Abstract
Background: Peripheral (plasma) and central (cerebrospinal fluid, CSF) measures of tau are higher in Alzheimer’s disease (AD) relative to prodromal stages and controls. While elevated CSF tau concentrations have been shown to be associated with lower grey matter density (GMD) in AD-specific regions, this correlation has yet to be examined for plasma in a large study.
Objective: Determine the neuroanatomical correlates of plasma tau using voxel-based analysis.
Methods: Cross-sectional data for 508 ADNI participants were collected for clinical, plasma total-tau (t-tau), CSF amyloid (Aβ42) and tau, and MRI variables. The relationship between plasma tau and GMD and between CSF t-tau and GMD were assessed on a voxel-by-voxel basis using regression models. Age, sex, APOE ɛ4 status, diagnosis, and total intracranial volume were used as covariates where appropriate. Participants were defined as amyloid positive (Aβ+) if CSF Aβ42 was <192 pg/mL.
Results: Plasma tau was negatively correlated with GMD in the medial temporal lobe (MTL), precuneus, thalamus, and striatum. The associations with thalamus and striatum were independent of diagnosis. A negative correlation also existed between plasma tau and GMD in Aβ+ participants in the MTL, precuneus, and frontal lobe. When compared to CSF t-tau, plasma tau showed a notably different associated brain atrophy pattern, with only small overlapping regions in the fusiform gyrus.
Conclusion: Plasma tau may serve as a non-specific marker for neurodegeneration but is still relevant to AD considering low GMD was associated with plasma tau in Aβ+ participants and not Aβ–participants.
Keywords: Alzheimer disease, magnetic resonance imaging, mild cognitive impairment, plasma, tau protein
INTRODUCTION
Understanding the underlying pathological processes of Alzheimer’s disease (AD) and developing reliable biomarkers are critical to identify the causes and pathogenesis of AD. Both in vivo and postmortem studies have shown that pathological tau is correlated with neurodegeneration, disease severity, and cognitive impairment [1]. Likewise, in vivo central measures of tau in cerebrospinal fluid (CSF) also correlate with postmortem tau pathology and are increased in AD patients relative to those in a prodromal stage of AD referred to as mild cognitive impairment (MCI) and cognitively normal controls (CN), aiding prediction of disease progression [2, 3].However, CSF collection is regarded as invasive, leading researchers to search for alternative methods to monitor MCI and AD such as blood-based biomarkers.
A recent meta-analysis reported plasma tau as the only blood-based biomarker to delineate AD from controls [4]. Fortunately, a new ultrasensitive technique was developed capable of detecting tau at low concentrations in plasma [5]. Similar to CSF, plasma tau concentrations were found to be significantly higher in AD relative to MCI and CN. Mean plasma tau was also higher in MCI compared to CN though this did not reach statistical significance [6–8]. The large overlap between AD, MCI, and CN suggested plasma tau may not be suitable as a diagnostic marker. Further, the correlation between plasma tau and CSF tau and hippocampal volume was weak or non-existent [6–8]. Mattsson et al. [8] did show evidence that plasma tau was associated with a greater longitudinal decline in hippocampal volume and Dage et al. [6] showed that high levels of plasma tau were associated with lower cortical thickness in AD-specific brain regions. These results suggest plasma tau may be correlated with atrophy of AD-specific regions as the disease progresses.
While the aforementioned plasma studies investigated specific measures of atrophy, our goal was to determine the neuroanatomical correlates of plasma tau using voxel-based analysis, which allows a more comprehensive assessment of whole brain atrophy. We investigated the association of plasma tau with atrophy using a voxel-based analysis in participants of the ADNI. We also aimed to determine if plasma and CSF tau concentrations were related to atrophy in similar brain regions. We hypothesize that plasma tau will be inversely correlated with grey matter density (GMD), a measure of grey matter atrophy [9], as seen in the previous reports of plasma tau and measures of atrophy. Secondly, we hypothesize that plasma total-tau (t-tau) and CSF t-tau will be related to similar regions of atrophy.
