Abstract
Objective
Cervical cancer is the second most common cancer among women worldwide, and over 85% of cervical cancers occur in developing countries such as China. Lack of resources for nationwide cervical cancer screening in China makes vaccination against oncogenic strains of HPV particularly important. Knowledge of age at sexual debut and sexual behavior is essential prior to implementation of a national vaccination program.
Methods and materials
A cross-sectional epidemiologic survey was conducted across 21 urban and rural sites in China to assess age at sexual debut and sexual behavior. 98.6% of the 11,852 recruited women aged 15–59 years were included in the analysis. Data were collected using a short, nurse-administered questionnaire and analyzed using standard descriptive statistics and survival analysis.
Results
In urban areas, more than ten percent of the 15–19 year old age group were already sexually active at the time of interview; this number increased to nearly 44% in the 20–24 year old age group. Chinese young women with an occupation were more likely to be sexually active compared to female students of the same age, irrespective of area of residence. The crude median sexual debut age for the youngest age group was 17 years, earlier than the sexual debut age reported by older cohorts. Younger age cohorts had an earlier menarche age than older cohorts and were more likely to have more sexual partners than older women, and more likely to have partners with more than one female partner.
Conclusion
There is a trend towards earlier sexual debut and riskier sexual behaviors in younger age groups of Chinese women. These findings suggest that HPV vaccination of women between the ages of 13 and 15 years, before the completion of national compulsory education, is likely to contribute to the prevention of HPV infection and cervical cancer in China.
Keywords: Human papillomavirus, HPV vaccine, Cervical cancer, Sexual debut, China
1. Introduction
Cervical cancer is the second most common cancer among women worldwide, with an estimated 529,000 new cases and 275,000 deaths occurring every year [1]. Over 85% of the cases occur in developing countries, where it accounts for 13% of female cancers [1]. In China, wide disparities in cervical cancer incidence and mortality exist, and are mainly attributable to the large population and the unequal economic development in different areas across the country [2]. To date, China does not have an established nationwide program for cervical cancer screening, and the majority of Chinese women have never been screened. This is particularly true for women in rural areas with poor access to health resources [2,3]. Furthermore, cervical cancer incidence has been increasing in young Chinese women, enlarging the overall burden of the disease in China [4].
Human papillomavirus (HPV) is the most common sexually transmitted virus [5] and is the main cause of cervical cancer [6–8]. HPV genotypes, or strains, are divided into low-risk and high-risk depending on the spectrum of lesions they induce. The low risk strains cause genital warts and low-grade squamous epithelial lesions, while the high risk, or oncogenic, HPV strains cause lesions that may progress to cervical cancer [8,9]. Two prophylactic vaccines against the highest-risk strains of human papillomavirus, Gardasil® (Merck & Co. Inc., Darmstadt, Germany) and Cervarix®, (GlaxoSmithKline Biologicals, Rixensart, Belgium) have been developed and approved in more than 100 countries around the world [10,11]. Numerous studies have been published on the efficacy of the vaccines, as well as issues related to policy and implementation in many countries [10–13]. There is great potential for HPV vaccines to significantly decrease the cervical cancer burden worldwide in generations to come.
Studies of HPV prevalence and incidence indicate that the most consistent predictor of infection is sexual activity, particularly the age of first sexual intercourse, or “sexual debut” [14]. As soon as girls begin having sex, their risk of infection with HPV increases dramatically, so the best vaccination strategy is to reach girls before first exposure. However, to date there are no reliable data on the age of sexual debut in Chinese women, or on the potential differences between women in rural and urban areas, making it difficult to plan for a nationwide HPV vaccination policy. As government approval for the HPV vaccines will likely be forthcoming after the completion of ongoing clinical trials, a comprehensive understanding of the current patterns of age of sexual debut among Chinese women is very important. Such data will help determine the appropriate age for HPV vaccination of Chinese females across the country when the vaccines are adopted in China in the near future. In this paper, we report the average age of sexual debut of Chinese women stratified by urban or rural area and age group.
