Abstract
Background and Methods
This cross-sectional study aimed at determining the prevalence and risk factors for severe anemia, severe microcytic anemia, and severe normocytic anemia among HIV-infected individuals aged >15 years. Univariate and multivariate analyses were performed to identify the risk factors for anemia.
Results
Data from 40 408 patients were analyzed, showing an overall prevalence of 22% for severe anemia. The risk of developing severe anemia increased by 49% among patients with a body mass index of <18.5 kg/m2, by approximately 2-fold among patients with the World Health Organization (WHO) stage III, and by 3-fold among patients with WHO stage IV illness. Severe normocytic anemia was uniquely increased among patients aged ≥50 years, among those with chronic diarrhea and Kaposi’s sarcoma, and those taking cotrimoxazole.
Conclusion
There was a high prevalence of severe anemia among adults infected with HIV. Focused identification of anemia should be based on the hemoglobin and mean corpuscular volume measurements.
Keywords: HIV, burden, determinants, severe anemia, adults
Introduction
Anemia is a common complication of HIV.1–3 The condition is often considered an inevitable consequence of HIV infection, with prevalence estimates ranging from 58% to 95% in different settings.3,4 In developed countries, the prevalence of severe anemia among HIV-infected individuals is between 1.4% and 2%, depending on the hemoglobin (Hgb) cutoff point and increases with advanced immunosuppression.4 Corresponding figures in developing countries are limited and also vary depending on the Hgb cutoff value chosen. According to recent data, in some Sub-Saharan African countries, the prevalence of severe anemia (defined as Hgb < 7.5 g/dL) at the initiation of antiretroviral (ARV) medication ranges from 3% to 8%.5 Using a less stringent cutoff (Hgb < 8 g/dL), a recent study conducted in an HIV care clinic in rural Tanzania revealed a 16.9% prevalence of severe anemia6 among HIV-infected individuals. In this study, 649 (77.4%) of 838 patients met the definition of anemia. Of the 649 patients with anemia, 254 (39.1%) had microcytosis and hypochromia, suggestive of iron deficiency, whereas 52 (8.0%) had hypochromia with normocytosis, and 18 (2.8%) had microcytosis with normochromia.
The etiology of anemia in HIV-infected patients is multifactorial, with opportunistic infections, nutritional deficiencies, certain medications (including cotrimoxazole and zidovudine [ZDV]), HIV itself, and infiltrative diseases of the bone marrow among the leading causes.7 Preexisting anemia may be worsened by any of these conditions.
Studies of the magnitude and determinants of severe anemia in the context of HIV infection are limited. The few available studies focused on general anemia or mild anemia and mainly target children and pregnant women.2,8,9 Severe anemia might also be common in the adult population and is clinically relevant because of the high mortality rate associated with it.10 Several factors are reportedly associated with mild anemia or anemia in general,4,6,11 but little is known about the specific factors associated with severe anemia and its morphological types as defined by the mean corpuscular volume (MCV), especially in HIV-infected adults.
Differentiating the types of anemia according to MCV can help determine the etiology of anemia and assist with its management. In sub-Saharan Africa, microcytic anemia (defined as anemia with MCV < 80 fl) is mainly due to iron deficiency, whereas normocytic anemia (defined as anemia with MCV of 80–100 fl) is usually due to anemia of chronic disease.3,12,13 Macrocytic anemia (defined as anemia with MCV > 100 fl) may be due to the deficiency of either cobalamin or folate.14
In this article, the authors report on the prevalence of severe anemia, severe microcytic anemia, and severe normocytic anemia and their associated risk factors among HIV-infected adults at the time of enrollment into a large urban HIV Care and Treatment program in Tanzania.
Methods
Design and Setting
A cross-sectional study was conducted at 12 HIV care and treatment clinics (CTCs) in Dar es Salaam, Tanzania. These clinics are supported by the Management and Development for Health (MDH) HIV Care and Treatment Program funded by the US President’s Emergency Plan for AIDS Relief (PEPFAR). The MDH-PEPFAR program has been enrolling participants into an HIV Care and Treatment Program since November 2004.
Study Population
For the prevalence of anemia, the authors included all HIV-infected males and nonpregnant females > 15 years of age who had the baseline Hgb among those enrolled into the MDH Program between November 2004 and December 2010. The authors examined risk factors for severe anemia among participants who had both Hgb and MCV during the same period.
