Abstract
Interferon-induced protein with tetratricopeptide repeats 1 (IFIT1) expression, involved in the regulation of translation, has been implicated to mediate resistance to chemotherapy and radiation in cancer cells in vitro. The purpose of this study was to evaluate the prognostic significance of IFIT1 protein expression in patients with breast cancer treated with Breast-Conserving Surgery and Radiation Therapy (BCS + RT). A tissue microarray was constructed with specimens from 282 women with node-negative, early-stage (I/II) breast cancer who were treated with BCS + RT. Immunohistochemistry was used to stain for the IFIT1 protein. Cytoplasmic IFIT1 protein expression levels were correlated with clinicopathologic factors, local relapse-free survival (LRFS), disease-free survival (DFS), and overall survival (OS). IFIT1 positivity was found in 123 (49%) of cases. The median follow-up time was 7.3 years. Eighty percent of the patients had T1 disease, 88% were human epidermal growth factor receptor 2 (HER2) negative, and 20% had triple-negative breast cancer (TNBC). IFIT1 positivity was associated with estrogen receptor negative status (p = 0.002), progesterone receptor negative status (p = 0.02), TNBC (p = 0.01), and HER2-positive status (p = 0.006). In univariate and multivariate analysis, IFIT1 positivity was associated with improved LRFS (p = 0.055 and p = 0.04, respectively). Using a log-rank test, IFIT1 positivity was found to be associated with improved LRFS (94% versus 85%, p = 0.046) but not DFS or OS at 10 years. On subset analysis of the TNBC patients, IFIT1 positivity was found to correlate with improved LRFS (100% versus 53%, p = 0.004) and DFS in (87% versus 49%, p = 0.048) at 10 years. Elevated IFIT1 protein expression is associated with improved LRFS. In addition, our data suggest that IFIT1 expression may help risk stratify patients with TNBC who may benefit from more aggressive therapy. As there is limited data on IFIT1 in breast cancer, additional work is needed to ascertain its significance.
Keywords: Early-stage breast cancer, interferon-induced protein with tetratricopeptide repeats 1, local control, molecular markers, radiation therapy
The management of breast cancer has been clearly impacted by the identification of both prognostic and therapy predicative molecular markers. Molecular markers used in genomic profiles allow for individualized treatments plans by identifying patients with disease that is sensitive or resistant to current therapies. Tailoring treatment improves outcomes as patients who are likely to have occult metastasis or resistant disease are given intensified treatment while overtreatment is avoided in patients who do not need treatment or show response to less toxic regimens.
Interferon-stimulated genes (ISG) have been identified as potential prognostic markers and possess a critical role in the suppression of malignant cells via cancer immunoediting, the process by which the immune system inhibits carcinogenesis (1–3). There are several hundred ISGs that are collectively involved in mechanisms of virus inhibition and resistance, cancer immunoediting, cell homeostasis and communication (4). ISG transcription is activated by the binding of interferon (IFN)-induced regulatory factors to interferon response elements (ISRE), which are present in the promoter region of all ISGs (5,6).
Interferon-induced protein with tetratricopeptide repeats 1 (IFIT1) is one of the first ISGs to be discovered and cloned. An in vitro study of the Nu61 squamous cell carcinoma cell line has demonstrated that IFIT1 is among 32 IFN-related DNA damage resistance (IRDS) genes that may regulate resistance against radiation- and chemotherapy-induced DNA damage (7). Weichselbaum et al. (7) created an IRDS classifier based on the mRNA expression of seven IRDS genes including IFIT1. This classifier was validated using independent breast cancer data sets and DNA microarrays. For each data set, the IRDS score improved predication accuracy for local regional control in patients treated with radiation after breast-conserving surgery. Specifically, the IRDS(−) group had a markedly better recurrence-free survival compared with IRDS(+) patients. This improvement in recurrence-free survival is a result of both fewer distant relapses among the IRDS(−) patients treated with adjuvant chemotherapy and lower local regional failure among IRDS(−) patients treated with adjuvant radiation therapy.
The treatment of early-stage breast cancer with radiation therapy has been shown to improve local control and increased local control improves overall survival (8,9). In addition, resistance to radiation therapy is implicated in local failure after breast-conserving surgery (10,11). Identifying molecular markers that predispose to radiation resistance can aid in developing tailored treatment plans for patients. IFIT1 is an interferon-stimulated protein that is involved in the regulation of translation and it has been implicated in mediating resistance to chemotherapy and radiation in cancer cells in vitro (7). The purpose of this study is to analyze the clinical, pathologic, and prognostic significance of IFIT1 in a cohort of early-stage, node-negative breast cancer patients treated with breast-conserving surgery and radiation therapy.
