Abstract
Aims
Heart failure remains a leading cause of morbidity, hospitalizations and deaths. We previously showed that overexpression of the enzyme ribonucleotide reductase (RNR) in cardiomyocytes increased levels of the myosin activator, 2-deoxy-adenosine triphosphate, catalyzed enhanced contraction and improved cardiac performance in rodent hearts. Here we used a swine model of myocardial infarction (MI) to test preliminarily a novel gene therapy for heart failure based on delivery of the human RNR enzyme complex under the control of a cardiac specific promoter via an adeno-associated virus serotype 6 vector - designated as BB-R12.
Methods and Results
We induced heart failure following MI in Yucatan minipigs by balloon occlusion of the left anterior descending artery. Two weeks later pigs received BB-R12 at one of three doses via antegrade coronary infusion. At two months post-treatment, left ventricular (LV) ejection fraction and systolic LV dimension (measured by echocardiography) improved significantly in the high-dose group, despite further deterioration in the saline controls. Hemodynamic parameters including LV end-diastolic pressure, +dP/dt, and −dP/dt all trended towards improvement in the high-dose group. We observed no difference in the histopathologic appearance of hearts or other organs from treated animals versus controls, nor did we encounter any safety or tolerability concerns following BB-R12 delivery.
Conclusion
These pilot results suggest cardiac-specific gene therapy using BB-R12 may reverse cardiac dysfunction by myosin activation in a large-animal heart failure model with no observed safety concerns. Thus further research into the therapeutic potential of BB-R12 for patients with chronic heart failure appears warranted.
Keywords: Gene therapy, heart failure, ribonucleotide reductase
INTRODUCTION
Gene therapy is a new option for treatment of heart failure (HF). Several gene therapies have been developed to improve cardiac performance in HF models by targeting calcium and adrenergic signaling, including the overexpression of sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA2a) which regulates calcium movement between the cytoplasm and the sarcoplasmic reticulum (SR),1–3 S100A which modifies SR calcium handling,4, 5 β-adrenergic receptor kinase (β-ARKct) which restores β-adrenergic receptor signaling,6, 7 small ubiquitin-related modifier 1 (SUMO-1) which regulates SERCA2a through post transcriptional modification,8 and an inhibitor of protein phosphatase 1 (I-1c) which regulates de-phosphorylation of phospholamban.9, 10 All of these gene therapies have employed adeno-associated virus (AAV) vectors to deliver their respective transgene. SERCA2a (Mydicar) is the most advanced of these gene therapies, and a clinical trial has shown it mediates beneficial effects on cardiac function as well as a decrease in hospitalizations.11, 12
In this study we sought to develop Ribonucleotide Reductase (RNR) gene therapy, which, in contrast, is independent of calcium and adrenergic signaling and instead, works to directly increase contractility by producing 2-deoxy-adenosine triphosphate (dATP) for myosin activity. Overexpression of RNR represents an entirely novel gene therapy concept for HF, where the gene product does not, in itself, produce the therapeutic effect. RNR is an essential enzyme that catalyzes the de novo synthesis of deoxyribonucleotide triphosphates (dNTPs), used principally for DNA synthesis and repair. The enzyme is a heterotetramer of two subunits, Rm1 and Rm2.13
Regnier et al. have demonstrated that small elevations of cytoplasmic levels of dATP increase force generation, cross bridge cycling and calcium sensitivity in skinned rat cardiomyocytes,14, 15 and in myocardium from failing human hearts.16 These results were obtained with increases in dATP concentration as small as ~1.5% of the total adenosine triphosphate nucleotide pool. Our groups have shown that overexpression of RNR in rat cardiomyocytes,17 transgenic mice18 and human-derived cardiomyocytes19 enhanced cardiac contraction via the increase of dATP. dATP is minimally available as a contractile substrate in mature cardiomyocytes because RNR transcription is down-regulated in non-replicating cells. In an effort to develop a treatment for HF, we have established a gene therapy vector to deliver human cDNA for Rm1 and Rm2 from a single transgene construct. This construct is designated BB-R12 and the up-regulation of expression of Rm1 and Rm2 occurs exclusively in cardiomyocytes under the control of the cardiac troponin T (cTnT) promoter. Transcription occurs via a single mRNA with the incorporation of a 2A sequence, resulting in the independent translation of both proteins that form the active heterotetrameric enzymes. By expressing the transgene restricted to the heart tissue via a cardiac specific promoter, we minimize the potential for non-cardiac effects and achieve direct targeting of the cardiac muscle myofibrils.