Data Collection and Management
At the first clinic visit, the attending physicians diagnosed and staged all patients according to the World Health Organization (WHO) clinical criteria. Other information collected at the initial visit included social and demographic characteristics, anthropometric measurements, previous ARV medication use, current cotrimoxazole use, history of tuberculosis (TB in the past), and current TB treatment. The initial laboratory workup at diagnosis and staging involved measurement of CD4 count (using FACS Calibur, Becton Dickinson [BD], Franklin Lakes, New Jersey), full blood picture (using ACT5 DIFF Hematology Analyzer, Beckman Coulter, Brea, California), biochemical tests (liver, lipid, and renal profiles using Cobas Integra 400 Plus Chemistry Analyzer, Roche, Basel, Switzerland), and hepatitis B sero status determined using SD Bioline antigen and antibodies (Standard Diagnostics, Inc, Suwon, Korea), respectively. All these machines and reagents were supplied by Roche Diagnostics (South Africa), through KAS Medics as a local agent. All patients’ demographic, clinical, laboratory, and therapeutic data were recorded by physicians and nurses on standard case report and National Care and Treatment Center forms. Data reviewers were stationed at each clinic to ensure adequacy and completeness of the data recording by the health care workers. Data collected were then entered into a secure computerized database designed solely for the purpose of data collection and analysis. Unique patient identifiers were used. The database was updated daily by professional data entry clerks. Weekly quality assurance checks of the database were performed by the data management team to ensure data accuracy. Data extracted for this analysis included age, sex, Hgb (g/dL), body mass index (BMI), WHO clinical stage, CD4 count (cells/mm3), history of previous ARV medication use, history of TB and current TB treatment, current use of cotrimoxazole, concurrent illnesses such as oral candidiasis, chronic diarrhea (>2 weeks), Kaposi’s sarcoma, wasting, syphilis, alanine transaminase (U/L), hepatitis B status, creatinine level (in mmol/L), and morphological classification of anemia based on MCV.
Outcome Measures
Severe anemia was defined as an Hgb level of <8.5 g/dL. This cutoff point reflects the level of severe anemia used for referral to district hospitals in Tanzania.15 Anemia was considered to be normocytic if the MCV level was between 80 and 100 fl, microcytic if the MCV was <80 fl, and macrocytic if it was >100 fl.14
Statistical Analysis
The prevalence of severe anemia at baseline was determined as the percentage of cases among patients enrolled in the treatment program during the study period. To identify predictors of severe anemia, univariate and multivariate analyses were performed using log binomial models to estimate relative risks (RR) and confidence intervals.16,17 In determining the risk factors for various types of severe anemia, separate models were run for multivariate analysis; one model included WHO stage but excluded components of the staging definition, namely TB treatment, oral candidiasis, chronic diarrhea, wasting, and Kaposi’s sarcoma. The other model included the latter individual conditions but excluded the WHO stage. In a few instances, the models did not converge and log Poisson models, which provide consistent but not fully efficient estimates of the prevalence ratio and its confidence intervals, were used.18 All available plausible predictors were included in the multivariate models if they were significant at a P value of <.20. The missing indicator method was used for covariates with missing values in the multivariate analyses.19 Stepwise restricted cubic splines were used to assess the nonlinearity of results and produce graphs of associations.20,21 The criterion for significance for all analyses was a 2-sided P value of <.05. All statistical analyses were performed with the statistical software package SAS release 9.1 (SAS Institute Inc, Cary, North Carolina).
Ethical Considerations
Patients were recruited for participation and enrolled in MDH-supported CTCs following written informed consent that was subject to ethical reviews by the Muhimbili University of Health and Allied Sciences (MUHAS) and the Harvard School of Public Health (HSPH) institutional review boards.
Results
Hemoglobin measurements at enrollment were available for a total of 46 430 HIV-infected adults. Of these, 6022 were excluded due to pregnancy. Hence, 40 408 patients met the inclusion criteria for analyzing the prevalence of overall severe anemia. A total of 38 656 participants who had both Hgb and MCV measurements were included for analysis of risk factors for various types of severe anemia.
The baseline characteristics of these 40 408 HIV-infected individuals with Hgb measurements are presented in Table 1. The age range for the study participants was 15 to 68 years, with a median of 37. The majority of study participants were female (66%) at WHO clinical stage III or IV (67%) and those (56%) with a baseline CD4 count of <200 cells/mm3.
Table 1.
Baseline Characteristics of HIV-Infected Adults at Enrollment (N = 40 408).
| Characteristics | N (%) |
|---|---|
| Gender | |
| Female | 26 920 (66) |
| Age group, years | |
| Median (minimum–maximum) | 37 (15–98) |
| <30 | 8922 (22) |
| 30≤40 | 17 460 (44) |
| 40≤50 | 9523 (24) |
| ≥50 | 4074 (10) |
| BMI group, kg/m2 | |
| <18.5 | 10 953 (29) |
| 18.5≤25 | 20 418 (53) |
| 25.0≤30 | 4925 (13) |
| ≥30 | 1947 (5) |
| WHO stage | |
| I | 5407 (14) |
| II | 7136 (18) |
| III | 17 522 (45) |
| IV | 8693 (22) |
| CD4 count, cells/mm3 | |
| <50 | 8045 (21) |
| 50≤200 | 13 259 (35) |
| ≥200 | 16 514 (44) |
| Others | |
| Previous ARV medication use | 4046 (10) |
| On TB treatment | 3654 (10) |
| Oral candidiasis | 2981 (8) |
| Chronic diarrhea | 2457 (7) |
| Wasting | 6628 (18) |
| Serum creatinine >1.2 mmol/L | 4013 (16) |
| Kaposi’s sarcoma | 291 (0.7) |
| On cotrimoxazole prophylaxis | 3636 (11) |
| Anemia categories | |
| Median(minimun–maximum) | 10.4 (1.1–42.9) |
| Hb <11 g/dL | 23 842 (59.0) |
| Hb <8.5 g/dL | 8894 (22.0) |
| Microcytica and Hgb <8.5 g/dL | 5937 (15.4) |
| Macrocyticb and Hgb <8.5 g/dL | 109 (0.3) |
| Normocyticc and Hgb <8.5 g/dL | 2476 (6.4) |
Abbreviation: Hgb, hemoglobin; ARV, antiretroviral; BMI, body mass index; MCV, mean corpuscular volume; TB, tuberculaosis; WHO, World Health Organization.