MATERIALS AND METHODS
Patient Characteristics
The 282 patients in this study were treated at the Yale University Department of Therapeutic Radiology, New Haven, CT between 1975 and 2005. The inclusion criteria were (a) node-negative, early-stage (I/II) cancer (b) treated with breast-conserving surgery followed by whole-breast radiation therapy (c) and the primary breast cancer tissue was available for study in paraffin-embedded blocks from the archives of the hospital or referring hospitals for processing into a tissue microarray.
Information about the patient’s clinical history was extracted from patient charts and assembled into a HIPAA compliant data base (12). The age of patients was defined by age at diagnosis. The size of the primary tumor was defined as the largest tumor diameter reported by the pathologist following surgery. Lymph node status was determined by histologic evidence of lymph node metastases. The primary end points of the study are local recurrence, distant metastasis-free survival (DMFS), and overall survival. Local recurrence was defined as clinical or biopsy-proven tumor recurrence in the ipsilateral breast. Distant metastases were defined as clinical evidence of distant disease based on clinical or radiographic evidence. The protocol was reviewed and approved by the Human Investigations Committee at the Yale University School of Medicine. This study was designed in accordance with the REMARK criteria for tumor marker studies (13).
All patients in this study were treated with breast-conserving surgery with or without axillary lymph node dissection, as clinically indicated and based on the standard practice patterns during the time period. After surgery, patients were treated with whole-breast radiation therapy. The median dose to the whole breast was 48 Gy, routinely followed by an electron cone down to the lumpectomy cavity for a total median dose of 64 Gy. Regional nodes were treated to a median dose of 46 Gy when clinically indicated. Adjuvant systemic chemotherapy and/or adjuvant hormone therapy was administered as clinically indicated in accordance with the practices of medical oncologists during this time interval.
Tissue Microarray
Distinct areas of tumor, separate from stroma and normal epithelium, on hematoxylin- and eosin-stained slides of the archived paraffin blocks of breast cancer tissue were identified by a pathologist and marked for subsequent analysis. Each tumor section was prepared in duplicate and extracted using a Tissue Microarrayer device (Beecher Instruments, Silver Spring, MD). Sections of the microarrays 5-μm thick were cut with a tape-based tissue transfer system (Intrumedics, Hackensack, NJ) and processed on to slides.
Immunohistochemistry
Immunohistochemistry was performed on 5-μm thick tissue sections prepared from formalin-fixed, paraffin-embedded tissue from the constructed tissue microarray block. Tissue sections were de-paraffinized and then quenched in 2% hydrogen peroxide-methanol solution. Samples were then pretreated to promote antigen retrieval with the DAKO Target Retrieval Solution (DAKO, Carpinteria, CA). A 3% hydrogen peroxide solution was used for endogenous peroxidase blocking. Slides were then incubated with antibody IFIT1 (Abcam CAT#AB7534, 1:200 dilution, Cambridge, MA). After incubation, the slides were washed in phosphate-buffered saline and a biotinylated secondary antibody was applied. Samples were then incubated with DAKO streptavidin-horseradish peroxidase using LSAB + Kit. 3,3-diaminobenzidine tetrahydrochloride dehydrate was applied as a chromogenic substrate. Finally, slides were counterstained with hematoxylin, dehydrated with ethanol, and mounted. A known positive case was included as positive control. For the negative control, the primary antibody was replaced with non-immune mouse serum.
Quantitative and qualitative assessment of all biomarkers stained was performed by a single, experienced pathologist (H.W.) who was blinded to patient outcomes. For cores that were uninterpretable because of tissue loss or lack of tumor cells, a score of not applicable was given. The intensity of IFIT1 staining was scored as 0 (no immunoreactivity), 1 (weak), 2 (moderate), or 3 (strong) in tumor cells. Cases scored with 0 and 1 were considered a group as negative expression levels, whereas cases scored with 2 and 3 were considered positive. This cutoff was chosen as it is routinely used in our laboratory and others as a typical cutoff for positive expression.
Statistical Analysis
The correlation between IFIT1 expression, conventional tumor markers, and patient characteristics was ascertained by standard chi-squared tests and Fisher’s exact test. Estimates of local relapse-free survival (LRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), cause-specific survival (CSS), and overall survival (OS) were calculated by the Kaplan–Meier product-limit method, and the differences were assessed by the log-rank test. Probabilities of survival were calculated from the date of breast cancer diagnosis to either the date at which relapse from breast carcinoma was clinically identified or the date of last contact. Multivariate survival analysis using Cox’s proportional hazard regression model was carried out to assess the independent contribution of each variable to survival. All tests of statistical significance were two sided. Values of p < 0.05 were considered statistically significant. Statistical analysis was carried out using SAS software (Version 9.1; SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
This study includes 282 women with early-stage, node-negative breast cancer treated with breast-conserving surgery and radiation therapy. The median age at diagnosis was 55 (range 25–88). Forty-two percent of the patients were under the age of 50 at the time of diagnosis. Eighty percent of the patients had T1 disease. Forty-six percent and 35% received adjuvant hormonal therapy and adjuvant chemotherapy, respectively. Sixty-three percent and 56% of the patients had estrogen receptor positive and progesterone receptor positive disease, respectively. Thirteen percent were HER2 positive, and 20% had triple-negative breast cancer (TNBC).