MATERIALS AND METHODS
Alzheimer’s Disease Neuroimaging Initiative (ADNI)
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and AD. Written informed consent was obtained from all participants according to the Declaration of Helsinki.
Plasma tau collection and quality control
Peripheral plasma tau concentrations were measured for 581 non-Hispanic Caucasian participants using the Single Molecule array (Simoa) technique with the Human Total Tau assay (Human Total Tau 2.0 kit, Quanterix Corp, Boston, MA, USA). This assay and the plasma tau characteristics for ADNI have been previously described [5, 8]. In brief, the assay uses two monoclonal antibodies which bind to the N-terminus and mid-region of tau, and measures both normal and phosphorylated tau protein. Values are given as pg/mL. A total of three samples had plasma concentration below the Limit of Detection (LOD) and 35 samples were below the Lower Limit of Quantification (LLOQ) and were removed from further analysis. An additional four samples had missing values. To reduce the possible effect of extreme outliers on statistical analysis, the mean and standard deviation (SD) of plasma tau were calculated; participants with a value more than three SDs above or below the mean value were regarded as outliers and removed from further analysis. This resulted in the removal of eight participants. Only participants with a MRI scan were included, leaving 508 participants for the current study (166 CN, 174 MCI, 168 AD).
CSF collection and quality control
CSF samples were available for 349 of the 508 ADNI-1 plasma tau participants with comparable demographic, clinical, and apolipoprotein (APOE) genotyping results to the full sample [10]. Briefly, a lumbar puncture was performed after an overnight fast and the CSF was collected into collection tubes. The CSF was transferred into polypropylene tubes, frozen on dry ice within 1 h of collection, and then shipped to the ADNI Biomarker Core laboratory at the University of Pennsylvania Medical Center on dry ice. Aliquots (0.5 ml) were prepared from these samples after 1 h of thawing at room temperature and stored in bar code-labeled polypropylene vials at – 80°C.
CSF analytes (Aβ1-42, t-tau and phosphorylated-tau (p-tau181p)) were measured using the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) with Innogenetics (INNO-BIA AlzBio3; Ghent, Belgium; for research use-only reagents) immunoassay kit-based reagents. To reduce the possible effect of outliers on statistical analysis, the mean and standard deviation of CSF analytes were calculated and participants with at least one analyte value more than three SD below or above the mean value of each of CSF variable were regarded as outliers and removed from the analysis. This resulted in 341 valid CSF samples. For the MRI-CSF study, only participants with a CSF value for both t-tau and p-tau were included in the CSF study leaving 331 participants available (91 CN, 158 MCI, 82 AD). Participants were classified as amyloid positive (Aβ+) if CSF Aβ1-42 <192 pg/mL.
MRI scan processing
All participants had baseline 1.5T magnetization-prepared rapid gradient-echo (MPRAGE) images downloaded from the ADNI LONI site (http://adni.loni.usc.edu/). Scan processing with voxel-based morphometry (VBM) in Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and quality control were done as previously described [11]. Briefly, scans were co-registered to a T1-weighted template and segmented into different tissue classes (grey matter, GM; white matter, WM; CSF). GM maps were normalized to Montreal Neurologic Institute (MNI) space without modulation as 1×1×1 mm voxels and smoothed with an 8 mm Gaussian kernel to create GMD images for further analysis. GMD is the average concentration of GM within voxels included in a region and therefore a reduction of GMD serves as a proxy of local atrophy [9].
