Abstract
SETTING
A large urban pediatric human immunodeficiency virus (HIV) clinic in Lilongwe, Malawi.
OBJECTIVE
To identify demographic and clinical risk factors for mortality in children co-infected with HIV and tuberculosis (TB).
DESIGN
A retrospective cohort study of HIV-infected children (aged <18 years) enrolled between October 2004 and October 2010 with at least one current or historical TB diagnosis. Descriptive statistics and logistic regression analyses were performed to determine factors associated with mortality.
RESULTS
A total of 1561 patients met the inclusion criteria, representing 32% of patients ever enrolled. Median age at TB diagnosis was 3.8 years (interquartile range 1.5–7.4); 60.9% had severe immune suppression and 47.6% of those with available data had some degree of acute malnutrition at TB diagnosis. Of the 1113 patients with known outcomes, 225 (20.2%) died. Children with TB-HIV co-infection not initiated on anti-retroviral therapy (ART) at any time were 8.8 times more likely to die compared to those initiated on ART 0–2 months after initiation of anti-tuberculosis treatment (adjusted OR 8.83, 95%CI 4.42–17.63). Severe immuno-suppression and World Health Organization Stage IV were also associated with mortality.
CONCLUSIONS
Pediatric TB-HIV co-infection is common and mortality is high in this cohort of Malawian children. Prompt initiation of ART should be emphasized in this high-risk patient population.
Keywords: pediatric, HIV, TB, co-infection, ART
The Human Immunodeficiency Virus (HIV) epidemic in sub-Saharan Africa has led to a large increase in tuberculosis (TB) incidence, as immunocompromised adults and children are at increased risk for reactivation of latent tuberculous and new infections.1 This has been disproportionately noted in women of child-bearing age, resulting in higher levels of TB exposure and infection in HIV-infected children in these households.2,3 In addition, children have faster rates of progression from infection to disease, and HIV infection accelerates that process, particularly in young children.4 Some high-burden African settings have reported a pediatric TB-HIV co-infection burden of 43.4% and above.5,6 In Malawi, high TB incidence (191 per 100 000 population in 2011) and HIV prevalence (11% in 2009), along with limited TB diagnostic tools and challenges in early infant diagnosis of HIV, make management of co-infected children a challenge, resulting in poor outcomes.7–10
The Malawi National TB Control Programme recommends a diagnostic approach focused on exposure history, clinical signs and symptoms, and chest radiography in children.11 Capacity for tuberculin skin test (TST), mycobacterial culture or pathology services in the country is minimal. Sputum microscopy is widely available, but rarely performed in young children incapable of providing adequate respiratory samples.
In the first and second editions of the Malawi anti-retroviral therapy (ART) guidelines, covering October 2003–April 2008, pulmonary TB (PTB) was a pediatric Stage III diagnosis for which clinicians could defer to the CD4 count to determine ART eligibility.12,13 These recommendations were consistent with World Health Organization (WHO) recommendations at the time.14 The third edition of the national guidelines released in April 2008 recommended ART for all HIV-infected children diagnosed with PTB, regardless of CD4, reflecting new advice from the WHO.15,16
National guidelines evolved from recommending deferred ART initiation until anti-tuberculosis treatment was completed, then until completion of the 2-month initiation phase of treatment, and finally to recommending ART initiation as early as 2 weeks after starting treatment (October 2010).12,13,15 Table 1 displays the pediatric anti-tuberculosis treatment and ART regimens during the time period of this study.
Table 1.
Malawi pediatric anti-tuberculosis and ART regimens, 2006–2010
| Regimen | |
|---|---|
| Anti-tuberculosis treatment | |
| Type of TB case | |
| New (first case) | 2RHZE/4RH (Regimen 1)* |
| Relapse, return after default, treatment failure, recurrent† | 2SRHZE/1RHZE/5RHE (Regimen 2)* |
| Meningitis | 2SRHZ/7RH* |
| Suspected or confirmed multidrug-resistant or extensively drug-resistant TB | Handled on a case-by-case basis with NTP |
| ART | |
| Standard first-line‡ | d4T/3TC/NVP |
| Standard second-line‡ | ABC/ddI/LPV-r |
R = rifampicin; H = isoniazid; Z = pyrazinamide; E = ethambutol; S = streptomycin. Numbers represent duration of therapy in months.
