Abstract
Background
Accumulating evidence suggests that posttraumatic stress disorder (PTSD) is associated with disrupted default mode network (DMN) connectivity, but findings across studies have not been uniform. Individual differences in relevant genes may account for some of the reported variability in the relationship between DMN connectivity and PTSD. In this study, we investigated this possibility using genome-wide association study (GWAS)-derived polygenic risk scores (PRSs) for relevant psychiatric traits. We hypothesized that the association between PTSD and DMN connectivity would be moderated by genetic risk for one or more psychiatric traits such that individuals with elevated polygenic risk for psychopathology and severe PTSD would exhibit disrupted DMN connectivity.
Methods
Participants were 156 white, non-Hispanic Veterans of the wars in Iraq and Afghanistan who were genotyped and underwent resting state functional magnetic resonance imaging and clinical assessment. PRSs for neuroticism, anxiety, major depressive disorder, and cross-disorder risk (based on five psychiatric disorders) were calculated using summary statistics from published large-scale consortia-based GWASs.
Results
Cross-disorder polygenic risk influenced the relationship between DMN connectivity and PTSD symptom severity such that individuals at greater genetic risk showed a significant negative association between PTSD symptom severity and connectivity between the posterior cingulate cortex and right middle temporal gyrus. Polygenic risk for neuroticism, anxiety, and major depressive disorder did not influence DMN connectivity directly or through an interaction with PTSD.
Conclusions
Findings illustrate the potential power of genome-wide PRSs to advance understanding of the relationship between PTSD and DMN connectivity, a putative neural endophenotype of the disorder.
Keywords: PTSD, fMRI, genetics, psychopathology, major depressive disorder
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric disorder that develops in response to exposure to a traumatic event. It is defined by re-experiencing of the trauma, avoidance of trauma-related stimuli and various symptoms of heightened arousal and negative affectivity. Converging findings from resting state functional magnetic resonance imaging (rs-fMRI) studies suggest that PTSD is associated with disrupted connectivity of the default mode network (DMN; Koch et al., 2016; Patel, Spreng, Shin, & Girard, 2012; Sripada et al., 2012). The DMN is an intrinsic functional connectivity network that is engaged primarily at rest or during internally-directed tasks such as mind-wandering, autobiographical memory, future thinking, self-mentalizing, and social processing of the self and others (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Andrews-Hanna, Smallwood, & Spreng, 2014; Gusnard, Akbudak, Shulman, & Raichle, 2001; Spreng & Grady, 2010). Its primary regions include the posterior cingulate cortex (PCC), medial prefrontal cortex, hippocampus, angular gyrus, and lateral temporal cortex (Raichle et al., 2001).
Rs-fMRI has attracted considerable interest from clinical neuroscientists as a paradigm for studying associations between psychopathology and perturbations of the functional neural connectome. Several prior rs-fMRI studies have shown PTSD to be associated with reduced functional connectivity in the DMN, suggesting the predominance of externally-focused and vigilant neural activity during rest (Bluhm et al., 2009; Kennis, van Rooij, van den Heuvel, Kahn, & Geuze, 2016; Koch et al., 2016; Qin et al., 2012; Sripada et al., 2012; Zhou et al., 2012). However, findings across studies have not been consistent, with some studies reporting positive or no associations between DMN connectivity and PTSD (Lanius et al., 2010; Patel et al., 2012; Reuveni et al., 2016). One possible source of variability in results across studies are genetic factors that influence functional connectivity (Glahn et al., 2010; Sheline et al., 2010; Wiggins et al., 2012; Wiggins et al., 2013). For example, Miller et al. (2016) recently found that serotonin receptor single nucleotide polymorphisms (SNPs) significantly moderated the relationship between PTSD and functional connectivity, whereby PTSD symptom severity was negatively associated with DMN connectivity between the PCC and right middle temporal gyrus only among patients homozygous for risk variants in the serotonin receptor gene HTR2A.
The aim of this study was to expand our search for genetic factors that moderate the association between PTSD and DMN connectivity using polygenic risk scores (PRSs). PRSs are derived from results of large-scale consortia-based genome-wide association studies (GWAS). They are summary measures of the additive effect of hundreds of thousands or millions of SNPs from across the entire genome and provide an index of an individual’s genetic propensity for the GWAS trait. PRSs are computed by taking SNPs with significance levels at and below a specified p-value threshold from the GWAS, weighting each SNP for each individual in an independent dataset based on its effect size from the GWAS, and then summing across the SNPs. The resulting PRS can be thought of as an individual’s genetic “risk” for the phenotype evaluated in the GWAS, with higher scores indicating greater genetic liability. PRSs have been shown to explain a larger proportion of the variance in the phenotype than SNPs from candidate genes or even peak SNPs from GWAS studies (Chatterjee, Shi, & García-Closas, 2016; Ridge, Mukherjee, Crane, & Kauwe, 2013).