Microcytic defined as MCV < 80.
Macrocytic defined as MCV > 100.
Normocytic defined as MCV = 80–100.
The overall prevalence of severe anemia (Hgb <8.5 g/dL) was 22%. The prevalence of microcytic severe anemia was 15.4% (accounting for 67% of all severe anemia cases) while that of severe normocytic anemia was 6.4%. Macrocytic severe anemia was rare, with a prevalence of only 0.3%.
Determinants of Severe Anemia
In the multivariate analyses (Table 2), the prevalence of severe anemia increased by 49% among patients with a BMI of <18.5kg/m2 compared to those with a normal BMI (18.5≤25 kg/m2), by approximately 2-fold among patients with WHO clinical stage III, and by 3-fold among patients with WHO clinical stage IV illness, compared to those with WHO clinical stage I. Also, the prevalence of severe anemia increased by 55% among patients with serum creatinine >1.2 mmol/L compared to those with normal renal function. In addition, the prevalence of severe anemia increased by 48% among patients with a CD4 count of <50 cells/mm3 and by 49% among those with a CD4 count of 50 to <200 cells/mm3 compared to those with higher CD4 counts. However, the relationship between CD4 and the risk of severe anemia was nonlinear (Figure 1). The prevalence of severe anemia decreased steeply up to a CD4 count of 400 to 480 cells/mm3 then slowly to 700 to 800 cells/mm3, after which point little additional benefit was observed. Likewise, the prevalence of severe anemia increased by 12% in those with oral candidiasis and by 35% among those with wasting. Male sex, age ≥50 years, being overweight or obese, and previous ARV medication use were protective against severe anemia. The TB treatment, Kaposi’s sarcoma, and use of cotrimoxazole prophylaxis were not significantly associated with the prevalence of severe anemia after controlling for other risk factors.
Table 2.
Risk Factors for the Prevalence of Severe Anemia (Hgb < 8.5 g/dL) among HIV-Infected Adults (N = 40 408).
| Characteristic | Hgb <8.5 N (%) | Univariate RR (95% CI) | P Value | Multivariate RR (95% CI) | P Value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 6562 (24.4%) | Reference | Reference | ||
| Male | 2332 (17.3%) | 0.71 (0.68–0.74) | <.001 | 0.62 (0.59–0.64) | <.001 |
| Age group, years (%) | <.001a | <.001a | |||
| <30 | 1951 (21.9%) | Reference | Reference | ||
| 30≤40 | 4045 (23.2%) | 1.10 (1.06–1.14) | 1.03 (0.99–1.08) | ||
| 40≤50 | 2061 (21.6%) | 0.98 (0.94–1.02) | 1.00 (0.995–1.05) | ||
| ≥50 | 727 (17.8%) | 0.79 (0.74–0.85) | 0.83 (0.77–0.89) | ||
| BMI group, kg/m2 (%) | <.001a | <.001a | |||
| <18.5 | 3824 (34.9%) | 2.03 (1.96–2.10) | 1.49 (1.43–1.55) | ||
| 18.5 to ≤25 | 3709 (18.2%) | Reference | Reference | ||
| 25.0 to ≤30 | 482 (9.8%) | 0.70 (0.67–0.73) | 0.60 (0.55–0.66) | ||
| ≥30 | 123 (6.3%) | 0.28 (0.23–0.33) | 0.43 (0.36–0.51) | ||
| WHO stageb | <.001a | <.001b | |||
| I | 409 (7.6%) | Reference | Reference | ||
| II | 857 (12.0%) | 0.50 (0.47–0.53) | 1.35 (1.21–1.52) | ||
| III | 4056 (23.2%) | 1.10 (1.06–1.14) | 2.20 (1.99–2.43) | ||
| IV | 3175 (36.5%) | 2.03 (1.95–2.10) | 2.77 (2.50–3.08) | ||
| CD4 count, cells/mm3 | <.001a | <.001a | |||
| <50 | 2561 (31.8%) | 1.63 (1.56–1.69) | 1.48 (1.40–1.56) | ||
| 50 to ≤200 | 3468 (26.2%) | 1.31 (1.26–1.36) | 1.49 (1.42–1.56) | ||
| ≥200 | 2242 (13.6%) | Reference | Reference | ||
| Others | |||||
| Previous ARV medication use | 701 (17.4%) | 0.77 (0.72–0.83) | <.001 | 0.82 (0.76–0.87) | <.001 |
| On TB treatmentc | 907 (24.8%) | 1.15 (1.08–1.22) | <.001 | 1.00 (0.94–1.06) | .94 |
| Oral candidiasisc | 1174 (39.4%) | 1.93 (1.83–2.02) | <.001 | 1.12 (1.07–1.17) | <.001 |
| Chronic diarrheac | 821 (33.4%) | 1.59 (1.49–1.68) | <.001 | 1.01 (0.96–1.06) | .74 |
| Wastingc | 2584 (39.0%) | 2.13 (2.05–2.21) | <.001 | 1.35 (1.29–1.41) | <.001 |
| Creatinine >1.2 mmol/L | 1398 (34.8%) | 1.72 (1.64–1.81) | <.001 | 1.55 (1.48–1.62) | <.001 |
| Kaposi’s sarcomac | 83 (28.5%) | 1.30 (1.08–1.56) | .007 | 0.96 (0.82–1.14) | .66 |
| On cotrimoxazole prophylaxis | 906 (24.9%) | 1.20 (1.13–1.27) | <.001 | 0.99 (0.94–1.05) | .76 |
Abbreviation: ARV, antiretroviral; BMI, body mass index; CI, confidence interval; Hgb, hemoglobin; MCV, mean corpuscular volume; RR, relative risk; TB, tuberculosis; WHO, World Health Organization.