2. Materials and methods
2.1. Study population and sampling method
This study was a multi-center, cross-sectional, epidemiologic survey. Due to a large population and unbalanced economic development, four-stage sampling was used to obtain a representative study population from seven different geographic regions of China, each of which was stratified into rural or urban based on economic development and population density. In total, seven urban and fourteen rural sites were included in this study. One urban site was selected from each geographic region from the 23 provincial general and women and children's hospitals that had participated in a previous cervical cancer study through the National Cervical Cancer Consortium of China. Two rural sites were selected from each geographic region from the 43 county hospitals enrolled in a national rural cervical cancer screening project supported by the Ministries of Finance and Health (MOF/H). In total, this study included seven urban and fourteen rural sites. In urban areas, subjects were selected by systematic sampling using a pre-assigned sampling interval from outpatients, inpatients, and their female relatives who were physically present at a study hospital on the day of the study. In rural areas, subjects aged 30–59 years were systematically selected from the MOF/H registry in each of the 14 counties using a pre-assigned sampling interval. Subjects aged 15–29 years were selected from the same counties as their older counterparts using cluster sampling; villages within each county were randomly selected, and then every woman of that age in the village was asked to participate in the study. Surveys were discontinued in each age group when the target sample size was reached. The ratio of urban to rural women and the proportion of subjects per age group approximated the Chinese female population aged 15–59 years in the year 2000 [15], which had an urban: rural ratio of 2:3 with 12.5% aged 15–19 years, 11.6% aged 20–24 years, 14.3% aged 25–29 years, 15.4% aged 30–34 years, 13.2% aged 35–39 years, 9.7% aged 40–44 years, 10.3% aged 45–59 years, 7.6% aged 50–54 years and 5.5% aged 55–59 years.
2.2. Informed consent
Before enrollment, all participants were given information about the objectives of the study, benefits and risks associated with participation, and assurance of the confidentiality of the information provided. All participants were given the opportunity to ask questions and receive answers. All participants signed an informed consent form according to international requirements and the study was approved by the Institutional Review Board of the Cancer Institute, Chinese Academy of Medical Sciences (CICAMS).
2.3. Data collection
Female nurses from local hospitals were trained to orally administer a questionnaire to survey the sexual behavior among different age groups of women in various areas of China. The interviewers varied in age and there was no pre-set relation between the interviewer's and interviewee's ages. All subjects were interviewed in a private area to avoid interruptions, and the survey information was recorded anonymously to encourage openness and honesty. Demographic characteristics, age of sexual debut, and information on sexual behaviors as well as reproductive, and contraceptive history were collected. The questionnaire has previously been validated in studies in China [14]. Completed questionnaires underwent regular quality control to check for missing or invalid responses.
2.4. Data management and analysis
Two local staff members separately entered survey information into an electronic database that was periodically sent to the data manager at CICAMS in Beijing. The datasets were double-checked for agreement. Statisticians at CICAMS analyzed the data using the Statistical Package for the Social Sciences (SPSS) version 13.0 (SPSS, Chicago, IL).
Our analysis focused on the age of first sexual activity as well as the proportion of respondents yet to experience sexual activity, by age cohort and area of residence (urban versus rural). Two methods were used to evaluate the age of sexual debut. Firstly, the standard summary statistics method considered only respondents who had already experienced sexual activity in the calculation of median and mean age of sexual debut. The cohort analysis (also termed survival analysis) considered all respondents at the time of interview and was used to also include those participants who had not yet experienced sexual activity [16,17]. The failure event was having a sexual experience. The cohort time was the sexual debut age for those with sexual experience and the current age of the respondent for those without sexual experience. The difference of cohort time between age groups was tested using the log-rank test. Because age was transformed as categorical data, Spearman rank correlation coefficients were used to estimate the relationship between age groups and age of sexual debut, as well as the relationship between age groups and the proportions not yet having experienced sexual activity.
Continuous variables were presented with their means and standard deviations when the data were fit for normal distribution, or medians and interquartile ranges when the data were fit for skewness distribution. The differences of these variables between urban and rural women were tested by t-tests or Z tests. Categorical data were presented with percentages and tested using chi-square tests.
3. Results
The study was carried out from May to December 2009. In total, 11,852 volunteers were interviewed from seven urban and 14 rural areas of China. After excluding the 171 ineligible cases (four were aged less than 15 years, and 167 did not come from the selected study sites), 11 681 women (98.6%) aged 15–59 years were included in the final analyses. See Table 1 for socio-demographic characteristics of the study population.
Table 1.