In practice, many recurrent cases are treated with Regimen 1 if they are thought to represent re-infection after successful treatment of a previous episode.
For patients on anti-tuberculosis treatment and first-line ART, there is no recommended switch from NVP to EFV. For patients needing simultaneous anti-tuberculosis treatment and second-line ART, double-dose LPV-r is usually recommended, as individual ritonavir is not available in the country for superboosting.
ART = antiretroviral therapy; TB = tuberculosis; NTP = National TB Control Programme; d4T = stavudine; 3TC = lamivudine; NVP = nevirapine; ABC = abacavir; ddI = didanosine; LPV-r = lopinavir/ritonavir; EFV = efavirenz.
In this context of pediatric HIV and TB care in Malawi, we conducted a retrospective review of a large cohort of TB-HIV co-infected children to identify risk factors for mortality. We also sought to describe this cohort receiving routine pediatric HIV care over a period of national guideline changes.
METHODS
Patients and setting
This was a retrospective cohort analysis of all HIV-infected pediatric patients (aged <18 years at enrolment) ever diagnosed with TB and enrolled at the Baylor College of Medicine–Abbott Fund Children’s Clinical Centre of Excellence (COE), Lilongwe, between October 2004 and October 2010. The COE provides primary HIV care to patients in the Lilongwe area and also acts as a regional and national referral center for complicated pediatric HIV cases, including patients with ART failure and Kaposi’s sarcoma. As of October 2010, 4874 HIV-infected pediatric patients had ever been enrolled, with an active caseload of 2461 patients, 1682 (68.3%) of whom were on ART. This represented approximately 8% of all children on ART in Malawi in 2010, making the COE the largest national pediatric ART provider.17
All patients enrolled have their demographic, medical and social history, and clinical data stored in an electronic medical record (EMR). The EMR was screened for patients with a TB diagnosis documented in their past medical history, WHO staging and prescription data for anti-tuberculosis treatment. HIV-exposed patients were excluded, as were those who had received only isoniazid preventive therapy (IPT).
The files of patients identified for inclusion were reviewed by two pediatricians (WCB and DO). Study data, including staging, ART regimen, TB history, acute nutritional status (according to national guidelines, which use weight-for-length, mid-upper arm circumference and edema assessments), and CD4 results closest to the time of TB diagnosis, were extracted and entered into an Access® 2007 database (Microsoft Corporation, Redmond, WA, USA) and merged with other demographic and clinical variables retrievable without chart review.18,19 CD4 results were stratified according to WHO age-based immune classifications, with the lower of the absolute and percentage results used to determine the degree of suppression.14
Tuberculosis diagnosis
There was no standardized algorithm for TB diagnosis and it was not always possible to determine how a diagnosis was made. Any patient treated for active TB was therefore included.
Ethics approval
Approval for the study was obtained from the Malawi National Health Sciences Research Committee and the Baylor College of Medicine Institutional Review Board.
Statistics
Stata®, version 11 (StataCorp LP, College Station, TX, USA) was used for analysis. Frequency tables were generated to describe the study population. Categorical variables were described using frequencies and all continuous variables were described using medians and interquartile ranges (IQRs). Nutritional status at the time of TB diagnosis was unavailable for 757 (48.5% of overall cohort) of the historical TB cases prior to enrolment at the COE, but as it was deemed to be an important variable for analysis, the missing data were imputed using information from related variables. Univariate logistic regression was performed on a subset of 1113 patients with ascertained mortality outcomes (alive or died) to determine factors associated with mortality; those who transferred out or were lost to follow-up (LTFU) were excluded from logistic regression analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) for unadjusted associations between mortality and each demographic or clinical factor were generated. The multivariate regression analysis was performed in a backward stepwise fashion, where variables were retained in the model if they were statistically significant at the 0.05 level. The variables included in the logistic regression analysis were age at TB diagnosis, sex, timing of ART and anti-tuberculosis treatment, type of TB, WHO disease stage, acute nutrition status and immune status.
