Abstract
The targeting specificity of tissue-specific Cre-recombinase transgenes is a key to interpreting phenotypes associated with their use. The Ocn-Cre and Dmp1-Cre transgenes are widely used to target osteoblasts and osteocytes, respectively. Here, we used high-resolution microscopy of bone sections and flow cytometry to carefully define the targeting specificity of these transgenes. These transgenes were crossed with Cxcl12gfp mice to identify Cxcl12-abundant reticular (CAR) cells, which are a perivascular mesenchymal stromal population implicated in hematopoietic stem/progenitor cell maintenance. We show that in addition to osteoblasts, Ocn-Cre targets a majority of CAR cells and arteriolar pericytes. Surprisingly, Dmp1-Cre also targets a subset of CAR cells, in which expression of osteoblast-lineage genes is enriched. Finally, we introduce a new tissue-specific Cre-recombinase, Tagln-Cre, which efficiently targets osteoblasts, a majority of CAR cells, and both venous sinusoidal and arteriolar pericytes. These data show that Ocn-Cre and Dmp1-Cre target broader stromal cell populations than previously appreciated and may aid in the design of future studies. Moreover, these data highlight the heterogeneity of mesenchymal stromal cells in the bone marrow and provide tools to interrogate this heterogeneity.
Keywords: BONE HISTOMORPHOMETRY, GENETIC ANIMAL MODELS, OSTEOBLASTS, STROMAL/STEM CELLS
Introduction
The bone marrow microenvironment contains a heterogeneous population of stromal cells that contribute to the regulation of hematopoiesis. Identifying these stromal cells and the signals they generate has important clinical implications for a number of hematopoietic diseases.(1,2) Mesenchymal stromal cells implicated in the maintenance of hematopoietic stem cells (HSCs) include endothelial cells, osteoblasts, CXCL12-abundant reticular (CAR) cells, mesenchymal stem cells (MSCs), and arteriolar pericytes.(3–6) The use of tissue-specific Cre-recombinase transgenes to delete genes of interest from defined stromal cell populations is an established and important technique in the field. Rigorously defining the targeting specificity of the Cre-recombinase transgenes is a key to the interpretation of such experiments.
Two Cre-recombinase transgenes that are commonly used to target osteolineage cells are Ocn-Cre and Dmp1-Cre. Osteocalcin (Ocn, Bglap) is a secreted protein implicated in bone and glucose metabolism.(7) Cell culture and in situ expression studies show that OCN expression is mostly limited to osteoblasts and osteocytes.(7,8) This has led to the widespread use of Ocn-Cre transgenes to specifically target osteoblasts and osteocytes.(9,10) Dentin matrix acidic phosphoprotein 1 (Dmp1) is expressed in odontoblasts, preosteocytes, and osteocytes.(11,12) Indeed, a transgene containing an 8-kb regulatory region of Dmp1 linked to GFP results in osteocyte-specific GFP expression in the bone marrow.(13) These data have led to the widespread use of Dmp1-Cre transgenes to specifically target osteocytes, although targeting of some osteoblasts also has been observed.(12,14,15) Further, a study by Kalajzic and colleagues(12) showed that a 10-kb Dmp1-Cre transgene targeted both osteoblasts and osteocytes, as well as a small population of undefined cells in the bone marrow.
In the present study, we used high-resolution microscopy of bone sections and flow cytometry to carefully define the targeting specificity of Ocn-Cre and Dmp1-Cre in the bone marrow. We showed that both the Ocn-Cre and Dmp1-Cre transgenes target a much broader population of bone marrow stromal cells than previously appreciated. We also characterized for the first time the spectrum of bone marrow stromal cells targeted by a Tagln-Cre transgene. We show that Tagln-Cre efficiently targets osteoblasts and perivascular stromal cells, but not endothelial cells.
Materials and Methods
Mouse strains
Ai9 (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J)(16) mice and Tagln-Cre (B6.129S6-Taglntm2(cre)Yec/J) mice were obtained from The Jackson Laboratories (Bar Harbor, ME, USA).(17) Ocn–Cre mice were a gift from Thomas Clemens (Johns Hopkins University, Baltimore, MD, USA).(18) Cxcl12gfp mice were a gift from Takashi Nagasawa (Kyoto University, Kyoto, Japan),(19) and Dmp1-Cre mice (containing the 9.6-kb murine Dmp1 promoter) were a gift from Roberto Civitelli (Washington University, St. Louis, MO, USA).(20) All mice used in this study were 8 to 10 weeks old. Both male and female mice were used equally in these studies. Genotyping primers are listed in Supporting Table 1. Mice were maintained under specific pathogen free (SPF) conditions, and all experimental procedures were performed according to methods approved by the Animal Studies Committee at Washington University.