METHODS
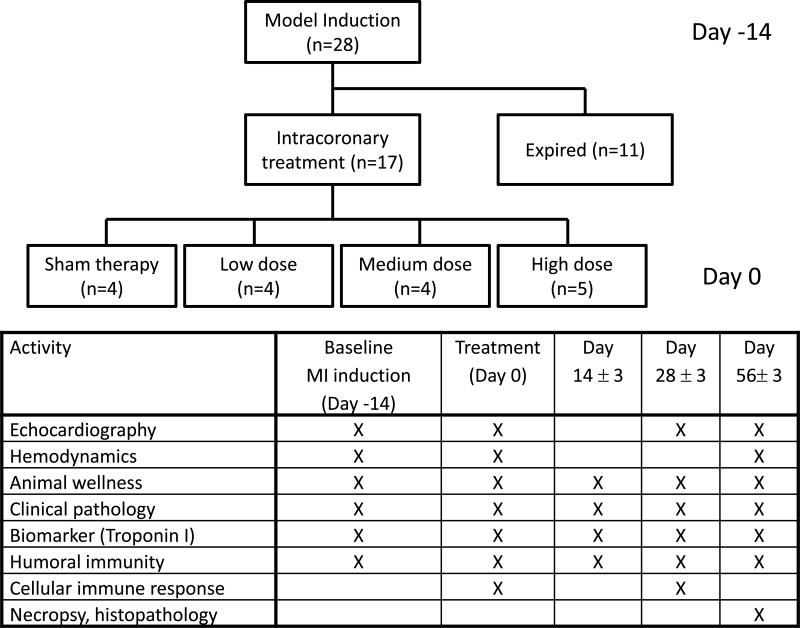
For full details of methods, please see the online supplement. Briefly, myocardial infarction (MI) was induced in Yucatan mini-pigs by 75 minutes of occlusion with a balloon catheter of the mid-left anterior descending artery. Consistent with other published porcine MI-HF gene therapy experiments7, BB-R12 or sham control was administered at 2 weeks post-MI (day 0) in an unblinded manner at 1 × 1013, 5 × 1012 and 1 × 1012 viral genome (VG), hereafter referred to as high, medium and low doses, respectively. Two-thirds of the study drug volume was infused antegrade into the left main coronary artery and the remainder into the proximal right coronary artery. Echocardiography, hemodynamics, animal wellness, pathology, immune response and biomarkers were assessed as shown in Figure 1. The timing of the cardiac assessments was based on results of prior rodent cardiac performance studies conducted in our laboratory. All values are reported as mean ± standard error of the mean (SEM). Data were analyzed mainly by a mixed effects regression model with factors for treatment group and time.
Figure 1. Study design.
MI was induced in 28 Yucatan mini-pigs by balloon occlusion of mid-left anterior descending artery. Two weeks later (day 0), 17 surviving pigs received antegrade coronary infusion of dilution buffer (sham), high (1 × 1013 VRG), medium (5 × 1012 VRG) or low (1 × 1012 VRG)-dose of BB-R12. We measured the listed parameters serially at the time points as shown.
RESULTS
Swine MI-HF model
The experimental design and flow of animals through the study is shown in Figure 1. Yucatan minipigs (35–45 kg) were screened for neutralizing antibodies to AAV6, and a total of 28 seronegative animals were enrolled in this study and underwent myocardial infarction (MI) induction (day -14). Eight pigs died from ventricular fibrillation during the MI induction procedure and an additional 3 pigs died after MI induction but prior to administration of BB-R12 gene therapy or placebo. A total of 17 pigs received intracoronary infusions of BB-R12 or formulation buffer (sham) as shown in Table 1. All treated animals survived until euthanasia at 56 days post-treatment.
Table 1.
Mean values of echocardiography and hemodynamics
| Sham N=4 |
High dose N=5 |
Medium dose N=4 |
Low dose N=4 |
|
|---|---|---|---|---|
| Echocardiography | ||||
| Left Ventricular Ejection Fraction: LVEF (%) | ||||
| Day -14 | 58.3 ±1.7 | 62.4±3.4 | 54.0±2.5 | 56.8±0.8 |
| Day 0 | 46.3±1.8 | 44.0±3.3 | 44.3±4.8 | 48.5±2.2 |
| Day 28 | 38.5±3.4 | 50.0±1.7* | 37.8±5.1 | 45.8±4.1 |
| Day 56 | 34.8±4.1 | 51.2±2.0** | 47.8±3.3* | 44.5±5.7 |
| Left Ventricular Fractional Shortening: LVFS (%) | ||||
| Day -14 | 30.6±4.8 | 31.2±3.3 | 26.3±3.7 | 33.0±8.0 |
| Day 0 | 17.7±5.6 | 25.1±4.9 | 17.7±4.9 | 30.1±1.0 |
| Day 28 | 23.5±3.8 | 25.4±4.0 | 19.1±2.4 | 22.3±5.3 |
| Day 56 | 12.4±2.0 | 31.8±3.5* | 22.6±4.4 | 23.8±4.0 |
| Left Ventricular End-Systolic Dimension: LVESD (mm) | ||||
| Day -14 | 29.4±2.0 | 26.3±1.0 | 27.8±1.9 | 23.7±3.9 |
| Day 0 | 38.7±3.1 | 33.9±2.4 | 40.7±6.9 | 29.8±2.7 |
| Day 28 | 39.2±2.7 | 36.8±2.8 | 42.0±2.4 | 35.7±4.9 |
