent results in a separation method that uses the same reversible and selective interactions that are present in many biological systems. This property has often been employed in affinity chromatography to purify, extract, or remove a given chemical or biochemical from a sample for either preparative work or analytical-scale applications (6). The use of a biologically-related agent as the stationary phase also gives this method the ability to study and model the interactions that occur between chemicals and biochemicals in living systems. This is true for both traditional affinity chromatography and high-performance affinity chromatography (HPAC), with the latter making use of HPLC supports and instrumentation to carry out an affinity-based separation or analysis (5–10).
This review will look at various formats that have been used in these methods to characterize the strength or rate of a biological interaction and the number and types of sites that are involved in these binding processes. It will also show how these methods can be used to study the interactions between several solutes for the same binding agent and to screen the interactions of many compounds with a given biological target (5–10). An emphasis will be placed on recent applications of these methods, and particularly those involving HPAC. Finally, recent trends in these methods and possible future directions for these techniques will be discussed.
General Approaches in Affinity Chromatography for Binding Studies
There are several methods by which biological interactions can be examined by affinity chromatography and HPAC (Table 1). One common approach is to use zonal elution (8,10). Zonal elution involves the injection of a small sample plug onto a chromatographic system, followed by separation of the peaks that result from this injection, as is illustrated in Figure 1A (11). This is the format that is most commonly used in other types of liquid chromatography for chemical measurement and identification. However, this format can also be used in affinity chromatography and HPAC to obtain information on a biological interaction by using the peak profile or retention time that is generated for a given compound with the immobilized binding agent (5,8,10).
Table 1.
Methods for examining biological interactions by affinity chromatography and HPAC.
| Technique | General principle | Applications |
|---|---|---|
| Zonal elution | Small plug of a chemical/biochemical is injected onto an affinity column; the profiles or retention times of the resulting peaks are used to provide information on how the injected solutes are interacting with the immobilized agent in the column | Characterization of overall binding Competition studies Site-specific studies Screening of drug candidates Characterization of binding sites |
| Frontal analysis | Solution of a chemical/biochemical is passed in a continuous manner through an affinity column; as sites in the column become occupied, a breakthrough curve forms that can provide information on the solute’s interactions with the immobilized agent in the column | Characterization of overall binding Competition studies Screening of drug candidates Characterization of binding sites |
| Plate height & peak profiling methods | Measurement and comparison of plate heights, such as for a solute and non-retained solute on an affinity column (containing an immobilized agent) and a control column | Measurement of dissociation rate constants for systems with weak-to-moderate binding |
| Peak decay method | Measurement of solute dissociation from a small affinity column under conditions that promote dissociation over rebinding of the solute to the immobilized agent in the column | Measurement of dissociation rate constants for systems with weak-to-strong binding |
| Split-peak method | Measurement of the non-retained (or retained) peak area for a solute on an affinity column under conditions where only part of the injected solute has sufficient time to bind to the immobilized agent | Measurement of association rate constants for systems with strong binding and slow dissociation rates |
| Ultrafast affinity extraction | Capture and measurement by a small affinity column of the non-bound form of a solute in the presence of a binding agent in solution | Measurement of binding strength and/or dissociation rate constants for systems with weak-to-strong binding |
Figure 1.
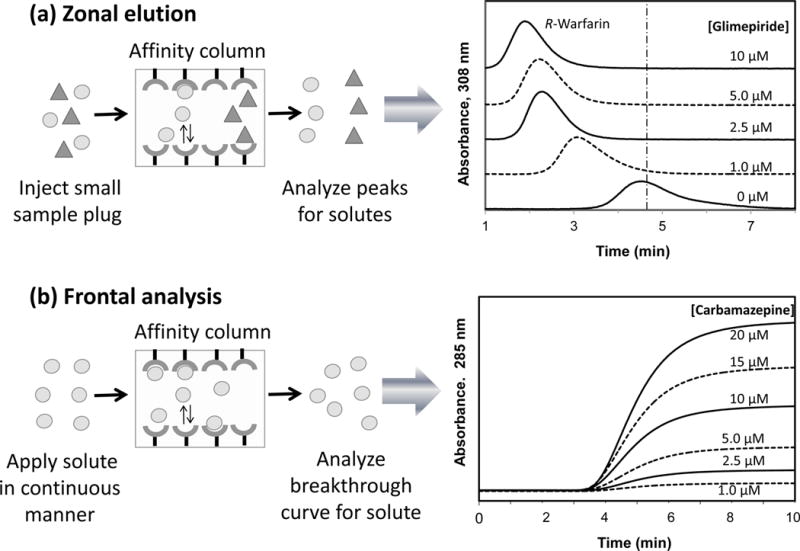
Examples of A) zonal elution and B) frontal analysis experiments for binding studies that were carried out by HPAC. The results given to the right in (A) illustrate the shift in retention that was observed for small injections of R-warfarin (i.e., a drug that binds to Sudlow site I of human serum albumin) onto a column containing immobilized human serum albumin in the presence of various concentrations of the glimepiride in the mobile phase; the vertical dashed line represents the mean position of the peak for R-warfarin in the absence of glimepiride. The results given to the right in (B) show the breakthrough curves that were obtained for the application of various concentrations of carbamazepine to a column that contained immobilized alpha1-acid glycoprotein. Adapted from Refs. (11,13) with permission from Elsevier.
As will be shown later, zonal elution can be employed with affinity chromatography and HPAC to determine the binding strength of a compound with an immobilized agent, the type of competition this compound may have with other chemicals for the binding agent, and the affinity and location of these binding sites. It is further possible in this type of experiment to look at the effects of pH, temperature, ionic strength and solution composition on a biological interaction (8,10). Some advantages of using zonal elution for such work are that it needs only a small amount of the injected compound for each experiment, the binding agent can often be reused for hundreds of studies, and it is possible to simultaneously look at more than one compound in a sample by using appropriate separation or detection conditions to examine and differentiate between these compounds as they elute from the column. This method also tends to give good precision and fast analysis times, especially when used in HPAC (8) (Figure 1A).