Flow cytometry
Bone marrow cells were harvested from mouse femurs by first uncapping the ends of the bone and then centrifuging at 3300 × g for 5 min to expel the bone marrow contents. These cells were then digested with 1.67 mg/mL of type II collagenase (Worthington Biochemical, Lakewood, NJ, USA) in phosphate-buffered saline (PBS) for 12 min at 37°C. Of note, the majority of osteoblasts are not recovered using this procedure (Supporting Fig. 1). The following antibodies were used: CD45 (30-F11), CD31 (390), and Ter119 (TER-119). Cells were analyzed on a Gallios flow cytometer (Beckman Coulter, Pasadena, CA, USA), and data analysis was done using FloJo version 10.0.7 software (TreeStar/FlowJo LLC, Ashland, OR, USA).
To sort Dmp1-Cre–targeted or Dmp1-Cre–non-targeted CAR cells, we first isolated platelet-derived growth factor receptor-beta (PDGFRβ)-positive stromal cells from the bone marrow of Dmp1-Cre ROSA26Ai9/+ Cxcl12gfp/+ mice using the AutoMacs Pro Separator system (Miltenyi Biotec, San Diego, CA, USA) and a biotinylated anti-PDGFRβ antibody (APB5). Cells were incubated with antibodies against Gr-1 (RB6-8C5), PDGFRβ (APB5), CD45 (30-F11), CD31 (390), and Ter119 (TER-119) and then incubated with brilliant violet 421-conjugated Streptavidin (405225; BioLegend, San Diego, CA, USA). CAR cells were identified as Cxcl12-GFPbright PDGRRβ+ Gr1− CD45− CD31− Ter119− cells. Dmp1-Cre–targeted CAR cells were tdTomatohigh. Cells were sorted using a MoFlo high-speed flow cytometer (Dako Cytomation, Carpinteria, CA, USA). All antibodies were obtained from eBioscience (San Diego, CA, USA), unless otherwise noted.
Immunostaining of bone sections
Mouse hindlimbs were fixed in PBS containing 4% paraformaldehyde, pH 7.4, for 24 hours at 4°C. Bones were then decalcified in PBS containing 14% EDTA, pH 7.4, for 7 days at 4°C. Following incubation in PBS containing 30% sucrose for 24 hours at 4°C, bones were embedded in Optimal Cutting Temperature Compound (Sakura Finetek, Torrance, CA, USA). These tissue blocks were cut into 12-μm sections using a Leica Cryo-Jane system (Leica Biosystems, Wetzlar, Germany). For immunostaining, the slides were blocked with 10% donkey serum, diluted in 0.1M Tris-Cl pH 7.5, 150 mM NaCl, and 0.1% Tween 20 (TNT) buffer for 1 hour at room temperature. Following blocking using the Avidin/Biotin Blocking Kit (SP-2001; Vector Laboratories, Burlingame, CA, USA), slides were then incubated in primary antibody overnight at 4°C and, where applicable, they were incubated with secondary antibody for 1 hour at room temperature. The following antibodies were used: rabbit anti-NG2 (AB5320; EMD Millipore, Billerica, MA, USA), rat anti-Sca1 (557403; BD Biosciences, San Jose, CA, USA), goat anti-VECadherin (AF1002; R&D Systems, Minneapolis, MN, USA), mouse anti-αSMA (1A4; Sigma Aldrich, St. Louis, MO, USA); AlexaFluor 488-conjugated donkey anti-rat IgG (Jackson ImmunoResearch, West Grove, PA, USA); DyLight649-conjugated donkey anti-rat IgG (Jackson ImmunoResearch), and biotin-conjugated donkey anti-goat IgG (Jackson ImmunoResearch). In some cases, slides were then incubated with streptavidin-DyLight 649 (Jackson ImmunoResearch) for 1 hour at room temperature. Finally, slides were mounted with ProLong Gold antifade reagent with DAPI (Life Technologies, Inc., Grand Island, NY, USA). Images were acquired with a LSM 700 microscope (Carl Zeiss Microscopy, Peabody, MA, USA) and processed using Volocity software (PerkinElmer, Waltham, MA, USA).