P value for trend.
The authors excluded TB treatment, oral candidiasis, chronic diarrhea, wasting, and Kaposi’s sarcoma from the model.
The authors excluded WHO staging from the model.
Figure 1.
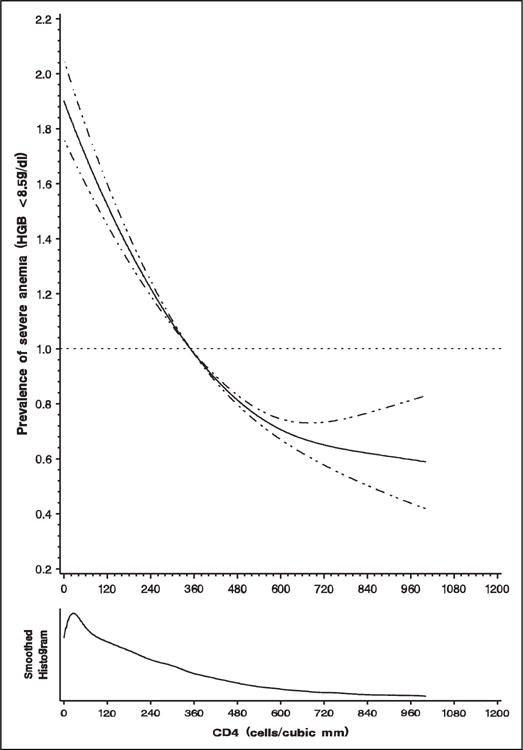
Prevalence of severe anemia (hemoglobin < 8.5 g/dL) in relation to CD4 count (cells/mm3).
Determinants of Severe Microcytic or Severe Normocytic Anemia
Severe microcytic anemia shared many of the same independent risk factors observed for severe anemia overall (Table 3), including low BMI (BMI <18.5 kg/m2 vs BMI 18.5≤25 kg/m2; multivariate relative risk [RR] 1.49; 95% confidence interval [CI] 1.41–1.57; P < .001), advanced HIV disease (stage IV vs stage I 2.50; 95% CI 2.21–2.82; P < .001), low CD4 count (RR 1.34; 95% CI 1.26–1.44; P < .001), oral candidiasis, (RR 1.18; 95% CI 1.17–1.27; P < .001), and raised serum creatinine (RR 1.49; 95% CI 1.39–1.58; P < .001). Protective factors for this subtype of severe anemia were identical to those observed for severe anemia overall.
Table 3.
