Abstract
Takayasu arteritis is a large-vessel vasculitis that often results in pulselessness due to fibrotic stenoses. Whereas minor calcification is sometimes seen with Takayasu arteritis, it rarely causes stenosis. Extensive calcification resulting in malperfusion is exceedingly rare and has been attributed to disorders in calcium trafficking in a chronic inflammatory state. We report an unusual case of rapidly progressive and extensive aortic calcification in the setting of Takayasu arteritis.
Large-vessel calcification is often associated with atherosclerotic disease or end-stage renal disease, but it may also be manifested as a result of hyperparathyroidism or chronic inflammatory states, as in the case of autoimmune vasculitides. Takayasu arteritis is a large-vessel vasculitis in which granulomatous inflammation often leads to vascular stenosis secondary to intimal fibrosis. Minor calcification may be associated with this chronic fibrotic reaction, but extensive, flow-limiting calcification is rare. We report a case of extensive large-vessel calcification in a young woman with Takayasu arteritis who has consented to its publication.
Case report
A previously healthy 13-year-old girl developed a prolonged febrile illness with associated elevated inflammatory markers. She was treated empirically with tapered glucocorticoids for 6 weeks, with resolution of symptoms and normalization of inflammatory markers. At the age of 16 years, carotid bruits were noted on a routine physical examination, and the erythrocyte sedimentation rate was >100 mm/h. She was diagnosed with Takayasu arteritis and treated with azathioprine (2 mg/kg daily) and glucocorticoids.
In 2005, at the age of 25 years, she developed bilateral lower extremity claudication. Physical examination revealed normal and symmetric radial pulses, but an abdominal bruit was present on auscultation and femoral pulses were not palpable. Computed tomography angiography revealed extensive calcification of her paravisceral aorta extending through her aortic bifurcation with severe stenosis of her common iliac arteries. Her erythrocyte sedimentation rate and C-reactive protein level were within normal limits. She had normal renal function, and parathyroid hormone levels were also within normal limits, ruling out primary hyperparathyroidism. She fulfilled four of the six 1990 American College of Rheumatology classification criteria for Takayasu arteritis1 (present: age <40 years, claudication, aortic bruit, angiographic abnormalities; absent: decreased brachial pulse, systolic difference >10 mm Hg between arms). The clinical impression was that her claudication was secondary to fibrotic stenoses from previously active Takayasu arteritis, and the patient was continued on azathioprine as a remission maintenance agent.
In 2009, she returned with disabling claudication, unable to walk more than 5 meters without rest. Whereas her inflammatory markers continued to be normal, she was severely osteopenic on dual-energy X-ray absorptiometry scan and was started on 2 g of calcium carbonate per day. Computed tomography angiography revealed progression of her aortoiliac calcification, now with near-occlusion of her bilateral common iliac arteries. She underwent bilateral common iliac artery angioplasty and stenting, resulting in normal pedal pulses and complete resolution of her symptoms.
In January 2013, azathioprine was discontinued because her disease was thought to be in prolonged remission. In June, her symptoms of claudication returned. On presentation to her primary care provider, she was noted to be hypertensive to 180/124 mm Hg in each arm. She was treated with aspirin and lisinopril and was referred for vascular surgery consultation. She continued to have normal and symmetric upper extremity pulses but absent femoral pulses. Serum inflammatory markers continued to be normal, but pull-back pressures during angiographic evaluation revealed a pressure gradient across her paravisceral calcified aorta of >100 mm Hg. Angiography revealed areas of dilation and stenosis in the bilateral proximal subclavian arteries, dilation of the ascending aorta and aortic arch, and stenosis of the bilateral brachial arteries with extensive collateral formation. Notably, there were sites of vascular disease in the branch arteries above the diaphragm in the absence of associated calcification (Fig 1). Whereas the aorta and iliac arteries had areas of dilation and stenosis, the mid-descending thoracic aorta through the aortic bifurcation was also heavily calcified, with near-occlusion of the aorta due to coral reef-type calcification at the level of the superior mesenteric artery. There was progression of calcific abdominal aortic disease compared with prior angiography from 2009 (Fig 2).