| Day 56 | 46.3±1.8 | 33.4±1.5 | 37.7±3.6* | 35.0±3.1 |
| Left Ventricular End-diastolic Dimension: LVEDD (mm) | ||||
| Day -14 | 42.4±1.0 | 38.5±1.9 | 37.9±3.2 | 35.2±3.6 |
| Day 0 | 47.0±1.7 | 45.7±2.4 | 48.7±5.5 | 42.8±4.5 |
| Day 28 | 51.1±1.2 | 49.3±2.1 | 52.1±3.3 | 45.4±3.8 |
| Day 56 | 52.8±1.9 | 49.2±1.8 | 48.6±3.5 | 45.8±2.5 |
| Hemodynamics | ||||
| +dP/dt (mmHg/sec) | ||||
| Day -14 | 989.0±64.1 | 1026.8±175.6 | 1104.5±160.7 | 1000.9±198.3 |
| Day 0 | 846.8±84.7 | 644.2±46.3 | 986.6±203.5 | 978.0±60.8 |
| Day 56 | 818.8±105.5 | 1159.8±139.3** | 963.1±151.7 | 1192.8±83.9 |
| −dP/dt (mmHg/sec) | ||||
| Day -14 | −762.7±259.5 | −870.8±223.2 | −904.5±86.4 | −1043.0±378.9 |
| Day 0 | −614.2±132.9 | −686.2±113.9 | −618.5±32.6 | −1027.3±110.9 |
| Day 56 | −666.6±86.3 | −1546.7±270.9* | −691.0±20.1 | −758.3±161.2 |
| Left Ventricular End-Diastolic Pressure: LVEDP (mmHg) | ||||
| Day -14 | 16.0±4.8 | 17.8±3.7 | 7.3±2.5 | 16.8±4.5 |
| Day 0 | 23.0±4.0 | 21.8±2.5 | 20.0±9.0 | 21.3±11.8 |
| Day 56 | 21.3±3.0 | 11.0±2.5 | 12.0±1.6 | 21.8±4.6 |
| Heart Rate (beats per minute) | ||||
| Day -14 | 73.5±10.6 | 74.4±7.6 | 84.8±7.4 | 66.3±3.8 |
| Day 0 | 66.0±2.0 | 78.8±4.0 | 80.0±8.7 | 73.5±2.9 |
| Day 56 | 94.0±18.1 | 94.2±8.3 | 103.5±10.4 | 95.0±4.3 |
| Aortic Pressure-Systolic (mmHg) | ||||
| Day -14 | 73.5±10.9 | 73.2±7.5 | 80.3±6.1 | 87.5±9.9 |
| Day 0 | 75.5±11.2 | 69.6±2.2 | 81.3±5.5 | 78.0±10.2 |
| Day 56 | 82.0±3.2 | 77.6±2.7 | 76.3±7.4 | 89.3±5.9 |
| Aortic Pressure-Diastolic (mmHg) | ||||
| Day -14 | 43.3±12.0 | 45.6±8.4 | 52.3±5.2 | 60.5±11.0 |
| Day 0 | 50.8±10.8 | 46.0±3.3 | 55.3±6.2 | 51.3±9.6 |
| Day 56 | 55.3±2.6 | 51.8±2.1 | 52.5±7.4 | 61.5±3.7 |
| Aortic Pressure-Mean (mmHg) | ||||
| Day -14 | 54.5±10.8 | 54.8±7.8 | 61.5±5.5 | 69.0±10.5 |
| Day 0 | 57.8±10.3 | 53.8±2.6 | 63.7±6.1 | 59.8±9.9 |
| Day 56 | 64.0±2.7 | 59.4±1.9 | 59.5±7.0 | 68.8±4.3 |
Data are shown as mean ± SEM.
p < 0.05,
p < 0.01 for differences between the sham group and each of the other groups, mixed effects regression model with factors for dosing group and time.
Effect of BB-R12 on cardiac systolic function
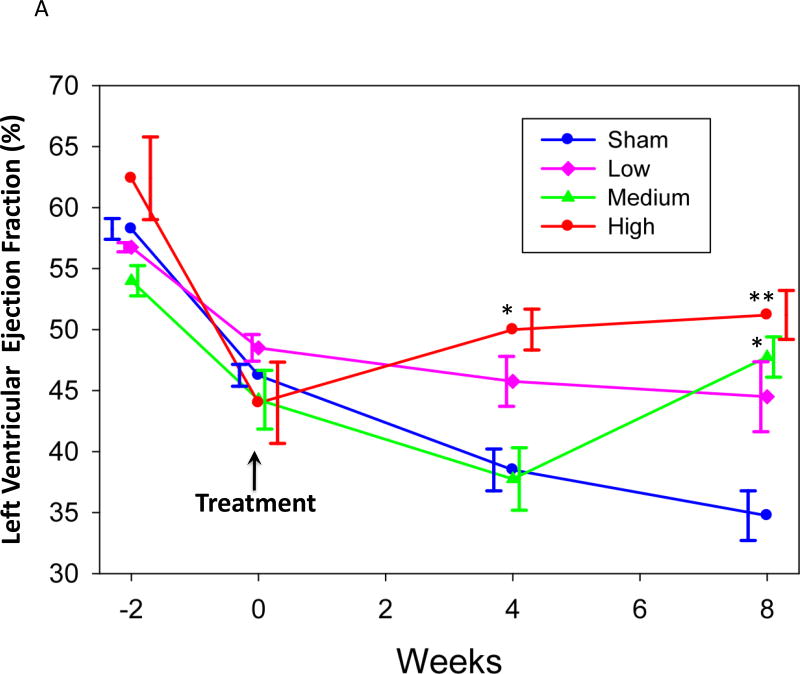
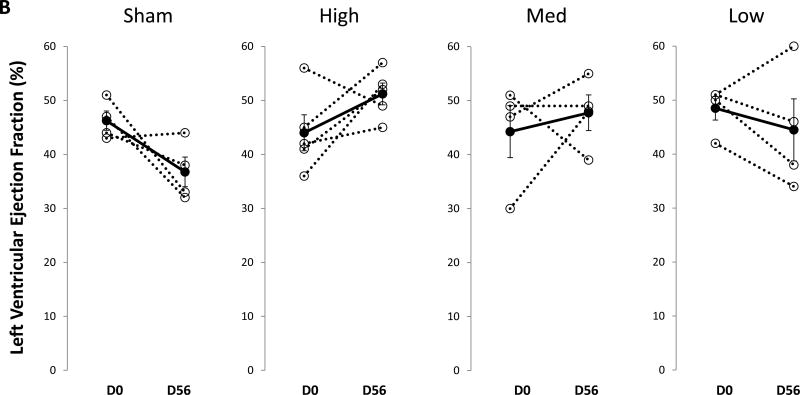
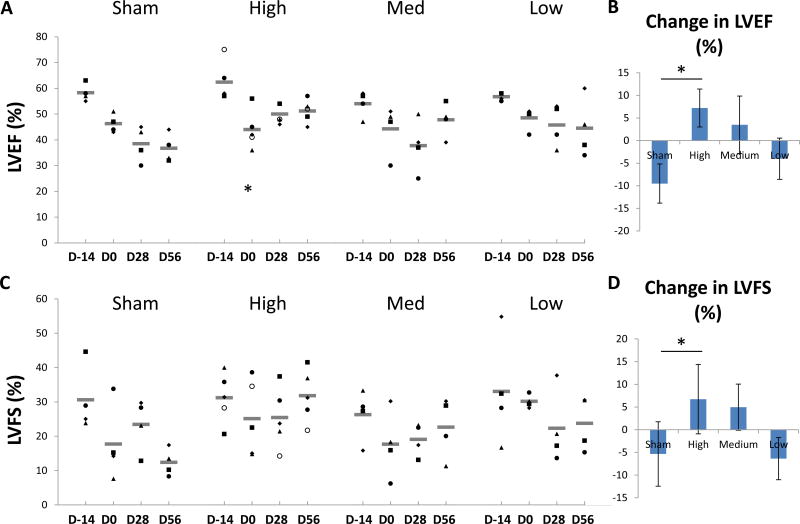
The mean values for each group and time point are shown in Table 1. All four groups had comparable left ventricular ejection fraction (LVEF) prior to infarction. By 14 days post-MI, mean LVEF had declined significantly in all groups, though the magnitude of the decline was variable. In the sham group LVEF continued to decline at 56 days post saline treatment (Table 1, Figure 2A). In contrast, LVEF in the high-dose group recovered after BB-R12 treatment at 28 and 56 days post-treatment (p = 0.04 and p=0..006 vs. control at each time, respectively). Interestingly, the medium-dose group did not respond at 28 days, but recovered at 56 days post-treatment and was then significantly higher than sham (P=0.03). The low-dose group showed showed no improvement from the time of gene delivery to 56 days.. The change of LVEF from day 0 to 56 was +7% for the high-dose group compared to −10% for the sham group (p<0.05) (Figure 2B and 3B).