Risk Factors for the Prevalence of Severe Microcytic Anemia ([(MCV < 80], Hgb <8.5 g/dL) among HIV-Infected Adults (N = 38656).a
| Characteristics | MCV<80; Hgb <8.5 N(%) | Univariate RR (95% CI) | P Value | Multivariate RR (95% CI) | P Value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 4662 (18.2%) | Reference | Reference | ||
| Male | 1275 (9.9%) | 0.55 (0.52–0.59) | <.001 | 0.48 (0.45–0.51) | <.001 |
| Age group, years (%) | <.001b | <.001ba | |||
| <30 | 1389 (16.3%) | Reference | Reference | ||
| 30≤40 | 2753 (16.5%) | 1.15 (1.08–1.21) | 1.02 (0.96–1.08) | ||
| 40≤50 | 1308 (14.4%) | 0.90 (0.84–0.96) | 0.96 (0.89–1.03) | ||
| ≥50 | 396 (10.2%) | 0.66 (0.59–0.74) | 0.67 (0.61–0.75) | ||
| BMI group, kg/m2 (%) | <.001b | <.001b | |||
| <18.5 | 2509 (24.0%) | 2.14 (1.98–2.31) | 1.49 (1.41–1.57) | ||
| 18.5≤25 | 2507 (12.8%) | Reference | Reference | ||
| 25.0≤30 | 349 (7.4%) | 0.69 (0.64–0.75) | 0.62 (0.56–0.69) | ||
| ≥30 | 81 (4.3%) | 0.30 (0.22–0.41) | 0.39 (0.31–0.48) | ||
| WHO stage | <.001b | <.001b | |||
| I | 317 (6.1%) | Reference | Reference | ||
| II | 603 (8.8%) | 0.58 (0.53–0.64) | 1.26 (1.10–1.44) | ||
| III | 2725 (16.2%) | 1.20 (1.13–1.27) | 2.02 (1.79–2.26) | ||
| IV | 2022 (24.5%) | 1.76 (1.65–1.89) | 2.50 (2.21–2.82) | ||
| CD4 count, cells/mm3 | <.001b | <.001b | |||
| <50 | 1632 (21.1%) | 1.54 (1.45–1.64) | 1.34 (1.26–1.44) | ||
| 50≤200 | 2292 (17.9%) | 1.28 (1.21–1.36) | 1.38 (1.30–1.46) | ||
| ≥200 | 1638 (10.3%) | Reference | Reference | ||
| Others | |||||
| Previous ARV medication use | 399 (10.6%) | 0.71 (0.63–0.79) | <.001 | 0.68 (0.62–0.75) | <.001 |
| On TB treatment | 586 (16.9%) | 1.11 (1.01–1.22) | .03 | 1.01 (0.94–1.09) | .77 |
| Oral candidiasis | 727 (27.0%) | 1.84 (1.70–1.99) | <.001 | 1.18 (1.11–1.27) | <.001 |
| Chronic diarrhea | 501 (21.4%) | 1.46 (1.32–1.60) | <.001 | 0.96 (0.88–1.04) | .27 |
| Wasting | 1627 (25.8%) | 2.01 (1.90–2.14) | <.001 | 1.30 (1.23–1.38) | <.001 |
| Creatinine >1.2 mmol/L | 854 (22.1%) | 1.60 (1.48–1.74) | <.001 | 1.49 (1.39–1.58) | <.001 |
| Kaposi’s sarcoma | 39 (14.3%) | 1.14 (1.04–1.25) | .015 | 0.73 (0.55–1.01) | .37 |
| On cotrimoxazole prophylaxis | 545 (15.9%) | 1.13 (1.09–1.16) | <.001 | 0.94 (0.87–1.02) | .12 |
Abbreviation: ARV, antiretroviral; BMI, body mass index; CI, confidence interval; Hgb, hemoglobin; MCV, mean corpuscular volume; RR, relative risk; TB, tuberculosis; WHO, World Health Organization.
The authors excluded TB treatment, oral candidiasis, chronic diarrhea, wasting, and Kaposi’s sarcoma from the model. The authors excluded WHO staging from the model.
P value for trend.
Similar risk factors were found to be associated with normocytic anemia (Table 4). In addition, the risk of severe normocytic anemia increased with age ≥50 years (RR 1.22; 95% CI 1.05–1.40; P < .001), chronic diarrhea (RR 1.22; 95% CI 1.08–1.38; P = .001), Kaposi’s sarcoma (RR 1.93; 95% CI 1.48–2.52; P < .001), and cotrimoxazole prophylaxis (RR 1.16; 95% CI 1.03–1.30; P = .01).
Table 4.
Risk Factors for the Prevalence of Severe Normocytic Anemia (MCV [80–100]; Hgb < 8.5 g/dL) among HIV-Infected Adults (N = 38 656).a
| Characteristics | MCV < 80; Hgb < 8.5 N (%) | Univariate RR (95% CI) | P Value | Multivariate RR (95% CI) | P Value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 1582 (6.2%) | Reference | Reference | ||
| Male | 894 (6.9%) | 1.11 (1.00–1.22) | .036 | 0.89 (0.82–0.96) | .005 |
| Age group, years (%) | <.001b | <.001b | |||
| <30 | 459 (5.2%) | Reference | Reference | ||
| 30 to <40 | 1082 (6.5%) | 1.03 (0.94–1.13) | 1.08 (0.97–1.20) | ||
| 40 to <50 | 629 (6.9%) | 1.10 (0.99–1.22) | 1.12 (0.99–1.26) | ||
| Above 50 | 291 (7.5%) | 1.20 (1.04–1.37) | 1.22 (1.05–1.40) | ||
| BMI group, kg/m2 (%) | <.001b | <.001b | |||
| <18.5 | 1095 (10.6%) | 2.14 (1.98–2.31) | 1.49 (1.36–1.62) | ||
| 18.5 ≤ 25 | 1027 (5.0%) | Reference | Reference | ||
| 25.0 ≤ 30 | 102 (2.2%) | 0.69 (0.64–0.75) | 0.51 (0.41–0.62) | ||
| ≥30 | 37 (2.0%) | 0.30 (0.22–0.41) | 0.56 (0.41–0.78) | ||
| WHO stage | <.001b | <.001b | |||
| I | 70 (1.4%) | Reference | Reference | ||
| II | 207 (3.0%) | 0.43 (0.36–0.51) | 1.75 (1.33–2.31) | ||
| III | 1146 (6.8%) | 1.32 (1.20–1.44) | 3.15 (2.45–4.05) | ||
| IV | 953 (11.5%) | 1.94 (1.74–2.17) | 4.14 (3.20–5.35) | ||
| CD4 count, cells/mm3 | <.001b | <.001b | |||
| <50 | 779 (10.1%) | 1.88 (1.70–2.07) | 1.94 (1.73–2.18) | ||
| 50 to <200 | 1026 (8.0%) | 1.44 (1.32–1.58) | 1.91 (1.72–2.13) | ||
| 200+ | 490 (3.1%) | Reference | Reference | ||