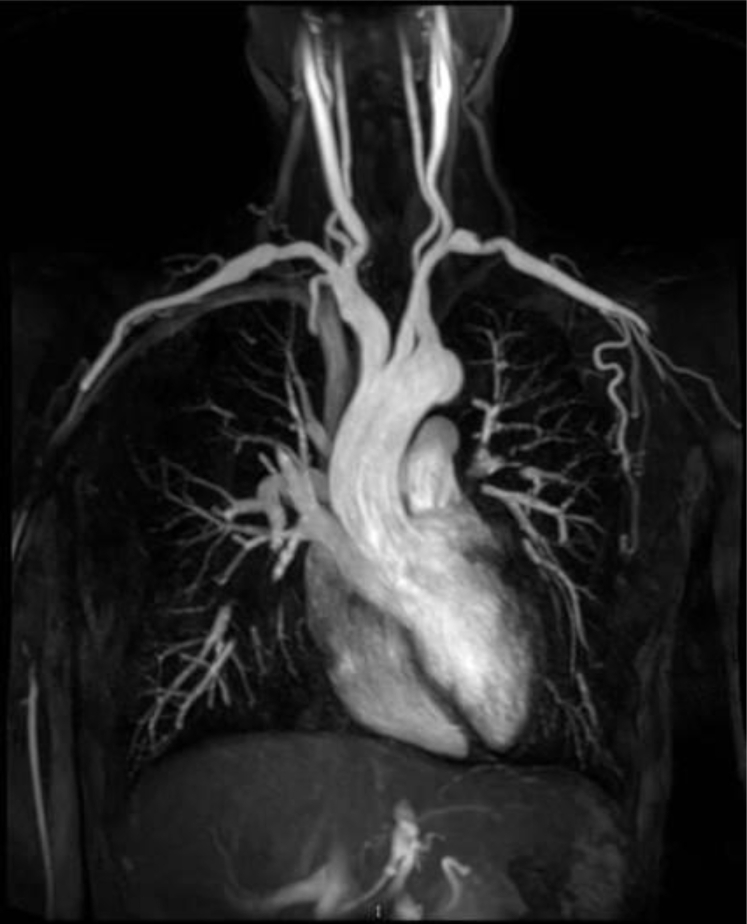
Fig 1.
Bilateral subclavian artery stenosis. Coronal reconstruction of magnetic resonance angiogram shows noncalcified stenosis of bilateral subclavian arteries suggestive of Takayasu arteritis.
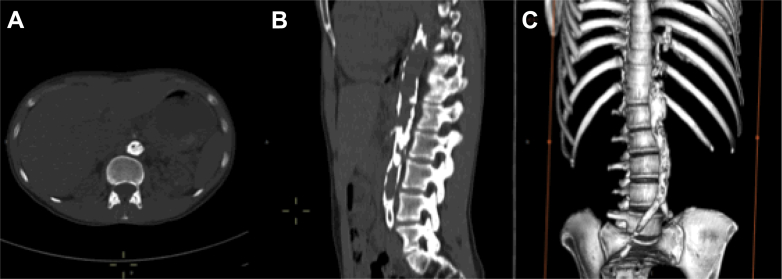
Fig 2.
Calcification of the abdominal aorta. Images from a non-contrast-enhanced computed tomography scan of the chest, abdomen, and pelvis. A, Axial view shows extensive calcification of the abdominal aorta with severe narrowing of the lumen of the aorta. B, Sagittal view shows the distribution of calcification throughout the abdominal aorta. C, A three-dimensional reconstruction shows the extent of calcification starting at the distal thoracic aorta and extending into the iliofemoral arteries.
Multidisciplinary review revealed no further option for medical management, and she was counseled on surgical options for her rapidly progressive disease. She continued nonoperative management while she sought a second opinion; however, in the subsequent 2 months, she developed symptoms of mesenteric ischemia including postprandial pain and weight loss.