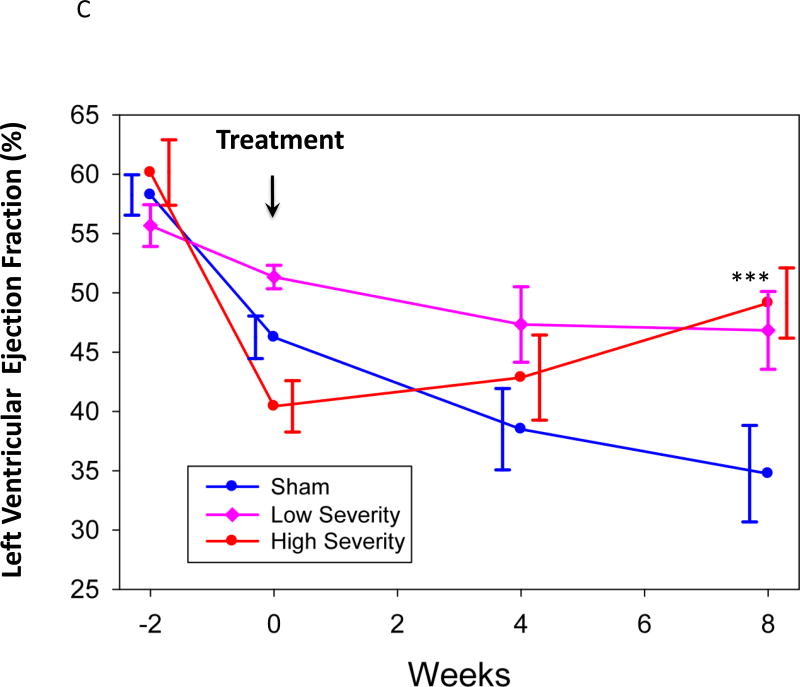
Figure 2. Left ventricular ejection fraction (LVEF) vs time.
(A) Mean LVEF for each dosing group at study time points are shown. *p < 0.05, **p<0.01 for differences vs sham, mixed effects regression model. (B) Individual and mean changes in LVEF from day 0 (D0) to day 56 (D56). (C) Mean LVEF for animals with high severity HF (defined as animals in the pooled treated group with LVEF below the median at Day 0 prior to treatment), low severity HF (similarly defined but with LVEF above the median) and sham treatment, ***p < 0.005 for differences vs sham, mixed effects regression model.
Figure 3. Left ventricular ejection fraction (LVEF) and fractional shortening (FS).
(A) Individual and mean LVEF at study time points for each group are shown. (B) Mean change (± SEM) in LVEF from Day 0 to Day 56. *p<0.05. (C) Individual and mean LVFS are shown. (D) Mean change in LVFS from Day 0 to Day 56.
An exploratory post-hoc analysis took into account the model variability in infarct size. All active-treated animals were pooled, then divided into two groups by the median decline in LVEF from Day -14 to Day 0 (high severity HF vs. low severity HF) and compared to the sham-treated group (Figure 2C). The sham-treated group showed significant deterioration of LVEF from Day 0 to Day 56 (p=0.02). The group with high severity HF showed significant improvement in LVEF at Day 56 (p=0.001) and a trend towards improvement at Day 28 (p=0.08), as well as a significant improvement compared to the sham-treated group (p=0.02), while the low severity HF group showed no change from Day 0 to Day 56 and no difference from control.
Similar findings occurred for the related measures of LV fractional shortening (LVFS). While the sham and low-dose groups showed a decline in LVFS at 56 days post-administration, both the high- and medium-dose groups showed increases (Figure 3C). At 56 days post-treatment, LVFS for the high-dose group showed a +7% increase, which was over the Day 0 function, compared with a −5% decrease in the sham. LVFS in high-dose group was significantly higher than the sham at 56 days after treatment (P=0.01).