For hematoxylin and eosin (H&E) staining, bone sections were air dried for 1 hour and then incubated with Hematoxylin Gill #3 (GHS316; Sigma-Aldrich) for 5 min followed by incubation with Eosin (HT110132; Sigma-Aldrich) for 3 min. Sections were then fixed by serial 5-min incubations in 50%, 70%, 95%, and 100% ethanol, followed by a 5-min incubation in xylene. Finally, slides were mounted with Permount mounting medium (Fisher Chemical, Pittsburgh, PA, USA). Images were acquired with a LSM 700 microscope (Carl Zeiss Microscopy).
RNA expression profiling
RNA was purified from sorted CAR cells using the Qiagen RNeasy Micro Kit (74004; Qiagen, Valencia, CA, USA). Libraries were generated using the NuGen Pico SL kit (NuGEN Technologies, San Carlos, CA, USA) and then hybridized to Affymetrix Mouse Gene 1.0 ST arrays (Affymetrix, Santa Clara, CA, USA). Gene set enrichment was performed using the GSEA software (Broad Institute, Cambridge, MA, USA). Differences in gene expression were determined using Significance Analysis of Microarrays (SAM; Stanford University, Stanford, CA, USA). Expression data has been submitted to Gene Expression Omnibus, record number GSE81399.
Statistical analyses
Unpaired t test was used to evaluate the significance of differences between two groups. All data are presented as mean ± SD.
Results
Ocn-Cre targets osteoblasts, a majority of CAR cells, and arteriolar pericytes
To characterize the targeting specificity of Ocn-Cre in postnatal mouse bones, we generated Ocn-Cre ROSA26Ai9/+ mice and Ocn-Cre ROSA26Ai9/+ Cxcl12gfp/+ mice. The Cxcl12gfp/+ transgene allows for the identification of CXCL12-GFPbright (CAR) cells, which are perivascular stromal cells in the bone marrow implicated in HSC maintenance.(3) The ROSA26Ai9/+ transgene allows for the identification of Ocn-Cre–targeted tdTomato+ cells. Immunostaining of the bone sections confirmed that the Ocn-Cre transgene efficiently targets osteoblasts (Fig. 1A, B; Supporting Fig. 2).(18) Surprisingly, we also observed that Ocn-Cre targets a substantial fraction of CXCL12-GFPbright cells (Fig. 1A, C). Of note, as expected, no tdTomato+ CAR cells were detected in control (Cxcl12gfp/+) mice (Supporting Fig. 3). Flow cytometry showed that Ocn-Cre targets 72.2% ± 4.0% (n = 3 mice) of CXCL12-GFPbright cells (Fig. 1D). To assess targeting of arteriolar pericytes, we stained bone sections from wild-type mice with antibodies against alpha-smooth muscle actin (αSMA) and NG2 (Fig. 2A, B). In these assays, arteriolar endothelial cells were identified by Sca1,(6) which is also expressed on hematopoietic stem/progenitor cells but not on CAR cells.(4) Whereas αSMA staining was limited to a subset of arteriolar pericytes, NG2 staining was observed in all arteriolar pericytes (Fig. 2A, B). Accordingly, all αSMA-positive arteriolar pericytes co-expressed NG2, while only 56.9% ± 11.1% (n = 3 mice) of NG2-positive arteriolar pericytes co-expressed αSMA. Immunostaining of bone sections from Ocn-Cre ROSA26Ai9/+ mice showed that Ocn-Cre targets 72.2% ± 13.3% (n = 3 mice) of NG2-postive arteriolar pericytes (Fig. 2C). Thus, in addition to osteoblasts, Ocn-Cre targets the majority of CAR cells and arteriolar pericytes in mice.
Fig. 1.