Frontal analysis is a second method that is often used in affinity chromatography and HPAC to characterize biological interactions (7,8,10). This technique is sometimes called frontal affinity chromatography (FAC) (10,12). In frontal analysis, a solution with a known concentration of a chemical or biochemical is passed in a continuous manner through a column that contains the desired binding agent, as is shown in Figure 1B. As the binding sites for the applied compound in the column become occupied, more of this compound will elute and produce a breakthrough curve (7,8,10). An example of such a curve is also provided in Figure 1B (13).
The mean position of the curve that is obtained in frontal analysis can be examined as a function of the concentration of the applied compound. This information can then be compared to various binding models to determine the types of interactions that are occurring in the column, the amount of binding sites that are present, and the equilibrium constants for these sites (7,8,10,12). The ability of frontal analysis to simultaneously provide information on both the amount of binding sites and the equilibrium constants for these sites is an important advantage of this method over zonal elution. Frontal analysis is also valuable for providing information on the overall model for an interaction between the applied compound and its binding agent. A disadvantage of this approach when compared to zonal elution is that frontal analysis usually requires more of the applied chemical and additional time because many solutions of a compound are often needed to carry out a binding study. However, the time needed for a frontal analysis study in HPAC can still be relatively fast (7), as illustrated is in Figure 1B.
Measurement and Comparison of Overall Binding
One way that affinity chromatography and HPAC can be used is to characterize the overall binding of a chemical or biochemical with an immobilized binding agent. Frontal analysis is often used for this purpose (7,8,10). An example is shown in Figure 2, in which HPAC was employed to look at interactions of the drug enantiomers R- and S-propranolol with low-density lipoprotein (LDL) (14). The data obtained by frontal analysis indicated that a simple non-saturable binding model (e.g., partitioning into the non-polar core of LDL) was present in the case of S-propranolol, while a mixture of saturable binding and non-saturable interactions was present for R-propranolol (e.g., binding with apolipoproteins plus partitioning into LDL) (14). A similar approach has been used to study the interactions of R/S-propranolol and verapamil with high-density lipoprotein (15) and the binding of R/S-propanolol with very low-density lipoprotein (16). HPAC and affinity chromatography have also been employed in examining the binding of various solutes and drugs with the transport proteins α1-acid glycoprotein (AGP) or human serum albumin (HSA) (10,17,18), the binding of urediofibrate-like dual agonists with peroxisome proliferator-activated receptors (19), and the interactions of many compounds with immobilized enzymes or lectins (12,20–22).
Figure 2.
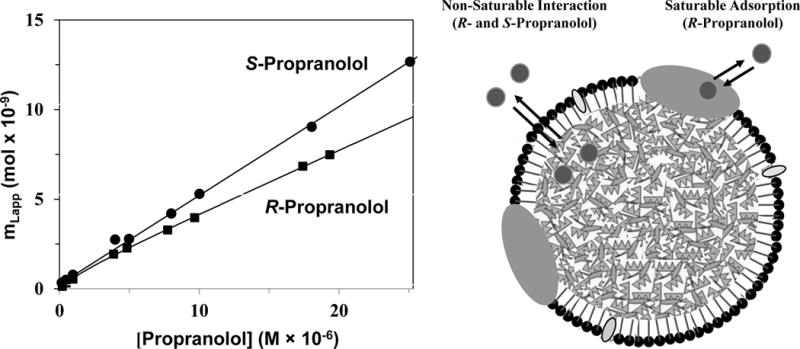
Comparison of data obtained by frontal analysis for examining the binding of R-propranolol and S-propranolol with an HPAC column containing immobilized low density lipoprotein (LDL) (left) and a general model showing the types of interactions each of these enantiomers had with LDL (right). The graph on the left is based on data obtained from Ref. (14).
Information on the overall interactions of a compound with an immobilized binding agent can also be provided by zonal elution. For instance, this can be accomplished by using the retention time (tR) or corresponding retention factor (k), where k = (tR – tM)/tM and tM is the column void time, for a given compound and binding agent. If the rates of association and dissociation between the compound and binding agent are relatively fast compared to the time the compound spends in the column, the values of tR and k will depend on both the number of binding sites for this compound in the column and equilibrium constants for these sites (8). This type of experiment has been used in HPAC to compare the binding of several sulfonylurea drugs and site-selective probes for HSA on columns that contained normal or glycated forms of this protein (i.e., as occur during diabetes) (23). A similar approach has been used to screen various drugs for their binding to HSA or AGP (13).
Site-Specific Binding and Competition Studies
Another use of zonal elution in HPAC and affinity chromatography has been in characterizing the competition that one compound may have with another for a given binding agent. This approach has been employed to examine the interactions of many drugs, hormones and other solutes with proteins or to measure the binding constants for a compound at a particular site on these binding agents (8,10). This experiment is usually carried out by placing a drug or competing agent at a known concentration in the mobile phase. A small amount of a second drug, solute or site-selective probe is then injected in the presence of this mobile phase and onto a column that contains the binding agent. As the injected compound goes though the column, it will compete for binding sites with the drug or solute that has been added to the mobile phase. During this process, the solute in the mobile phase will affect the retention of the injected compound if these two chemicals share binding sites or have allosteric effects between their binding regions. The resulting data can be used to determine the types of interactions that are present between these chemicals and the equilibrium constants for these processes (8).
An example of this type of study is provided in Figure 3 (11), as was conducted by using HPAC. In this case, the binding of a drug (i.e., glimepiride) at a specific site on an immobilized protein (i.e., normal HSA or a glycated form of HSA) was examined by placing the drug into the mobile phase while injections were made of a second compound that was known to bind at only a specific region on the protein (i.e., L-tryptophan, which binds to Sudlow site II of HSA). In this type of study, the retention factor for the injected compound would be expected to change as the concentration of the drug is varied in the mobile phase if these two solutes compete directly at the selected site (8). In this situation, a plot of 1/k versus the concentration of the drug should give a linear relationship with a positive slope. The linear relationships that were obtained (Figure 3) made it possible to determine the equilibrium constants for glimepiride at Sudlow site II on each type of HSA that was examined (11,24). The same method has been used to examine the competition of other site-specific probes and drugs during their interactions with the normal or modified HSA and with AGP (17,18,25–27). Similar approaches have been used to investigate additional interactions, including multi-site binding and allosteric effects (8,28–32).