| Others | |||||
| Previous ARV medication use | 224 (5.9%) | 0.92 (0.78–1.08) | .30 | ||
| On TB treatment | 269 (7.8%) | 1.22 (1.06–1.42) | .007 | 0.89 (0.77–1.02) | .10 |
| Oral candidiasis | 334 (11.8%) | 2.07 (1.82–2.35) | <.001 | 1.08 (0.96–1.21) | .19 |
| Chronic diarrhea | 275 (11.7%) | 2.14 (1.87–2.45) | <.001 | 1.22 (1.08–1.38) | .001 |
| Wasting | 785 (12.5%) | 2.46 (2.23–2.71) | <.001 | 1.48 (1.35–1.62) | <.001 |
| Creatinine >1.2 mmol/L | 485 (12.4%) | 2.34 (2.08–2.64) | <.001 | 1.83 (1.65–2.02) | <.001 |
| Kaposi’s sarcoma | 40 (14.7%) | 2.45 (1.79–3.35) | <.001 | 1.93 (1.48–2.52) | <.001 |
| On cotrimoxazole prophylaxis | 304 (8.9%) | 1.51 (1.31–1.74) | <.001 | 1.16 (1.03–1.30) | .01 |
Abbreviation: ARV, antiretroviral; BMI, body mass index; CI, confidence interval; Hgb, hemoglobin; MCV, mean corpuscular volume; RR, relative risk; TB, tuberculosis; WHO, World Health Organization.
The authors excluded TB treatment, oral candidiasis, chronic diarrhea, wasting, and Kaposi’s sarcoma from the model. We excluded WHO staging from the model.
P value for trend.
Discussion
The authors found that severe anemia is common among HIV-infected adults in this large cohort of patients from urban Tanzania. In this study, the overall prevalence of severe anemia was high when compared to the reports from other studies.5,6,22 As in other studies, the prevalence of anemia depended upon several factors including the stage of HIV disease, age, sex as well as the definition of anemia used.4,23 This high prevalence may be due to the moderate to severe immunosuppression in most patients and the relatively high cutoff level the authors used to define severe anemia. The prevalence of cutoff points of 7.5 g/dL and 8 g/dL was found to be 12.4% and 16.8%, respectively. The authors opted to use the cutoff point of 8.5 g/dL, as it is commonly used in Tanzania for referral to district hospitals in Tanzania. So the study confirms the previous findings that as HIV progresses, the prevalence and severity of anemia increase.
The finding that severe microcytic anemia was the most common type of anemia among these Tanzanian study patients is similar to the results of a study done by Johannessen et al6 among 838 HIV-infected adults in rural Tanzania. In this study, 54% of all HIV-infected patients with severe anemia had microcytosis. However, in this study the proportion of microcytosis was higher (67%). The predominance of microcytic anemia in Tanzanian patients with HIV suggests that severe HIV-associated anemia in this setting may be more related to iron deficiency than to HIV-associated chronic inflammation as it is in Western countries.6 Iron deficiency is highly prevalent in Tanzania, accounting for 61% of all anemia.24
In this study, underweight (BMI <18 kg/m2) was associated with severe anemia. Malnutrition significantly contributes to anemia due to insufficient levels of iron, folate, and B12 for erythrocyte production as well as the risk of increased infections. In one study, it was shown that nutrient supplements can correct anemia in HIV-infected adults, though the correction is more quickly achieved in HIV-negative undernourished adults than in those who are HIV positive.25 Surprisingly, the study conducted in rural Tanzania6 did not show a significant association between mild anemia (Hgb < 12 g/d) and low BMI. This may imply that malnutrition is more likely to be a marker of severe anemia rather than mild anemia.6
The observation that oral candidiasis is an independent risk factor for severe anemia among HIV-infected patients is in agreement with the findings from a multicenter cohort study of HIV-positive and -negative female participants from the United States.26 The same findings were reported by Subbaraman et al27 in southern India.
The authors also observed that a history of TB was associated with a lower prevalence of severe anemia among HIV-infected adults. This is contrary to other studies27–29 that clearly showed that TB is an independent risk factor for anemia/severe anemia. However, currently having had TB treatment in the study was not significantly associated with severe anemia. One of the possible explanations for this discrepancy is that, in this study, a history of TB (but not currently having TB) could indicate that these patients received closer care of their medical conditions, including immediate correction of anemia. A study by Lee et al30 revealed that treating TB in some patients with HIV leads to complete resolution of anemia.