It is now well-established that risk for PTSD is influenced by genetic factors that are not unique to PTSD, but rather, shared with genetic risk for other psychiatric disorders such as major depression (Koenen et al., 2008; Sartor et al., 2012; Solovieff et al., 2014) and anxiety (Chantarujikapong et al. 2001). For example, Solovieff and colleagues (2014) found that polygenic risk for depression was associated with PTSD symptom severity. In another study, using data from a twin study of Vietnam veterans, Wolf et al. (2010) modeled the relative strength of genetic and environmental influences on the common factors underlying PTSD and the internalizing and externalizing spectra of psychopathology. Analyses revealed that 41% of the variance in the internalizing latent variable was accounted for by one genetic factor, while a second, distinct, genetic factor explained 40% of the variance in the externalizing latent variable. PTSD was primarily associated with the internalizing spectrum. Based on this pattern of results, the authors concluded that the first genetic factor likely corresponded conceptually to the inherited/temperamental component of trait negative emotionality/neuroticism (i.e., the primary personality substrate for the internalizing disorders) which has also been implicated in other research as the primary temperamental vulnerability for PTSD (Breslau & Schultz, 2013; Miller, 2003; Miller, Grief, & Smith, 2003; Miller, Kaloupek, Dillon, & Keane, 2004).
Similarly, the Cross-Disorder Group of the Psychiatric Genomics Consortium (PGC; 2013) examined shared genetic vulnerability across five psychiatric disorders: major depressive disorder, bipolar disorder, schizophrenia, autism spectrum disorder, and attention hyperactivity deficit disorder. Results suggested genetic commonalities between the three adult disorders (i.e., major depressive disorder, bipolar disorder, and schizophrenia) and between autism spectrum disorder, schizophrenia and bipolar disorder. In another study, Bulik-Sullivan et al. (2015) examined shared genetic vulnerabilities across 24 psychiatric traits and found genetic commonalities between anorexia nervosa, obesity, schizophrenia, major depressive disorder, and/or bipolar disorder (among others).
The goal of this study was to leverage the scientific and statistical power of the genome-wide PRSs derived from these studies to advance understanding of the relationship between PTSD and a putative neural endophenotype of the condition, disrupted DMN connectivity. Although there is strong evidence for an association between disruptions in DMN connectivity and PTSD, there is minimal research that examines the link between PRSs and PTSD or DMN connectivity (see Supplemental Table S1 for additional details). However, as suggested by the foregoing twin, personality, and molecular genetic GWAS study findings, there is support for shared genetic risk across psychiatric disorders and in particular, genetic commonalities between PTSD and neuroticism, anxiety, and depression. As such, we focused on polygenic scores for neuroticism, anxiety, major depressive disorder and cross-disorder risk and evaluated the hypothesis that the association between PTSD and DMN connectivity would be moderated by PRSs for one or more of these traits. Moreover, given the high rate of comorbidity between PTSD and mild traumatic brain injury (mTBI) in this cohort, and prior evidence for an association between mTBI and DMN connectivity (Johnson et al., 2012; Vakhtin et al., 2013), we also examined the potential influence of this comorbid condition.
Methods and Materials
Participants
Participants were military Veterans of Operations Enduring Freedom, Iraqi Freedom, and New Dawn recruited through outreach events in the Boston community and consecutively enrolled into the Translational Research Center for TBI and Stress Disorders (TRACTS) located on the Jamaica Plain campus of VA Boston Healthcare System. To avoid genetic ancestry confounds, we focused our analyses on all genotype-confirmed white, non-Hispanic veterans with neuroimaging, clinical, and genotype data. The final sample included 156 male (92.3%) and female (7.7%) Veterans ages 19–57 (M/SD = 31.2/8.1). Fifty-five percent of the sample met DSM-IV criteria for current posttraumatic stress disorder (PTSD; symptom severity M/SD = 44.1/27.6). Table 1 includes medication information for the sample.
Table 1.
Medication information of sample
| Type of Medication | No., % |
|---|---|
| Antihypertensives | 14, 9.0 |
| Cholesterol | 5, 3.2 |
| Antidepressants | 35, 22.4 |
| Antiepileptics | 10, 6.4 |
| Sedative/Hypnotics | 9, 5.8 |
| Pain | 55, 35.3 |
| Antipsychotics | 4, 2.6 |
| Proton Pump Inhibitors | 9, 5.8 |
| Antihistamine/Antiallergy | 18, 11.5 |
| Stimulants | 5, 3.2 |
| Other | 37, 23.7 |
| Total | 103, 66.0 |
Note: Many individuals were taking more than one medication.
Total number is the number of subjects taking at least one medication. Other includes medications for thyroid, asthma, steroid contraception, erectile dysfunction, androgen steroids, muscle relaxant, acid reflux, antiviral, antimetabolites, antibiotics, vitamins, and supplements.
Exclusionary criteria included history of seizures unrelated to head injury, vascular disease, heart conditions, serious mental illness such as bipolar disorder or other psychotic disorders, active suicidal or homicidal ideation, cognitive disorder due to a general medical condition, and incompatibility with MRI due to ferromagnetic objects or pregnancy. All study procedures were reviewed and approved by the local Institutional Review Board.
