Abstract
Susceptibility to rheumatoid arthritis (RA) is associated with HLA-DRB1 alleles coding a 5-amino acid sequence motif called the shared epitope (SE). To explore the potential mechanisms that lead to RA susceptibility, we analyze the in vitro effect of peptides bearing different HLA-DR4 sequences on human peripheral blood-derived cells. Three 15-mer peptides were used: 65-79*0401 (an HLA-DRB1*04:01-coded sequence carrying the SE motif, QKRAA); 65-79*0402 (an HLA-DRB1*04:02-coded sequence carrying a SE-negative motif, DERAA); 65-79*0403 (an HLA-DRB1*04:03-coded sequence carrying a SE-negative motif, QRRAE). We found that CD4 TH17 cells are regulated by peptide treatment with gender bias. In male-derived T cells, all peptide treatments significantly reduced TH17 cell differentiation in vitro when compared to no peptide treatment, and to female samples. TH17 differentiation in samples not treated with peptides, either in the presence or absence of TH17-polarizing cytokines, was higher in males than in females; however, in unfractionated PBMC after treatment with TH17 polarizing cytokines, IL-17A positive cells were more abundant in females than in males. In addition, SE-positive females showed a significantly higher percentage of IL-17A-positive cells compared to SE-negative females. In conclusion, donor’s SE status and gender may both influence TH17 immune polarization.
Keywords: MHC class II, PBMC, TH17, cytokines, immune polarization
2. INTRODUCTION
It is well established that particular HLA-DRB1 alleles play enhancing or protective effects on the susceptibility to rheumatoid arthritis (RA) (1). Specifically, HLA-DRB1 alleles that code a sequence motif called “shared epitope” (SE), containing one of the sequences QKRAA, QRRAA or RRRAA in residues 70 to 74 of the DRβ chain confer susceptibility to severe RA (2, 3). In contrast, HLA-DRB1 alleles that code the sequence DERAA in the same region confer protection against aggressive disease (4, 5). The mechanistic basis of these associations has not yet been elucidated.
RA is a chronic inflammatory condition in which several tissues, particularly the joint synovial layers, are targeted (6). There is recruitment of immune cells and activation of bone remodeling that can lead to deformed joints and disability. In addition, multi-systemic damage is produced because other tissues and organs are affected by the inflammatory milieu and oxidative stress (1, 2, 6, 7).
Both the innate and adaptive arms of the immune system play key roles in pathogenesis of RA. Recent evidence suggests that a TH17-skewed immune response plays a role in the pathogenesis of several autoimmune diseases, RA in particular. IL-17A is more abundantly found in joints, tissues, and in blood of RA patients compared to healthy controls (8, 9), and therapeutic effects have been observed in clinical trials using neutralizing antibodies against this cytokine (10, 11).
While it is presently unknown if the SE directly affects TH17 cells in humans, our group has recently demonstrated that this sequence motif acts as a signal transduction ligand that facilitates TH17 polarization in mice (2, 12–14). In vitro studies have shown that SE ligand, either as linear peptides or as natively folded tetrameric HLA-DR proteins, were able to trigger production of reactive oxygen species (ROS) and nitric oxide (NO) (15). The cell surface receptor with which the SE ligand interacts was identified as calreticulin (16–18). Activation of calreticulin-mediated signaling by the SE ligand in mouse dendritic cells (DC) was found to lead to polarization of T helper cells towards TH17 cells (12).
While activated T cells in both humans and mice express membrane-associated calreticulin (19, 20), only human T cells express MHC class II molecules on their surface (21). Therefore, it is conceivable that activated human T cells may be regulated directly by the SE-calreticulin pathway. Accordingly, in this study we have undertaken to determine the effect of the SE ligand, and control peptides derived from SE-negative HLA-DR molecules on human cells. Given the well-documented female predominance in RA, we also sought to determine the gender effect.
3. MATERIALS AND METHODS
3.1. Cell culture conditions and isolation of blood derived human PBMC and CD4+ T cells
PBMC and/or pure CD4+ T cells from unidentified healthy donors (Table 1), whose gender and other demographic details were unknown were obtained from leukopack filters kindly provided to us by Dr. Davenport from the Blood Bank, Department of Pathology at University of Michigan Hospital. Filters were rinsed out of blood cells by flushing them with 150 ml of MACS rinsing buffer (PBS and EDTA 2mM) containing BSA (0.5%) (Miltenyi, Cambridge, MA, USA). Then, cells were collected after centrifugation at 1,250 rpm and subjected to a density gradient over Ficoll-Paque PLUS solution (GE Healthcare, Fairfield, CT, USA) for 30 min at 1,800 rpm. The PBMC were recovered from the interface of the gradient and washed twice with MACS buffer. Next, PBMC were counted, resuspended in a concentration of 1 × 106 cells per 200 µl (96-well plate) in appropriate cell culture media described below and incubated at 37°C in 5% CO2 for up to 8 days. CD4+ cells were negatively purified from PBMC using human T cell isolation kit from Miltenyi (Cambridge, MA, USA).
Table 1.