Demographic characteristics of participants.
| Characteristics | Urban | Rural | Total | Statistics, P |
|---|---|---|---|---|
| Number interviewed (number, %) | 4761 (100.0) | 6920 (100.0) | 11,681 (100.0) | |
| Age (year, Mean ± SD) | 33.9 ±11.6 | 34.0 ±11.9 | 34.0 ±11.8 | Z = −0.45, P = 0.651 |
| Age group (number, %) | ||||
| 15–19 | 594 (12.5) | 940 (13.6) | 1534 (13.1) | χ2 = 12.49, P =0.131 |
| 20–24 | 612 (12.9) | 810 (11.7) | 1422 (12.2) | |
| 25–29 | 705 (14.8) | 945 (13.7) | 1650 (14.1) | |
| 30–34 | 682 (14.3) | 1061 (15.3) | 1743 (14.9) | |
| 35–39 | 649 (13.6) | 889 (12.8) | 1538 (13.2) | |
| 40–44 | 464 (9.7) | 687 (9.9) | 1151 (9.9) | |
| 45–49 | 452 (9.5) | 681 (9.8) | 1133 (9.7) | |
| 50–54 | 356 (7.5) | 531 (7.7) | 887 (7.6) | |
| 55–59 | 247 (5.2) | 376 (5.4) | 623 (5.3) | |
| Marital status (number, %) | ||||
| Unmarried | 1283 (26.9) | 1383 (20.0) | 2666 (22.8) | χ2 =143.50, P < 0.0001 |
| Married | 3317 (69.7) | 5403 (78.1) | 8720 (74.7) | |
| Divorced/widow | 157 (3.3) | 101 (1.5) | 258 (2.2) | |
| Othersa | 4 (0.1) | 33 (0.5) | 37 (0.3) | |
| Level of education (number, %) | ||||
| Primary school and below | 159 (3.3) | 2353 (34.0) | 2512 (21.5) | χ2 =4646.00, P < 0.0001 |
| Junior middle school | 645(13.5) | 2814 (40.7) | 3459 (29.6) | |
| Senior middle school | 1555 (32.7) | 1439 (20.8) | 2994 (25.6) | |
| College and above | 2402 (50.5) | 314 (4.5) | 2716 (23.3) | |
| Career (number, %) | ||||
| Student | 773 (16.2) | 959 (13.9) | 1732 (14.8) | χ2 =12.63, P = 0.0003 |
| Others | 3988 (83.8) | 5961 (86.1) | 9949 (85.2) | |
| Menarche age (year, Mean ± SD) | 12.7 ±1.5 | 13.7 ±1.8 | 13.3 ±1.8 | Z = −32, P < 0.0001 |
SD, standard deviation.
Including cohabitation, separation or remarriage.
3.1. Sexual debut
At the time of interview, the proportion of young women aged 15–19 years who had experienced sexual activity with a partner ranged from 4.5% among rural respondents to 10.8% among urban respondents (Table 2). The proportions increased to 62.2% and 44.4% of rural and urban women, respectively, in the 20–24 year-old age group. After age 34 years, almost all women in both urban and rural areas of China reported having experienced sexual activity with a partner.
Table 2.
Statistics of sexual debut in China, stratified by age group and geographic region.
| Age group (years) | Urban | Rural | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Total number | Without sexual activitya (number, %) | Sexual debut age | Total number | Without sexual activitya (number, %) | Sexual debut age | |||||||
|
|
|
|||||||||||
| Standard analysisb | Cohort analysise | Standard analysisb | Cohort analysise | |||||||||
|
|
|
|
|
|||||||||
| Medianc | Mean±SD | Medianf | Mean | Mediand | Mean±SD | Mediang | Mean | |||||
| 15–19 | 594 | 530 (89.2) | 17 | 16.4±1.3 | – | 18.7 | 940 | 898 (95.5) | 17 | 16.9 ±0.9 | – | 18.8 |
| 20–24 | 612 | 340 (55.6) | 19 | 19.4±1.8 | 23 | 21.8 | 810 | 306 (37.8) | 19 | 19.3 ±1.7 | 21 | 20.8 |
| 25–29 | 705 | 110 (15.6) | 22 | 21.6±2.5 | 22 | 22.7 | 945 | 57 (6.0) | 21 | 20.6 ±2.4 | 21 | 21.1 |
| 30–34 | 682 | 16 (2.3) | 22 | 22.0 ±2.9 | 22 | 22.3 | 1061 | 7 (0.7) | 20 | 20.6 ±2.6 | 20 | 20.6 |
| 35–39 | 649 | 4 (0.6) | 22 | 22.0 ±2.9 | 22 | 22.1 | 889 | 0 (0.0) | 21 | 20.8 ±2.9 | 21 | 20.8 |
| 40–44 | 464 | 2 (0.4) | 22 | 21.7±2.8 | 22 | 21.8 | 687 | 1 (0.1) | 20 | 20.5 ±2.7 | 20 | 20.5 |
| 45–49 | 452 | 5 (1.1) | 22 | 22.0 ±2.6 | 22 | 22.3 | 681 | 5 (0.7) | 21 | 20.7 ±2.6 | 21 | 20.9 |
| 50–54 | 356 | 4 (1.1) | 23 | 22.4 ±2.8 | 23 | 22.7 | 531 | 3 (0.6) | 21 | 21.0±2.8 | 21 | 21.2 |
| 55–59 | 247 | 0 (0.0) | 23 | 22.4 ±3.2 | 23 | 22.4 | 376 | 7 (1.9) | 20 | 20.3 ±3.1 | 20 | 21.0 |
| Total | 4761 | 1011 (21.1) | 22 | 21.7±2.9 | 22 | 22.5 | 6920 | 1284 (18.6) | 20 | 20.5 ±2.7 | 21 | 21.1 |
SD, standard deviation.