RESULTS
A total of 1561 co-infected patients were identified, representing 32% of all HIV-infected children ever enrolled. As of October 2010, 225 (14.4%) of these patients were known to have died, while 888 (56.9%) were alive and in care (Figure). The median age at TB diagnosis for the 1452 patients with a known diagnosis date was 3.8 years (IQR 1.5–7.4). Of those with CD4 data, 60.9% were severely immune-suppressed. Nearly half (47.6%) of the 804 patients with known nutritional status at TB diagnosis had some degree of acute malnutrition. TB was diagnosed after initiation of ART in 159 patients; for 139 this was in the first 12 months after ART. A comparison of key demographic and clinical variables for patients with known vs. unknown mortality outcomes is shown in Table 2.
Figure.
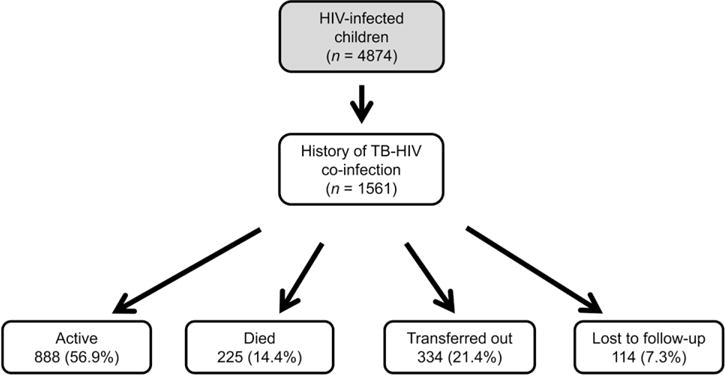
Study enrolment and outcomes in Malawian TB-HIV co-infected children, October 2004–October 2010. TB = tuberculosis; HIV = human immunodeficiency virus.
Table 2.
Description of a Malawian pediatric TB-HIV co-infected cohort
| Known outcomes
|
Unknown outcomes
|
||||
|---|---|---|---|---|---|
| Characteristic | Alive n (%) | Died n (%) | Lost to follow-up n (%) | Transferred out n (%) | Total |
| Age at TB diagnosis | |||||
| ≤12 months | 92 (10.4) | 57 (25.3) | 13 (11.4) | 39 (11.7) | 201 (12.9) |
| 1–3 years | 245 (27.6) | 64 (28.4) | 35 (30.7) | 101 (30.2) | 445 (28.5) |
| >3–5 years | 128 (14.4) | 18 (8.0) | 26 (22.8) | 49 (14.7) | 221 (14.1) |
| >5–10 years | 235 (26.4) | 33 (14.8) | 23 (20.2) | 80 (24.0) | 371 (23.8) |
| >10–18 years | 133 (15.0) | 28 (12.4) | 8 (7.0) | 45 (13.4) | 214 (13.7) |
| Unknown | 55 (6.2) | 25 (11.1) | 9 (7.9) | 20 (6.0) | 109 (7.0) |
| Sex | |||||
| Male | 440 (49.6) | 134 (59.6) | 65 (57.0) | 176 (52.7) | 815 (52.2) |
| Female | 448 (50.4) | 91 (40.4) | 49 (43.0) | 158 (47.3) | 746 (47.8) |
| Type of TB | |||||
| PTB* | 828 (93.2) | 197 (87.6) | 110 (96.5) | 301 (90.1) | 1436 (92.0) |
| Lymph node TB | 32 (3.6) | 15 (6.7) | 3 (2.6) | 17 (5.1) | 67 (4.3) |
| Abdominal TB | 8 (0.9) | 5 (2.2) | 0 | 8 (2.4) | 21 (1.3) |
| Miliary TB | 6 (0.7) | 3 (1.3) | 0 | 4 (1.2) | 13 (0.8) |
| TB meningitis | 6 (0.7) | 1 (0.4) | 0 | 0 | 7 (0.4) |
| Unknown | 8 (0.9) | 4 (1.8) | 1 (0.9) | 4 (1.2) | 17 (1.1) |
| Maximum WHO Stage† | |||||
| I | 5 (0.6) | 0 | 1 (0.9) | 3 (0.9) | 9 (0.6) |
| II | 18 (2.0) | 0 | 4 (3.5) | 6 (1.8) | 28 (1.8) |
| III | 680 (76.6) | 99 (44.0) | 90 (79.0) | 250 (74.9) | 1119 (71.7) |
| IV | 185 (20.8) | 126 (56.0) | 19 (16.6) | 75 (22.4) | 405 (25.9) |
| Immune suppression‡ | |||||
| None | 213 (24.0) | 14 (6.3) | 14 (12.2) | 50 (15.0) | 291 (18.6) |
| Mild | 85 (9.6) | 10 (4.4) | 10 (8.8) | 28 (8.4) | 133 (8.5) |
| Advanced | 97 (10.9) | 10 (4.4) | 10 (8.8) | 28 (8.4) | 145 (9.3) |
| Severe | 479 (53.9) | 150 (66.7) | 49 (43.0) | 208 (62.2) | 886 (56.8) |
| Unknown (no CD4 count) | 14 (1.6) | 41 (18.2) | 31 (27.2) | 20 (6.0) | 106 (6.8) |