Demographics of the samples used in this study
| Donor's ID and cell type |
Gender | HLA class II | |||
|---|---|---|---|---|---|
| DRB1 | DQB1 | DPB1 | SE | ||
| PBMC | |||||
| 1 | Male | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND |
| 3 | Female | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND | ND |
| 5 | Female | 04:07g 07:01 | 02:02 03:01 | 04:01 04:01 | SE−/− |
| 6 | Female | 04:04 14:01g | 03:02 05:03 | 03:01 04:01 | SE+/− |
| 7 | Male | 01:01 13:03 | 03:01 05:01 | 02:01 02:01 | SE+/− |
| 8 | Male | 07:01 15:01 | 02:02 06:02 | 04:01 11:01 | SE−/− |
| 9 | Female | 03:01 07:01 | 02:01 03:03 | 04:01 04:01 | SE−/− |
| 10 | Male | 07:01 13:02 | 02:02 06:09 | 05:01 05:01 | DERAA+/− |
| 11 | Male | 04:01 15:01 | 03:01 06:02 | 04:01 04:01 | SE+/− |
| 12 | Male | 07:01 08:04 | 02:02 04:02 | 04:01 14:01 | SE−/− |
| 13 | Male | 04:03 14:06 | 03:01 03:02 | 02:01 04:01 | SE+/− |
| 14 | Male | 07:01 07:01 | 02:02 03:03 | 11:01 04:01 | SE−/− |
| 15 | Female | 01:01 04:01 | 03:02 05:01 | 04:01 04:01 | SE+/+ |
| 16 | ND | ND | ND | ND | ND |
| 17 | Female | ND | ND | ND | SE −/− |
| 18 | Female | ND | ND | ND | RRAA+ SE+/− |
| CD4 | |||||
| 1 | Female | ND | ND | ND | ND |
| 2 | Male | ND | ND | ND | ND |
| 3 | Female | ND | ND | ND | ND |
| 4 | Male | ND | ND | ND | KRAA− SE−/− |
| 5 | ND | ND | ND | ND | KRAA+ SE+/− |
| 6 | Female | ND | ND | ND | KRAA+ SE+/− |
| 7 | Male | ND | ND | ND | ND |
| 8 | Male | ND | ND | ND | KRAA− SE−/− |
| 9 | Male | ND | ND | ND | KRAA− SE−/− |
| 10 | Male | ND | ND | ND | RRAA+ SE+/− |
| 11 | Female | ND | ND | ND | KRAA− SE−/− |
| 12 | Female | ND | ND | ND | RRAA+ SE+/− |
| 13 | Male | ND | ND | ND | RRAA+ SE+/− |
PBMC or CD4+ cells (with a purity yield of at least 97%) were cultured in IMDM media containing: HEPES, glutamine, 10% serum replacement KnockOut factor, nonessential amino acids, sodium pyruvate (1mM), and antibiotics penicillin and streptomycin at 50 U/ml each (GIBCO, Life Technologies, Grand Island, NY, USA). CD4+ cells were cultured at 37°C in 5% CO2 for 8 days at initial concentration of 8–9 × 105 cells per well in 200 ul of a 96-well plate. Induction of TH17/IL-17A positive cells was achieved using cell culture media containing a cocktail of the following cytokines: IL-1beta (10 ng/ml), IL-2 (20 U/ml), IL-23 (10 ng/ml), and TGF-beta (3 ng/ml) in the presence of neutralizing anti-IFN-gamma (10 µg/ml) and anti-IL-4 (10 µg/ml) antibodies. In addition to cytokines and antibodies, CD4+ cells were polyclonaly activated with T-cell activation beads conjugated with anti-CD2, anti-CD3, and anti-CD28 antibodies (Miltenyi Cambridge, MA, USA) at 2:1 beads/cell ratio. A control of non- polarizing media was devoid of cytokines and neutralizing antibodies but contained T-cell activation beads was used in certain experiments. All cytokines and neutralizing antibodies were purchased from BioLegend (San Diego, CA, USA) unless otherwise stated. Three different 15-mer peptides (from Bioworld, Dublin, OH, USA) were used for culture stimulation in this study: 65-79*0401 (KDLLEQKRAAVDTYC) containing the SE sequence motif, coded by an RA-associated allele DRB1*04:01; 65-79*0402 (KDILEDERAAVDTYC), containing SE negative sequence, coded by an RA-protective allele DRB1*04:02; 65-79*0403 (KDLLEQRRAEVDTYC) containing SE negative sequence, coded by a RA-neutral allele DRB1*04:03.
3.2. Flow cytometry
Staining for intracellular IL-17A cytokine (either in PBMC or CD4+ cells) was carried out in V bottom 96 well plates after stimulation of cells with fresh media containing stimulation cocktail plus protein transport inhibitors (500 x) solution from eBioscience (San Diego, CA, USA) for 4.5 h. Then, around 2 × 106 cells were pooled, washed by centrifugation at 1,250 rpm with MACS rinsing buffer containing BSA 0.5%, and stained with surface markers (either CD4 or CD3) or the respective fluorescent isotype controls for 30 min at 4°C in MACS buffer. After a wash with MACS buffer cells were fixed using intracellular fixing buffer from eBioscience (San Diego, CA, USA) for 20 min in dark at room temperature. Then, cells were washed twice by centrifugation at 1,700 rpm with permeabilization and washing buffer from eBioscience (San Diego, CA, USA) and stained with intracellular marker (IL-17A) or the respective fluorescent isotype controls for 30 min at 4°C in permeabilization and washing buffer. Next, cells were washed twice by centrifugation at 1,700 rpm, resuspended in cell staining buffer from eBiosciences (San Diego, CA, USA) and analyzed by flow cytometry in the Flow Cytometry Core facility at the University of Michigan, Medical School using a BD FACSCalibur flow cytometer. Subsequently, FlowJo software was used to quantify percentage of positive cells in each sample setting gates to reach 0.5% in the right and left upper gates of the graph plots with the respective fluorescent isotype negative controls. Fluorescently label antibodies and respective isotype fluorochrome controls were purchase from BioLegend (San Diego, CA, USA).
3.3. Gender and HLA class II genotyping
Genomic DNA (gDNA) was isolated from blood-derived cells (approximately 5 million cells) from each donor using DNeasy Blood and Tissue kit from Qiagen (Valencia, CA, USA) following instructions provided by manufacturer’s manual. Next, gDNA was used as template for q-RT-PCR genotype analysis starting with a gDNA concentration of 3,000 gene copies and doing subsequently 2 based 10 dilutions (for 300 and 30 gene copies), respectively.
The primers for amplification of HLA-DRB1 sequence were adapted from a published patent (22) as follow: DRβ (amino acid residues 16–23) forward: ATTTCTTCAATGGGACGGAGC; DRβ (amino acid residues 87–94) reverse: CGCCGCTGCACTGTGAAGCTCTC; each primer was used at 500 nM final concentration. The sequence for probe specific for KRAA and RRAA alleles were as described (22) adding an extra G at the 3’ end: AGAAGCGGGCCGCGG and ACAGGCGGGCCGCGG (with FAM, ZEN, and Iowa Black modifications included) respectively, and used at 200 nM final concentration. The q-RT-PCR for genotyping was done using fast TaqMan program for 20 µl per well (5 µl template) with settings advised for TaqMan Universal Master Mix II containing UNG. Undetermined Ct throughout gDNA dilutions used was considered negative SE genotype donor and Ct values ranging from 28.5 up to 33.71 (for 3,000 and 30 copies of the gene, respectively) were considered positive SE (KRAA or RRAA) donor.