Image analysis
To evaluate the relationship between central (CSF) and peripheral (plasma) measures of tau and GMD, voxel-wise linear regression models in SPM8 were used. Covariates included in the regression models were age, sex, APOE status (ɛ4 carrier or ɛ4 non-carrier), and total intracranial volume. Analyses were done with and without diagnosis as an additional covariate. An explicit GM mask was applied to the MRI scans to restrict the search area for the statistical analysis. Significant results were displayed at a voxel-wise p < 0.05 (family-wise error (FWE) corrected for multiple comparisons) and with a minimum cluster size (k) of 100 voxels. If no brain regions survived correction for multiple comparisons, then a slightly less stringent voxel-wise p-value of 0.001 (uncorrected) was used. To determine if there were any overlapping brain regions significantly correlated to plasma tau and CSF, a composite image was created in SPM8. Anatomical regions were defined by using the x-y-z coordinates for the most significant voxel within each cluster. These coordinates were entered into the Talairach daemon (http://www.talairach.org/daemon.html) to receive the anatomical names for the GM regions closest to that coordinate [12].
Statistical analysis
SPSS V24.0 was used for all statistical analyses. CSF t-tau and p-tau values were log transformed in order to achieve normal distribution. Plasma tau values were normally distributed and thus absolute values were used for analysis; results were unchanged when transformed plasma values were used. The association of sex, APOE ɛ4 status, and Aβ positivity with diagnostic group was assessed using a Pearson chi-squared test. ANOVA was used to assess the relationship of age, education, Mini-Mental State Exam (MMSE), Clinical Dementia Rating Scale – Sum of Boxes (CDR-SB), plasma tau, and CSF analytes with diagnostic status. Post-hoc pairwise differences among diagnostic groups were assessed using a Bonferroni correction for multiple comparisons. The MarsBaR toolbox in SPM8 was used to extract mean GMD from significant clusters from the voxel-wise results for further characterization of the results [13].
Regional analysis
We attempted to replicate the analysis in Dage et al. in the ADNI cohort data using a similar method to that described in the manuscript [6]. Briefly, MRI scans from all participants were processed using Freesurfer version 5.1 to extract mean bilateral cortical thickness from the entorhinal cortex and the inferior temporal, middle temporal, and fusiform gyri [14, 15]. These cortical thickness estimates were averaged to create an AD signature cortical thickness measure, as in Dage et al. [6]. Abnormal cortical thickness was defined as a mean thickness below the 90th percentile of the AD participants as previously described [6, 16], resulting in an abnormal cortical thickness cut-off of <2.77 mm. As in Dage et al. [6], we sought to investigate the association between plasma tau and the probability of having abnormal cortical thickness, as defined above, in MCI and CN participants (n = 339), as well as in the full sample. A logistic regression was used to estimate this association both unadjusted (model 1) and adjusted for age, sex, education, and APOE ɛ4 status (model 2), as was done by Dageet al. [6].
RESULTS
Demographic and clinical characteristics
There was a near equal number of participants in each diagnostic group with 166 CN, 174 MCI, and 168 AD. Significant differences among diagnostic groups were observed for all demographic and clinical characteristics examined except for age (Table 1). As expected, the AD group had the highest percentage of APOE ɛ4 carriers (67.9%), followed by the MCI group consisting of 54% APOE ɛ4 carriers. Significant differences in the mean MMSE and CDR-SB among the diagnostic groups, with the AD group showing the most impairment and the MCI group showing intermediate impairment between AD and CN (p < 0.001). Plasma tau, CSF t-tau, and CSF p-tau were significantly different between groups, with the AD group showing significantly higher concentrations compared to MCI and CN (p = 0.002, p < 0.001, and p < 0.001, respectively). Similar to previous reports, the mean plasma tau concentrations in the MCI and CN groups were nearly equal. The number of Aβ+ participants was also significantly different among diagnostic groups (p < 0.001). There was no significant difference in demographic and clinical characteristics for the 35 ADNI participants whose samples were excluded compared to those included in the present study (Supplementary Table 1). However, as expected plasma tau was significantly different between the included/excluded groups since the excluded plasma tau samples all had a value <1. Plasma tau levels between diagnostic groups for the excluded samples was not statistically different (p = 0.202).