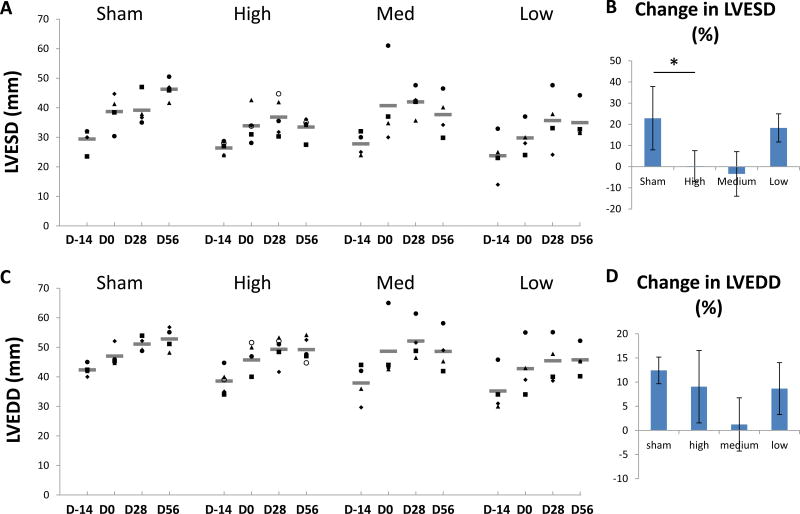
BB-R12 effect on LV dimensions
In all groups, LV end-systolic dimension (LVESD) increased from pre-infarction to the time of treatment at day 0 (Figure 4A). In the sham group, LVESD increased progressively and peaked at 56 days post-saline injection (Figure 4B). In the high- and medium-dose treated groups, however, the LVESD at 56 days post-treatment was similar or smaller to that at Day 0. In the high-dose treated group, LVESD at 56 days post-treatment was significantly shorter than the sham (P=0.01, Table 1), reflecting enhanced contractility. In contrast, left ventricular end-diastolic dimension (LVEDD) showed progressive dilation during 56 days after gene delivery for all groups (Figure 4C). There was no significant difference in LVEDD between sham and treated groups at 56 days after treatment.
Figure 4. Left ventricular end-systolic (LVESD) and end-diastolic dimensions (LVEDD).
(A) Individual and mean LVESD at study time points for each group are shown. (B) Mean change (± SEM) in LVESD from Day 0 to Day 56. (C) Individual and mean LVEDD are shown. (D) Mean change in LVEDD from Day 0 to Day 56.
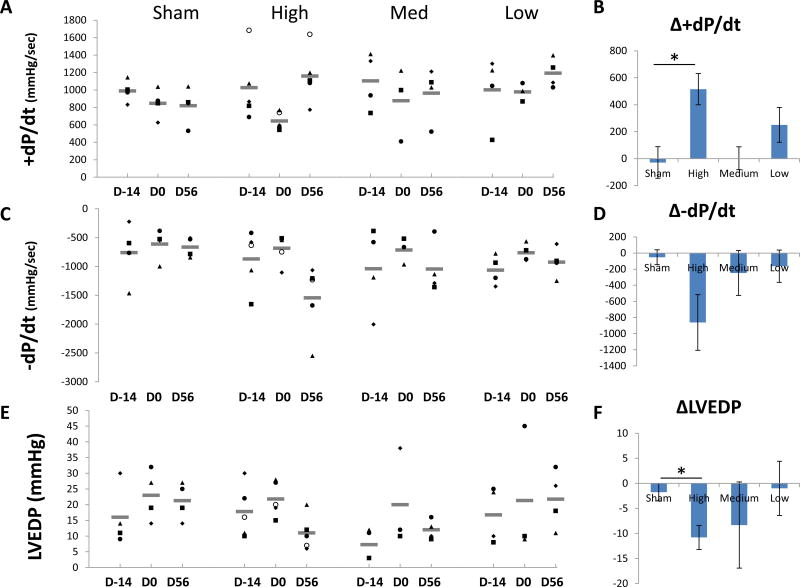
BB-R12 effects on +dP/dt, −dP/dt and end-diastolic pressure
The mean values for hemodynamic measurements are shown in Table 1. At 14 days following MI (Day 0), the mean values of maximum rate of pressure rise (+dP/dt), a parameter of systolic function, maximum rate of pressure decline (−dP/dt), a parameter of early diastolic function, and LV end-diastolic pressure (LVEDP) showed changes consistent with the onset of HF in all groups and these changes persisted in the sham group. At 56 days after treatment, there was improvement in these measures in the high- and medium-dose treated groups compared to the sham, although there was no difference in blood pressure and heart rates (Figure 5). The mean change in +dP/dt from day 0 to 56 was significantly increased in high-dose group compared to sham group, reflecting the positive inotropic effect of BB-R12 (P=0.004, Figure 5A, 5B). There were similar improvements in the negative rate of pressure change (-dP/dt) in response to BB-R12 treatment (Figure 5C, 5D). The mean LVEDP increased at 14 days following MI in all groups which persisted in the sham and low-dose treated groups. In the high- and medium-dose treated groups, MI-induced increases in LVEDP were reversed at Day 56 following treatment with BB-R12 (Figure 5E, 5F). The LVEDP change from day 0 to 56 was significantly improved in high-dose group compared to sham group (P=0.04, Figure 5F).
Figure 5. Hemodynamic measurements.
(A) Individual and mean +dP/dt at study time points for each group are shown. (B) Mean change (± SEM) in +dP/dt from Day 0 to Day 56. *p<0.05. (C) Individual and mean −dP/dt are shown. (D) Mean change in −dP/dt from Day 0 to Day 56. (E) Individual and mean change in left ventricular end-diastolic pressure (LVEDP) at study time points for each group are shown. (F) Mean change in LVEDP from Day 0 to Day 56.
The other measured hemodynamic parameters such as pulmonary capillary wedge pressure, pulmonary arterial pressure, central venous pressure and cardiac output in the treated groups were not significantly different from the sham group after 56 days (Supplemental Table 1).