For gender determination gDNA (5 µg per reaction) was used as a template in a SYBR green based q-RT-PCR. Primers for DYS14 (multi copy locus for testis Y-linked specific protein) are described in (23) and they were used at 40 nM final concentration. The primers for 18s as an internal control were used at a 20nM final concentration. The q-RT-PCR for gender determination was done using settings advised in the Fast SYBR Green Master mix (AB Applied Biosystems, Life Technologies, Grand Island, NY, USA) and 7.5 µl of gDNA as template with 20 ul total reaction volume. A StepOne thermocycler device (Applied Biosystems, Life Technologies, Grand Island, NY, USA) was used. There was a clear difference between males and females. For instance deltadeltaCt values calculated were less than 1 × 10^-5 for females and over 0.2 for males. The primers and probes were purchased from IDT Integrated DNA Technologies (Coralville, IA, USA).
HLA class II genotyping was performed by the University of Michigan Histocompatibility Laboratory. Analyses were done by low- and high-resolution PCR-based allelic typing technology (24). These methodologies are used routinely to analyze donor compatibility for transplantation.
3.4. Cytokine quantification
Human IL-17A and IFN-gamma were quantified in PBMC culture supernatants by ELISA techniques following provider’s instructions. For IL-17A quantification an Optimax kit from IMGENEX (San Diego, CA) was used. IFN-gamma was quantified using a BIOLEGEND (San Diego, CA) standard ELISA kit.
3.5. Statistical analysis
The data were analyzed using IBM SPSS Statistics version 20 and GraphPad Prism version 5. Linear mixed models were used to analyze the data due to repeated measures on the same individuals being present in data sets. Models included variables of interest as covariates as well as random intercepts for individuals as a way to control for the dependence present in the data from the repeated measures.
4. RESULTS
4.1. T cell polarizing effects on PBMC by HLA-DR4-derived peptides
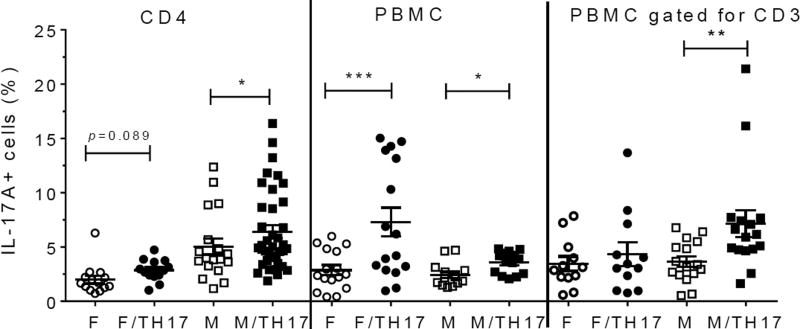
PBMCs were collected from 18 healthy donors (8 males, 7 females, and 3 samples with undetermined gender which were not included in the data analysis, Table 1). As can be seen in Figure 1, the percentage of IL-17A cells in peptide-untreated PBMC differed depending on the gender, the type of cells analyzed, and the response to TH17 polarizing cytokines. Contrary to pure CD4 cells, where male samples showed a higher percentage of TH17 cells either with or without TH17 polarizing cytokines (see below), in PBMC samples, IL-17A positive cells from females were more abundant after TH17 polarizing cytokines treatment (Figure 1). However, upon gating PBMC for CD3, the percentage of IL17-A cells showed a similar trend to the one obtained with pure CD4 cells (Figure 1).
Figure 1. Influence of gender on the percentages of IL-17A positive cells in unstimulated cells.
Purified CD4 T cells form females (n=5) or males (n=7), and PBMC from females (n=7) or males (n=8) were incubated for 8 days in the presence or absence of TH17-polarizing cytokines and the percentage of IL-17A positive cells was analyzed by flow cytometry. Data represent mean ± SEM of percentage of IL-17A-positive cells. Due to repeated measures on the same individuals, the p values were calculated by linear mixed model with percentage of IL-17A positive cells as outcome, gender, cytokines and gender-cytokines interaction as covariates. The marginal mean comparisons were done to compare cytokines within gender and gender within cytokines. *, p<0.05; **, p<0.01 and ***, p<0.001. Abbreviations: F, female; M, male; F/TH17, female cells treated with TH17-polarizing cytokines; M/TH17, male cells treated with TH17-polarizing cytokines.