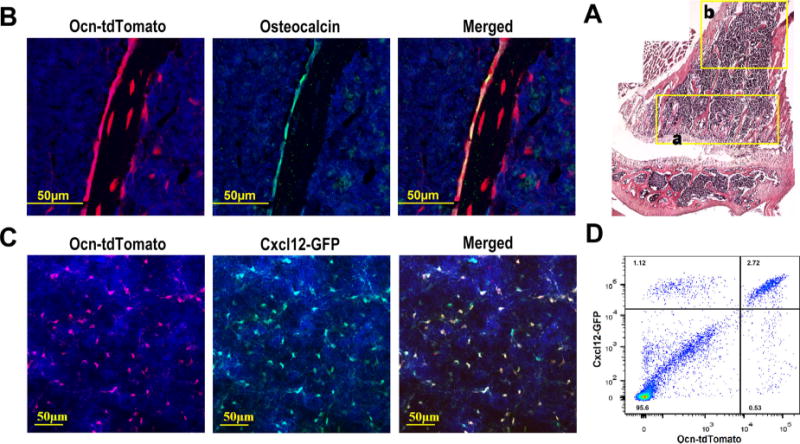
Ocn-Cre targets osteoblasts and a majority of CAR cells. (A) Composite image of H&E stained sections from the femur of an Ocn-Cre ROSA26Ai9/+ mouse. (B) Representative photomicrographs of the metaphyseal region (region “a” in A) of a femur section stained for osteocalcin (green) to mark osteoblasts and DAPI (blue) to highlight nuclei; cells that had undergone Cre-mediated recombination express tdTomato (red). (C) Representative photomicrographs taken from the diaphyseal region (similar to region “b” in A) of a femur section from an Ocn-Cre ROSA26Ai9/+ Cxcl12gfp/+ mouse. Cells that express CXCL12 also express GFP (green). Counterstaining with DAPI highlights nuclei (blue). (D) Representative dot plots showing GFP and tdTomato expression in lineage (CD45, CD31, and Ter119) negative stromal cells harvested from Ocn-Cre ROSA26Ai9/+ Cxcl12gfp/+ mice. Original magnification, ×200 except for A, which is ×100.
Fig. 2.
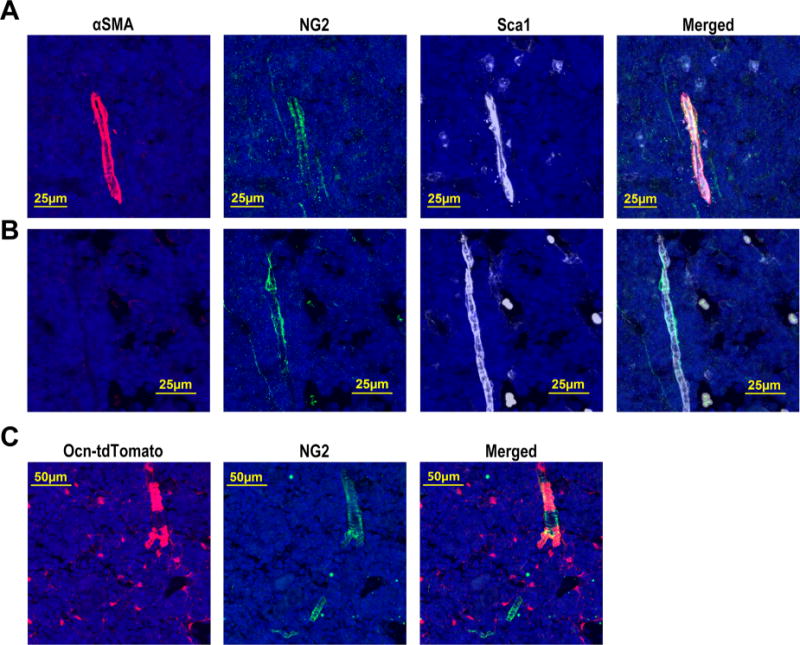
Ocn-Cre targets the majority of arteriolar pericytes. Representative photomicrographs of the diaphyseal region (similar to region “b” in Fig. 1A) of a femur section from a wild-type mouse stained for αSMA (red), NG2 (green), Sca1 (white), and DAPI (blue). (A) Images showing αSMA+ NG2+ arteriolar pericytes around Sca1+ arteriolar endothelial cells. (B) Images showing Αsma− NG2+ arteriolar pericytes around Sca1+ arteriolar endothelial cells. (C) Representative photomicrographs of the diaphyseal region of a femur section from an Ocn-Cre ROSA26Ai9/+ mouse stained for NG2 (green) and DAPI (blue). TdTomato (red) represents cells targeted by Ocn-Cre. Original magnification, ×200.
Dmp1-Cre targets osteoblasts and a subset of CAR cells
To characterize the targeting specificity of Dmp1-Cre in postnatal mouse bones, we generated Dmp1-Cre ROSA26Ai9/+ mice and Dmp1-Cre ROSA26Ai9/+ Cxcl12gfp/+ mice. As reported,(15,20) the Dmp1-Cre transgene efficiently targets all osteoblasts (Fig. 3A, B; Supporting Fig. 4). Surprisingly, Dmp1-Cre also targets a subset of CAR cells (Fig. 3A, C). Interestingly, Dmp1-Cre–targeted CAR cells were not enriched near the endosteum or osteoblasts, but were distributed throughout the bone marrow (Supporting Fig. 5A, B). By flow cytometry 29.2% ± 1.7% (n = 3 mice) of CAR cells are targeted by Dmp1-Cre (Fig. 3D). In contrast to Ocn-Cre, NG2+ arteriolar pericytes were rarely targeted by Dmp1-Cre (Fig. 3E). Thus, Dmp1-Cre targets all osteoblasts and a subset of CAR cells but few arteriolar pericytes.