Table 1.
Demographic and clinical characteristics
| CN | MCI | AD | p-value | |
| N | 166 | 174 | 168 | – |
| Age (years) | 75.2 (5.1) | 74.1 (7.6) | 75.3 (7.3) | 0.789 |
| Sex (M, F) | 95, 71 | 115, 59 | 87, 81 | 0.025 |
| Education (years) | 16.1 (2.7) | 15.8 (3) | 14.8 (3.1) | < 0.001 |
| APOE ɛ4 (% ɛ4 positive) | 27.1% | 54% | 67.9% | < 0.001 |
| MMSE | 29.1 (1) | 26.9 (1.8) | 23.2 (2) | < 0.001 |
| CDR-SB | 0 (0.1) | 1.6 (0.9) | 4.3 (1.6) | < 0.001 |
| Plasma tau pg/mL | 2.71 (1) | 2.81 (1.2) | 3.13 (1.3) | 0.002 |
| CSF t-tau pg/mL* | 65.7 (24.7) | 97.6 (48.6) | 121.2 (52.4) | < 0.001 |
| CSF p-tau pg/mL* | 22.3 (10.5) | 34.3 (16.2) | 41.5 (18.5) | < 0.001 |
| Amyloid (–/+)** | 59, 32 | 41, 116 | 5, 76 | < 0.001 |
CN, cognitively normal; MCI, mild cognitive impairment; AD, Alzheimer’s disease; M, male; F, female; APOE, apolipoprotein; MMSE, mini-mental state exam; CDR-SB, clinical dementia rating-sum of boxes; CSF, cerebrospinal fluid. Amyloid + if CSF Aβ ≤192 pg/mL. Mean (standard deviation). Significant p-values <0.05 are italicized. *n = 91 CN, 158 MCI, 82 AD. **missing 1 MCI and 1 AD.
Plasma tau association with cortical thickness
A recent report by Dage et al. found that high levels of plasma tau were associated with lower cortical thickness in the entorhinal, inferior temporal, middle temporal, and fusiform gyri of MCI and controls [6]. However, we did not find a significant association of plasma tau levels with abnormal cortical thickness in either the unadjusted or adjusted models (Table 2).
Table 2.
Association of plasma tau levels with cortical thickness in AD-specific brain regions
| Abnormal cortical | Model 1 | Model 2 | |||||
| thickness (<2.77) | |||||||
| n | OR (95% CI) | p-value | n | OR (95% CI) | p-value | ||
| CN+MCI | Continuous | 339 | 0.95 (0.78–1.16) | 0.62 | 339 | 0.94 (0.77 – 1.15) | 0.55 |
| Quartiles | |||||||
| 1 | 339 | reference | 339 | reference | |||
| 2 | 0.79 (0.44–1.42) | 0.43 | 0.72 (0.39 – 1.32) | 0.29 | |||
| 3 | 0.89 (0.5–1.6) | 0.69 | 0.86 (0.47–1.57) | 0.61 | |||
| 4 | 0.93 (0.51–1.73) | 0.83 | 0.88 (0.47–1.67) | 0.7 | |||
| CN+MCI+AD | Continuous | 507 | 1.12 (0.96–1.31) | 0.16 | 507 | 1.1 (0.93–1.3) | 0.27 |
| Quartiles | |||||||
| 1 | 507 | reference | 507 | reference | |||
| 2 | 1 (0.61–1.66) | 0.98 | 0.87 (0.52–1.47) | 0.61 | |||
| 3 | 0.96 (0.58–1.59) | 0.88 | 0.85 (0.5–1.43) | 0.53 | |||
| 4 | 1.49 (0.89–2.5) | 0.13 | 1.36 (0.79–2.32) | 0.27 | |||
CN, cognitively normal; MCI, mild cognitive impairment; AD, Alzheimer’s disease; OR, odds ratio; CI, confidence interval. Model 1 is unadjusted. Model 2 is adjusted for age, sex, education, and APOE ɛ4 status. The cortical thickness measure is an average of the entorhinal, fusiform, inferior temporal, and middle temporal regions of interest.