Figure 3.
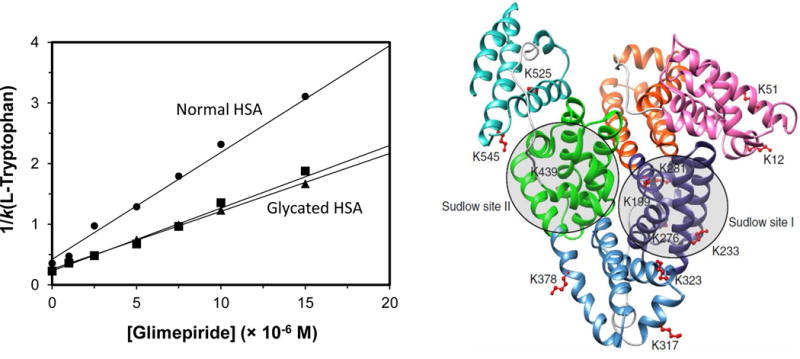
Use of zonal elution-based competition studies in HPAC to examine the direct competition of a drug or solute with an injected probe for specific binding sites on an immobilized protein, as illustrated by the image on the left for the competition of glimepiride with L-tryptophan at Sudlow site II on columns that contained normal HSA (●) or two glycated preparations of this protein (■,▲). The image on the right shows of modification in glycated HSA and the location of the two major drug binding sites on this protein, Sudlow sites I and II. Reproduced from Refs. (11,24) with permission from Elsevier.
An alternative approach that can be used to detect allosteric effects or direct competition is to employ a plot of k0/(k - k0) versus the reciprocal of the competing agent’s concentration in the mobile phase (33). In this type of plot, k0 is the retention factor for the injected solute in the presence of the competing agent, and k is the retention factor in the presence of a given concentration of the same competing agent. This plot results in a linear relationship for systems with either direct competition at a single site or that have simple allosteric effects between the mobile phase additive and injected compound. The response of this plot can be used to obtain the association equilibrium constant for the competing agent at its binding site and the coupling constant for the effect of this competing agent on binding by the injected compound with the immobilized agent. An important advantage of this method is it can be used to examine both directions of an allosteric effect between two compounds that bind to the same agent (33,34). This approach has been used to study the allosteric interactions between warfarin and tamoxifen on HSA (34), the effect of tolbutamide on the binding of R-warfarin with HSA (27), and the allosteric effects that occur as S-propranolol and warfarin bind to AGP (17).
Frontal analysis coupled with mass spectrometry (FAC-MS) is another approach that has been employed to examine the competition of compounds for binding agents in affinity columns (32,35). In this method, a solute and competing agent are both placed into the mobile phase and applied simultaneously to the column. The breakthrough curve observed for the solute is then viewed as a function of the concentration of the competing agent to look for the presence of direct competition or allosteric effects (32,35). This method can also be used to compare a series of competing agents and to obtain the equilibrium constants between these agents and the immobilized agent (19,35–38). FAC-MS has been used in this format with open-tubular capillaries to compare various ureidofibrate-like dual agonists for their binding to domains of peroxisome proliferator-activated receptors (19). A similar method has been used to determine the binding constants for several flavonoids with the histone deacetylase SIRT6 and to characterize the ability of these agents to displace quercetin from SIRT6 (35).
Screening Drug Candidates
A related application of HPAC and affinity chromatography has been as a tool for screening drug candidates and potential binding partners for biological targets. This work has often involved the use of FAC-MS (12). This technique has been employed to screen mixtures of drug candidates for their binding to enzymes, lectins, antibodies and receptors (12,37–41). The chemicals that have been screened by this approach have included peptides, oligosaccharides and enzyme inhibitors (12,37–41).
Mass spectrometry has also been combined with zonal elution and affinity columns for the high-throughput screening of binding by chemicals to an immobilized agent. The use of this combination with systems that have weak-to-moderate interactions (i.e., association equilibrium constants of 105–106 M−1 or less) is known as weak affinity chromatography-mass spectrometry (5,42–45). This is illustrated in Figure 4, where this technique was used to test various drug fragments for their binding to the molecular chaperone heat shock protein 90 (HSP90, a potential target for the treatment of cancer and other diseases) (42). Weak affinity chromatography-mass spectrometry has also been used to examine the binding of drugs to albumin (18,46). In addition, this method has been employed to look at the interactions of mixtures containing various compound fragments with enzymes such as kinases or proteases (43–45).
Figure 4.

Use of weak affinity chromatography (WAC) to screen the binding of drug fragments, as illustrated by employing a column containing the immobilized ATPase domain of heat shock protein 90 (HSP90). A), chromatograms obtained for fragments 46–62, out of a total of 111 drug fragments tested. B), results obtained for all 111 drug fragments when screened for their binding to HSP90 by using WAC, nuclear magnetic resonance (NMR) spectroscopy, surface plasmon resonance (SPR) spectroscopy, a fluorescence polarization (FP) assay, or a thermal shift assay (Tm), with some results also being included based on X-ray crystallography and isothermal titration calorimetry (ITC). The hits for binding are indicated by green (or dark gray) and non-hits are represented by red (or light gray). Adapted from Ref. (42) with permission from the American Chemical Society.
Characterization of Binding Sites
The use of affinity chromatography and HPAC in competition studies is one way in which the interaction sites of a particular drug or solute can be identified and studied on a protein or other type of binding agent (8,32). However, it is possible to also use these methods to obtain even more information about the nature of this interaction and binding regions. An example is the use of various temperatures during zonal elution or frontal analysis studies to determine the changes in enthalpy and entropy that occur during the retention of a compound by an immobilized agent. Changes in the pH, ionic strength or polarity of the mobile phase can be employed in a similar manner to see whether acid/base interactions, hydrogen bonding, coulombic forces, and/or non-polar interactions contribute to this binding (8).