| Acute nutrition status§ | |||||
| Normal | 476 (53.6) | 91 (40.4) | 59 (51.8) | 189 (56.6) | 815 (52.2) |
| Mild malnutrition | 70 (7.8) | 15 (6.7) | 6 (5.2) | 13 (3.9) | 104 (6.7) |
| Moderate malnutrition | 183 (20.7) | 30 (13.3) | 15 (13.2) | 64 (19.2) | 292 (18.7) |
| Severe malnutrition | 159 (17.9) | 89 (39.6) | 34 (29.8) | 68 (20.3) | 350 (22.4) |
| Sequence of anti-tuberculosis treatment and ART | |||||
| ART 0–2 months after start of anti-tuberculosis treatment | 137 (15.4) | 44 (19.5) | 11 (9.7) | 51 (15.3) | 243 (15.6) |
| ART 2–6 months after start of anti-tuberculosis treatment | 173 (19.5) | 31 (13.8) | 15 (13.2) | 52 (15.6) | 271 (17.4) |
| ART >6 months after start of anti-tuberculosis treatment | 335 (37.7) | 48 (21.4) | 12 (10.5) | 86 (25.7) | 481 (30.8) |
| ART before start of anti-tuberculosis treatment | 132 (14.9) | 27 (12.0) | 3 (2.6) | 36 (10.8) | 198 (12.7) |
| No ART | 111 (12.5) | 75 (33.3) | 73 (64.0) | 109 (32.6) | 368 (23.5) |
| Total | 888 (100) | 225 (100) | 114 (100) | 334 (100) | 1561 (100) |
Patients with concurrent PTB and lymph node TB were classified as PTB.
Patients with a distant history of TB could be WHO Clinical Stage I or II, depending on other history and examination findings.
Based on CD4 result closest in time to anti-tuberculosis treatment, classification per WHO guidelines.
Includes imputed data for 757 patients with missing historical nutritional data at the time of TB diagnosis.
TB = tuberculosis; HIV = human immunodeficiency virus; PTB = pulmonary TB; WHO = World Health Organization; ART = antiretroviral therapy.
Cross-tabulation of ART and immune status showed that 29% of children not on ART and 64% of children on ART had severe immunosuppression near the time of TB diagnosis. Two-by-two cross-tabulation of ART status and outcome showed that TB-HIV co-infected children who had never been started on ART had a more than three-fold higher likelihood of dying compared to those on ART (OR 3.50, 95%CI 2.45– 4.99, P < 0.001).
In univariate analysis, age <12 months, male sex, PTB, ART initiation >2 months after anti-tuberculosis treatment, no ART, WHO HIV Stage IV, severe malnutrition, and severe immune suppression were associated with greater mortality (P < 0.05; Table 3). In multivariate analysis, male sex, WHO HIV Stage IV, no ART and severe immunosuppression remained associated with increased risk of mortality (P < 0.05), after controlling for the other variables in the model. Children with HIV and TB co-infection not initiated on ART at any time were almost nine times more likely to die than those initiated on ART 0–2 months after initiation of anti-tuberculosis treatment (adjusted OR [aOR] 8.83, 95%CI 4.42–17.63, P < 0.001). In addition, children with severe immunosuppression were six times more likely to die than children with no immunosuppression (aOR 6.02, 95%CI 2.98–12.17, P < 0.001).