Clinical and Demographic Assessment
PTSD symptom severity was assessed with the Clinician-Administered PTSD Scale (CAPS) for DSM-IV (Blake et al., 1995) and diagnoses were confirmed via consensus of three doctoral-level psychologists. For the purposes of this study, we analyzed a continuous measure of PTSD symptom severity within the last 30 days prior to the assessment. The CAPS is a structured interview that assesses each DSM-IV PTSD symptom with two items that reflect the frequency and intensity of symptoms (each on a 0–4 scale) with higher scores indicative of greater symptom severity. TBI was evaluated with the Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L; Fortier et al., 2014), a semi-structured interview developed for combat Veterans. The BAT-L queries participants on self-reported lifetime history of head trauma including blast exposure before, during, and after deployment. Three doctoral-level psychologists met to determine consensus as to whether criteria for TBI had been met and whether symptoms following the injury were due to TBI as opposed to psychiatric disturbance.
Trauma exposure was assessed with the Traumatic Life Events Questionnaire (TLEQ; Kubany et al., 2000), a self-report measure of 22 traumatic events such as natural disasters, exposure to warfare, robbery involving a weapon, and physical abuse. Individuals reported the frequency of each event and whether fear, helplessness, or horror was present. Traumatic events were counted as present/absent and the number of different types of traumatic events was summed for each individual (range between 0 and 22). Alcohol use was measured with Lifetime Drinking History (LDH; Skinner & Sheu, 1982), a retrospective interview that queries participants on patterns of alcohol use, abuse, and dependence.
MRI Acquisition and Processing
Imaging data were acquired on a 3 Tesla Siemens TIM Trio scanner with a 12-channel head coil. Two Magnetization Prepared Rapid Gradient Echo (MP-RAGE) T1-weighted structural scans were acquired with the following parameters: TR = 2530, TE = 3.32 ms, flip angle = 7°, 1 × 1 × 1 mm voxels. These structural scans were averaged to create a single high contrast-to-noise image and were further used in surface reconstruction, functional connectivity seed placement, and registration. During the same session, two six-minute rs-fMRI scans were collected using the following parameters: gradient echo echo-planar imaging, TR = 3000, TE = 30ms, flip angle = 90°, 3 × 3 × 3.75 mm voxels, 38 slices. Participants were instructed to keep their eyes open and stay awake during rs-fMRI data collection.
Data were processed using a combination of FreeSurfer (http://surfer.nmr.mgh.harvard.edu), Analysis of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov/afni), and the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) FSL (http://www.fmrib.ox.ac.uk/fsl) software packages, but primarily followed the FSFAST processing stream (http://freesurfer.net/fswiki/FsFast). Preprocessing of rs-fMRI data involved motion correction, time shifting, concatenation of scans, regression of motion from time series, regression of the global mean and the average time courses from the white matter and ventricles, and band pass filtering between 0.01 and 0.10 Hz. Data were first sampled to and smoothed on the surface and then registered to a surface-based template (Fischl, Sereno, Tootell, Dale, 1999). The bilateral superior third of the isthmus of the cingulate (posterior portion, corresponding to the PCC– a core hub of the DMN; Andrews-Hanna et al., 2010; Figure 1A) was used as the DMN seed region and derived from the surface-based parcellations in each participant’s native space (Fischl et al., 2002). Group-level connectivity maps were assessed in the entire TRACTS cohort (without restrictions to ancestry) and were clustered at a vertex-wise threshold of p < 10−20. This analysis yielded four regions of interest in the DMN from each hemisphere identified as: bilateral isthmus of the cingulate, angular gyrus, middle temporal gyrus, and medial prefrontal cortex (Figure 1B).
Figure 1. DMN regions.
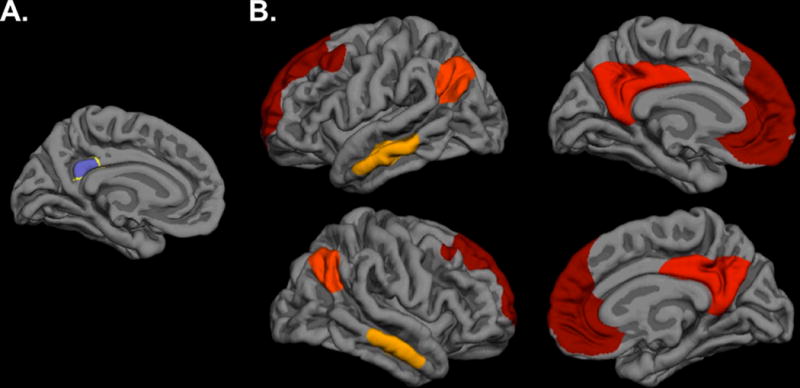
(A) Bilateral seed region of the superior third of the isthmus of the cingulate, or PCC. (B) Medial and lateral views of the eight significant DMN regions with a bilateral PCC seed used in analyses, including bilateral isthmus of the cingulate, middle temporal gyrus, angular gyrus, and medial prefrontal cortex. Each color represents one of the four regions, bilaterally (red=medial prefrontal cortex, dark orange=isthmus of the cingulate, light orange=angular gyrus, yellow=middle temporal gyrus). DMN=default mode network; PCC=posterior cingulate cortex.