Low CD4 count (<200 cells/mm3) was associated with increased risk of anemia. This is consistent with the results from previous studies1,26,27 in which patients who had CD4 count of <200 cells/mm3 had a higher prevalence of severe anemia than those with CD4 count of <200 cells/mm.3
Severe microcytic and normocytic anemia shared many of the same independent risk factors observed for severe anemia. Individuals aged ≥50 years, however, had an increased risk of severe normocytic anemia and a decreased risk of severe microcytic anemia. These findings may be explained by the fact that chronic diseases and cancers, the major contributors to normocytic anemia, are more common at ≥50 years of age.
This analysis also suggests that taking prophylactic cotrimoxazole increases the risk of severe normocytic but not microcytic anemia in HIV-infected adults. This finding differs from the results reported by Sullivan et al4 in the United States. This may be due to the fact that administration of cotrimoxazole can cause drug-associated aplastic anemia or immune-mediated destruction of a specific population of blood cells, thereby leading to the development of normocytic anemia. However, in the case of the US study, the cutoff point for anemia was <10 g/dL which was not categorized by the level of MCV. The finding is more specifically related to the association between taking prophylactic cotrimoxazole and severe normocytic anemia and not mild anemia in general.
The findings that being a male and being overweight or obese were associated with a reduced risk of all 3 categories of anemia (overall severe anemia, severe microcytic anemia, and severe normocytic anemia) are consistent with other previous studies.4,11 Men were less likely than women to be severely anemic, possibly because they are not exposed to menstrual blood loss and multiple deliveries.
The strength of this study lies in its large and diverse patient population in a resource-limited country that allowed us to assess severe anemia prevalence and risk factors with considerable precision. In addition, examining the various risk factors for subtypes of severe anemia based on MCV levels enabled us to identify additional unique factors that are specific to normocytic anemia. In this study, the authors also adjusted for many confounders of severe anemia, thus increasing the validity and the strength of the associations reported.
The findings of this study were limited by a number of factors. First, the authors lacked information on serum iron, ferritin, total iron-binding capacity, folate, cobalamin level, and bone marrow, which would have given us more insight into risk factors for microcytic anemia and micronutrient deficiencies in HIV-infected adults. It is also difficult to establish whether the risk factors preceded the onset of severe anemia, or whether they reflect determinants of survival among HIV-infected individuals with severe anemia. Even with these limitations, this study provides a comprehensive evaluation of the risk factors for severe anemia in HIV-infected patients. This study will serve as a reference for future recommendations to improve care of HIV-infected adults and as a starting point for further etiological studies on the pathophysiology of HIV-related anemia in African adults.
In conclusion, the authors observed a high prevalence of severe anemia among adults infected with HIV. Microcytic anemia was more prevalent, suggesting a major role of iron deficiency. Advanced HIV disease and being underweight were associated with all types of anemia, but age ≥50 years, chronic diarrhea, Kaposi’s sarcoma, and cotrimoxazole were uniquely and exclusively associated with severe normocytic anemia. There is a need to have a focused approach of identifying patients with anemia based on both the hemoglobin and the MCV measurements, since the risk factors for severe anemia differ among various morphological types.
Acknowledgments
The authors thank MDH, Dar es Salaam City Council, MUHAS, HSPH, and the Ministry of Health and Social Welfare in Tanzania for the guidance and collaboration in implementing a national HIV Care and Treatment Program in Dar es Salaam, Tanzania. The authors would also like to thank all the patients and staff of the MDH supported Care and Treatment Program who contributed to these findings.
Funding
The author(s) disclosed the receipt of the following financial support for the research, authorship, and/or publication of this article: HIV clinics in this study were funded through collaboration between the Government of Tanzania and the US President’s Emergency Plan for AIDS Relief (PEPFAR), HSPH, and by the Ministry of Health and Social Welfare. The author was supported by a grant from National Institutes of Health (NIH) as a Fogarty International Clinical Research Fellow through grant number R24 TW007988-05. The research reported work in this publication was supported by the Fogarty International Center of the National Institutes of Health under award number U2RTW008254.