Voxel-based MRI analysis
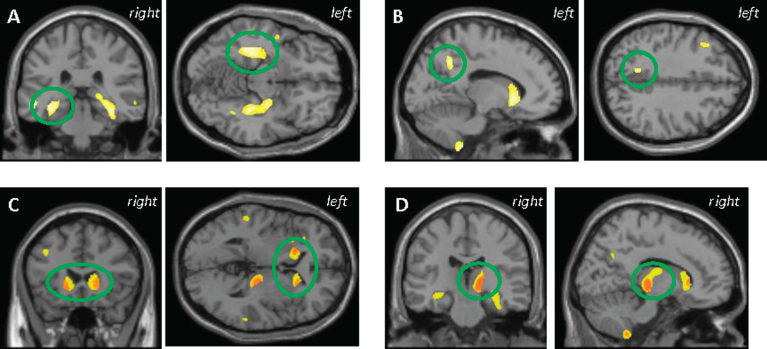
Across all participants, a significant association between higher plasma tau and lower GMD in several brain regions was observed, including in the middle, inferior, and superior temporal gyrus, parahippocampus, hippocampus, fusiform, uncus, precuneus, thalamus, caudate, putamen, and middle and inferior frontal gyrus (Fig. 1A-D, voxel-wise p < 0.001 (uncorrected), k = 100 voxels). As would be expected, AD participants had lower mean GMD in the larger clusters identified (MTL and striatum; Supplementary Figure 1A). When controlling for diagnosis, a significant association between plasma tau and GMD was still observed in the right thalamus and bilaterally in the striatum (Fig. 1C, D). No significant clusters were observed in the positive/unexpected direction (data notshown).
Fig.1.
VBM analysis in all participants showed higher plasma tau was correlated with lower grey matter density (GMD) in the (A) medial temporal lobe, (B) precuneus, (C) striatum, and (D) thalamus. C and D represent the anatomic overlap (orange) of regions of GM atrophy associated with higher plasma tau using only age, sex, APOE ɛ4 status, and total intracranial volume as covariates (yellow) and with the addition of diagnosis as a covariate (red). Results are displayed at p < 0.001 (uncorrected) and at a threshold (k) of 100 voxels.
Amyloid-positive participants
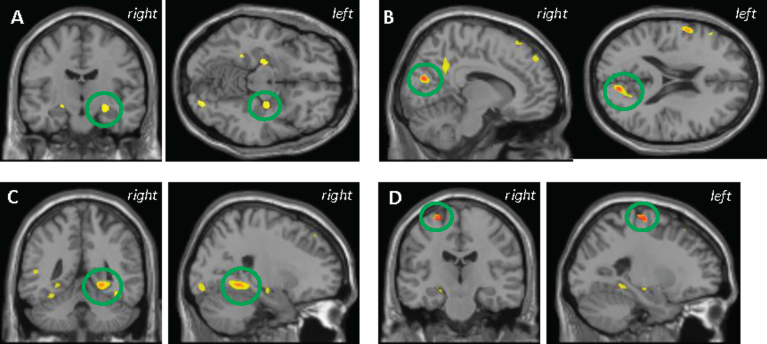
There was no significant association between plasma tau and GMD in the Aβ- participants; however, higher plasma tau was significantly correlated with lower GMD in the Aβ+ participants in the fusiform, hippocampus, parahippocampus, precuneus, and premotor cortex (Fig. 2A-D, voxel-wise p < 0.001 (uncorrected), k = 100 voxels), as well as the frontal and parietal lobes, pre- and post-central gyri, and the globus pallidus. Notably, in the MTL cluster for MCI and AD, Aβ+ participants showed lower mean GMD compared to Aβ–participants (Supplementary Figure 1B). After diagnosis was added as a covariate, many of the same regions of low GMD remained significantlycorrelated with higher plasma tau including in the precuneus, parahippocampus, and premotor cortex (Fig. 2B-D). No significant clusters were observed in the positive/unexpected direction (data not shown).