Fig. 3.
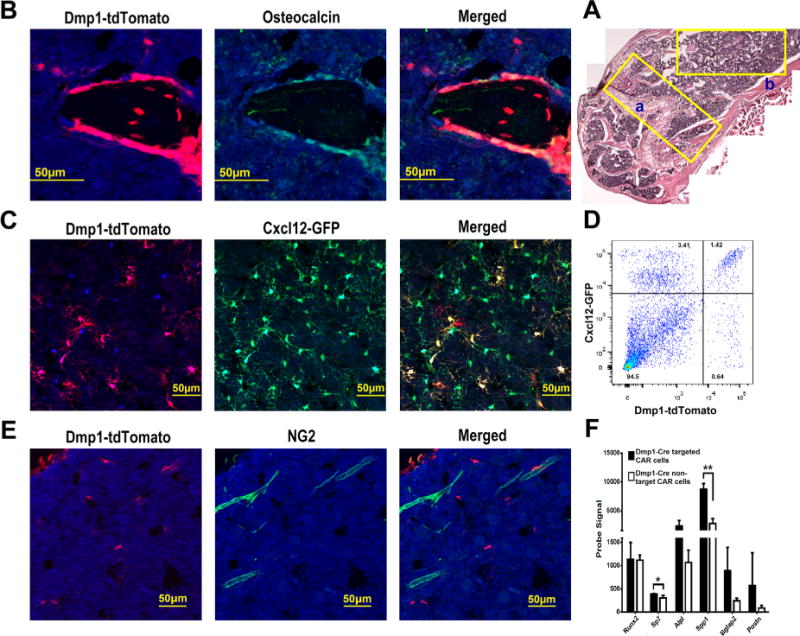
Dmp1-Cre targets all the osteoblasts and a subset of CAR cells but no arteriolar pericytes. (A) Composite image of H&E-stained sections from the femur of a Dmp1-Cre ROSA26Ai9/+ mouse. (B) Representative photomicrographs of the metaphyseal region (region “a” in A) of a femur section that was stained for osteocalcin (green) and DAPI (blue). Dmp1-Cre targeted cells express tdTomato (red). (C) Representative photomicrographs taken from the diaphyseal region (similar to region “b” in A) of a femur section from a Dmp1-Cre ROSA26Ai9/+ Cxcl12gfp/+ mouse; cells that express CXCL12 also express GFP (green). (D) Representative dot plot showing GFP and tdTomato expression in lineage (CD45, CD31, and Ter119) negative stromal cells harvested from Dmp1-Cre ROSA26Ai9/+ Cxcl12gfp/+ mice. (E) Representative photomicrographs taken from the diaphyseal region of a femur section from a Dmp1-Cre ROSA26Ai9/+ Cxcl12gfp/+ mouse stained for NG2 (green) and DAPI (blue). (F) RNA expression profiling of sorted Dmp1-Cre targeted (tdTomato+) or non-targeted (tdTomato−) CAR cells was performed. Shown are probe signals for the indicated genes (n = 3 mice). All data represent the mean ± SD. *p < 0.05; **p < 0.01 (unpaired t test). Original magnification, ×200 except for A, which is ×100.
To characterize the Dmp1-Cre–targeted subset of CAR cells, we sorted tdTomato+ (Dmp1-Cre–targeted) and tdTomato− CAR cells (Dmp1-Cre–non-targeted) and performed RNA expression profiling. Gene set enrichment analysis showed that Dmp1-Cre–targeted CAR cells were highly enriched for a previously identified group of genes involved in osteoblast maturation or bone development (Supporting Fig. 6A). Indeed, expression of genes associated with mature osteoblasts such as Bglap2 (Ocn) and Postn (periostin) are increased nearly fourfold compared to non-targeted CAR cells (Fig. 3F). In contrast, expression of early osteoblast lineage genes, including Sp7 (osterix) and Runx2 were normal or only minimally elevated (Fig. 3F). Expression of key HSC maintenance genes (Cxcl12, Kitl, and Angpt1) or key B lymphoid factor genes (Igf1, Flt3l, or BAFF) was similar in Dmp1-Cre–targeted and Dmp1-Cre–non-targeted CAR cells (Supporting Fig. 6B, C). However, expression of interleukin-7 (IL-7), which is required for pro-B cell maintenance, was significantly reduced in Dmp1-Cre–targeted CAR cells.