rs = 0.63, P < 0.0001.
Participants with at least one sexual experience.
rs = 0.21, P < 0.0001.
rs= 0.10, P < 0.001.
All participants in age group.
Log Rank Test, χ2 =59.94, P < 0.0001.
Log Rank Test, χ2 =103.94, P < 0.0001.
Age at sexual debut was obtained using standard frequency calculation and cohort analysis techniques by age group and stratified by rural or urban area (also shown in Table 2). The crude median age of first sexual activity with a partner was 17 years in the youngest age group (15–19 years) and 19 years in the 20–24 year age group for both rural and urban women. Starting with the 25–29 year-old age group, the crude median age of first sexual activity was 1–3 years younger for rural women than for urban women, with the largest difference appearing in the 55–59 year-old age group with rural women in this age cohort experiencing sexual debut 3 years earlier than their urban counterparts. As expected, crude median age at first sexual activity was lower or equal to that obtained by cohort analysis for all age groups since women who had not yet experienced sexual activity were included.
The cumulative probability of sexual activity at each year of age among young women aged 15–19 and 20–24 years old was calculated for women in both urban and rural areas (Fig. 1). In urban areas, the probability of sexual activity among women aged 15–19 years was slightly higher than among those aged 20–24 years, whereas the relationship was inversed in rural areas. However, the value of this calculation is limited, because 22.3% of the 15–19 year-old women were not yet 19 years old at the time of interview, and the probability of sexual activity at a specific age could not be calculated in women who had not yet reached that age.
Fig. 1.
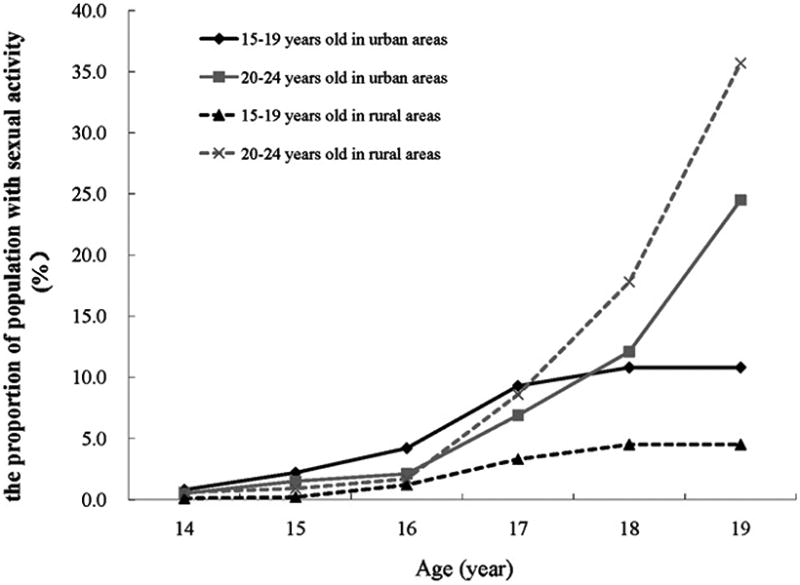
The proportion of the population with sexual activity in urban and rural women aged 15–19 and 20–24 years old.
Table 3 shows the prevalence of sexual activity among the two youngest age groups surveyed, broken down into students and non-students. While only 0.6% of 15–19 year-old students in rural areas and 4.0% in urban areas were sexually active, among non-students of the same age these percentages were 23.1% in rural areas and 38.8% in urban areas. In the 20–24 year-old age group, the difference between students and non-students was even more pronounced, with a 71.7% difference between students and women of other occupations in rural areas, and a 46.7% difference for urban women. This suggests that as Chinese women quit school they were more likely to become sexually active; in the 15–19 year-old age group, this affected a higher percentage of urban women, but in the 20–24 year-old age group, more rural workers had started sexual activity.