Table 3.
Factors associated with mortality in Malawian TB-HIV co-infected children
| Univariate regression
|
Multivariate regression
|
|||
|---|---|---|---|---|
| Variable | OR (95%CI) | P value | aOR (95%CI)* | P value |
| Age at TB diagnosis | ||||
| <12 months | 2.94 (1.74– 4.97) | <0.001† | 1.69 (0.89–3.22) | 0.112 |
| 1–3 years | 1.24 (0.76–2.03) | 0.39 | 1.03 (0.57–1.86) | 0.920 |
| >3–5 years | 0.67 (0.35–1.27) | 0.22 | 0.78 (0.36–1.68) | 0.521 |
| >5–10 years | 0.67 (0.39–1.15) | 0.15 | 0.70 (0.37–1.31) | 0.259 |
| >10–18 years | Reference | Reference | ||
| Sequence of anti-tuberculosis treatment and ART | ||||
| ART 0–2 months after start of anti-tuberculosis treatment | Reference | Reference | ||
| ART 2–6 months after start of anti-tuberculosis treatment | 0.45 (0.33–0.93) | 0.025† | 0.88 (0.49–1.59) | 0.673 |
| ART >6 months after start of anti-tuberculosis treatment | 0.45 (0.28–0.70) | 0.001† | 0.72 (0.39–1.33) | 0.292 |
| ART before start of anti-tuberculosis treatment | 0.64 (0.37–1.09) | 0.099 | 1.23 (0.65–2.31) | 0.524 |
| No ART | 2.10 (1.34–3.29) | 0.001† | 8.83 (4.42–17.63) | <0.001† |
| Sex | ||||
| Male | 1.50 (1.11–2.02) | 0.008† | 1.70 (1.15–2.52) | 0.008† |
| Female | Reference | Reference | ||
| Type of TB | ||||
| Pulmonary TB | 1.94 (1.17–3.22) | 0.013† | 1.56 (0.83–2.93) | 0.169 |
| Other TB | Reference | Reference | ||
| Maximum WHO Stage | ||||
| Stage III | Reference | Reference | ||
| Stage IV | 4.67 (3.43–6.37) | <0.001† | 4.48 (2.91–6.90) | <0.001† |
| Acute nutrition status | ||||
| Normal | Reference | Reference | ||
| Mild malnutrition | 1.12 (0.61–2.04) | 0.710 | 1.51 (0.74–3.11) | 0.259 |
| Moderate malnutrition | 0.86 (0.55–1.34) | 0.500 | 1.00 (0.58–1.71) | 0.988 |
| Severe malnutrition | 2.93 (2.08– 4.13) | <0.001† | 1.06 (0.63–1.80) | 0.822 |
| Immune suppression | ||||
| None | Reference | Reference | ||
| Mild | 1.78 (0.77– 4.19) | 0.179 | 1.86 (0.74– 4.73) | 0.189 |
| Advanced | 1.57 (0.67–3.66) | 0.297 | 1.84 (0.70– 4.82) | 0.217 |
| Severe | 4.76 (2.69–8.43) | <0.001† | 6.02 (2.98–12.17) | <0.001† |
Adjusted for all other covariates in the model.
Statistically significant.
TB = tuberculosis; HIV = human immunodeficiency virus; OR = odds ratio; CI = confidence interval; aOR = a djusted OR; ART = antiretroviral therapy; WHO = World Health Organization.
DISCUSSION
This study presents data from a large pediatric TB-HIV co-infected cohort using retrospective data from routine patient care. This is operational research reflecting conditions in the field, thereby facilitating the evaluation of national health policies.
The most striking findings were the high overall mortality (20% of patients with known outcomes) and the significant difference in mortality between TB-HIV co-infected patients who never started ART compared to those who did. We have previously reported a mortality of 4.8% in our overall cohort of infected children who started ART.20 Those co-infected patients who never received ART fell predominantly into one of two groups: patients deemed ineligible for ART due to pre-2008 guidelines, which permitted using CD4 counts to determine eligibility for WHO Stage III PTB patients, and those who needed ART but never started due to clinical instability or lack of social/care giver readiness. We were not able to determine with certainty which patients in our cohort fell into each category, but a subset analysis of those with available CD4 data showed that 29.8% of patients who did not start ART had severe immune suppression. At least these patients, and possibly others, were ART-eligible and likely not started on treatment due to lack of clinical stability or social readiness.