Tagln-Cre targets osteoblasts, a majority of CAR cells, and both arteriolar and venous sinusoidal pericytes
Arteriolar pericytes have been implicated in HSC maintenance and can be readily identified in the bone marrow as Nestin-GFPbright or NG2+ periarteriolar cells.(6) However, a recent study reported that a substantial number of functional HSCs localize to venous sinusoids in the central bone marrow.(21) In an effort to better visualize and isolate sinusoidal pericytes, we tested targeting by the Tagln-Cre transgene. Tagln encodes for transgelin (SM22a) and is expressed in smooth muscle cells and cardiomyocytes.(22–24) Tagln is also expressed in osteoblasts.(25) Accordingly, Tagln-Cre targets all osteoblasts (Fig. 4A, B; Supporting Fig. 7). Analyzing Tagln-Cre ROSA26Ai9/+ Cxcl12gfp/+ mice, we observed that Tagln-Cre and CXCL12-GFP mark overlapping, but distinct, bone marrow stromal cell populations (Fig. 4B, C). Whereas Tagln-Cre targets the great majority of CXCL12-GFP+ CAR cells that line venous sinusoids which are marked by vascular endothelial cadherin (VE-cadherin),(6,26) it does not efficiently target those CXCL12-GFP+ CAR cells that are not in direct contact with sinusoids (Fig. 4D, yellow arrows). Conversely, Tagln-Cre targets a population of perisinusoidal cells that are CXCL12-GFP dim/negative (Fig. 4D, red arrows), presumably representing non-CAR venous pericytes. Moreover, Tagln-Cre, but not CXCL12-GFP, marks periarteriolar pericytes (Fig. 4E). Indeed, Tagln-Cre targeted nearly all NG2+ arteriolar pericytes (Fig. 4E, F). Flow cytometry showed that Tagln-Cre targets 74.9% ± 5.2% (n = 3 mice) of CAR cells (Fig. 4G). Conversely, 16.6% ± 2.3% (n = 3 mice) of Tagln-Cre–targeted stromal cells were CXCL12-GFP dim/negative. Collectively, these data show that Tagln-Cre efficiently targets all osteoblasts, a majority of CAR cells, and both venous and arteriolar pericytes.
Fig. 4.
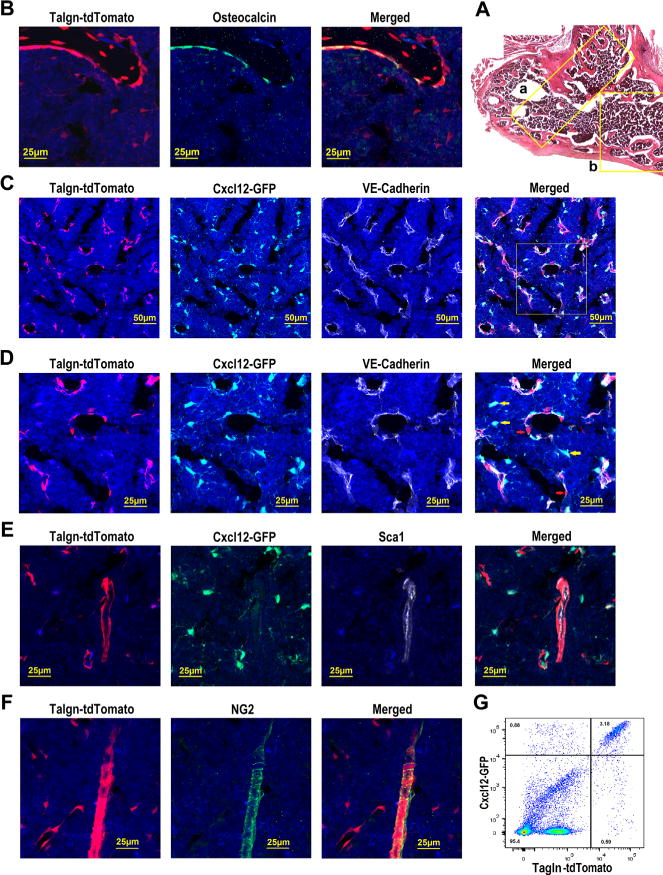
Tagln-Cre targets osteoblasts, a majority of CAR cells, and both venous sinusoidal and arteriolar pericytes. (A) Composite image of H&E-stained sections from the femur of a Tagln-Cre ROSA26Ai9/+ mouse. (B) Representative photomicrographs of the metaphyseal region (region “a” in A) of a femur section that was stained for osteocalcin (green) and DAPI (blue). (C) Representative photomicrographs taken from the diaphyseal region (similar to region “b” in A) of a femur section from a Tagln-Cre ROSA26Ai9/+ Cxcl12gfp/+ mouse stained for VE-cadherin (white) to mark all endothelial cells; cells that express CXCL12 also express GFP (green). (D) Enlarged images of the boxed region in C. (E) Representative photomicrographs taken from the diaphyseal region of a femur section from a Tagln-Cre ROSA26Ai9/+ Cxcl12gfp/+ mouse stained for Sca1 (white) to mark arteriolar endothelial cells. (F) Representative photomicrographs taken from the diaphyseal region of a femur section from Tagln-Cre ROSA26Ai9/+ mouse stained for NG2 (green) to mark arteriolar pericytes. (G) Representative dot plot of lineage (CD45, CD31, and Ter119) negative stromal cells from a Tagln-Cre ROSA26Ai9/+ Cxcl12gfp/+ mouse showing GFP and tdTomato expression. Original magnification, ×200 except for A, which is ×100.