Table 3.
Proportion of study participants aged 15–24 years old experiencing sexual activity, stratified by occupation.
| Age group (years) | Region | Students | Other occupations | Total | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Number (%) | Number having sexual activity (%) | Number (%) | Number having sexual activity (%) | Number (%) | Number having sexual activity (%) | ||
| 15–19 | Urban | 478 (80.5) | 19 (4.0) | 116 (19.5) | 45 (38.8) | 594 (100.0) | 64 (10.8) |
| Rural | 780 (83.0) | 5 (0.6) | 160 (17.0) | 37 (23.1) | 940 (100.0) | 42 (4.5) | |
| Total | 1258 (82.0) | 24 (1.9) | 276 (18.0) | 82 (29.7) | 1534 (100.0) | 106 (6.9) | |
| 20–24 | Urban | 226 (36.9) | 34 (15.0) | 386 (63.1) | 238 (61.7) | 612 (100.0) | 272 (44.4) |
| Rural | 168 (20.7) | 9 (5.4) | 642 (79.3) | 495 (77.1) | 810 (100.0) | 504 (62.2) | |
| Total | 394 (27.7) | 43 (10.9) | 1028 (72.3) | 733 (71.3) | 1422 (100.0) | 776 (54.6) | |
| Total | Urban | 704 (58.4) | 53 (7.5) | 502 (41.6) | 283 (56.4) | 1206 (100.0) | 336 (27.9) |
| Rural | 948 (54.2) | 14 (1.5) | 802 (45.8) | 532 (66.3) | 1750 (100.0) | 546 (31.2) | |
| Total | 1652 (55.9) | 67 (4.1) | 1304 (44.1) | 815 (62.5) | 2956 (100.0) | 882 (29.8) | |
3.2. Sexual behavior
We collected information on sexual behavior characteristics, including age at menarche, number of lifetime sexual partners, contraceptive method, number of pregnancies, abortions and deliveries, extra-marital sexual activity of the woman's partner, and genital cleaning practices. Our data show that age at menarche has been steadily decreasing in China. The youngest respondents reported a mean age at menarche of 12.2 years for urban girls and 12.8 years for urban girls. Women aged 55–59 years reported that they did not start menstruating until a mean age of 13.4 years for urbanites and 14.8 years for rural dwellers (Fig. 2). At the time of interview, 84–93% of respondents had only one lifetime sexual contact. A greater proportion of urban women (16%) had more than one lifetime sexual partner compared to rural women (6.6%) (χ2 = 213.30, P < 0.0001). A higher proportion of young urban women had multiple sexual partners than older urban women (see Figure, Supplemental Digital Content 1). In each consecutively younger age group, the percentage of women having multiple sexual partners increased significantly for urban women (P < 0.0001). The proportions of urban and rural women acknowledging that their partner had extra-marital relationships were only slightly different (4.2% versus 3.3%, χ2 = 5.47, P = 0.020), but more urban than rural women were unsure whether their husband was sexually promiscuous (27.4% versus 14.7%, χ2 = 231.20, P < 0.0001). Women of younger age groups reported that their husbands had extra-marital relationships more often than women from older age groups. When asked about contraception, a high proportion of urban women reported having used condoms and IUDs, while a higher proportion of rural women reported having used IUDs and tubal ligations. Oral contraceptives were used more frequently by urban women than rural women (6.8% versus 2.8%, χ2 = 87.41, P < 0.0001). Urban women were more likely to clean the perineum both before and after sexual intercourse, and the youngest age group in both urban and rural areas was less likely to clean the perineum after sexual intercourse than older women. For data on sexual behaviors, see Table, Supplemental Digital Content 2.
Fig. 2.