Another way to characterize a particular site on a binding agent is to compare the retention or affinities of this agent for a series of related compounds. This approach can be used to provide a general picture of the structural features that are most important to the binding process and on the types of forces that create such binding (8,47,48). This type of data can also be used to create a quantitative structure-retention relationship, in which the retention factors measured for a series of related molecules are compared to parameters that describe various structural and physical features of these compounds. Once the structural/physical parameters that are most important to this retention are identified, this information can be used to develop a model of the binding site and in how it interacts with such compounds (49–51). Quantitative structure-retention relationships have been used with HPAC to study the binding of benzodiazepines to HSA (50), the interactions of beta-adrenolytic drugs, antihistamines, and other drugs with AGP (8,10,51–54), and the ability of various organic molecules to take part in skin permeation through the use of a column that contained keratin (49).
A complementary approach that can be used is to see how the retention of a solute or group of solutes is altered as a change is made in the structure of a binding agent in an affinity column. For instance, this method has been employed in HPAC to look at how modifying specific amino acids (e.g., Cys-34 or Trp-214) on HSA affects the ability of this protein to bind to various drugs (55–58). This approach has also been used to see how the glycation of HSA changes the binding by this protein to sulfonylurea drugs and other pharmaceutical agents, as is illustrated in Figure 3 (11,25,26). This latter work has recently been performed with samples of in vivo glycated HSA from individual patients, making this approach a possible future tool for personalized medicine (25).
Kinetic Methods
Yet another way affinity chromatography and HPAC can be used is to provide data on the kinetics of a biological interaction. Many techniques have been developed for acquiring such information (7,8,59). One method that can be used for a system with weak-to-moderate binding is to make band-broadening measurements (59). This can be done by measuring and comparing the plate heights for a solute at one or several flow rates on an affinity column and on an inert control column. The difference in these plate height values is then employed to determine the portion of the plate height that is a result of stationary phase mass transfer (Hk). This term can then be directly related to the dissociation rate constant (kd) for the solute in its interaction with the immobilized binding agent (7,59).
In a version of this technique that is known as the plate height method, a plot is made of Hk as a function of the flow rate (or linear velocity) and the retention factor of the solute. This plot is then used to provide the value of kd for the immobilized agent with the injected solute (7). This approach has been used to characterize the association and dissociation rates of HSA with solutes and drugs such as D/L-tryptophan and R/S-warfarin (60,61). Another form of this technique is peak profiling, which is based on the difference in the total plate heights that are acquired for a solute on an affinity column and a control column. This difference may be examined at either a single flow rate or at several flow rates and is again used to find the value of the dissociation rate constant (59). This second approach has been utilized to determine the dissociation rate constants of HSA for carbamazepine, imipramine, and L-tryptophan (62,63). In addition, this method has been used with a second column containing a chiral stationary phase to determine the dissociation rates for HSA with some chiral metabolites of phenytoin (64).
The peak decay method is an alternative approach for examining the rate of dissociation for biological systems (7,59). This technique uses small affinity columns and conditions that promote dissociation over rebinding of a retained solute. One form of this method involves the application of a solute to an affinity column, to which the solute has moderate-to-strong binding, followed by the application of a displacing agent to quickly elute the retained solute. The result is an elution profile that forms a first-order decay curve and in which the slope provides the dissociation rate constant for the solute (7,59). An alternative approach that can be used with systems that have weak-to-moderate strength interactions is to use small affinity columns and fast flow rates, which can allow this type of study to be conducted without the need for a displacing agent (59,65). In addition, a step change in a factor such as the mobile phase pH can sometimes be used to promote rapid release of the solute, as might be used to compare elution conditions for an affinity column (17,66).
The peak decay method has been used in HPAC with small affinity columns to determine the dissociation rates of AGP for amitriptyline, lidocaine, and nortriptyline (67). This method has also been used with affinity monolith columns to determine the dissociation rate constants for HSA with a number of drugs (67,68). A pH step change has been employed with the peak decay method to characterize the dissociation rate of thyroxine from antibodies and aptamers that can bind to this hormone (69).
The split-peak method is a technique that can be used with affinity columns to look at systems with strong binding and relatively slow rates of dissociation (7,8,59). In this technique conditions are used in which only part of an injected solute has sufficient time to bind to the affinity column. These conditions are typically created by using a small column and/or a high flow rate. The result is a situation in which the injection of even a pure sample of the solute results in two peaks, one of which is highly retained and the second of which elutes as a non-retained fraction. The change in the relative size of these peaks is then examined as the injection flow rate is varied and used to determine the association rate constant or mass transfer rate for the solute in the column (7,8,59).
The split-peak method has been used in several studies to examine the binding of immunoglobulins to small affinity columns that contain protein A or protein G (59,70). This method has been used to optimize a clinical assay based on HPAC for the rapid measurement of IgG-class antibodies in serum by using protein A columns (59). Forms of this method have also been employed to examine the binding of HSA to immobilized anti-HSA antibodies and to characterize the rate of binding by thyroxine with antibodies or aptamers (59,66).
Ultrafast affinity extraction is a newer method that can examine both the rate and degree of binding for a biological interaction (59,71–75). This method looks at an interaction in solution through the use of a small affinity column that can rapidly capture the non-bound form of a solute in an injected mixture of this solute and a soluble binding agent. The relative size of the captured fraction is used to determine the rate or extent of the biological interaction. The immobilized agent used to capture the free form of the solute may be an antibody (72,74,75) or a more general binding agent such as HSA (i.e., which can be used to retain many drugs) (71,73). Conditions are used so that the time the sample spends in the column is small or comparable to the time needed for dissociation of the solute from its soluble binding agent. This often involves the use of affinity microcolumns and flow rates that produce sample residence times in the column that span from the low millisecond range up to a few seconds (59).