For the former group, ART eligibility guidelines have since evolved to include all TB patients, as numerous studies have shown that ART improves TB outcomes and mortality in HIV-infected children, regardless of immune status.21–24 For the latter group, who were identified as needing ART but were never initiated on it, increased emphasis on timely ART initiation after TB diagnosis is needed, with consideration of reduced requirements for pre-ART care giver education, especially if the child is unwell. New national ART guideline revisions in 2011 allowing for more timely, and even simultaneous, initiation of ART and anti-tuberculosis treatment will help, as increasing evidence links earlier ART initiation to reduced mortality in TB-HIV co-infected adults and children.25–27 While other risk factors for mortality in TB-HIV co-infected adults have been well established, and include severe immunodeficiency, advanced age, and malnutrition, similar risk factors in children have not been well established.28,29 A unique and important consideration when comparing our outcomes with those of other studies is our chosen starting point of enrolment into HIV care, and not the TB clinic. We do not know how many co-infected children in our area were diagnosed with TB and were either never tested for HIV or never enrolled into HIV care, and how many of those children died. If we had been able to include such patients, we would likely have seen an even greater difference in mortality between those who were and were not enrolled in HIV care and received ART.
Our cohort included 139 patients diagnosed with TB in the 12 months after ART initiation. We lacked sufficient data to determine exactly how many of these cases met definitions for immune reconstitution inflammatory syndrome (IRIS). Regional studies have reported incidences of TB IRIS of 7.4–15.4% in children starting ART.30,31 Other patients in our cohort likely had active TB that was missed before initiating ART. A Ugandan pediatric study demonstrated a 70% reduction in new TB cases diagnosed after ART initiation with implementation of standardized pre-ART TST screening.32 The lack of TB screening tools such as TST, gastric lavage and sputum induction is a major barrier to improved recognition of TB in Malawi.
Our analysis was suggestive of an increased risk of mortality in TB-HIV co-infected infants (although not conclusive in multivariate analysis, with an adjusted OR of 1.69, 95%CI 0.89–3.22), and adds to the literature that demonstrates an increased risk of severe disease and mortality in TB-HIV co-infected infants.33,34 This vulnerability likely results from several factors, including poorly controlled HIV replication in infants not on ART, greater likelihood of TB exposure due to close contact with source mothers and immune immaturity, which is known to lead to rapid progression from TB infection to disease and higher mortality, even in non-HIV-infected children.5 Regardless of the cause, this is a high-risk sub-group that warrants more frequent and higher-level follow-up and emphasis on timely ART initiation.
Male sex was associated with a statistically significantly increased risk of mortality in multivariate analysis. There were no differences in care provision at the COE based on sex, and we cannot explain these results. However, we doubt that sex would truly have a bearing on mortality outcomes in the context of pediatric TB-HIV co-infection.
Transfer-out and LTFU patients (respectively 21.4% and 7.3% of the cohort) were excluded from mortality analyses, and this may have biased the estimates of mortality risk. During the time of this study, the COE had the policy of actively transferring stable patients out to ART centers closer to their homes, so the majority of those patients were likely still alive and on ART. However, it is customary to assume that a fairly high proportion of LTFU patients have died, meaning our true mortality was possibly higher than reported.35
This retrospective study had several limitations. There were gaps in our data, particularly in identifying the exact dates of TB diagnosis before clinic enrolment and nutritional status for historical TB diagnoses, requiring data imputation for logistic regression analysis. We also had to assume that all patients were vertically infected, including adolescents (13.7% of our cohort), and established that this was reasonable given observed local pediatric transmission patterns. If some patients were horizontally infected and had a historical TB diagnosis, there is a chance they did not have true TB-HIV co-infection. These missing data and limitations were partially offset by our rigorous data collection process and the breadth of clinical information included, coupled with the relatively large sample size. Furthermore, due to inherent challenges in diagnosing TB in children, particularly in Malawi, there were likely cases of other lung disease misdiagnosed as TB, and also cases where anti-tuberculosis treatment was used without strong diagnostic evidence in critically ill patients who showed no improvement on ART.