BB-R12 does not evoke a significant humoral or cellular immune response
The development of a humoral immune response was monitored using an ELISA for the viral capsid protein. Titers of anti-AAV6 antibodies are shown in Supplemental Table 2. Most samples contained no appreciable antibody levels above the minimum detectable level (<1:800). In some treated animals at some time points, there was a transient and unremarkable titer of 1:800. All samples from sham-treated animals showed a titer of <1:800.
The cellular immune response was measured using a γ-interferon ELISPOT assay and the results are shown in Supplemental Table 3. Cytotoxic T lymphocyte (CTL) response to both viral capsid protein and Rm1 transgene were negative or equivocal at 2 weeks post-administration of BB-R12. Both controls, phorbol ester stimulated CTLs cells and cells from animals immunized with peptide libraries, showed significant positive responses.
Effects of BB-R12 on blood counts, chemistries or biomarkers
There were no significant differences between sham and treated groups in clinical pathology at 14, 28 or 56 days after gene delivery. The results of this analysis suggest no adverse effects on blood composition, blood chemistry, or liver enzymes following BB-R12 treatment. Sporadic elevations of serum troponin were observed between Day 14 and Day 56 in multiple groups, including the sham-treated animals (Supplemental Table 4).
Pathology and biodistribution
Overall, myocardial infarction led to similar lesions in the heart in all of the test groups and the sham control group. The percentage of fibrosis in the central infarct area in the sham and treated groups were similar (Supplemental Figure 1). The severity, frequency and distribution of the lesions in the treated hearts were similar to that seen in the sham group. There were no organ lesions or histopathologic changes in the treatment groups attributed to the BB-R12 therapy. No significant findings were observed by animal wellness monitoring across all treatment groups from the gene delivery to the endpoint. There was no significant difference in the mean AAV copy number per µg of DNA in the heart (left ventricle, left atrium and right ventricle), lung and liver between the treated groups, though the number of tissue samples analyzed was small (Supplemental Figure 2). No viral DNA was detected in the sham group.
DISCUSSION
In this pilot study, we observed persistent improvement in cardiac performance following a single anterograde coronary infusion of BB-R12 in a swine MI-HF model. Our findings at 2 months following therapy included 1) improvements in LVEF, LVFS and LVESD; 2) improvements in hemodynamic measurements including +dP/dt, −dP/dt and LVEDP, and 3) no safety or toxicological concerns were observed. The small number of study animals and inherent variability of the porcine MI-HF model limit the conclusions that can be drawn, but consistent improvements across multiple measures of cardiac performance, especially in the animals with the most severe HF are encouraging. Thus, these findings extend our previous observations in rodent and ex vivo experiments to a standard large-animal model of HF.
Gene therapies that are in development involve manipulation of either calcium handling (e.g. SERCA2a) or calcium indirectly via adrenergic signaling, and have been reported to improve HF in large-animal models.1–10 In this study, we observed improvement in multiple key contractile parameters in a large-animal MI/HF model using a gene therapy that works via a very different mechanism, cardiac myosin activation via cardiac-specific elevation of cytosolic dATP. Our group has shown that small amounts of dATP catalyze enhanced cardiac performance through myosin activation.14–16 The mechanism involves facilitation of actin-myosin cross-bridge cycling. Faster cycling allows more myosin heads (the force generators) to interact with actin during each cardiac contraction. Enhanced contractility has been seen across the range of physiologic levels of calcium (enhanced calcium sensitivity) but dATP has no effect on calcium transients.15,17
The increase in dATP is achieved through the enhanced expression of RNR, which is normally down regulated in mature cardiac muscle cells, and the increased expression of which results in the synthesis of dATP in cardiomyocytes. Though not measured directly in this pilot study, we have previously demonstrated that up-regulation of RNR is linked to increased dATP levels and this, in turn, to enhanced cardiac performance.17 Expression of RNR using the cTnT promoter facilitates cardiomyocyte-specific transcription and minimizes possible off target effects with a level of therapeutic specificity not achievable using small molecule drugs or gene therapies using constitutively expressed regulatory elements. Interestingly, even though contraction is stronger and faster, chemo-mechanical modeling suggests the weaker hydrostatic interaction between dATP and myosin in the binding pocket (vs. ATP) may result in a faster release of the hydrolysis product dADP, thus myosin detachment rate and relaxation is also faster.20 Consequently the kinetics of cell, tissue and organ relaxation appear matched to the enhanced contractility.
Direct targeting of the contraction machinery of the cardiac muscle cell by intracellular production of a superior myosin substrate is a new therapeutic paradigm to treat HF and is independent of both calcium and adrenergic signaling. Omecamtiv, a small molecule drug in clinical development, represents a new class of inotropic agents, cardiac myosin activators.21 Omecamtiv works directly on myosin and stimulates myocardial ATPase by strengthening myosin/actin crossbridge formation, thus increasing left ventricular systolic function independent of calcium while decreasing filling pressure without increasing heart rate or oxygen consumption.22, 23 Omecamtiv has reached later-stage clinical testing, which validates myosin activation as a target for inotropic therapy. However, omecamtiv is delivered by repeated intravenous infusions or chronic oral dosing, does not appear to affect the rate of ventricular pressure development and increases systolic ejection time in a dose dependent manner, leading to the prolongation of systole.22 In contrast dATP and BB-R12, requires a one-time administration, and appears to increase left ventricular function with no prolongation of systolic ejection time or shortening of diastole.