Ultrafast affinity extraction has been utilized for systems that range from weak-to-strong binding (59,72,75). For instance, this method has been used with columns that contained immobilized antibodies for determining the free fraction of warfarin in mixtures of this drug with HSA (74) and, in combination with flow-based displacement immunoassays, the free fractions of thyroxine and phenytoin in clinical samples (72,75). This method has been used with columns containing HSA to measure the equilibrium constants for a soluble form of this protein with R- or S-warfarin, S-ibuprofen and imipramine (71). Ultrafast affinity extraction has also been used in a two-column system and with a chiral stationary phase to measure the free fractions and binding constants of R- and S-warfarin in drug-protein mixtures and serum (Figure 5) (73).
Figure 5.
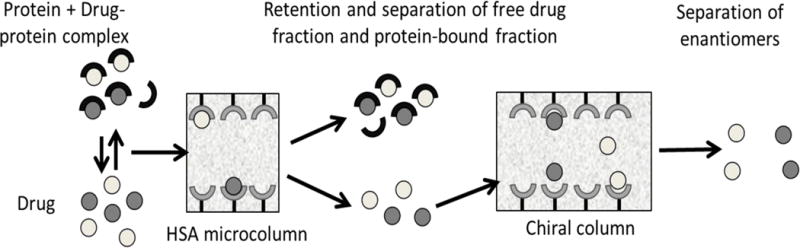
Scheme for the simultaneous isolation of a free drug fraction and separation of the various chiral forms of a drug in this fraction by using ultrafast affinity extraction and an HPLC chiral stationary phase. This particular example uses an affinity microcolumn containing immobilized HSA for ultrafast affinity extraction. Reproduced from Ref. (73) with permission from The Royal Society of Chemistry.
Conclusion and Future Directions
This review discussed a variety of ways in which affinity chromatography has been used to examine biological interactions of interest in clinical or pharmaceutical analysis. Particular attention was given to more recent work and techniques that have involved the use of HPAC. The general principles behind techniques such as zonal elution and frontal analysis were described, showing how these approaches can be used to examine the strength of a biological interaction, the number and types of sites that are involved in these processes, and the effects of one solute on another during these interactions. Several means for examining the kinetics of a biological interaction by HPAC and affinity chromatography were also considered. Systems that have been examined by these methods have ranged from the binding of drugs and hormones to proteins or receptors to the analysis of antibody-antigen, enzyme-inhibitor and sugar-lectin interactions (6–10,12,18).
There are many features of these methods that have made them attractive for such work. For instance, as shown in this review, these techniques can be used with many binding agents or formats and can be coupled with numerous detection methods, spanning from absorbance measurements to mass spectrometry (8–10,12). This is often possible using approaches with label-free detection, although these methods can also be combined with suitable tags for the study of trace analytes (8,72,75). The speed and precision of these methods, especially when used in HPAC, are other valuable features. The ability to often reuse the same immobilized binding agent (i.e., in many cases hundreds of injections) is another useful feature of this approach (8–10).
Many of the recent developments in this field will probably continue and further expand the capabilities of these methods. For instance, it is expected that columns based on monoliths and supports for ultra-performance liquid chromatography will continue to be adapted for use in HPAC and affinity chromatography (6,10,20–22). This should allow even more rapid assays to be created with these methods and will provide greater ease in coupling affinity columns with mass spectrometry or other analytical techniques (5,12,19,38–41). Further work in the miniaturization of affinity columns and systems is also anticipated (5,10,23,37). This work has already led to the possibility of carrying out binding studies on relatively exotic binding agents (e.g., lipoproteins, receptors or modified forms of proteins) and even binding agents that have been obtained from individual patients (e.g., glycated HSA) (14–16,25–27,36). These efforts, in turn, have resulted in the proposed use of HPAC as a tool in personalized medicine (25).
Another trend that is expected to continue is the creation of new formats for affinity-based binding assays. A specific example that was described is ultrafast affinity extraction, which can provide a direct measure of the strength or rate of biological interactions in solution and which can even examine these processes directly in clinical samples (59,72–75). The combination of these recent tools and formats with those that are already available should result in even more future applications for affinity chromatography and HPAC in the characterization of biological interactions for clinical studies or pharmaceutical analysis.
Acknowledgments
Portions of this work were supported by the National Institutes of Health under grants R01 GM044931 and R01 DK069629.
Footnotes
Disclaimer: This is an un-copyedited authored manuscript copyrighted by the American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.
References
- 1.Burtis CA, Ashwood ER, Bruns DE, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 5th. St Louis (MO): Saunders; 2012. [Google Scholar]
- 2.Clarke W, editor. Contemporary practice in clinical chemistry. 3rd. Washington (DC): AACC Press; 2016. [Google Scholar]
- 3.Williams MA, Daviter T, editors. Protein-ligand interactions, methods and applications. New York (NY): Springer; 2013. [Google Scholar]
- 4.Cantor CR, Schimmel PR. Biophysical chemistry, part 2: techniques for the study of biological structure and function, part 2. San Francisco (CA): Freeman; 1980. [Google Scholar]
- 5.Ohlson S, Duong-Thi MD. Emerging technologies for fragment screening. In: Erlanson DA, Jahnke W, editors. Fragment-based drug discovery: lessons and outlook. 1st. Weinheim (Germany): Wiley-VCH Verlag; 2016. pp. 173–95. [Google Scholar]
- 6.Hage DS, editor. Handbook of affinity chromatography. 2nd. Boca Raton (FL): CRC Press; 2006. [Google Scholar]
- 7.Chaiken IM. Analytical affinity chromatography. Boca Raton (FL): CRC Press; 1987. [Google Scholar]