Genotyping
Generation of the genotype data for the TRACTS cohort, including DNA extraction, genotyping, and data cleaning, has been described in detail elsewhere (Miller et al., 2015), and will only be summarized briefly here. DNA was isolated from peripheral blood samples hybridized to Illumina HumanOmni2.5–8 microarrays (Illumina, San Diego, CA) according to manufacturer’s protocol and imaged using the Illumina iScan System. Samples for which the call rate was <0.99 were repeated. In the resulting data, all individuals had call rates >0.994, and all samples passed a concordance check between sex and X chromosome homozygosity performed in PLINK v1.07 (Purcell et al., 2007). Ancestry was confirmed by a principal component analysis performed in EIGENSTRAT (Price et al., 2006) based on 100,000 common (minor allele frequency >0.05) SNPs in a joint analysis using reference-population data from the 1000 Genomes Project (2010; 2012). An additional principal component analysis was performed within the white, non-Hispanic subsample for use as covariates.
PRS Calculation
Polygenic scores for neuroticism, anxiety, major depressive disorder, and cross-disorder risk were calculated using results from published GWAS studies. The PRSs for neuroticism, anxiety, and major depressive disorder were based on the GWAS meta-analysis of neuroticism conducted by the Genetics of Personality Consortium (available for download at http://www.tweelingenregister.org/GPC/; de Moor et al., 2015), GWAS meta-analysis of anxiety disorders conducted by the Anxiety Neuro Genetics STudy (available for download at https://www.med.unc.edu/pgc/results-and-downloads; Otowa et al., 2016), and a GWAS mega-analysis of major depressive disorder conducted by the PGC (available for download at https://www.med.unc.edu/pgc/downloads/; Ripke et al., 2013), respectively. Similarly, the polygenic scores for cross-disorder risk were computed based on a list of reference alleles and effect sizes from a GWAS of five major psychiatric disorders (i.e., major depressive disorder, bipolar disorder, schizophrenia, autism spectrum disorder, and attention hyperactivity deficit disorder) conducted by the Cross-Disorder Group of the PGC (2013), available for download at https://www.med.unc.edu/pgc/downloads. PRSs were calculated using the -score option in PLINK, which computes a linear function of the additively coded reference alleles weighted by the log odds ratio estimates from the relevant GWAS. As noted earlier, PRSs are computed using specified p-value thresholds. Since there is no a-priori method to determine which threshold yields the most predictive score, we examined PTSD using six different p-value thresholds representing a range of polygenicity: p < 0.05, p < 0.10, p < 0.20, p < 0.30, p < 0.40, and p < 0.50.
Statistical Analysis
The R (http://www.R-project.org) and SPSS, version 22 (IBM Corp., Armonk, NY) platforms were used for statistical analyses. Analyses examining the relationships between neuroticism, anxiety, major depressive disorder, or cross-disorder PRSs and DMN connectivity were conducted using hierarchal linear regression models. Models were run separately for each respective PRS (i.e., neuroticism, anxiety, major depressive disorder, or cross-disorder), in which the eight functional connectivity variables (four for each hemisphere) were regressed onto PTSD symptom severity, along with covariates of age, sex, the first three ancestry substructure principal components, and alcohol use. In each model, the six PRS thresholds for each respective trait and each PTSD severity by PRS (at the six different thresholds) interaction were evaluated in six separate regressions (one for each p–value threshold). A Monte-Carlo null simulation with 10,000 replicates in which PRSs were randomly permuted between subjects was used to compute multiple-testing corrected significance for each model. This procedure adjusted for the six PRS thresholds examined while accounting for the correlations between the eight functional connectivity measures1. We then performed secondary analyses using the PRS thresholds that showed the strongest interaction with PTSD symptom severity. Secondary analyses examined whether TBI diagnosis, number of TBIs, or trauma exposure accounted for the relationship between PTSD symptom severity, functional connectivity and polygenic risk using the same approach. We also tested the main effects and interactions with polygenic risk of TBI diagnosis, number of TBIs, and trauma exposure on functional connectivity for the strongest polygenic risk threshold by PTSD symptom severity interactions.