Gene therapy using BB-R12 increases cardiac function by turning a small number of transduced cardiomyocytes into cellular factories that generate dATP. dATP increases the contraction of both the transduced cell and adjacent cardiac muscle cells by passive diffusion of the nucleotide throughout the heart via gap junctions. Lundy et al. showed that small numbers of ex-vivo RNR-infected cardiomyocytes directly injected into 3 loci in rat hearts had positive overall effects on cardiac function, demonstrating that a small proportion of transduced cells distributed in a limited number of sites can increase cardiac function.19 This amplification of the effect of RNR up-regulation may indicate that the number of transduced cells required to produce a therapeutic effect may be lower than for other gene therapies. We speculate that the delayed time-course of improvement of cardiac performance observed in the medium-dose treated group may be consistent with the gradual accumulation of cytosolic dATP over time. In prior rodent and ex vivo experiments, observed increases in contractility affected the entire heart. However, the potential risks of inducing regional increases in contractility warrant further assessment.
In this small, pilot study, no adverse effects of BB-R12 therapy were observed. No treatment-related deaths or adverse events were observed, and gross pathology and histopathology showed no difference from sham treated animals. Blood chemistry was unremarkable and no elevation of liver enzymes was observed. A low level, transient humoral response to AAV6 capsid protein was observed in a minority of animals in response to therapy. Cellular immune responses showed no appreciable CTL response to either the capsid protein or transgene product. It should be noted that the amino acid sequences of the transgene are identical between pigs and humans and therefore these results are reassuring that a cellular immune response to the transgene product was unlikely.
Nowakowski et al. studied transgenic mice that expressed RNR constitutively in all cells, and observed no documentable cardiac pathology (hypertrophy, fibrosis etc.), but found a marked, lifelong increase in cardiac performance.18 Based on this work, we expected that cardiac-specific transgene expression would likely produce a safe therapeutic. Our pilot study observations are consistent with this expectation. It should be noted that RNR is regulated allosterically by dATP, and additional post-translational regulatory mechanisms also play a role, working collectively to keep dATP levels low.13, 24 The buildup of abnormal levels of dATP is further limited by the passive diffusion out of the producer cells and into adjacent coupled cells through gap junctions. However, small amounts of dATP (<10% of the total ATP pool) show increases in force development and cell contraction at the myofibril level.25
Study limitations
Despite the encouraging results, there are important limitations of the current pilot study that can be addressed in subsequent studies to assess further the therapeutic potential for BB-R12 in the treatment of HF. Our observations must be considered preliminary until confirmed by subsequent additional research. The timing of BB-R12 administration following MI and the timing of serial assessments in our study may not be predictive of other situations and longer-term follow-up. The results from this model of recent onset ischemic HF may not be predictive of established ischemic HF or other forms of HF. The doses tested and delivery protocol used were selected empirically based on our prior work and similar published studies. Further studies may provide information on optimization of dose, delivery, efficacy and safety. We did not use continuous ECG recordings to monitor for arrhythmias, but there were no animal deaths following gene therapy. We did not measure myocardial oxygen consumption or coronary blood flow and these should be assessed as part of further safety assessments. The neurohormonal effects of BB-R12, ie, on BNP and catecholamine levels, should be evaluated in future experiments. Lastly, more extensive evaluation of viral and transgene persistence and expression in the heart and other organs will be needed as part of further safety evaluations.
CONCLUSION
Results from transgenic animals and small animal studies have shown that over-expression of RNR in the heart leads to improved cardiac performance via synthesis of dATP. The current study extends these observations to a relevant, standard large-animal model of HF with encouraging preliminary results. We recorded persistent improvement in multiple measures of HF severity after treatment with BB-R12 with no observed adverse effects. As the first gene therapy with a mechanism of action that is independent of calcium regulation, these findings justify further evaluation of the therapeutic potential of BB-R12 to treat HF.
Supplementary Material
Acknowledgments
FUNDING
Funding for this study was provided by BEAT Biotherapeutics, Seattle, WA. SK was supported by fellowships from Banyu Life Science Foundation International and the American Heart Association (14POST20150045).
Footnotes
Conflict of interest: ST, JL and GGM are employed at BEAT Biotherapeutics. MAL, CEM and MR are co-founders and equity holders in BEAT Biotherapeutics. SK, HR and JC: NONE.
References
- 1.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF, Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. Journal of the American College of Cardiology. 2008;51(11):1112–9. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Beeri R, Chaput M, Guerrero JL, Kawase Y, Yosefy C, Abedat S, Karakikes I, Morel C, Tisosky A, Sullivan S, Handschumacher MD, Gilon D, Vlahakes GJ, Hajjar RJ, Levine RA. Gene delivery of sarcoplasmic reticulum calcium ATPase inhibits ventricular remodeling in ischemic mitral regurgitation. Circulation Heart failure. 2010;3(5):627–34. doi: 10.1161/CIRCHEARTFAILURE.109.891184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz MG, Fargnoli AS, Williams RD, Steuerwald NM, Isidro A, Ivanina AV, Sokolova IM, Bridges CR. Safety and efficacy of high-dose adeno-associated virus 9 encoding sarcoplasmic reticulum Ca(2+) adenosine triphosphatase delivered by molecular cardiac surgery with recirculating delivery in ovine ischemic cardiomyopathy. The Journal of thoracic and cardiovascular surgery. 