- 8.Hage DS, Chen J. Quantitative affinity chromatography: practical aspects. In: Hage DS, editor. Handbook of affinity chromatography. 2nd. Boca Raton (FL): CRC Press; 2006. pp. 595–628. [Google Scholar]
- 9.Winzor DJ. Quantitative affinity chromatography: recent theoretical developments. In: Hage DS, editor. Handbook of affinity chromatography. 2nd. Boca Raton (FL): CRC Press; 2006. pp. 629–62. [Google Scholar]
- 10.Zheng X, Li X, Beeram S, Podariu M, Matsuda R, Pfaunmiller EL, et al. Analysis of biomolecular interactions using affinity microcolumns: a review. J Chromatogr B. 2014;968:49–63. doi: 10.1016/j.jchromb.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuda R, Li Z, Zheng X, Hage DS. Analysis of multi-site drug-protein interactions by high-performance affinity chromatography: binding of glimepiride to normal or glycated human serum albumin. J Chromatogr A. 2015;1408:133–44. doi: 10.1016/j.chroma.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schriemer DS. Biosensor alternative: frontal affinity chromatography. Anal Chem. 2004;76:440A–8A. doi: 10.1021/ac041684m. [DOI] [PubMed] [Google Scholar]
- 13.Jackson Bi C, Vargas-Badilla A, Li J, Rada R, Anguizola GJ, et al. Entrapment of alpha1-acid glycoprotein in high-performance affinity columns for drug-protein binding studies. J Chromatogr B. 2016;1021:188–96. doi: 10.1016/j.jchromb.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobansky MR, Hage DS. Identification and analysis of stereoselective drug interactions with low density lipoprotein by high-performance affinity chromatography. Anal Biochem Chem. 2012;403:563–71. doi: 10.1007/s00216-012-5816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Sobansky MR, Hage DS. Analysis of drug interactions with high-density lipoprotein by high-performance affinity chromatography. Anal Biochem. 2010;397:107–14. doi: 10.1016/j.ab.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobansky MR, Hage DS. Analysis of drug interactions with very low density lipoprotein by high-performance affinity chromatography. Anal Biochem Chem. 2014;406:6203–11. doi: 10.1007/s00216-014-8081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anguizola J, Bi C, Koke M, Jackson A, Hage DS. On-column entrapment of alpha1-acid glycoprotein for studies of drug-protein binding by high-performance affinity chromatography. Anal Bioanal Chem. 2016;408:5745–56. doi: 10.1007/s00216-016-9677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hage DS, Anguizola JA, Jackson AJ, Matsuda R, Papastavros E, Pfaunmiller E, et al. Chromatographic analysis of drug interactions in the serum proteome. Anal Methods. 2011;3:1449–60. doi: 10.1039/C1AY05068K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temporini C, Pochetti G, Fracchiolla G, Piemontese L, Montanari R, Moaddel R, et al. Open tubular columns containing the immobilized ligand binding domain of peroxisome proliferator-activated receptors α and γ for dual agonists characterization by frontal affinity chromatography with mass spectrometry detection. J Chromatogr A. 2013;1284:36–43. doi: 10.1016/j.chroma.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tetala KKK, Chen B, Visser GM, van Beek TA. Single step synthesis of carbohydrate monolithic capillary columns for affinity chromatography of lectins. J Sep Sci. 2007;30:2828–35. doi: 10.1002/jssc.200700356. [DOI] [PubMed] [Google Scholar]
- 21.Kovarik P, Hodgson RJ, Covey T, Brook MA, Brennan JD. Capillary-scale frontal affinity chromatography/MALDI tandem mass spectrometry using protein-doped monolithic silica columns. Anal Chem. 2005;77:3340–50. doi: 10.1021/ac048263p. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson RJ, Chen Y, Zhang Z, Tleugabulova E, Long H, Zhao X, et al. Protein-doped monolithic silica columns for capillary liquid chromatography prepared by the sol−gel method: applications to frontal affinity chromatography. Anal Chem. 2004;76:2780–90. doi: 10.1021/ac0352124. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AJ, Anguizola J, Pfaunmiller EL, Hage DS. Use of entrapment and high-performance affinity chromatography to compare the binding of drugs and site-specific probes with normal and glycated human serum albumin. Anal Bioanal Chem. 2013;405:5833–41. doi: 10.1007/s00216-013-6981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, Debolt E, et al. Review: glycation of human serum albumin. Clin Chim Acta. 2013;425:64–76. doi: 10.1016/j.cca.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anguizola J, Joseph KS, Barnaby OS, Matsuda R, Alvarado G, Clark W, et al. Development of affinity microcolumns for drug-protein binding studies in personalized medicine: interactions of sulfonylurea drugs with in vivo glycated human serum albumin. Anal Chem. 2013;85:4453–60. doi: 10.1021/ac303734c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda R, Anguizola J, Joseph KS, Hage DS. Analysis of drug interactions with modified proteins by high-performance affinity chromatography: binding of glibenclamide to normal and glycated human serum albumin. J Chromatogr A. 2012;1265:114–22. doi: 10.1016/j.chroma.2012.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph KS, Hage DS. Characterization of the binding of sulfonylurea drugs to HSA by high-performance affinity chromatography. J Chromatogr B. 2010;878:1590–8. doi: 10.1016/j.jchromb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergström M, Åström E, Påhlsson P, Ohlson S, editors. Elucidating the selectivity of recombinant forms of Aleuria aurantia lectin using weak affinity chromatography. J Chromatogr B. 2012;885–886:66–72. doi: 10.1016/j.jchromb.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Engström HA, Johansson R, Koch-Schmidt P, Gregorius K, Ohlson S, Bergström M. Evaluation of a glucose sensing antibody using weak affinity chromatography. Biomed Chromatogr. 2008;22:272–7. doi: 10.1002/bmc.924. [DOI] [PubMed] [Google Scholar]
- 30.Winzor DJ. Determination of binding constants by affinity chromatography. J Chromatogr A. 2004;1037:351–67. doi: 10.1016/j.chroma.2003.11.092. [DOI] [PubMed] [Google Scholar]