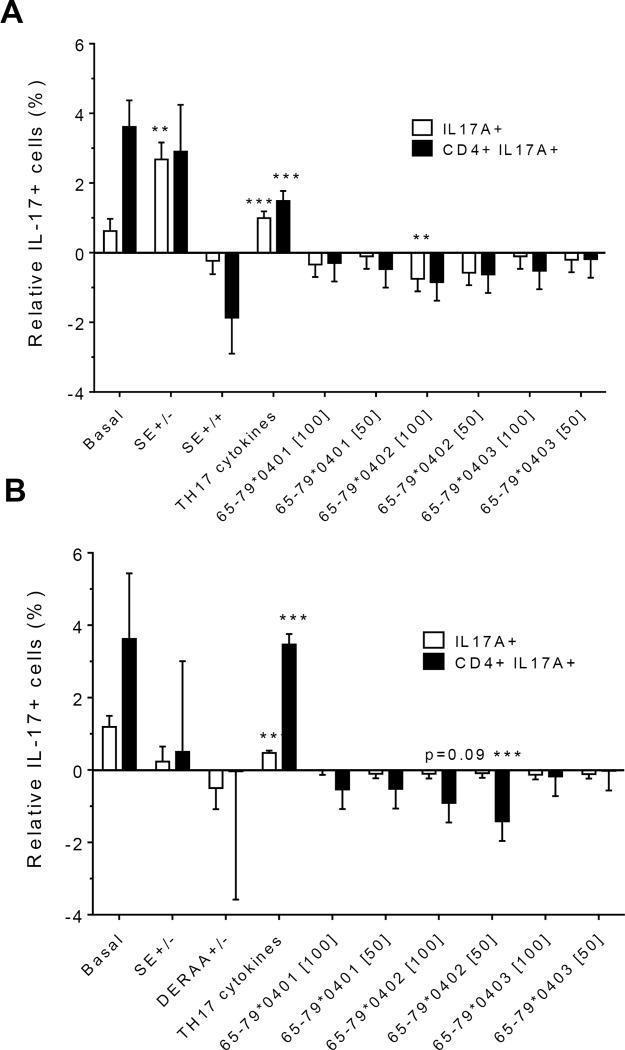
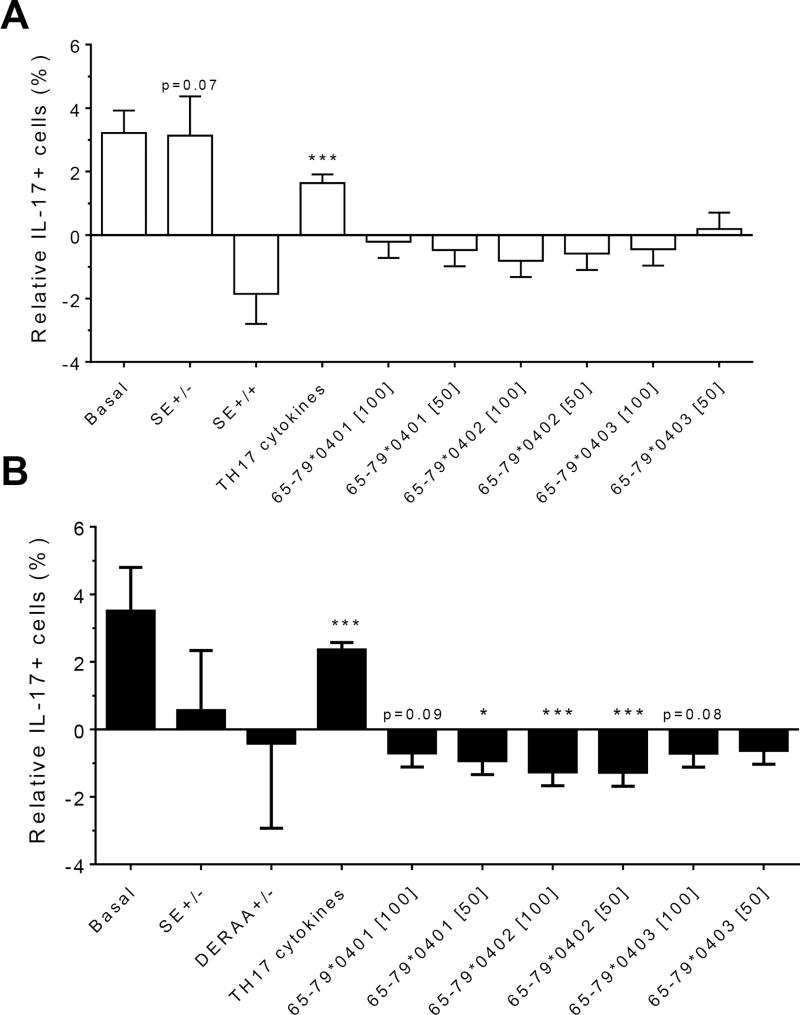
Because PBMCs contain mixed cell populations, the flow cytometer analysis was done using linear mixed models, a linear regression analysis which includes random and fixed effects of repeated observations. This analysis was performed while accounting for percentages of IL-17A single-positive cells, as well as CD4, IL-17A double-positive cells. As shown in figures 2A (females) and 2B (males), treatment with TH17 polarizing cytokines significantly increased the percentage of IL-17A-positive cells, compared to cultures without cytokines, irrespective of cell type or gender. The percentage of IL-17A-positive cells in females was significantly inhibited by 65-79*0402 peptide treatment in single-positive cells, but in male samples, the inhibitory effect reaches significance only in CD4, IL-17A double-positive cells (Figures 2A and 2B). An intriguing gender effect was found in which female PBMC samples carrying SE-positive alleles showed a significantly higher percentage of IL-17A single-positive cells, compared to SE-negative females (Figure 2A). Gating PBMCs for CD3 showed that the main targets of peptides inhibition in males were CD3+ cells. This subset was not affected by the peptides in female samples (compare Figures 3A versus 3B). These findings are consistent with the known association of RA with both female gender and the SE.
Figure 2. Effect of HLA-DR4-derived peptides on IL-17A-positive cell differentiation in vitro.
Percentage of IL-17A single positive, or IL-17A+ CD4+ double positive cells were determined by flow cytometry in cells treated with different concentrations (µg/ml) of different peptides. Data represent mean ± SEM of estimated fixed effects of TH17 cell percentages, modeled by linear mixed model analysis (SPSS, IBM), using a gender-stratified data file and considering the effects of SE status, as well as the presence of TH17 polarizing cytokines or peptides. The basal value reflects the average percentage of IL-17A positive cells obtained in a condition were all the parameters are set to zero, namely in the absence of SE TH17-polarizing cytokines and peptide. Individual values represent the change compared to the basal value. **, p<0.01; ***, p<0.001.
Figure 3. Effect of HLA-DR4-derived peptides on IL-17A+, CD3+ double-positive cell differentiation in vitro.
PBMC were incubated with different peptides at different concentrations, with or without TH17-polarizing cytokines. The percentage of IL-17A+, CD3+ double-positive cells was determined by flow cytometry and analyzed statistically as in Figure 2. *, p<0.05; ***, p<0.001 .
4.2. Polarizing effects of HLA-DR4-derived peptides on purified CD4-T cells
CD4 T cells were negatively purified from 13 unidentified healthy donors (7 males, 5 females and 1 sample with undetermined gender which was not included in analysis, Table 1). Peptides were incubated with pure CD4 cells in the presence of T-cell activation beads, in the presence or absence of TH17-polarizing cytokines cocktail for 8 days and analyzed by flow cytometry.