2014;148(3):1065–1073.e2. doi: 10.1016/j.jtcvs.2014.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Muller OJ, Most P. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Science translational medicine. 2011;3(92):92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber C, Neacsu I, Krautz B, Schlegel P, Sauer S, Raake P, Ritterhoff J, Jungmann A, Remppis AB, Stangassinger M, Koch WJ, Katus HA, Muller OJ, Most P, Pleger ST. Therapeutic safety of high myocardial expression levels of the molecular inotrope S100A1 in a preclinical heart failure model. Gene therapy. 2014;21(2):131–8. doi: 10.1038/gt.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz MG, Fargnoli AS, Swain JD, Tomasulo CE, Ciccarelli M, Huang ZM, Rabinowitz JE, Bridges CR. AAV6-betaARKct gene delivery mediated by molecular cardiac surgery with recirculating delivery (MCARD) in sheep results in robust gene expression and increased adrenergic reserve. The Journal of thoracic and cardiovascular surgery. 2012;143(3):720–726.e3. doi: 10.1016/j.jtcvs.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, Katus HA, Most P, Muller OJ. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. European heart journal. 2013;34(19):1437–47. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilemann L, Lee A, Ishikawa K, Aguero J, Rapti K, Santos-Gallego C, Kohlbrenner E, Fish KM, Kho C, Hajjar RJ. SUMO-1 gene transfer improves cardiac function in a large-animal model of heart failure. Science translational medicine. 2013;5(211):211ra159. doi: 10.1126/scitranslmed.3006487. [DOI] [PubMed] [Google Scholar]
- 9.Fish KM, Ladage D, Kawase Y, Karakikes I, Jeong D, Ly H, Ishikawa K, Hadri L, Tilemann L, Muller-Ehmsen J, Samulski RJ, Kranias EG, Hajjar RJ. AAV9.I-1c delivered via direct coronary infusion in a porcine model of heart failure improves contractility and mitigates adverse remodeling. Circulation Heart failure. 2013;6(2):310–7. doi: 10.1161/CIRCHEARTFAILURE.112.971325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa K, Fish KM, Tilemann L, Rapti K, Aguero J, Santos-Gallego CG, Lee A, Karakikes I, Xie C, Akar FG, Shimada YJ, Gwathmey JK, Asokan A, McPhee S, Samulski J, Samulski RJ, Sigg DC, Weber T, Kranias EG, Hajjar RJ. Cardiac I-1c Overexpression With Reengineered AAV Improves Cardiac Function in Swine Ischemic Heart Failure. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22(12):2038–45. doi: 10.1038/mt.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124(3):304–13. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M, Hajjar RJ. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circulation research. 2014;114(1):101–8. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 13.Kashlan OB, Cooperman BS. Comprehensive model for allosteric regulation of mammalian ribonucleotide reductase: refinements and consequences. Biochemistry. 2003;42(6):1696–706. doi: 10.1021/bi020634d. [DOI] [PubMed] [Google Scholar]
- 14.Regnier M, Rivera AJ, Chen Y, Chase PB. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circulation research. 2000;86(12):1211–7. doi: 10.1161/01.res.86.12.1211. [DOI] [PubMed] [Google Scholar]
- 15.Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophysical journal. 2004;87(3):1815–24. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moussavi-Harami F, Razumova MV, Racca AW, Cheng Y, Stempien-Otero A, Regnier M. 2-Deoxy adenosine triphosphate improves contraction in human end-stage heart failure. Journal of molecular and cellular cardiology. 2014;79c:256–263. doi: 10.1016/j.yjmcc.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korte FS, Dai J, Buckley K, Feest ER, Adamek N, Geeves MA, Murry CE, Regnier M. Upregulation of cardiomyocyte ribonucleotide reductase increases intracellular 2 deoxy-ATP, contractility, and relaxation. Journal of molecular and cellular cardiology. 2011;51(6):894–901. doi: 10.1016/j.yjmcc.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowakowski SG, Kolwicz SC, Korte FS, Luo Z, Robinson-Hamm JN, Page JL, Brozovich F, Weiss RS, Tian R, Murry CE, Regnier M. Transgenic overexpression of ribonucleotide reductase improves cardiac performance. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):6187–92. doi: 10.1073/pnas.1220693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundy SD, Murphy SA, Dupras SK, Dai J, Murry CE, Laflamme MA, Regnier M. Cell-based delivery of dATP via gap junctions enhances cardiac contractility. Journal of molecular and cellular cardiology. 2014;72:350–9. doi: 10.1016/j.yjmcc.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowakowski SG, Adamek N, Geeves MA, Gay E, Kolwicz SC, Murry CE, Daggett V, Regnier M. 2-deoxy-ATP Alters Myosin Structure to Enhance Cross-Bridge Cycling and Improve Cardiac Function. Biophysical journal. 2013;104(2):17a. [Google Scholar]
- 21.Teerlink JR. A novel approach to improve cardiac performance: cardiac myosin activators. Heart failure reviews. 2009;14(4):289–98. doi: 10.1007/s10741-009-9135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, Schiller NB, Malik FI, Wolff AA. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378(9792):667–75. doi: 10.1016/S0140-6736(11)61219-1. [DOI] [PubMed] [Google Scholar]
- 23.Morgan BP, Muci A, Lu PP, Qian X, Tochimoto T, Smith WW, Garard M, Kraynack E, Collibee S, Suehiro I, Tomasi A, Valdez SC, Wang W, Jiang H, Hartman J, Rodriguez HM, Kawas R, Sylvester S, Elias KA, Godinez G, Lee K, Anderson R, Sueoka S, Xu D, Wang Z, Djordjevic N, Malik FI, Morgans DJ., Jr Discovery of omecamtiv mecarbil the first, selective, small molecule activator of cardiac Myosin. ACS medicinal chemistry letters. 2010;1(9):472–7. doi: 10.1021/ml100138q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairman JW, Wijerathna SR, Ahmad MF, Xu H, Nakano R, Jha S, Prendergast J, Welin RM, Flodin S, Roos A, Nordlund P, Li Z, Walz T, Dealwis CG. Structural basis for allosteric regulation of human ribonucleotide reductase by nucleotide-induced oligomerization. Nature structural & molecular biology. 2011;18(3):316–22. doi: 10.1038/nsmb.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoffstall B, Clark A, Chase PB. Positive inotropic effects of low dATP/ATP ratios on mechanics and kinetics of porcine cardiac muscle. Biophysical journal. 2006;91(6):2216–26. doi: 10.1529/biophysj.105.079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.