Fig.2.
VBM analysis in the amyloid positive participants showed higher plasma tau was correlated with lower grey matter (GM) density in the (A) hippocampus, (B) precuneus, (C) parahippocampus, and (D) BA 6 premotor cortex. B-D also represent the anatomic overlap (orange) of regions of GM atrophy associated with higher plasma tau using only age, sex, APOE ɛ4 status, and total intracranial volume as covariates (yellow) and with the addition of diagnosis as a covariate (red). Results are displayed at p < 0.001 (uncorrected) and at a threshold (k) of 100 voxels.
CSF-Plasma tau comparison
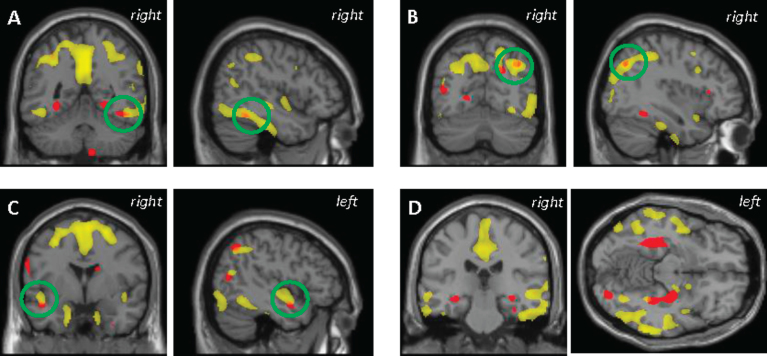
Peripheral measures of tau protein were of total-tau only, thus, we sought to compare the regional atrophy associated with plasma tau to that associated with CSF t-tau only. At p < 0.05 (FWE corrected), higher CSF t-tau was associated with lower GMD in the precuneus, temporal gyrus, and fusiform gyrus in all participants (Supplementary Figure 2). However, the uncorrected results were used for comparison with the plasma tau results, as this threshold was used in the plasma tau analyses described above. Central and peripheral measures of tau protein were associated with GMD in some overlapping, but largely different brain regions. Across all participants, the temporal pole, fusiform, and angular gyrus were brain regions in which both higher CSF t-tau and plasma tau were associated with lower GMD (Fig. 3A-D). As several reports have previously shown, higher CSF t-tau was associated with lower GMD in cortical structures known to be affected in persons with AD. However, as above, in the present study plasma tau was predominantly associated with subcortical structures. Within the Aβ+ participants, no significant overlap was observed between the association of GMD with plasma tau and the association of GMD with CSF t-tau (data not shown).
Fig.3.
Anatomical overlap of grey matter atrophy (orange) associated with higher plasma tau (red) and that associated with higher CSF t-tau (yellow). Results are displayed at p < 0.001 (uncorrected) and at a threshold (k) of 100 voxels.
DISCUSSION
The main goal of this study was to determine if plasma tau was associated with cortical atrophy in a population at risk for AD or already manifesting signs of clinical AD. We found higher plasma tau was correlated with lower GMD in several AD-specific brain regions, including the MTL and precuneus, as well as in the thalamus and striatum. Further investigation into only Aβ+ participants also revealed an association between higher plasma tau concentrations and lower GMD in MTL structures, the precuneus, and the premotor cortex. In Aβ–participants, no significant association between plasma tau and GMD was observed for any analysis. These findings suggest that plasma tau may reflect neurodegeneration in both AD-specific regions and more generally. Additionally, we failed to replicate the correlation of atrophy as measured by cortical thickness described by Dage et al. [6].