Discussion
Ocn-Cre has been widely used to target osteoblasts in past studies.(7,9,10) Our data show that Ocn-Cre targets not only osteoblasts, but also more than 70% of CAR cells and arteriolar pericytes. CAR cells are mesenchymal progenitors that have adipogenic and osteogenic capacity in vitro.(27) However, only a small subset of CAR cells contributes to osteoblast development in vivo.(3) Whether the Ocn-Cre–targeted subset of CAR cells is fated to osteoblast differentiation is unclear. Of note, we did not observe preferential localization of Ocn-Cre–targeted CAR cells to the endosteal region. CAR cells constitutively produce high levels of multiple cytokines and chemokines that regulate hematopoiesis, including CXCL12 and stem cell factor.(27) Indeed, CAR cells have been implicated in the maintenance of HSCs and B lymphoid progenitors.(28,29) Thus, phenotypes reported using Ocn-Cre need to be interpreted in light of our data showing targeting of CAR cells and arteriolar pericytes, in addition to osteoblasts.
Dmp1-Cre has been widely used to target osteocytes.(12,14,30) Several Dmp1-Cre transgenes have been described. In this study, we show that the 10-kb Dmp1-Cre transgene not only efficiently targets osteoblasts, but also targets a subset of CAR cells. The results are consistent with a prior study by Kalajzic and colleagues(12) showing that the 10-kb Dmp1-Cre transgene targets a small population of undefined cells in the bone marrow, in addition to osteoblasts and osteocytes. Of note, the same group also reported that an 8-kb Dmp1-Cre transgene, which is thought to be more osteocyte-restricted, targets, at least a subset of, osteoblasts.(12) Whether the 8-kb Dmp1-Cre transgene targets a subset of CAR cells will require further study. Our data show that the 10-kb Dmp1-Cre transgene targets approximately 30% of CAR cells. Expression profiling of this subset of CAR cells shows higher expression of genes associated with mature osteoblasts, suggesting that Dmp1-Cre–targeted CAR cells may be enriched for osteoprogenitors. Functional studies are needed to confirm this possibility.
We report for the first time the spectrum of bone marrow stromal cells that are targeted by a Tagln-Cre transgene. Prior studies in non-bone tissues had shown transgelin expression in cardiomyocytes and vascular smooth muscle cells.(22–24) Consistent with its expression in osteoblasts,(25) Tagln-Cre efficiently targets osteoblasts. Interestingly, Tagln-Cre appears to target a majority of CAR cells. Specifically, it targets those CAR cells that are closely associated with venous sinusoids (ie, venous sinusoidal pericytes). Conversely, Tagln-Cre does not efficiently target CAR cells that are more distant from sinusoids. Finally, Tagln-Cre efficiently targets arteriolar pericytes, which, despite evidence for high CXCL12 expression,(6) do not express high-level GFP in Cxcl12gfp mice. Thus, the Tagln-Cre represents an important new tool for investigators to efficiently target both venous sinusoidal and arteriolar pericytes in the bone marrow.