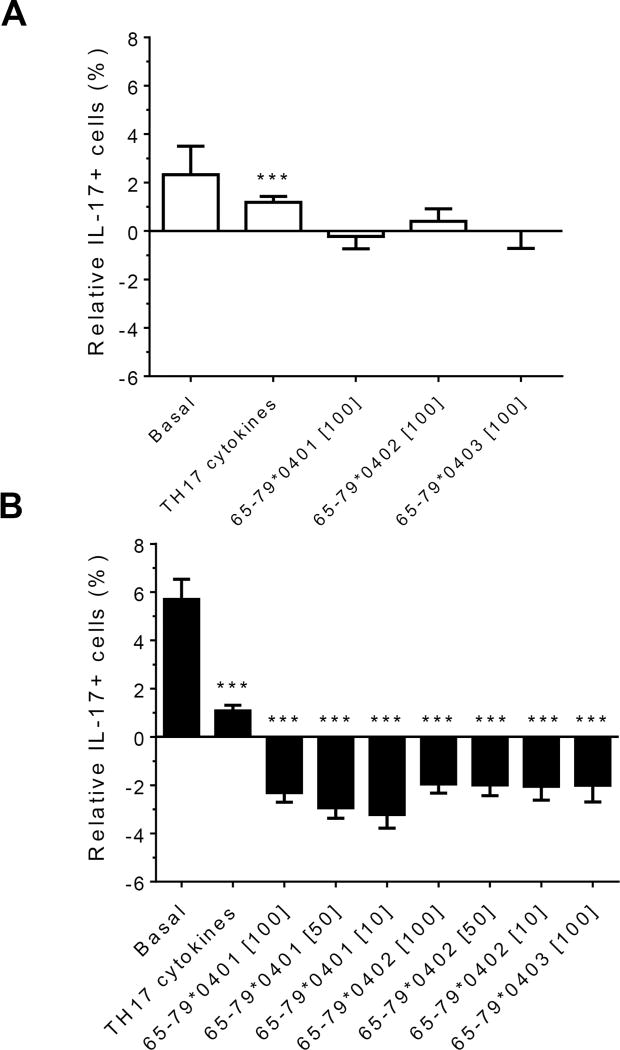
Modeling the data with linear mixed models, considering effects of gender, peptide treatment, SE status, TH17 polarizing cytokines treatment, and an interaction between peptides treatment and gender, we observed significant inhibitory effect of peptides on TH17-positive cells, mainly in males (Figures 4A and 4B). As shown in figure 4A, TH17 cell abundance in females was not affected significantly by peptide treatment. In conclusion, purified male CD4 T cells show a more noticeable TH17 regulation by HLA-DR4-derived peptides than female cells.
Figure 4. Gender effect on in vitro TH17 differentiation in the presence of HLA-DR4-derived peptides.
Purified CD4 T cells were incubated with different peptides at different concentrations, with or without TH17-polarizing cytokines. The percentage of IL-17A cells was determined by flow cytometry and analyzed statistically as in Figure 2. ***, p<0.001.
4.3. Effects of HLA-DR4-derived peptides on cytokine secretion
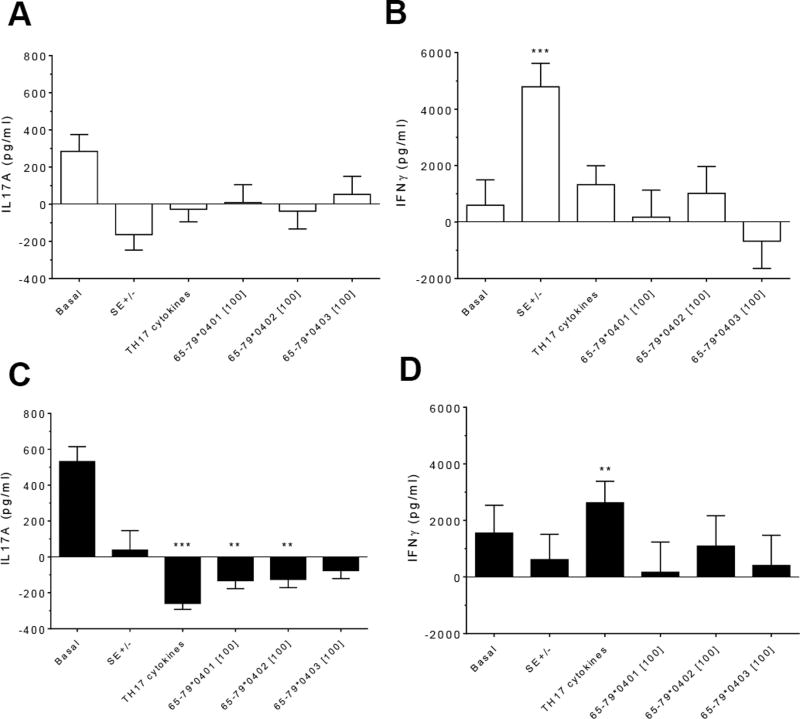
To better characterize the immune effects of HLA-DR4-derived peptides, we determined their impact on the production of two T cell subset-associated cytokines: IL-17A and IFNγ. Consistent with the findings shown above, peptides 65-79*0401 and 65-79*0402 produce significant inhibitory effects on IL-17A and IFNγ production only in males but not in females (Figures 5A and 5C). Interestingly, SE-positive females showed a significantly higher IFNγ secretion, as compared to SE negative females (Figures 5B and 5D). No such effect could be seen in males. Different from data observed in flow cytometry experiments, TH17 polarizing culture medium increased IL-17A production in males compared to incubation without such media (Figures 5C).
Figure 5. Effect of HLA-DR4-derived peptides on cytokine production.
PBMCs from 3 females (A and B) and 6 males (C and D) were incubated for 8 days with the peptides at 100 µg/ml with or without TH17-polarizing cytokines. Concentrations of IL-17A (A and C) and IFNγ (B and D) in supernatants were quantified by ELISA. Data represent mean ± SEM of concentration of cytokines (pg/ml) modeled by linear mixed model analysis (SPSS, IBM), using a gender stratified data file and considering main effects of SE status and treatment with TH17 polarizing cytokines or peptides. The basal value reflects the average cytokine concentration obtained in a condition were all the parameters are set to zero as discussed in the legend to Figure 2. **, p<0.01; ***, p<0.001.
5. DISCUSSION
The SE motif has been previously demonstrated to enhance susceptibility for RA in humans (25, 26) and mice (1, 27–29). Our group has recently concluded that in mice, the mechanism by which SE works seems to be independent of antigen presentation, but rather related to the ability of this 5 amino acids sequence motif to act as a ligand that stimulates innate immune signaling events, which facilitate TH17 polarization and osteoclastogenesis (7, 12, 15, 17, 30). Whether the SE ligand has similar effects in humans is currently unknown.