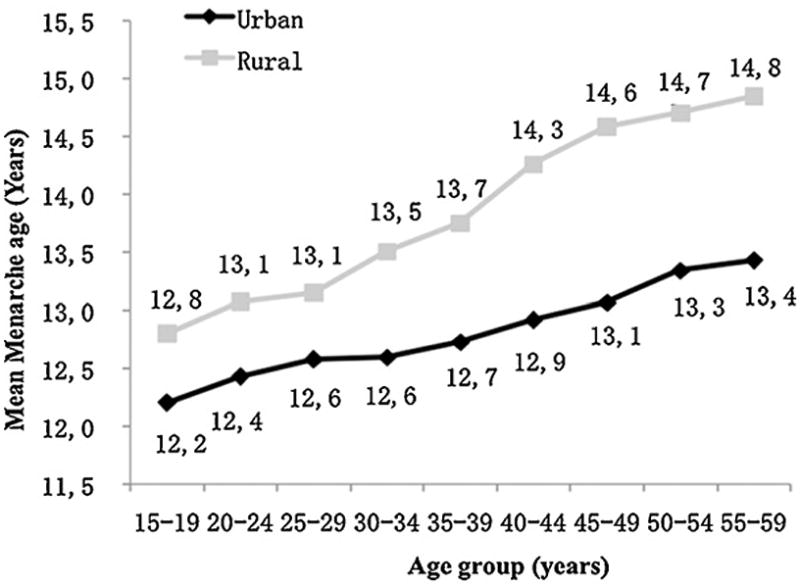
The trend of menarche age by age group and geographic region.
4. Discussion
The percentage and behavior of sexually active young people influences the circulation of HPV and the incidence of infections [18,19]. Our data show that the age of sexual debut among Chinese women was earlier in younger age groups than it was for older age groups in both urban and rural women. Beginning sexual intercourse at younger ages than in the past puts young women at risk for HPV earlier in life and increases the pool of at-risk sexually active young people. Furthermore, the trends in sexual behavior exhibited by young Chinese women, including having more sexual partners, having sexual partners who have multiple partners, and not cleaning the genitals after sexual intercourse, reveal that younger women have a less inhibited and more risky sexual lifestyle than previous generations. Condom use was not decreased among young Chinese women compared to older women as in some European studies [20], which is possibly attributable to governmental family planning policies. However, while consistent condom use does appear to reduce the risk of cervical HPV infection in young women [16,21], condoms are not fully protective against HPV acquisition since the virus can be transmitted through non-penetrative sexual contact [22,23]. Together, earlier sexual debut and riskier sexual behavior may indicate that the human papilloma virus enjoys a greater circulation in younger Chinese women than in older women. This in turn increases the probability of infection by high-risk genotypes and ultimately the risk of cervical cancer [20].
While the median age at sexual debut for younger cohorts was the same for urban and rural women, our data suggest that this has not always been the case. As the cohorts increased in age, the sexual debut median age of rural women was 1–3 years earlier than that of urban women in most cohorts above age 25 years, and was 3 years earlier in the 55–59-year-old cohort. The mean ages, determined by cohort analysis and thus perhaps more reflective of actual sexual debut age than the summary statistics method, diverged in the 20–24-year-old age group, and a 1–2 year difference was maintained in all older cohorts. This effect in older cohorts could be due to the fact that traditionally, rural Chinese women married earlier in life than urban women, and thus commenced sexual activity earlier. This effect could be diminishing now as rural women marry later [24] and as urban women havemore risky and earlier sexual activity, including sexual partners before marriage, a trend which our data show is true for both urban and rural dwellers but is stronger among urbanites (see Table, Supplementary Digital Content 2). Thus, two modern trends combined with traditional patterns have resulted in a similar age of sexual debut among young cohorts of rural and urban Chinese women.
Since the current HPV vaccines are prophylactic and not therapeutic [25], vaccination of women before sexual debut is the most cost-effective strategy to prevent cervical cancer. Our findings suggest that HPV vaccination between the ages of 13 and 15 years should be considered in China. Although the current age of sexual debut was higher than 15 years, the downward trend suggests that sexual debut will occur even earlier for girls younger than those included in the study. The downward trend of age at menarche also suggests that girls might begin sexual activity earlier in coming years.
Furthermore, as shown in Table 3, eighteen percent of both urban and rural 15–19-year-olds were no longer in school, and 29.7% of that group were sexually active (compared to the 1.9% of students of the same age who were sexually active). Vaccinating girls before age 15 years would prevent approximately 58,000 cases of CIN3 or cervical cancer in the lifespan of that cohort, based on the following parameters: the HPV acquired infection rate in three years after sexual debut is 50% [26], the prevalences of HPV 16 and 18 among HPV positive women are 23% and 9%, the persistent prevalences of HPV 16 and 18 are 29.4% and 22%, and the progression rates to CIN3 or worse of persistent HPV 16 and 18 are 46% and 27% during 12-year follow-up [27]. In the short-term, urban girls would benefit more from early vaccination since a higher percentage of urban 15–19-year-olds started sexual activity after leaving school, but eventually the benefit would become equal since in the next oldest age cohort of workers, more rural girls had started sexual activity. Another element to consider is that rural women have less consistent access to healthcare, and vaccinating them while still in school would ensure access to HPV vaccination.