- 31.Kakita H, Nakamura K, Kato Y. High-performance affinity chromatography of a chick lectin on an adsorbent based on hydrophilic polymer gel. J Chromatogr. 1991;543:315–26. doi: 10.1016/s0021-9673(01)95784-x. [DOI] [PubMed] [Google Scholar]
- 32.Hage DS. High-performance affinity chromatography: a powerful tool for studying serum protein binding. J Chromatogr B. 2002;768:3–30. doi: 10.1016/s0378-4347(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Hage DS. Quantitative analysis of allosteric drug-protein binding by biointeraction chromatography. Nature Biotechnol. 2004;22:1445–8. doi: 10.1038/nbt1022. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Hage DS. Quantitative studies of allosteric effects by biointeraction chromatography: analysis of protein binding to low solubility drugs. Anal Chem. 2006;78:2672–83. doi: 10.1021/ac052017b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh N, Ravichandran S, Norton DD, Fugmann SD, Moaddel R. Synthesis and characterization of a SIRT6 open tubular column: predicting deacetylation activity using frontal chromatography. Anal Biochem. 2013;436:78–83. doi: 10.1016/j.ab.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moaddel R, Wainer IW. The preparation and development of cellular membrane affinity chromatography columns. Nature Protocols. 2009;4:197–205. doi: 10.1038/nprot.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schriemer DC, Bundle DR, Li L, Hindsgaul O. Micro-scale frontal affinity chromatography with mass spectrometric detection: a new method for the screening of compound libraries. Angew Chem Int Ed. 1998;37:3383–97. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3383::AID-ANIE3383>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Palcic MM, Mo H, Goldstein IJ, Hindsgaul O. Rapid determination of the binding affinity and specificity of the mushroom Polyporus squamosus lectin using frontal affinity chromatography coupled to electrospray mass spectrometry. Glycobiology. 2001;11:141–7. doi: 10.1093/glycob/11.2.141. [DOI] [PubMed] [Google Scholar]
- 39.Calleri E, Fracchiolla G, Montanari R, Pochetti G, Lavecchia A, Loiodice F, et al. Frontal affinity chromatography with MS detection of the ligand binding domain of PPARγ receptor: ligand affinity screening and stereoselective ligand–macromolecule interaction. J Chromatogr A. 2012;1232:84–92. doi: 10.1016/j.chroma.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B, Palcic MM, Schriemer DC, Alarez-Manilla G, Pierce M, Hindsgaul O. Frontal affinity chromatography coupled to mass spectrometry for screening mixtures of enzyme inhibitors. Anal Biochem. 2001;299:173–82. doi: 10.1006/abio.2001.5417. [DOI] [PubMed] [Google Scholar]
- 41.Slon-Usakiewicz JJ, Ng W, Dai JR, Pasternak A, Redden PR. Frontal affinity chromatography with MS detection (FAC-MS) in drug discovery. Drug Discov Today. 2005;10:409–16. doi: 10.1016/S1359-6446(04)03360-4. [DOI] [PubMed] [Google Scholar]
- 42.Meiby E, Simmonite H, le Strat L, Davis B, Matassova N, Moore JD, et al. Fragment screening by weak affinity chromatography: comparison with established techniques for screening against HSP90. Anal Chem. 2013;85:6756–66. doi: 10.1021/ac400715t. [DOI] [PubMed] [Google Scholar]
- 43.Duong-Thi MD, Meiby E, Bergström M, Fex T, Isaksson R, Ohlson S, editors. Weak affinity chromatography as a new approach for fragment screening in drug discovery. Anal Biochem. 2011;414:138–46. doi: 10.1016/j.ab.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Meiby E, Knapp S, Elkins JM, Ohlson S. Fragment screening of cyclin G-associated kinase by weak affinity chromatography. Anal Bioanal Chem. 2012;404:2417–25. doi: 10.1007/s00216-012-6335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duong-Thi MD, Bergström M, Fex T, Isaksson R, Ohlson S, editors. High-throughput fragment screening by affinity LC-MS. J Biomol Screening. 2013;18:160–71. doi: 10.1177/1087057112459271. [DOI] [PubMed] [Google Scholar]
- 46.Ohlson S, Shoravi S, Fex T, Isaksson R. Screening for transient biological interactions as applied to albumin ligands: a new concept for drug discovery. Anal Biochem. 2006;359:120–3. doi: 10.1016/j.ab.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Loun B, Hage DS. Characterization of thyroxine-albumin binding using high-performance affinity chromatography. II. Comparison of the binding of thyroxine, triiodothyronines and related compounds at the warfarin and indole sites of human serum albumin. J Chromatogr B. 1995;665:303–14. doi: 10.1016/0378-4347(94)00547-i. [DOI] [PubMed] [Google Scholar]
- 48.Ohlson S, Bergström M, Påhlsson P, Lundblad A. Use of monoclonal antibodies for weak affinity chromatography. J Chromatogr A. 1997;758:199–208. doi: 10.1016/s0021-9673(96)00733-9. [DOI] [PubMed] [Google Scholar]
- 49.Turowski M, Kaliszan R. Keratin immobilized on silica as a new stationary phase for chromatographic modelling of skin permeation. J Pharm Biomed Anal. 1997;15:1325–33. doi: 10.1016/s0731-7085(96)02009-2. [DOI] [PubMed] [Google Scholar]
- 50.Kaliszan R, Kaliszan A, Noctor TAG, Purcell WP, Wainer IW. Mechanism of retention of benzodiazepines in affinity, reversed-phase and adsorption high-performance liquid chromatography in view of quantitative structure retention relationships. J Chromatogr A. 1992;609:69–81. [Google Scholar]
- 51.Kaliszan R, Nasal A, Turowski M. Quantitative structure-retention relationships in the examination of the topography of the binding site of antihistamine drugs on α1-acid glycoprotein. J Chromatogr A. 1996;722:25–32. doi: 10.1016/0021-9673(95)00523-4. [DOI] [PubMed] [Google Scholar]
- 52.Karlsson A, Aspegren A. Enantiomeric separation of amino alcohols on protein phases using statistical experimental design: A comparative study. J Chromatogr A. 2000;866:15–23. doi: 10.1016/s0021-9673(99)01040-7. [DOI] [PubMed] [Google Scholar]
- 53.Fitos I, Visy J, Simonyi M, Hermansson J. Chiral high-performance liquid chromatographic separations of vinca alkaloid analogues on α1-acid glycoprotein and human serum albumin columns. J Chromatogr A. 1992;609:163–71. doi: 10.1016/0021-9673(92)80159-r. [DOI] [PubMed] [Google Scholar]