There are six isoforms of tau protein, all present and hyperphosphorylated, in neurofibrillary tau tangles found in the AD brain. Antibodies for both the plasma and CSF assays bind to the mid-region of the tau protein, thus giving measures of total-tau protein. While phosphorylated tau is the pathological tau species in tauopathies, development of a Simoa assay targeted at p-tauThr231 is still underway.
Plasma tau in AD quantified using the Simoa technique has only recently been investigated. The findings from these studies have been similar, with plasma tau significantly higher in AD compared to MCI and controls, but no significant difference between MCI and controls [6–8]. However, there was high overlap in plasma tau levels between these diagnostic groups which suggested that plasma tau will not be a suitable biomarker for AD. Mattsson et al. [8] also showed that higher plasma tau was associated with worsening cognition, atrophy, and hypometabolism over time suggesting evaluation of plasma tau may provide insight into brain changes over time.
The MTL is one of the first brain regions that shows tau pathology and the earliest to degenerate in AD patients [17]. Thus, our observation that high plasma tau is associated with cortical atrophy in several MTL structures including the parahippocampus and hippocampus across all participants and in Aβ+ participants suggests that this peripheral measure of tau may be reflective of CSF and brain tau pathology as well as brain atrophy. Although a different method was used to investigate the brain integrity, our findings are similar to but not replicative of that of Dage et al. who showed higher plasma tau was associated with lower cortical thickness in the entorhinal, inferior temporal, middle temporal, and fusiform [6]. The mean plasma tau levels for MCI and CN were nearly equivalent in this study which could account for our failure to replicate. We also observed a correlation between higher plasma tau and lower GMD in the precuneus. A functional decline in the precuneus occurs early in the course of AD. Specifically, the precuneus along with the hippocampus are both core components of the default mode network, an important functional resting-state network that is impaired early in AD [18]. Thus, the atrophy in the precuneus associated with plasma tau could be reflective of structural deterioration in the default mode network early in AD.
Even more striking was the inverse association of plasma tau with GMD in the thalamus and the striatum across all participants, especially given that these areas are affected later in the disease course. These were the only brain regions that were also independent of diagnosis, suggesting that plasma tau may reflect neurodegeneration unrelated to AD diagnosis. Alternatively, in the Aβ+ participants, the association of plasma tau and GMD in the parahippocampus and precuneus was independent of diagnosis. These findings suggest that plasma tau may be reflecting neurodegeneration specific to AD pathology. Studies evaluating plasma tau in other tauopathies may shed light on this hypothesis.
Our initial hypothesis was that plasma tau and CSF t-tau would be related to similar regions of atrophy, especially if the two tau measures were truly reflective of AD neurodegeneration. Unexpectedly, plasma tau and CSF t-tau had very little overlap, with plasma tau mapping more to subcortical structures and CSF t-tau mapping more to cortical structures. Only small regions of overlap were observed in the temporal pole, fusiform, and angular gyrus. These results suggest that CSF and plasma measures of tau protein may reflect related but somewhat different pathological substrates of AD. This could be due to differences in the assays, tau isoforms detected by the assays, or in the variability of the measurements. There may also be differences in how tau, released from neurons, is cleared from brain interstitial fluid to CSF and plasma. Further work is needed to further elucidate the differences between plasma and CSF tau.
We have shown here that plasma tau may be a non-specific marker for neurodegeneration. However, plasma tau may still be relevant to AD since we observed associations of higher plasma tau with AD-specific brain atrophy in Aβ+ participants but not in Aβ–participants. Taking into account these findings and those described by Mattsson et al. [8], we believe plasma tau may be useful as a screening tool for detecting early AD-related neurodegeneration in cognitively normal or mildly symptomatic older adults, rather than as a diagnostic tool per say [8]. Future studies are needed to determine if plasma tau may be useful to predict future cognitive decline and increased longitudinal atrophy, and if plasma tau is associated with cerebral plaque and neurofibrillary tangle deposition. Additionally, future studies in which plasma tau could be used in combination with other biomarkers in a staged approach would be of interest. In this way, sufficient sensitivity and specificity may be achieved. However, since the plasma tau assay is a relatively non-invasive and an inexpensive test that could potentially be done in primary care rather than specialist settings, this technique could serve as a first-line screening tool to detect changes in asymptomatic older individuals and select individuals for more expensive or invasive (e.g., imaging, CSF) tests to more specifically identify AD-based pathology.