Results
Results revealed a significant interaction between PTSD symptom severity and cross-disorder polygenic risk on DMN connectivity between the PCC and right middle temporal gyrus at the p < 0.05 and 0.10 thresholds (p < 0.05 threshold: B = −0.0007, p = 0.0009, corrected p < 0.02; p < 0.10 threshold: B = −0.0005, p = 0.0006, corrected p = 0.01), such that at higher levels of cross-disorder polygenic risk there was a stronger negative relationship between PTSD symptom severity and functional connectivity2. No other regions survived multiple comparison correction (all corrected p’s > 0.1), although there was also a trend level effect for an interaction between cross-disorder polygenic risk and PTSD symptom severity for the connectivity between the PCC and left middle temporal gyrus at the p < 0.05 threshold (B = −0.0005, p = 0.004, corrected p = 0.06). There were no significant main effects for either cross-disorder polygenic risk or PTSD symptom severity (all p’s > 0.1). Analyses of neuroticism, anxiety, and major depressive disorder polygenic risk revealed no significant main effects or interactions with PTSD symptom severity on the functional connectivity between the PCC and any of the eight regions of the DMN after multiple comparison correction (all p’s > 0.08)3. Main effects of covariates are discussed in the Supporting Information.
To further examine the nature of the interaction between cross-disorder polygenic risk and PTSD symptom severity with connectivity between the PCC and right middle temporal gyrus, we performed Pearson correlations in which we divided the sample into two groups based on cross-disorder polygenic risk using a median split (low and high polygenic risk). Results revealed a significant negative correlation for the high cross-disorder risk group using both the p < 0.05 (r = −0.3, p = 0.02, Figure 2A) and p < 0.10 (r = −0.3, p = 0.01, Figure 2B) thresholds, indicating that those with high cross-disorder polygenic risk and severe PTSD symptoms showed reduced functional connectivity between the PCC and right middle temporal gyrus. There were no significant associations in the low cross-disorder risk group at either threshold (p < 0.05 threshold: r = 0.2, p = 0.1, Figure 2A; p < 0.10 threshold: r = 0.2, p = 0.1, Figure 2B).
Figure 2. Cross-disorder polygenic risk influences DMN functional connectivity in those with greater PTSD symptom severity.
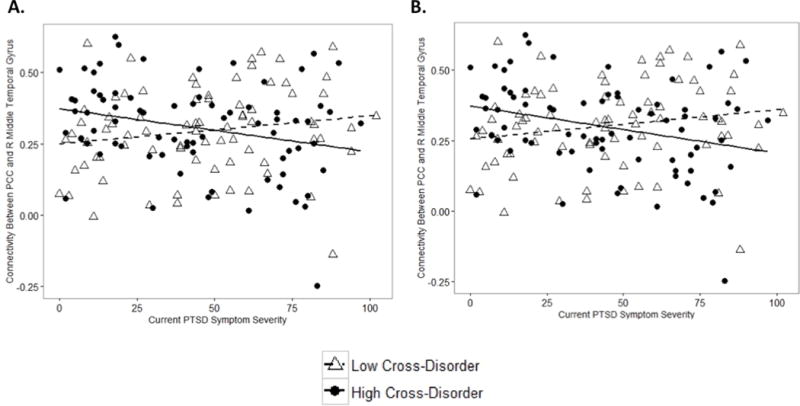
There was a significant interaction between PTSD symptom severity and cross-disorder polygenic risk score at both p <0.05 and 0.10 thresholds for DMN functional connectivity. Individuals with high cross-disorder polygenic risk (as determined by a median split) at (A) the p < 0.05 threshold and (B) the p < 0.10 threshold had reduced functional connectivity between the PCC and the right middle temporal gyrus with increasing PTSD symptom severity. There was no significant association between PTSD symptom severity and PCC-right middle temporal gyrus connectivity with low cross-disorder polygenic risk at either threshold. DMN=default mode network; PCC=posterior cingulate cortex; PTSD=posttraumatic stress disorder; R=right.
Finally, to determine whether TBI (diagnosis or number of TBIs) or trauma exposure accounted for the observed association between PTSD symptom severity, cross-disorder polygenic risk, and functional connectivity between the PCC and right middle temporal gyrus, we performed a series of analyses that included the main effects of TBI diagnosis, number of TBIs, or trauma exposure in the model. The pattern of results did not change (see Supplementary Tables S2, S3, and S4). Moreover, none of these variables independently contributed to the variance explained in the connectivity between the PCC and right middle temporal gyrus. We also examined whether there was an interaction between cross-disorder polygenic risk and these variables on the functional connectivity between the PCC and right middle temporal gyrus. There was no significant interaction between TBI diagnosis or number of TBIs with cross-disorder polygenic risk on the functional connectivity between the PCC and right middle temporal gyrus (all p’s > 0.5). There was a trend level effect for the interaction between cross-disorder polygenic risk and trauma exposure (β = −1.00, p = 0.066), although this would not have survived multiple comparison correction with all eight DMN connectivity variables in the model.