- 54.Gyimesi-Forras K, Szasz G, Gergely A, Szabo M, Kokosi K. Optical resolution of a series of potential cholecystokinin antagonist 4(3H)-quinazolone derivatives by chiral liquid chromatography on alpha(1)-acidglycoprotein stationary phase. J Chromatogr Sci. 2000;38:430–4. doi: 10.1093/chromsci/38.10.430. [DOI] [PubMed] [Google Scholar]
- 55.Noctor TAG, Wainer IW. The in situ acetylation of an immobilized human serum albumin chiral stationary phase for high-performance liquid chromatography in the examination of drug–protein binding phenomena. Pharmaceut Res. 1992;9:480–4. doi: 10.1023/a:1015884112039. [DOI] [PubMed] [Google Scholar]
- 56.Chattopadhyay A, Tian T, Kortum L, Hage DS. Development of tryptophan-modified human serum albumin columns for site-specific studies of drug–protein interactions by high-performance affinity chromatography. J Chromatogr B. 1998;715:183–90. doi: 10.1016/s0378-4347(98)00140-6. [DOI] [PubMed] [Google Scholar]
- 57.Bertucci C, Nanni B, Raffaelli A, Salvadori P. Chemical modification of human albumin at Cys34 by ethacrynic acid: structural characterisation and binding properties. J Pharm Biomed Anal. 1998;18:127–36. doi: 10.1016/s0731-7085(98)00163-0. [DOI] [PubMed] [Google Scholar]
- 58.Zheng X, Podariu M, Bi C, Hage DS. Development of enhanced capacity affinity microcolumns by using a hybrid of protein cross-linking/modification and immobilization. J Chromatogr A. 2015;1400:82–90. doi: 10.1016/j.chroma.2015.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng X, Bi C, Li Z, Podariu M, Hage DS. Analytical methods for kinetic studies of biological interactions: a review. J Pharm Biomed Anal. 2015;113:163–80. doi: 10.1016/j.jpba.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loun B, Hage DS. Chiral separation mechanisms in protein-based HPLC columns. 2. Kinetic studies of (R)- and (S)-warfarin binding to immobilized human serum albumin. Anal Chem. 1996;68:1218–25. doi: 10.1021/ac950827p. [DOI] [PubMed] [Google Scholar]
- 61.Yang J, Hage DS. Effect of mobile phase composition on the binding kinetics of chiral solutes on a protein-based HPLC column: interactions of D- and L-tryptophan with immobilized human serum albumin. J Chromatogr A. 1997;766:15–25. doi: 10.1016/s0021-9673(96)01040-0. [DOI] [PubMed] [Google Scholar]
- 62.Schiel JE, Ohnmacht CM, Hage DS. Measurement of drug-protein dissociation rates by high-performance affinity chromatography and peak profiling. Anal Chem. 2009;81:4320–33. doi: 10.1021/ac9000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tong Z, Schiel JE, Papastavros E, Ohnmacht CM, Smith QR, Hage DS. Kinetic studies of drug-protein interactions by using peak profiling and high-performance affinity chromatography: examination of multi-site interactions of drugs with human serum albumin columns. J Chromatogr A. 2011;1218:2065–71. doi: 10.1016/j.chroma.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tong Z, Hage DS. Characterization of interaction kinetics between chiral solutes and human serum albumin by using high-performance affinity chromatography and peak profiling. J Chromatogr A. 2011;1218:6892–7. doi: 10.1016/j.chroma.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Schiel JE, Hage DS. Non-competitive peak decay analysis of drug-protein dissociation by high-performance affinity chromatography and peak profiling. J Sep Sci. 2009;32:1632–41. doi: 10.1002/jssc.200900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfaunmiller E, Moser AC, Hage DS. Biointeraction analysis of immobilized antibodies and related agents by high-performance immunoaffinity chromatography. Methods. 2012;56:130–5. doi: 10.1016/j.ymeth.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoo MJ, Hage DS. High-throughput analysis of drug dissociation from serum proteins using affinity silica monoliths. J Sep Sci. 2011;34:2255–63. doi: 10.1002/jssc.201100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo MJ, Hage DS. Use of peak decay analysis and affinity microcolumns containing silica monoliths for rapid determination of drug-protein dissociation rates. J Chromatogr A. 2011;218:2072–8. doi: 10.1016/j.chroma.2010.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson MA, Moser AC, Hage DS. Biointeraction analysis by high-performance affinity chromatography: kinetic studies of immobilized antibodies. J Chromatogr B. 2010;878:165–71. doi: 10.1016/j.jchromb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hage DS, Walters RR, Hethcote HW. Split-peak affinity chromatographic studies of the immobilization-dependent adsorption kinetics of protein A. Anal Chem. 1986;58:274–9. doi: 10.1021/ac00293a003. [DOI] [PubMed] [Google Scholar]
- 71.Mallik R, Yoo MJ, Briscoe CJ, Hage DS. Analysis of drug-protein binding by ultrafast affinity chromatography using immobilized human serum albumin. J Chromatogr A. 2010;1217:2796–803. doi: 10.1016/j.chroma.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohnmacht CM, Schiel JE, Hage DS. Analysis of free drug fractions using near infrared fluorescent labels and an ultrafast immunoextraction/displacement assay. Anal Chem. 2006;78:7547–56. doi: 10.1021/ac061215f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng X, Yoo MJ, Hage DS. Analysis of free fractions for chiral drugs using ultrafast extraction and multi-dimensional high-performance affinity chromatography. Analyst. 2013;138:6262–5. doi: 10.1039/c3an01315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clarke C, Choudhuri AR, Hage DS. Analysis of free drug fractions by ultra-fast immunoaffinity chromatography. Anal Chem. 2001;73:2157–64. doi: 10.1021/ac0009752. [DOI] [PubMed] [Google Scholar]
- 75.Clarke W, Schiel JE, Moser A, Hage DS. Analysis of free hormone fractions by an ultrafast immunoextraction/displacement immunoassay: studies using free thyroxine as a model system. Anal Chem. 2005;77:1859–66. doi: 10.1021/ac040127x. [DOI] [PubMed] [Google Scholar]