One potentially relevant difference between humans and mice is that activated T lymphocytes express MHC class II molecules in the former, but not the latter species. Additionally, mice do not have an exact match for SE sequence (21, 31, 32). Strikingly, transgenic mice engineered to express SE-positive HLA class II molecules are more prone to inflammatory and autoimmune diseases than non-transgenic mice (33). Additionally, a higher level of expression of MHC class II lymphocytes from synovial fluid of RA patients than healthy donors has been described as well (34). In light of these considerations, the study described here was designed as the first step toward characterization of SE effects on in vitro TH17 polarization in humans.
Analyzing pure CD4 cell samples, we found a significant gender bias both in terms of constitutive percentages of TH17 cells, and the effect of HLA-DR-derived peptides on TH17 differentiation in vitro. These findings might be relevant for therapeutic decisions in the future, when IL-17A-targeted drugs become widely available. The consequences of these findings could affect the selection of treatments of choice for female versus male RA patients, and could also affect decisions about future research and development of new drugs to regulate TH17 immune polarization in other human diseases.
It has been previously reported that naïve T cells derived from males secrete more IL-17 than cells derived from females (35). In addition, cells from females are more prone to produce TH1-polarized immune response compared to male cells (36). These gender-associated immune effects have been purported to be mediated by regulatory actions of the peroxisome proliferator-activated receptor alpha and gamma (35, 36). Consistent with those reports, our findings suggest that in CD4 cells, the percentage CD4, TH17 double-positive cells show a gender bias as well, since females had lower percentage of TH17 cells than males. Previously, it has been described that males have significantly higher percentage of effector memory T cells but, different from the present study, in that report TH17 percentages were not analyzed (37).
Different from purified CD4 T cells, in unfractionated PBMC populations, higher percentages of IL-17A positive cells were present in females compared to males after TH17 polarizing cytokines treatment (Figure 1), suggesting that the subset that accounts for IL-17A-positive cells in females is mainly non-CD4 cells. In this context it is noteworthy that in the RA joint environment, mast cells and other innate immune cells have been identified as major subsets in which IL-17A positivity can be found (38–40).
Autoimmune diseases - in which TH17 cells have been shown to play a key role – are generally more common in females than in males (26, 41). This relationship is seemingly difficult to reconcile with the finding that males produce more IL-17A compared to females (35), as well as our finding that males have higher percentage of TH17 cells than females. However, when accounting for total IL-17A-positive cells in the PBMC samples, females did have higher numbers of such cells. The mechanisms involved in this gender disparity are probably complex. One possible mechanism may involve regulatory T cells. It has been recently reported that healthy females have lower percentage of peripheral blood regulatory T cells compared to healthy males (42).
Importantly, in this study we have observed a gender biased response when the effects of HLA-DR-derived peptides where analyzed. Those peptides induced lower percentage of IL-17A cells in males compared to females. Our data indicate that peptide 65-79*0401 was more active and specific in pure CD4, and in CD3-gated PBMCs. In contrast, peptides containing the DERAA sequence (65-79*0402) had a broader inhibitory effect on both pure CD4-positive cells and PBMCs (Figures 3 and 5). These data are potentially consistent with the long observed protective effect of DERAA-expressing HLA-DRB1 alleles against severe RA (43). Interestingly, DERAA inhibitory effects were dependent both on the cell type and gender. The main target of 65-79*0402 inhibitory effect was found to be IL-17A single-positive cells in females, and CD4, IL-17A double-positive cells in males (Figure 2). Also, PBMC from SE-positive females had higher percentage of IL-17A single-positive cells, compared to SE-negative female donors (Figure 2). Importantly, this difference was not seen in males. Together, these data suggest that in females, the main contributors to the larger IL-17A positive cell population are non-CD4 cells.
The inhibitory effect of HLA-DR4-derived peptides on the abundance of CD4, TH17-positive cells in males suggests that such peptides may conceivably induce apoptosis of these cells. Enhanced apoptosis has been described in activated human T cells following a T-T interaction, and was attributed to MHC class II/T cell receptor-mediated signaling (44, 45). Interestingly, it has been demonstrated that the density of MHC class II molecules expressed on APC can skew helper T cell polarization in vivo in mice. When the density of MHC class II molecules is diminished, there is an enhanced TH1 polarization, compared to APC expressing normal levels of MHC class II molecules (46). Thus, immune polarization can be regulated in response to a variety of stimuli, including cell-cell interaction and MHC class II density. The data shown here suggest that HLA-DR4-derived peptides might act as ligands and regulate TH17 polarization as well.
Consistent with their effect on cytokine production (Figure 5), HLA-DR4-derived peptides induced an inhibitory effect on the CD3+/IL-17A+ subpopulation of PBMC (Figure 3). However, given the heterogeneous nature of PBMC, we cannot rule out the possibility that immunosuppressive cytokines (e.g. IL-10, IL-4, or IL-27) may have been responsible for inhibiting TH17 cell differentiation. Consistent with this scenario, it has been previously reported that in mice, male-derived APC produce higher levels of IL-10 than female APC (47–49). In addition, cell-cell interaction between APC and T lymphocytes can skew helper T cell polarization, as reported by Evans et al. (50).
In summary, in this study we demonstrate for the first time that polarization of TH17 cells in humans is regulated by a combined effect of HLA-DRB1 allele products and gender. The relevance of these findings to the epidemiology and pathogenesis of RA, as well as the nature of molecular mechanisms that mediate these regulatory effects require further investigation.