It is difficult to compare the results of our survey with published data as there has not been a nationwide survey of sexual debut and behavior in China in over 13 years. A survey of around 10,000 women published in 1997 [28] used different age categories for analysis, but around 76% of college-age women reported becoming sexually active between the ages of 17and 22 years, which is consistent with our data. The same study reported a median age of menarche of 13 years, slightly higher than what was found in our study, suggesting it is indeed decreasing in China. Other more recent reports do not stratify age of sexual debut or sexual behavior data by age group, limiting the comparability with our study [29,30].
One of the strengths of this survey was that it covered a large population from different areas of China, providing a high level of representativeness. Another strength was that the age of sexual debut was calculated by two established methods – a standard summary statistics calculation that enabled comparison with previously published data, and a cohort analysis technique that accounted for censored subjects and the fact that not all subjects contributed to the data in the same way (i.e., the older the subject, the more information was provided). Finally, the stratification into urban and rural groups allowed comparisons that will be important for the customization of vaccination program design and implementation in China. Limitations included the sensitive nature of the topic, and potential information bias resulting from self-reporting. Poor recall of their age of sexual debut among older women could explain some of the differences found between young and older cohorts. Findings may also have been distorted by different definitions of sexual intercourse, as the survey did not specify between genital–genital, oral–genital, and anal–genital sex. Different types of sexual activity can affect HPV transmission; for example, the risk of acquiring HPV through oral-genital sex is very low [21]. However, this distortion was likely small, if present at all, due to very traditional ideas of sexual behavior in China. It is also important to keep in mind that for future Chinese HPV vaccination campaigns, our sexual debut data should be considered along with HPV prevalence and genotype information, which is not included in this study.
Cervical cancer prevention through prophylactic vaccination against oncogenic HPV types should target adolescents before the onset of sexual activity. The results of this survey support vaccination of Chinese girls between the ages of 13 and 15 years, before girls leave school and enter the workplace. Our data show that younger cohorts tend to start earlier and will acquire more sexual partners than older cohorts; thus early vaccination is clearly recommended. Vaccinating well before the median age of sexual debut will ensure that the majority of adolescents are vaccinated before exposure to HPV to most effectively decrease their risk of developing cervical cancer. This timing will ultimately have a larger effect on rural girls since a higher percentage of them commence sexual activity after entering the workforce and they are consistently harder to reach with healthcare interventions. Additional research is needed to determine the most appropriate vaccine delivery strategy, but this study lays the groundwork for future public health HPV vaccination campaigns in China.
Supplementary Material
Acknowledgments
The project was made possible by the efforts of twenty-one collaborative hospitals and by the participation of women from Beijing, Nanjing, Xinjiang, Liaoning, Tianjin, Shanxi, Inner Mongolia, Shandong, Hunan, Henan, Fujian, Guangdong, Jiangxi, Yunnan, Chongqing, and Gansu, as well as by funding from GlaxoSmithKline Biologicals in Rixensart, Belgium. Cervarix is a registered trademark of the GlaxoSmithKline group of companies, and Gardasil is a registered trademark of the Merck & Co. Inc. (Darmstadt, Germany). This work was partially supported by the National Institutes of Health, Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental & Craniofacial Research, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases Health, and NIH Office of Women's Health and Research through the International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act.