Discussion
This study used PRSs to examine genomic sources of variation in patterns of neural network connectivity associated with PTSD. On the basis of previous twin, personality, and molecular genetic GWAS studies suggesting that broad classes of psychiatric disorders share common genetic vulnerabilities, we examined PRSs for neuroticism, anxiety, major depressive disorder, and cross-disorder. Analyses revealed that cross-disorder polygenic risk significantly moderated the relationship between PTSD symptom severity and DMN connectivity, such that individuals at high cross-disorder genetic risk and severe PTSD symptoms showed reduced functional connectivity between the PCC and right middle temporal gyrus. PRSs for neuroticism, anxiety, and major depressive disorder were not significantly associated with DMN functional connectivity. Moreover, secondary analyses showed that the DMN connectivity effects were not attributable to TBI or trauma exposure. These results align with findings of recent studies suggesting that a broad spectrum of psychiatric disorders may be influenced by common genetic vulnerabilities (Bulik-Sullivan et al., 2015; Cross-Disorders Group PGC, 2013) and extend them by showing that these genetic traits are relevant to the association between PTSD symptom severity and a putative neural endophenotype of the disorder, disrupted DMN connectivity.
Our finding of reduced DMN connectivity between the PCC and middle temporal gyrus in a subset of patients with PTSD is consistent with prior rs-fMRI studies (Kennis et al., 2016; Koch et al., 2016; Sripada et al., 2012; Zhou et al., 2012). The PCC is widely implicated in self-monitoring and -reflection (Johnson et al., 2002; Vogt & Laureys, 2005) and previous studies have found disrupted connectivity between this region and the middle temporal gyrus in patients with PTSD (Bluhm et al., 2009; Qin et al., 2012). The middle temporal gyrus is part of the dorsal medial prefrontal cortex subsystem of the DMN which is associated with self-referential judgements and the present mental state (Andrews-Hanna et al., 2010). Disrupted self-referential processing is a common clinical presentation of many psychiatric disorders including major depressive disorder, autism spectrum disorder, and schizophrenia (Bora, Yucel, & Pantelis, 2009; Harvey, Lee, Horan, Ochsner, & Green, 2011; Lombardo, Barnes, Wheelwright, & Baron-Cohen, 2007; Mor & Winquist, 2002). Therefore, it is possible that altered functional connectivity in a DMN subsystem important for self-referential processing might act as a mechanism for shared clinical symptoms between PTSD and its comorbidities in individuals with severe PTSD and high cross-disorder genetic risk. This interpretation of our findings suggests that genetic vulnerability to psychopathology in combination with severe PTSD symptoms may place an individual at greater risk for comorbid disorders via this DMN mechanism. However, more research is needed to confirm this hypothesis.
As illustrated in Figure 2, individuals with low cross-disorder genetic risk showed a diverging pattern of functional connectivity and PTSD symptom severity from those with high cross-disorder genetic risk. Specifically, results revealed a non-significant trend toward increased functional connectivity between the PCC and right middle temporal gyrus in individuals with low cross-disorder genetic risk and high PTSD symptom severity, which likely contributed to the overall interaction. These results are consistent with previous work by Lanius et al. (2010) who found a positive association between DMN connectivity and PTSD symptom severity, and suggest that genetic vulnerabilities may be a possible source of variability in studies investigating PTSD symptom severity and DMN connectivity. However, it will be important for future work to continue to investigate modulatory genetic factors in the relationship between PTSD and functional connectivity.
Interestingly, we did not find any main effects of PTSD symptom severity or PRSs on DMN connectivity. This is in contrast to previous studies that have found significant PTSD effects on DMN connectivity (Bluhm et al., 2009; Kennis et al., 2016; Koch et al., 2016; Qin et al., 2012; Sripada et al., 2012; Zhou et al., 2012), but is consistent with a recent study that reported no PTSD group differences in DMN connectivity (Reuveni et al., 2016). Recent work by Miller and colleagues (2017) suggests that PTSD is associated with specific disruptions in the medial temporal lobe subsystem of the DMN, which includes regions such as the hippocampus and ventromedial prefrontal cortex. It is possible that the absence of a bivariate association between PTSD and DMN connectivity in the current study is related to this reported specificity of DMN alterations in PTSD, which was not tested here. Another possibility is that individual differences in genetics play a role in moderating the association between PTSD and DMN connectivity, which thereby limits bivariate associations between the two directly. This is consistent with our previous work (Miller et al., 2016) and highlights the importance of genetic factors in PTSD-associated neural outcomes. To our knowledge, this is the first study to examine the association between DMN connectivity and PRSs of neuroticism, anxiety, depression, and cross-disorder. Our results suggest that genetic risk for these disorders alone may not be sufficient for disruptions in functional connectivity, but instead may require additional vulnerabilities such as severe PTSD. However, it will be important for future work to confirm these results.
These findings should be considered in light of several limitations. First, this was a cross-sectional study and while the genetic PRSs obviously represent stable genetic traits, causal inferences about the direction of the association between PTSD and DMN connectivity are less clear. Second, this was a white, non-Hispanic, predominately male sample with high levels of combat-related trauma. It is unknown whether these findings will extend to samples from other ancestries, female samples or non-military samples with other trauma profiles. Replication from additional samples is needed to confirm these results. Third, it is possible that the PRSs of depression and neuroticism are not as strong a measure of genetic risk as the PRSs of other disorders used in this study. However, both depression and neuroticism PRSs were computed from large meta- and mega-analyses equating to sample sizes > 9000 with equally impressive replication cohorts. Future work would benefit from further investigation of depression and neuroticism PRSs in PTSD. Fourth, given the size of our sample and the number of tests conducted, there is a possibility for an increase in Type I error. However, we used multiple comparison correction in our initial analyses of DMN connectivity and polygenic risk to reduce the likelihood of this increase in error. Moreover, these analyses were comparable to previously published work with similar sample sizes investigating genetics, clinical disorders, and neuroimaging measures (Costafreda et al., 2013; Hayes et al., 2017; Miller et al., 2016; Miller et al., 2015; Whitwell et al., 2012). Nonetheless, it will be important for other studies with larger sample sizes to replicate these findings.