Acknowledgments
Dr. Blanco was supported by the Diversity Postdoctoral Fellowship Award from Janssen Research & Development and University of Michigan
Footnotes
AUTHORSHIP CONTRIBUTIONS
LPB, W-PF-L and JH designed the experimental approach. LPB performed the experiments, and wrote the manuscript. LPB and MP analyzed and interpreted the data.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
References
- 1.Bax M, van Heemst J, Huizinga T, Toes R. Genetics of rheumatoid arthritis: what have we learned? Immunogenetics. 2011;63(8):459–466. doi: 10.1007/s00251-011-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Almeida DE, Ling S, Holoshitz J. New insights into the functional role of the rheumatoid arthritis shared epitope. FEBS Letters. 2011;585(23):3619–3626. doi: 10.1016/j.febslet.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis & Rheumatism. 1987;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 4.Carrier N. The DERAA HLA-DR alleles in patients with early polyarthritis: protection against severe disease and lack of association with rheumatoid arthritis autoantibodies. Arthritis and rheumatism. 2009;60(3):698–707. doi: 10.1002/art.24353. [DOI] [PubMed] [Google Scholar]
- 5.Mackie SL, Taylor JC, Martin SG, Wordsworth P, Steer S, Wilson AG, Worthington J, Emery P, Barrett JH, Morgan AW. A spectrum of susceptibility to rheumatoid arthritis within HLA-DRB1: stratification by autoantibody status in a large UK population. Genes Immun. 2012;13(2):120–128. doi: 10.1038/gene.2011.60. [DOI] [PubMed] [Google Scholar]
- 6.McInnes IB, Schett G. The Pathogenesis of Rheumatoid Arthritis. New England Journal of Medicine. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 7.Blanco LP, Ling S, Holoshitz J. Oxidative stress in rheumatoid arthritis: new insights. In: Dichi I, Colado Simao A, Wander Bregano J, Cecchini R, editors. Role of Oxidative Stress in Chronic Diseases. Science Publishers Hampshire, USA; 2013. [Google Scholar]
- 8.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell–derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis & Rheumatism. 1999;42(5):963–970. doi: 10.1002/1529-0131(199905)42:5<963::aid-anr15>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. The Journal of Clinical Investigation. 1999;103(9):1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miossec P, van den Berg WB. IL-17 as a future therapeutic target for rheumatoid arthritis. Nature Reviews Rheumatology. 2009;5(10):549+. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 11.Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, Sloan-Lancaster J. LY2439821, a humanized anti–interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis & Rheumatism. 2010;62(4):929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 12.De Almeida DE, Ling S, Pi X, Hartmann-Scruggs AM, Pumpens P, Holoshitz J. Immune Dysregulation by the Rheumatoid Arthritis Shared Epitope. The Journal of Immunology. 2010;185(3):1927–1934. doi: 10.4049/jimmunol.0904002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holoshitz J, De Almeida DE, Ling S. A role for calreticulin in the pathogenesis of rheumatoid arthritis. Annals of the New York Academy of Sciences. 2010;1209(1):91–98. doi: 10.1111/j.1749-6632.2010.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holoshitz J, Liu Y, Fu J, Joseph J, Ling S, Colletta A, Sharma P, Begun D, Goldstein S, Taichman R. An HLA-DRB1–Coded Signal Transduction Ligand Facilitates Inflammatory Arthritis: A New Mechanism of Autoimmunity. The Journal of Immunology. 2012 doi: 10.4049/jimmunol.1202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling S, Lai A, Borschukova O, Pumpens P, Holoshitz J. Activation of nitric oxide signaling by the rheumatoid arthritis shared epitope. Arthritis & Rheumatism. 2006;54(11):3423–3432. doi: 10.1002/art.22178. [DOI] [PubMed] [Google Scholar]
- 16.Ling S, Cheng A, Pumpens P, Michalak M, Holoshitz J. Identification of the Rheumatoid Arthritis Shared Epitope Binding Site on Calreticulin. PLoS ONE. 2010;5(7):e11703. doi: 10.1371/journal.pone.0011703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling S, Pi X, Holoshitz J. The Rheumatoid Arthritis Shared Epitope Triggers Innate Immune Signaling via Cell Surface Calreticulin. The Journal of Immunology. 2007;179(9):6359–6367. doi: 10.4049/jimmunol.179.9.6359. [DOI] [PubMed] [Google Scholar]
- 18.Ling S, Cline EN, Haug TS, Fox DA, Holoshitz J. Citrullinated calreticulin potentiates rheumatoid arthritis shared epitope signaling. Arthritis & Rheumatism. 2012 doi: 10.1002/art.37814. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arosa FA, de Jesus O, Porto G, Carmo AM, de Sousa M. Calreticulin Is Expressed on the Cell Surface of Activated Human Peripheral Blood T Lymphocytes in Association with Major Histocompatibility Complex Class I Molecules. Journal of Biological Chemistry. 1999;274(24):16917–16922. doi: 10.1074/jbc.274.24.16917. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee PP, Vinay DS, Mathew A, Raje M, Parekh V, Prasad DVR, Kumar A, Mitra D, Mishra GC. Evidence That Glycoprotein 96 (B2), a Stress Protein, Functions as a Th2-Specific Costimulatory Molecule. The Journal of Immunology. 2002;169(7):3507–3518. doi: 10.4049/jimmunol.169.7.3507. [DOI] [PubMed] [Google Scholar]
- 21.Mangalam AK, Rajagopalan G, Taneja V, David CS. HLA Class II Transgenic Mice Mimic Human Inflammatory Diseases. In: Frederick WA, editor. Advances in Immunology. Academic Press; 2008. [DOI] [PubMed] [Google Scholar]
- 22.Baxter-Lowe LA, Gorsky JA. In: Method for HLA typing. Google, editor. The Blood Center Research Foundation, Inc.; 1995. [Google Scholar]
- 23.Horlitz M, Lucas A, Sprenger-Haussels M. Optimized Quantification of Fragmented, Free Circulating DNA in Human Blood Plasma Using a Calibrated Duplex Real-Time PCR. PLoS ONE. 2009;4(9):e7207. doi: 10.1371/journal.pone.0007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlich H. HLA DNA typing: past, present, and future. Tissue Antigens. 2012;80(1):1–11. doi: 10.1111/j.1399-0039.2012.01881.x. [DOI] [PubMed] [Google Scholar]