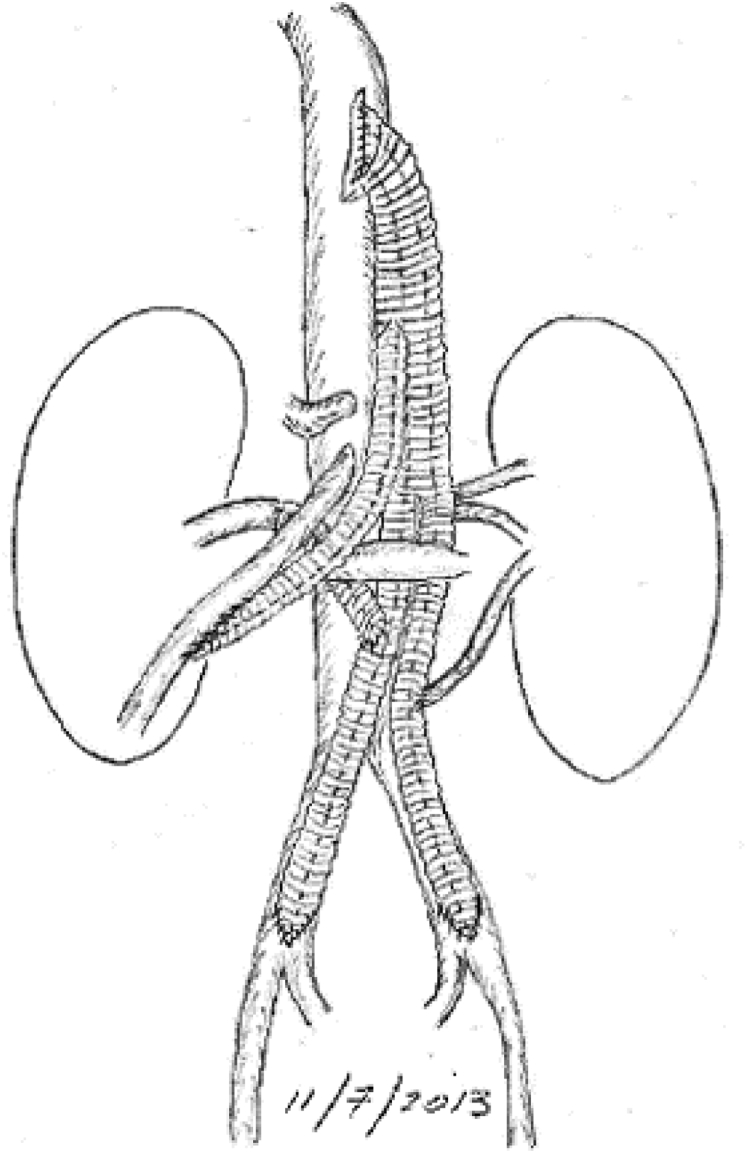
After extensive discussion of the surgical options including aortic endarterectomy and bypass, she underwent descending thoracic aortobi-iliac bypass and superior mesenteric artery and right renal artery bypass and reimplantation of her left renal artery. This was completed through separate midline laparotomy and seventh interspace thoracotomy incisions to reduce morbidity. A custom Dacron graft was fashioned on the back table, and the proximal anastomosis was sewn in an end-to-side fashion using a side-biting DeBakey-Bahnson clamp. The graft was tunneled retrodiaphragmatically into the retroperitoneum, and the iliac anastomoses were completed in and end-to-side fashion, restoring flow to the legs. The visceral branches were then sewn in succession to minimize ischemia time (Fig 3). She tolerated the procedure well and was discharged without complication. Follow-up at 1 month revealed ankle-brachial indices >1 and resolution of her claudication and symptoms of mesenteric ischemia.
Fig 3.
Illustration of surgical reconstruction.
(Courtesy B.W. Starnes.)
Pathologic examination of the diseased aortoiliac and right renal artery revealed adventitial fibrosis with severe disruption of the elastic architecture consistent with the diagnosis of Takayasu arteritis. The “healthy tissue” was also subjected to histologic analysis; von Kossa staining exhibited mineralized deposits in the adventitial layer. Both pieces of tissue were essentially devoid of inflammation, giant cells, granulomas, or endarteritis obliterans.
Dermal fibroblasts were isolated and cultured from the patient, and the mineralization process was compared with fibroblasts isolated from healthy controls and from patients with the medial vascular calcification disease of arterial calcification due to deficiency of CD73 (ACDC). The patient's cells showed an abnormally high expression of nonspecific alkaline phosphatase, an enzyme that plays a key role in mineralization and hydroxyapatite formation. Even more striking was the patient's baseline activity of alkaline phosphatase, which exceeded that of the ACDC cell line even after it was artificially stimulated under osteogenic conditions for 5 days. Furthermore, the fibroblasts from the patient showed evidence of calcification at baseline, whereas both control and ACDC fibroblasts did not.
Discussion
Takayasu arteritis is characterized by granulomatous inflammation with intimal fibrosis, resulting in loss of pulses and malperfusion due to fibrous stenoses. Calcification has been reported in 29% to 54% of cases2 and is commonly considered a sequela of prior inflammation. Extensive calcification resulting in stenosis and malperfusion is rare and previously described only in case reports.3 Vascular calcification has also been described in the setting of rheumatoid arthritis and systemic lupus erythematosus,4 which suggests a link between chronic inflammation and vascular calcification.