CONCLUSIONS
Pediatric TB-HIV co-infection is common, and mortality was high in this cohort. Better TB diagnostic tools for children, along with national guidelines and clinic protocols that prioritize prompt initiation of ART and close follow-up of co-infected patients, with special attention to infants and those with advanced clinical stage and severe immunosuppression, should lead to improved outcomes. Future research can evaluate the impact of a new national pre-ART IPT program and of universal ART for all children aged <5 years if implemented.
Acknowledgments
The authors thank the clinical and Monitoring and Evaluation teams at the Baylor College of Medicine–Abbott Fund Children’s Clinical Centre of Excellence; the Baylor College of Medicine International Pediatric AIDS Initiative senior leadership, including G Schutze, S Wanless, N Calles, M Mizwa and M Kline; and their colleagues at the HIV and TB Departments of the Malawi Ministry of Health.
MMK was supported in part by grant D43 TW01036, and DO was supported in part by grant mR24 TW007988 from the Fogarty International Center of the National Institutes of Health, Bethesda, MD, USA.
Support for this paper was provided by the Baylor College of Medicine Children’s Foundation Malawi Tingathe Program with funds from the United States Agency for International Development (USAID) Cooperative Agreement number 674-A-00-10-00093-00. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Footnotes
Conflict of interest: none declared.
References
- 1.Swaminathan S. Tuberculosis in HIV-infected children. Paediatr Respir Rev. 2004;5:225–230. doi: 10.1016/j.prrv.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Lawn SD, Bekker LG, Middlekoop K, et al. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 3.Marais BJ, Schaaf HS. Childhood tuberculosis: an emerging and previously neglected problem. Infect Dis Clin North Am. 2010;24:727–749. doi: 10.1016/j.idc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Feja K, Saiman L. Tuberculosis in children. Clin Chest Med. 2005;26:295–312. doi: 10.1016/j.ccm.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Newton SM, Brent AJ, Anderson SA, et al. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldacker C, Tweya H, Keiser O, et al. Characteristics of adults and children diagnosed with tuberculosis in Lilongwe, Malawi: findings from an integrated HIV/TB clinic. Trop Med Int Health. 2012;17:1108–1116. doi: 10.1111/j.1365-3156.2012.03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Global tuberculosis report, 2012. Geneva, Switzerland: WHO; 2012. (WHO/HTM/TB/2012.6). [Google Scholar]
- 8.Joint United Nations Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic, 2010. Geneva, Switzerland: UNAIDS; 2010. [Google Scholar]
- 9.Harries AD, Hargreaves NJ, Graham SM, et al. Childhood tuberculosis in Malawi: nationwide case-finding and treatment outcomes. Int J Tuberc Lung Dis. 2002;6:424–431. [PubMed] [Google Scholar]
- 10.Weismuller MM, Graham SM, Claessens NJM, Meijnen S, Salaniponi FM, Harries AD. Diagnosis of childhood tuberculosis in Malawi: an audit of hospital practice. Int J Tuberc Lung Dis. 2002;6:432–438. [PubMed] [Google Scholar]
- 11.Malawi Ministry of Health. National Tuberculosis Control Programme manual. 6th. Lilongwe, Malawi: Malawi Ministry of Health; 2007. [Google Scholar]
- 12.Malawi Ministry of Health. Treatment of AIDS, guidelines for the use of antiretroviral therapy in Malawi. 1st. Lilongwe, Malawi: Malawi MoH; 2003. [Google Scholar]
- 13.Malawi Ministry of Health. Treatment of AIDS, guidelines for the use of antiretroviral therapy in Malawi. 2nd. Lilongwe, Malawi: Malawi MoH; 2006. [Google Scholar]
- 14.World Health Organization Regional Offi ce for South-East Asia, UNICEF. Management of HIV infection and antiretroviral therapy in infants and children: a clinical manual. New Delhi, India: WHO SEARO; 2006. (WHO Technical Publication No. 51). [Google Scholar]