- 25.Holoshitz J. The rheumatoid arthritis HLA-DRB1 shared epitope. Current opinion in rheumatology. 2010;22(3):293–8. doi: 10.1097/BOR.0b013e328336ba63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nussinovitch U, Shoenfeld Y. The role of gender and organ specific autoimmunity. Autoimmunity Reviews. 2012;11(6–7):A377–A385. doi: 10.1016/j.autrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Behrens M, Smart M, Luckey D, Luthra H, Taneja V. To B or not to B: Role of B cells in pathogenesis of arthritis in HLA transgenic mice. Journal of Autoimmunity. 2011;37(2):95–103. doi: 10.1016/j.jaut.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taneja V, Behrens M, Basal E, Sparks J, Griffiths MM, Luthra H, David CS. Delineating the Role of the HLA-DR4"Shared Epitope" in Susceptibility versus Resistance to Develop Arthritis. The Journal of Immunology. 2008;181(4):2869–2877. doi: 10.4049/jimmunol.181.4.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taneja V, Behrens M, Mangalam A, Griffiths MM, Luthra HS, David CS. New humanized HLA–DR4–transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis & Rheumatism. 2007;56(1):69–78. doi: 10.1002/art.22213. [DOI] [PubMed] [Google Scholar]
- 30.Ling S, Li Z, Borschukova O, Xiao L, Pumpens P, Holoshitz J. The rheumatoid arthritis shared epitope increases cellular susceptibility to oxidative stress by antagonizing an adenosine-mediated anti-oxidative pathway. Arthritis Research & Therapy. 2006;9(1):R5. doi: 10.1186/ar2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko HS, Fu SM, Winchester RJ, Yu DT, Kunkel HG. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. The Journal of Experimental Medicine. 1979;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu DT, Winchester RJ, Fu SM, Gibofsky A, Ko HS, Kunkel HG. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. The Journal of Experimental Medicine. 1980;151(1):91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangalam A, Rodriguez M, David C. Role of MHC class II expressing CD4+ T cells in proteolipid protein91-110-induced EAE in HLA-DR3 transgenic mice. European Journal of Immunology. 2006;36(12):3356–3370. doi: 10.1002/eji.200636217. [DOI] [PubMed] [Google Scholar]
- 34.Smith MD, Roberts-Thomson PJ. Lymphocyte surface marker expression in rheumatic diseases: evidence for prior activation of lymphocytes in vivo. Annals of the Rheumatic Diseases. 1990;49(2):81–87. doi: 10.1136/ard.49.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, Akkermann R, Stanczyk FZ, Prat A, Steinman L, Dunn SE. Peroxisome proliferator-activated receptor (PPAR)alpha and -gamma regulate IFN-gamma and IL-17A production by human T cells in a sex-specific way. Proceedings of the National Academy of Sciences. 2012;109(24):9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, Crowell A, Loh J, Oksenberg J, Steinman L. Peroxisome proliferator–activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell–mediated autoimmunity. The Journal of Experimental Medicine. 2007;204(2):321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J, Greer J, Hull R, O'Sullivan J, Henderson R, Read S, McCombe P. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immunity & Ageing. 2010;7(1):4. doi: 10.1186/1742-4933-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moran EM, Heydrich R, Ng CT, Saber TP, McCormick J, Sieper J, Appel H, Fearon U, Veale DJ. IL-17A Expression Is Localised to Both Mononuclear and Polymorphonuclear Synovial Cell Infiltrates. PLoS ONE. 2011;6(8):e24048. doi: 10.1371/journal.pone.0024048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Cutting Edge: Mast Cells Express IL-17A in Rheumatoid Arthritis Synovium. The Journal of Immunology. 2010;184(7):3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 40.Appel H, Maier R, Wu P, Scheer R, Hempfing A, Kayser R, Thiel A, Radbruch A, Loddenkemper C, Sieper J. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Research & Therapy. 2011;13(3):R95. doi: 10.1186/ar3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barragán-Martínez C, Amaya-Amaya J, Pineda-Tamayo R, Mantilla RD, Castellanos-de la Hoz J, Bernal-Macías S, Rojas-Villarraga A, Anaya J-M. Gender Differences in Latin-American Patients With Rheumatoid Arthritis. Gender Medicine. 2012;9(6):490–510.e5. doi: 10.1016/j.genm.2012.10.005. doi: http://dx.doi.org/10.1016/j.genm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Afshan G. CD4 CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clinical laboratory. 2012;58(5–6):567–71. [PubMed] [Google Scholar]
- 43.Feitsma AL, van der Helm-van Mil AHM, Huizinga TWJ, de Vries RRP, Toes REM. Protection against rheumatoid arthritis by HLA: nature and nurture. Annals of the Rheumatic Diseases. 2008;67(Suppl 3):iii61–iii63. doi: 10.1136/ard.2008.098509. [DOI] [PubMed] [Google Scholar]
- 44.Holling TM, Schooten E, van Den Elsen PJ. Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men. Human Immunology. 2004;65(4):282–290. doi: 10.1016/j.humimm.2004.01.005. doi: http://dx.doi.org/10.1016/j.humimm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Truman J-P, Ericson ML, Choqueux-Séébold CJM, Charron DJ, Mooney NA. Lymphocyte programmed cell death is mediated via HLA class II DR. International Immunology. 1994;6(6):887–896. doi: 10.1093/intimm/6.6.887. [DOI] [PubMed] [Google Scholar]
- 46.DiMolfetto L, Neal HA, Wu A, Reilly C, Lo D. The Density of the Class II MHC T Cell Receptor Ligand Influences IFN-gamma/IL-4 Ratios in Immune Responsesin Vivo. Cellular Immunology. 1998;183(1):70–79. doi: 10.1006/cimm.1997.1231. [DOI] [PubMed] [Google Scholar]
- 47.Hussain S, Stohlman SA. Peritoneal macrophage from male and female SJL mice differ in IL-10 expression and macrophage maturation. Journal of Leukocyte Biology. 2012;91(4):571–579. doi: 10.1189/jlb.0711351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilcoxen SC, Kirkman E, Dowdell KC, Stohlman SA. Gender-Dependent IL-12 Secretion by APC Is Regulated by IL-10. The Journal of Immunology. 2000;164(12):6237–6243. doi: 10.4049/jimmunol.164.12.6237. [DOI] [PubMed] [Google Scholar]
- 49.Hunter Christopher A, Kastelein R. Interleukin-27: Balancing Protective and Pathological Immunity. Immunity. 2012;37(6):960–969. doi: 10.1016/j.immuni.2012.11.003. doi: http://dx.doi.org/10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proceedings of the National Academy of Sciences. 2007;104(43):17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]