This study highlights the complexity and heterogeneity of mesenchymal stromal cells in the bone marrow. Nestin-GFP+, LepR+, and CAR cells represent overlapping but not identical populations of perivascular mesenchymal stromal cells.(3,5,29,31) Bulk cell analysis of each of these populations shows high-level expression of genes that regulate hematopoiesis, including factors that regulate HSCs (eg, kit ligand) and B lymphopoiesis (eg, IL-7).(3,28,29,31) Our study suggests that there is considerable heterogeneity within the CAR cell population. For example, the Dmp1-Cre–targeted subset of CAR cells, in addition to being enriched for osteoblast genes, expresses a lower level of IL-7. IL-7–producing stromal cells in the bone marrow are required for the maintenance of Pro-B cells,(28) suggesting that Dmp1-Cre–targeted CAR cells likely do not contribute to this specific stage of B cell development.
In summary, we have rigorously defined the targeting specificities in the bone marrow for the three Cre-recombinase transgenes. Ocn-Cre and Dmp1-Cre target broader stromal cell populations than previously appreciated, and this data should be incorporated into the design of future studies. These data further highlight the heterogeneity of mesenchymal stromal cells in the bone marrow, and suggest that the Cre-recombinase transgenes used in this study could be used to interrogate this heterogeneity.
Supplementary Material
Acknowledgments
This work was supported by RO1 HL60772 (to DCL) and P50 CA171963 (to DCL). We thank Amy Schmidt for technical assistance and Jackie Tucker-Davis for animal care.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Authors’ roles: JZ and DCL conceived and designed the experiments, analyzed the data, and wrote the manuscript. JZ performed the experiments.
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvi LM, Link DC. Cellular complexity of the bone marrow hematopoietic stem cell niche. Calcif Tissue Int. 2014;94(1):112–24. doi: 10.1007/s00223-013-9805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan-Speranza TC, Conigrave AD. Osteocalcin: an osteoblast-derived polypeptide hormone that modulates whole body energy metabolism. Calcif Tissue Int. 2015;96(1):1–10. doi: 10.1007/s00223-014-9931-y. [DOI] [PubMed] [Google Scholar]
- 8.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgers TA, Hoffmann MF, Collins CJ, et al. Mice lacking pten in osteoblasts have improved intramembranous and late endochondral fracture healing. PLoS One. 2013;8(5):e63857. doi: 10.1371/journal.pone.0063857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Z, Zylstra-Diegel CR, Schumacher CA, et al. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A. 2012;109(33):E2197–204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin C, D’Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007;86(12):1134–41. doi: 10.1177/154405910708601202. [DOI] [PubMed] [Google Scholar]
- 12.Kalajzic I, Matthews BG, Torreggiani E, Harris MA, Divieti Pajevic P, Harris SE. In vitro and in vivo approaches to study osteocyte biology. Bone. 2013;54(2):296–306. doi: 10.1016/j.bone.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalajzic I, Braut A, Guo D, et al. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35(1):74–82. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Z, Huang J, Cao L, Liang Y, Han X, Quarles LD. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS One. 2014;9(8):e104154. doi: 10.1371/journal.pone.0104154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–41. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Zhong W, Cui T, et al. Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler Thromb Vasc Biol. 2006;26(3):e23–4. doi: 10.1161/01.ATV.0000202661.61837.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Xuan S, Bouxsein ML, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–12. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 19.Ara T, Itoi M, Kawabata K, et al. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170(9):4649–55. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86(4):320–5. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- 21.Acar M, Kocherlakota KS, Murphy MM, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526(7571):126–30. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umans L, Cox L, Tjwa M, et al. Inactivation of Smad5 in endothelial cells and smooth muscle cells demonstrates that Smad5 is required for cardiac homeostasis. Am J Pathol. 2007;170(5):1460–72. doi: 10.2353/ajpath.2007.060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assinder SJ, Stanton JA, Prasad PD. Transgelin: an actin-binding protein and tumour suppressor. Intl J Biochem Cell Biol. 2009;41(3):482–6. doi: 10.1016/j.biocel.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 24.El-Bizri N, Guignabert C, Wang L, et al. SM22alpha-targeted deletion of bone morphogenetic protein receptor 1A in mice impairs cardiac and vascular development, and influences organogenesis. Development. 2008;135(17):2981–91. doi: 10.1242/dev.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120(7):2423–31. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inra CN, Zhou BO, Acar M, et al. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature. 2015;527(7579):466–71. doi: 10.1038/nature15530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omatsu Y, Sugiyama T, Kohara H, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–99. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Komori T. Mouse models for the evaluation of osteocyte functions. J Bone Metab. 2014;21(1):55–60. doi: 10.11005/jbm.2014.21.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