Although there were underlying fibrous stenoses from burned out Takayasu arteritis, the amount of associated vascular calcification and the degree of osteoporosis in this young woman suggest that there may be other processes at work. The absence of active inflammation was supported by negative serum inflammatory markers, and histologic evidence of “burned out” disease further suggested other contributing factors to the progressive vascular disease. The association between osteoporosis and vascular calcification is well described in the literature5 and has been attributed to estrogen deficiency, lipid metabolism,6 and variations in calcium trafficking and the osteoprotegerin/receptor activator of nuclear factor κB ligand (RANKL) systems.7 A genetic basis has also been described as ACDC, which is caused by a nonsense mutation in NT5E, the gene coding for CD73.8 Whereas our patient demonstrated increased osteogenic activity in situ and in cultured dermal fibroblasts, it is not clearly linked to any of these previously described mechanisms.
Reconstructive surgery for Takayasu arteritis should be performed in the quiescent phase of disease.9 Good outcomes from angioplasty and reconstructive surgery are possible in Takayasu arteritis, but revision surgery is often needed.10, 11 In these difficult cases, surgical bypass is an effective treatment for extensive aortic calcification and resultant malperfusion, but unfortunately the underlying pathophysiologic mechanism remains poorly understood. As a result, the underlying disease process may progress, causing further stenosis and potentially restenosis of the vascular grafts. As more is understood about calcium trafficking and its dysregulation in states of chronic inflammation, we hope it will lead to treatment for the process underlying this highly morbid and rapidly progressive disease.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Arend W.P., Michel B.A., Bloch D.A., Hunder G.G., Calabrese L.H., Edworthy S.M., et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–1134. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 2.Yamato M., Lecky J.W., Hiramatsu K., Kohda E. Takayasu arteritis: radiographic and angiographic findings in 59 patients. Radiology. 1986;161:329–334. doi: 10.1148/radiology.161.2.2876459. [DOI] [PubMed] [Google Scholar]
- 3.Gujadhur A., Smith E.R., McMahon L.P., Spanger M., Chuen J., Holt S.G. Large vessel calcification in Takayasu arteritis. Intern Med J. 2013;43:584–587. doi: 10.1111/imj.12116. [DOI] [PubMed] [Google Scholar]
- 4.Yiu K.H., Wang S., Mok M.Y., Ooi G.C., Khong P.L., Lau C.S., et al. Relationship between cardiac valvular and arterial calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 2011;38:621–627. doi: 10.3899/jrheum.100844. [DOI] [PubMed] [Google Scholar]
- 5.Lampropoulos C.E., Papaioannou I., D'Cruz D.P. Osteoporosis—a risk factor for cardiovascular disease? Nat Rev Rheumatol. 2012;8:587–598. doi: 10.1038/nrrheum.2012.120. [DOI] [PubMed] [Google Scholar]
- 6.Demer L.L. Vascular calcification and osteoporosis: inflammatory responses to oxidized lipids. Int J Epidemiol. 2002;31:737–741. doi: 10.1093/ije/31.4.737. [DOI] [PubMed] [Google Scholar]
- 7.Bezerra M.C., Calomeni G.D., Caparbo V.F., Gebrim E.S., Rocha M.S., Pereira R.M.R. Low bone density and low serum levels of soluble RANK ligand are associated with severe arterial calcification in patients with Takayasu arteritis. Rheumatology (Oxford) 2005;44:1503–1506. doi: 10.1093/rheumatology/kei045. [DOI] [PubMed] [Google Scholar]
- 8.St Hilaire C., Ziegler S.G., Markello T.C., Brusco A., Groden C., Gill F., et al. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhtyar C., Guillevin L., Cid M.C., Dasgupta B., de Groot K., Gross W., et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2009;68:318–323. doi: 10.1136/ard.2008.088351. [DOI] [PubMed] [Google Scholar]
- 10.Matsuura K., Ogino H., Matsuda H., Minatoya K., Sasaki H., Yagihara T., et al. Surgical outcome of aortic arch repair for patients with Takayasu arteritis. Ann Thorac Surg. 2006;81:178–182. doi: 10.1016/j.athoracsur.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 11.Fields C.E., Bower T.C., Cooper L.T., Hoskin T., Noel A.A., Panneton J.M., et al. Takayasu's arteritis: operative results and influence of disease activity. J Vasc Surg. 2006;43:64–71. doi: 10.1016/j.jvs.2005.10.010. [DOI] [PubMed] [Google Scholar]