Conclusion
We examined PRSs of PTSD-related traits to advance the understanding of the relationship between PTSD and a hypothesized neural endophenotype of the disorder, disrupted DMN connectivity. Our findings contribute to a growing body of literature on rs-fMRI in PTSD and suggest that individual differences in genetic risk for psychopathology may modify the association between PTSD symptom severity and DMN connectivity. The present findings illustrate the potential power of genome-wide PRSs to advance understanding of the relationship between psychiatric disorders and the putative neural endophenotypes of those conditions.
Supplementary Material
Acknowledgments
Research and preparation of this manuscript was supported by National Institutes of Mental Health (NIMH) training grant (T32MH019836-01) awarded to Terence Keane, Ph.D. supporting DRM, NIMH grant R21MH102834 awarded to MWM, and the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development National Network Research Center (B9254-C). Research reported in this publication was also supported by the National Institute On Aging of the National Institutes of Health (NIH) grant R03AG051877 awarded to EJW and a Presidential Early Career Award for Scientists and Engineering as administered by U.S. Department of VA Office of Research and Development, PECASE 2013A award to EJW. This work was further supported with resources and the use of facilities at the Neuroimaging Research for Veterans Center, VA Boston Healthcare System and the Pharmacogenomics Analysis Laboratory, Research and Development Service, Central Arkansas VA Healthcare System, Little Rock, Arkansas. The authors would like to thank Drs. Fortier, Amick, Kenna, Corbo, and Mr. Musto as well as the entire team of TRACTS investigators for their assistance with data collection and management. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the United States government, NIMH, or NIH.
Footnotes
These analyses were comparable to previously published work with similar sample sizes investigating genetics, PTSD, and/or neuroimaging measures (Miller et al., 2016; Miller et al., 2015).
These results were not accounted for by candidate SNPs (rs977003 and rs7322347) implicated in the association between DMN connectivity and PTSD symptom severity in previous work (Miller et al., 2016).
Because schizophrenia and bipolar are two of the largest constituent GWASs contributing to the cross-disorder polygenic risk score, it is possible that these disorders influenced the results. Therefore, we examined the association between PTSD symptom severity, DMN functional connectivity and PRSs for schizophrenia and bipolar. Details and results of this analysis are reported in the supporting information.
The authors declare that they have no conflicts of interest or financial disclosures.
References
- 1000 Genomes Project Consurtium. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1000 Genomes Project Consurtium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Lanius RA. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: Meta-analysis. Schizophr Res. 2009;109(1):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Breslau N, Schultz L. Neuroticism and post-traumatic stress disorder: A prospective investigation. Psychol Med. 2013;43(08):1697–1702. doi: 10.1017/S0033291712002632. [DOI] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, Robinson EB. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, True WR. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Research. 2001;103(2):133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Shi J, García-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17:392–406. doi: 10.1038/nrg.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, McCann P, Saker P, Cole JH, Cohen-Woods S, Farmer AE, Fu CH. Modulation of amygdala response and connectivity in depression by serotonin transporter polymorphism and diagnosis. Journal of Affective Disorders. 2013;150(1):96–103. doi: 10.1016/j.jad.2013.02.028. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consoritum (PGC) Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. The Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor MH, van den Berg SM, Verweij KJ, Krueger RF, Luciano M, Vasquez AA, Amin N. Genome-wide association study identifies novel locus for neuroticism and shows polygenic association with Major Depressive Disorder. JAMA Psychiatry. 2015;72(7):642. doi: 10.1001/jamapsychiatry.2015.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Amick MM, Grande L, McGlynn S, Kenna A, Morra L, McGlinchey RE. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: Evidence of research utility and validity. J Head Trauma Rehabil. 2014;29(1):89–98. doi: 10.1097/HTR.0b013e3182865859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, Blangero J. Genetic control over the resting brain. Proc Natl Acad Sci U S A. 2010;107(3):1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Lee J, Horan WP, Ochsner K, Green MF. Do patients with schizophrenia benefit from a self-referential memory bias? Schizophr Res. 2011;127(1):171–177. doi: 10.1016/j.schres.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Logue MW, Sadeh N, Spielberg JM, Verfaellie M, Hayes SM, Milberg WP. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain. 2017:aww344. doi: 10.1093/brain/aww344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self‐reflection. Brain. 2002;125(8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: Resting-state fMRI study. Neuroimage. 2012;59(1):511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis M, van Rooij SJ, van den Heuvel MP, Kahn RS, Geuze E. Functional network topology associated with posttraumatic stress disorder in veterans. Neuroimage Clin. 2016;10:302–309. doi: 10.1016/j.nicl.