Footnotes
Authors’ Note
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Fangman JJ, Scadden DT. Anemia in HIV-infected adults: epidemiology, pathogenesis and clinical management. Curr Hematol Rep. 2005;4(2):95–96. [PubMed] [Google Scholar]
- 2.O’Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, Fawzi WW. Anemia is an independent predictor of mortality and immunological progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr. 2005;40(2):219–220. doi: 10.1097/01.qai.0000166374.16222.a2. [DOI] [PubMed] [Google Scholar]
- 3.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV Infection: Clinical Impact and evidence-based management strategies. Clin Infect Dis. 2004;38(10):1454–1457. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91(1):301–308. [PubMed] [Google Scholar]
- 5.Zhou J, Jaquet A, Bissagnene E, et al. Short-term risk of anaemia following initiation of combination antiretroviral treatment in HIV-infected patients in countries in sub-Saharan Africa, Asia-Pacific, and central and South America. J Int AIDS Soc. 2012;15(1):5. doi: 10.1186/1758-2652-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannessen A, Naman E, Gundersen SG, Bruun JN. Antiretroviral treatment reverses HIV-associated anemia in rural Tanzania. BMC Infect Dis. 2011;11:190. doi: 10.1186/1471-2334-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wills TS, Nadler JP, Somboonwit C, et al. Anemia prevalence and associated risk factors in a single-center ambulatory HIV clinical cohort. AIDS Read. 2004;14(6):305–310. [PubMed] [Google Scholar]
- 8.Adesina O, Oladokun A, Akinyemi O, Akingbola T, Awolude O, Adewole I. Risk of anaemia in HIV positive pregnant women in Ibadan, south west Nigeria. Afr J Med Med Sci. 2011;40(1):67–73. [PubMed] [Google Scholar]
- 9.Okechukwu A, Gambo D, Okechukwu O. Prevalence of anaemia in HIV-infected children at the University of Abuja Teaching Hospital, Gwagwalada. Niger J Med. 2010;19(1):50–57. doi: 10.4314/njm.v19i1.52480. [DOI] [PubMed] [Google Scholar]
- 10.Johnnessen A, Naman E, Ngowi B, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mugisha JO, Shafer LA, Van der Paal L, et al. Anemia in a rural Ugandan HIV cohort: prevalence at enrolment, incidence, diagnosis and associated factors. Trop Med Int Health. 2008;13(6):788–794. doi: 10.1111/j.1365-3156.2008.02069.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewis SM, Bain BJ, Bates I. Dacie and Lewis Practical Hematology. 10th. Vol. 133. London, UK: Churchill Livingstone Elservier; 2010. pp. 162–163.pp. 206 [Google Scholar]
- 13.Nyesigire RE, Bajunirwe F, Kiwanuka J. Anaemia in HIV-infected children: severity, types and effect on response to HAART. BMC Pediatr. 2012;12:170. doi: 10.1186/1471-2431-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mary Lynn R, Sutin D. Geriatric Medicine and Gerontology. London, UK: Churchill Livingstone, Edinburg; 2003. Blood disorders and their management in old age; pp. 1229–1123. [Google Scholar]
- 15.Massawe SN, Urassa EN, Mmari M, et al. The complexity of pregnanc anemia in Dar-es-Salaam. Gynecol Obstet Invest. 47(2):76–82. doi: 10.1159/000010067. [DOI] [PubMed] [Google Scholar]
- 16.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol. 1986;123(1):174–184. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 17.Skov T, Deddens J, Petersen MR, Endahl L. Prevalence proportion ratios: estimation and hypothesis testing. Int J Epidemiol. 1998;27(1):91–95. doi: 10.1093/ije/27.1.91. [DOI] [PubMed] [Google Scholar]
- 18.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen OS. Theoretical Epidemiology. New York, NY: Wiley; 1985. [Google Scholar]
- 20.Smith M, Patricia L. Splines as a useful and convenient statistical tool. Am Stat. 1979;33(2):57. [Google Scholar]
- 21.Harrell M, Frank E, Lee J, et al. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1998;80(15):1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 22.Mocroft A, Kirk O, Barton SE, et al. Anemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. AIDS. 1999;13(8):943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 23.Cartmell E, Natalal H, Francois I, et al. Nutritional and clinical status of children admitted to the malnutrition ward, Maputo central hospital: a comparison of data from 2001 and 1983. J Trop Pediatr. 2005;51(2):102–105. doi: 10.1093/tropej/fmh088. [DOI] [PubMed] [Google Scholar]
- 24.Tatala S, Svanberg U, Mduma B. Low dietary iron availability is a major cause of anemia: a nutrition survey in the Lindi District of Tanzania. Am J Clin Nutr. 1998;68(1):171–178. doi: 10.1093/ajcn/68.1.171. [DOI] [PubMed] [Google Scholar]
- 25.Simpore J, Zongo F, Kabore F, et al. Nutrition rehabilitation of HIV-infected and HIV-negative undernourished adults utilizing spirulina. Ann Nutr Metab. 2005;49(6):373–380. doi: 10.1159/000088889. [DOI] [PubMed] [Google Scholar]
- 26.Semba RD, Shah N, Klein RS, et al. Prevalence and cumulative incidence of and risk factors for anemia in a multicenter cohort study of human immunodeficiency virus-infected and -uninfected women. Clin Infect Dis. 2002;34(2):260–266. doi: 10.1086/338151. [DOI] [PubMed] [Google Scholar]
- 27.Subbaraman R, Devaleenal B, Selvamuthu P, et al. Factors associated with anemia in HIV-infected individuals in southern India. Int J STD AIDS. 2009;20(7):489–492. doi: 10.1258/ijsa.2008.008370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngowi BJ, Mfinanga SG, Bruun JN, Morkve O. Pulmonary tuberculosis among people living with HIV/AIDS attending care and treatment in rural northern Tanzania. BMC Public Health. 2008;8:341. doi: 10.1186/1471-2458-8-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calis JC, Phiri KS, Faragher EB, et al. Severe anemia in Malawian adults. N Engl J Med. 2008;358(9):888–899. doi: 10.1056/NEJMoa072727. [DOI] [PubMed] [Google Scholar]
- 30.Lee SW, Kang YA, Yoon YS, et al. The prevalence and evolution of anemia in patients with tuberculosis. J Korean Med Sci. 2006;21(6):1028–1033. doi: 10.3346/jkms.2006.21.6.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]