One study limitation is the lack of a commensurate replicate data set. This tau quantification method is relatively new and hopefully independent data sets will become available. Additionally, accumulatedp-tau is the main hallmark in AD and other tauopathies but there is not yet a technique for measuring p-tau in plasma, thus yielding a second limitation to this study. New techniques to assess p-tau in plasma could be extremely beneficial. A third limitation is that our data is cross-sectional only. Longitudinal plasma tau data would provide us the information needed to better assess changes in plasma tau over time and the association between changing plasma tau measures and rate of neurodegeneration. Lastly, we excluded participants with plasma tau values below the lower limit of quantification, although the results were not significantly different when these participants were included (data not shown).
In conclusion, high concentrations of plasma tau were associated with lower GMD in both AD-specific and non-AD-related brain regions. Future replication and longitudinal studies will be important to fully elucidate the contribution of plasma tau as a possible biomarker in AD and other tauopathies.
Supplementary Material
ACKNOWLEDGMENTS
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Additional research was supported by NIH grants (R01 AG19771, P30 AG10133, R36 AG053445, and K01 AG049050), the Alzheimer’s Association, the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative, and the Indiana Clinical and Translational Science Institute.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-1114r2).
Appendix
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-161114.
REFERENCES
- [1]. Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol 71, 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TL, Ances BM (2016) Tau and Abeta imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med 8, 338ra366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR Jr, Feldman HH, Bokde AL, Alexander GE, Scheltens P, Vellas B, Dubois B, Weiner M, Hampel H, North American Alzheimer’s Disease Neuroimaging Initiative (ADNI) (2012) Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging 33, 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, Holtta M, Rosen C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol 15, 673–684. [DOI] [PubMed] [Google Scholar]
- [5]. Randall J, Mortberg E, Provuncher GK, Fournier DR, Duffy DC, Rubertsson S, Blennow K, Zetterberg H, Wilson DH (2013) Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: Results of a pilot study. Resuscitation 84, 351–356. [DOI] [PubMed] [Google Scholar]
- [6]. Dage JL, Wennberg AM, Airey DC, Hagen CE, Knopman DS, Machulda MM, Roberts RO, Jack CR Jr, Petersen RC, Mielke MM (2016) Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement 12, 1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, Hansson O (2013) Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, Palmqvist S, Baker D, Tan Hehir CA, Jeromin A, Hanlon D, Song L, Shaw LM, Trojanowski JQ, Weiner MW, Hansson O, Blennow K, ADNI Investigators (2016) Plasma tau in Alzheimer disease. Neurology 87, 1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11, 805–821. [DOI] [PubMed] [Google Scholar]
- [10]. Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Risacher SL, Kim S, Shen L, Nho K, Foroud T, Green RC, Petersen RC, Jack CR Jr, Aisen PS, Koeppe RA, Jagust WJ, Shaw LM, Trojanowski JQ, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative (ADNI) (2013) The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI). Front Aging Neurosci 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Brett M, Anton J-L, Valabregue R, Poline J-B (2002) Region of interest analysis using an SPM toolbox Human Brain Mapping, Sendai, Japan, 2002. [Google Scholar]
- [14]. Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- [15]. Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207. [DOI] [PubMed] [Google Scholar]
- [16]. Jack CR Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Rocca WA, Boeve BF, Petersen RC (2012) An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol 71, 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A 101, 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-161114.