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depress Anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Fu QJ, Ertel K, Lyons MJ, Eisen SA, True WR, Tsuang MT. Common genetic liability to major depression and posttraumatic stress disorder in men. J Affect Disord. 2008;105(1):109–115. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubany ES, Leisen MB, Kaplan AS, Watson SB, Haynes SN, Owens JA, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychol Assess. 2000;12(2):210. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS One. 2007;2(9):e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, Verfaellie M. Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017 doi: 10.1016/j.bpsc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Personality and the etiology and expression of PTSD: A three‐factor model perspective. Clin Psychol: Science and Practice. 2003;10(4):373–393. [Google Scholar]
- Miller MW, Greif JL, Smith AA. Multidimensional Personality Questionnaire profiles of veterans with traumatic combat exposure: Externalizing and internalizing subtypes. Psychol Assess. 2003;15(2):205. doi: 10.1037/1040-3590.15.2.205. [DOI] [PubMed] [Google Scholar]
- Miller MW, Kaloupek DG, Dillon AL, Keane TM. Externalizing and internalizing subtypes of combat-related PTSD: A replication and extension using the PSY-5 scales. J Abnorm Psychol. 2004;113(4):636. doi: 10.1037/0021-843X.113.4.636. [DOI] [PubMed] [Google Scholar]
- Miller MW, Sperbeck E, Robinson ME, Sadeh N, Wolf EJ, Hayes JP, McGlinchey R. 5-HT2A gene variants moderate the association between PTSD and reduced default mode network connectivity. Front Neurosci. 2016;10:299. doi: 10.3389/fnins.2016.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Wolf EJ, Sadeh N, Logue M, Spielberg JM, Hayes JP, Carter WC. A novel locus in the oxidative stress-related gene ALOX12 moderates the association between PTSD and thickness of the prefrontal cortex. Psychoneuroendocrinology. 2015;62:359–365. doi: 10.1016/j.psyneuen.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N, Winquist J. Self-focused attention and negative affect: a meta-analysis. Psychol Bull. 2002;128(4):638. doi: 10.1037/0033-2909.128.4.638. [DOI] [PubMed] [Google Scholar]
- Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, Fanous A. Meta-analysis of genome-wide association studies of anxiety disorders. Molecular Psychiatry. 2016;21(10):1391–1399. doi: 10.1038/mp.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Daly MJ. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin LD, Wang Z, Sun YW, Wan JQ, Su SS, Zhou Y, Xu JR. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Res. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni I, Bonne O, Giesser R, Shragai T, Lazarovits G, Isserles M, Levin N. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum Brain Mapp. 2016;37(2):589–599. doi: 10.1002/hbm.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge PG, Mukherjee S, Crane PK, Kauwe JS. Alzheimer’s disease: Analyzing the missing heritability. PLoS One. 2013;8(11):e79771. doi: 10.1371/journal.pone.0079771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, Breen G, Sullivan PF. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, Nelson EC. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry. 2012;69(3):293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Fagan A. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci. 2010;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43(11):1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Solovieff N, Roberts AL, Ratanatharathorn A, Haloosim M, De Vivo I, King AP, Koenen KC. Genetic association analysis of 300 genes identifies a risk haplotype in SLC18A2 for post-traumatic stress disorder in two independent samples. Neuropsychopharmacology. 2014;39(8):1872–1879. doi: 10.1038/npp.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22(6):1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I. Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakhtin AA, Calhoun VD, Jung RE, Prestopnik JL, Taylore PA, Ford CC. Changes in intrinsic functional brain networks following blast-induced mild traumatic brain injury. Brain Injury. 2013;27(11):1304–1310. doi: 10.3109/02699052.2013.823561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandex M, Parisi JE. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012;135(3):794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Bedoyan JK, Peltier SJ, Ashinoff S, Carrasco M, Weng SJ, Monk CS. The impact of serotonin transporter (5-HTTLPR) genotype on the development of resting-state functional connectivity in children and adolescents: A preliminary report. Neuroimage. 2012;59(3):2760–2770. doi: 10.1016/j.neuroimage.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, Bedoyan JK, Carrasco M, Welsh RC, Martin DM, Monk CS. The impact of serotonin transporter genotype on default network connectivity in children and adolescents with autism spectrum disorders. NeuroImage: Clin. 2013;2:17–24. doi: 10.1016/j.nicl.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Miller MW, Krueger RF, Lyons MJ, Tsuang MT, Koenen KC. Posttraumatic stress disorder and the genetic structure of comorbidity. J Abnorm Psychol. 2010;119(2):320. doi: 10.1037/a0019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Z, Qin LD, Wan JQ, Sun YW, Su SS, Xu JR. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PLoS One. 2012;7(10):e46833. doi: 10.1371/journal.pone.0046833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