Footnotes
Conflict of interest: One author, Dr. Schmidt, works for GlaxoSmithKline Biologicals (Rixensart, Belgium); no other authors on this manuscript have any conflicts of interest related to this work.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.canep.2012.01.009.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 doi: 10.1002/ijc.25516. E-pub. [DOI] [PubMed] [Google Scholar]
- 2.Shi JF, Qiao YL, Smith JS, Dondog B, Bao YP, Dai M, et al. Epidemiology and prevention of human papillomavirus and cervical cancer in China and Mongolia. Vaccine. 2008;26(Suppl. 12):M53–9. doi: 10.1016/j.vaccine.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Zhang YZ, Ma JF, Zhao FH, Xiang XE, Ma ZH, Shi YT, et al. Three-year follow-up results of visual inspection with acetic acid/Lugol's iodine (VIA/VILI) used as an alternative screening method for cervical cancer in rural areas. Chin J Cancer. 2010;29(1):4–8. doi: 10.5732/cjc.009.10687. [DOI] [PubMed] [Google Scholar]
- 4.Zhao FH, Hu SY, Zhang SW, Chen WQ, Qiao YL. Cervical cancer mortality in 2004–2005 and changes during last 30 years in China. Zhonghua Yu Fang Yi XueZaZhi. 2010;44(5):408–12. [PubMed] [Google Scholar]
- 5.Burchell AN, Winer RL, de Sanjose S, Franco EL. Epidemiology and transmissiondynamics of genital HPV infection. Vaccine. 2006;24(Suppl. 3):S52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 7.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.zurHausen H. Oncogenic DNA viruses. Oncogene. 2001;20(54):7820–3. doi: 10.1038/sj.onc.1204958. [DOI] [PubMed] [Google Scholar]
- 9.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. Proof of Principle Study Investigators. A controlled trial of human papillomavirus type 16 vaccine. N Engl J Med. 2002;347(21):1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 10.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomized double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 11.Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, Bosch FX, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst. 2004;96:604–15. doi: 10.1093/jnci/djh104. [DOI] [PubMed] [Google Scholar]
- 12.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 13.Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51:1–78. [PubMed] [Google Scholar]
- 14.Li J, Li LK, Ma JF, Wei LH, Niyazi M, Li CQ, et al. Knowledge and attitudes about human papillomavirus(HPV) and HPV vaccines among women living in metropolitan and rural regions of China. Vaccine. 2009;27(8):1210–5. doi: 10.1016/j.vaccine.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Ministry of Health (MOH) Chinese Health Statistics Yearbook. Beijing, China: Peking Union Medical Press; 2007. p. 337. [Google Scholar]
- 16.Crochard A, Luyts D, di Nicola SM, Goncalves AG. Self-reported sexual debut and behavior in young adults aged 18–24 years in seven European countries: implications for HPV vaccination programs. Gynecol Oncol. 2009;115:S7–14. doi: 10.1016/j.ygyno.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Zaba B, Pisani E, Slaymaker E, Boerma JT. Age at first sex: understanding recent trends in African demographic surveys. Sex Transm Infect. 2004:ii28–35. doi: 10.1136/sti.2004.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sellors JW, Karwalajtys TL, Kaczorowski J, Mahony JB, Lytwyn A, Chong S, et al. Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ. 2003;168(4):421–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Hildesheim A, Herrero R, Wacholder S, Rodriquez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16, 18 L1 virus-like particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 20.Panatto D, Amicizia D, Lugarini J, Sasso T, Sormani MP, Badolati G, et al. Sexual behavior in Ligurian (Northern Italy) adolescents and young people; suggestions for HPV vaccination policies. Vaccine. 2009;27:A6–10. doi: 10.1016/j.vaccine.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 21.Winer RL, Hughes JP, Feng Q, O'Reilly S, Kiviat NB, Holmes KK, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 22.Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ. 2004;82:454–61. [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez BY, Wilkens LR, Zhu X, Thompson P, McDuffle K, Shvetsov YB, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–94. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin XY, Li SZ, Feldman MW. Marriage form and age at first marriage: a comparative study in three counties in contemporary rural China. Soc Biol. 2005;52:18–46. doi: 10.1080/19485565.2002.9989097. [DOI] [PubMed] [Google Scholar]
- 25.Harper DH, Franco EL, Wheeler CM, Mosicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4–5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 26.Moscicki AB. Impact of HPV infection in adolescent populations. J Adolesc Health. 2005;37(Suppl. 6):S3–9. doi: 10.1016/j.jadohealth.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102(19):1478–88. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Ng ML, Zhou LP, Haeberle EJ. Sexual behavior in modern China: report on the nation-wide survey of 20,000 men and women. New York: Continuum; 1997. p. 586. [Google Scholar]
- 29.Gu C, Chan CWH, Twinn S. How sexual history and knowledge of cervical cancer and screening influence Chinese women's screening behavior in mainland China. Cancer Nurs. 2010;33(6):445–53. doi: 10.1097/NCC.0b013e3181e456dc. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Wei C, Buchholz ME, Martin MC, Smith BD, Huang ZJ, et al. Prevalence and risks for sexually transmitted infections among a national sample of migrants versus non-migrants in China. Int J STD AIDS. 2010;21:410–5. doi: 10.1258/ijsa.2009.008518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