- 15.Malawi Ministry of Health. Treatment of AIDS: guidelines for the use of antiretroviral therapy in Malawi. 3rd. Lilongwe, Malawi: Malawi MoH; 2008. [Google Scholar]
- 16.World Health Organization. Recommendations for a public health approach. Geneva, Switzerland: WHO; 2009. Antiretroviral therapy for HIV infection in infants and children: towards universal access. [PubMed] [Google Scholar]
- 17.Malawi Ministry of Health. Results up to 31st December 2010. Lilongwe, Malawi: Malawi MoH; 2010. Malawi Antiretroviral Treatment Programme quarterly report. [Google Scholar]
- 18.Kim MH, Cox C, Dave A, et al. Prompt initiation of ART with therapeutic food is associated with improved outcomes in HIV-infected Malawian children with malnutrition. J Acquir Immune Defic Syndr. 2012;59:173–176. doi: 10.1097/QAI.0b013e3182405f8f. [DOI] [PubMed] [Google Scholar]
- 19.Malawi Ministry of Health. Guidelines for the management of severe acute malnutrition. Lilongwe, Malawi: Malawi MoH; 2006. [Google Scholar]
- 20.Kabue MM, Buck WC, Wanless SR, et al. Mortality and clinical outcomes in HIV-infected children on antiretroviral therapy in Malawi, Lesotho, and Swaziland. Pediatrics. 2012;130:e591–e599. doi: 10.1542/peds.2011-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinson NA, Moultrie H, van Niekerk R, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis. 2009;13:862–867. [PMC free article] [PubMed] [Google Scholar]
- 22.Braitstein P, Nyandiko W, Vreeman R, et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J. 2009;28:626–632. doi: 10.1097/INF.0b013e31819665c5. [DOI] [PubMed] [Google Scholar]
- 23.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walters E, Cotton MF, Rabie H, et al. Clinical presentation and outcome of tuberculosis in human immune deficiency virus infected children on anti-retroviral therapy. BMC Pediatr. 2008;8:1. doi: 10.1186/1471-2431-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Health. Clinical management of HIV in children and adults. 1st. Lilongwe, Malawi: Malawi MoH; 2011. [Google Scholar]
- 26.Török ME, Farrar JJ. When to start antiretroviral therapy in HIV-associated tuberculosis. N Engl J Med. 2011;365:1538–1540. doi: 10.1056/NEJMe1109546. [DOI] [PubMed] [Google Scholar]
- 27.Pensi T, Hemal A, Banerjee T. Simultaneous HAART improves survival in children co-infected with HIV and TB. Trop Med Int Health. 2012;17:52–58. doi: 10.1111/j.1365-3156.2011.02884.x. [DOI] [PubMed] [Google Scholar]
- 28.Semba RD, Darnton-Hill I, de Pee S. Addressing tuberculosis in the context of malnutrition and HIV co-infection. Food Nutr Bull. 2010;31(Suppl):S345–S364. [PubMed] [Google Scholar]
- 29.Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15:871–885. doi: 10.5588/ijtld.10.0352. [DOI] [PubMed] [Google Scholar]
- 30.Smith K, Kuhn L, Coovadia A, et al. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS. 2009;23:1097–1107. doi: 10.1097/QAD.0b013e32832afefc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orikiiriza J, Bakeera-Kitaka S, Musiime V, et al. The clinical pattern, prevalence, and factors associated with immune reconstitution inflammatory syndrome in Ugandan children. AIDS. 2010;24:2009–2017. doi: 10.1097/QAD.0b013e32833b260a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakeera-Kitaka S, Conesa-Botella A, Dhabangi A, et al. Tuberculosis in human immunodeficiency virus infected Ugandan children starting on antiretroviral therapy. Int J Tuberc Lung Dis. 2011;15:1082–1086. doi: 10.5588/ijtld.10.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis. 2009;48:108–114. doi: 10.1086/595012. [DOI] [PubMed] [Google Scholar]
- 34.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(Suppl 3):S184–S194. doi: 10.1086/651490. [DOI] [PubMed] [Google Scholar]
- 35.Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are ‘lost to follow-up’ in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]