As of September 2008, there was a median follow-up time of 7.3 years (range, 1.0–25.9 years). The ipsilateral breast relapse-free rate for the whole cohort at 5 and 10 years was 94% and 90%, DMFS of 94% and 89%, and OS of 94% and 88%, respectively. Additional clinical and pathologic information can be found in Table 1.
Table 1.
Patient Clinical and Pathologic Characteristics
| n (%) | |
|---|---|
| Age (years) | |
| ≤ 50 | 118 (42) |
| >50 | 164 (58) |
| Race | |
| White | 236 (84) |
| Black | 36 (13) |
| Other | 10 (4) |
| Smoking history | |
| Negative | 140 (66) |
| Positive | 72 (34) |
| Unknown | 70 |
| Tumor stage | |
| T1 | 226 (80) |
| T2 | 53 (19) |
| Other/Unknown | 3 (2) |
| Surgical margin status | |
| Negative | 240 (93) |
| Close/Positive | 18 (7) |
| Unknown | 24 |
| Received adjuvant hormone therapy | |
| No | 151 (54) |
| Yes | 128 (46) |
| Received adjuvant chemotherapy | |
| No | 183 (65) |
| Yes | 97 (35) |
| Estrogen receptor status | |
| Negative | 99 (37) |
| Positive | 171 (63) |
| Unknown | 12 |
| Progesterone receptor status | |
| Negative | 105 (44) |
| Positive | 135 (56) |
| Unknown | 42 |
| HER2 status | |
| Negative | 218 (88) |
| Positive | 31 (13) |
| Unknown | 33 |
| Triple-negative status | |
| No | 201 (80) |
| Yes | 51 (20) |
| Unknown | 30 |
| IFIT1 cytoplasmic expression | |
| Negative | 126 (51) |
| Positive | 123 (49) |
| Unknown | 33 |
Immunostaining
In the breast cancer tissue, the predominant staining for IFIT1 was cytoplasmic, but there was some nuclear staining. Staining of the sections was heterogeneous, ranging from a few tumor cells to almost all cells. IFIT1 expression was positive in 123 (49%) of the 282 patients, using the criteria previously described in the methods. Figure 1 shows representative immunostaining.
Figure 1.
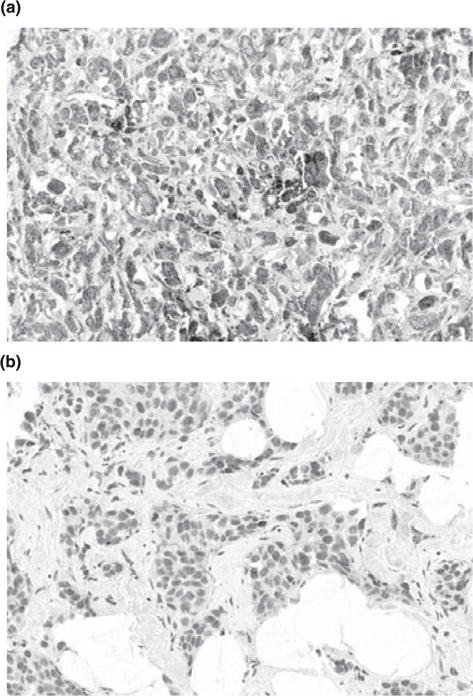
Representative slides of positive (a) and negative (b) cases with IFIT1 immunostaining.
IFIT1 Expression Correlation with Clinical-Pathologic and Patient Outcomes
IFIT1 expression was compared with known clinical-pathologic features of the patients using chi-square analysis and Fisher’s exact test. IFIT1-positive status is associated with nonwhite race (p = 0.004), estrogen and progesterone receptor negative status (p = 0.002 and 0.02, respectively), TNBC (p = 0.0125), HER2-positive status (p = 0.006), and receiving adjuvant chemotherapy (p < 0.05). IFIT1 did not significantly correlate with any other variables in Table 2.
Table 2.
Chi-Square Analysis
| Characteristic | IFIT1 Negative n, (%) (%) | IFIT1 Positive n, (%) | p |
|---|---|---|---|
| Age (years) | |||
| ≤ 50 | 46 (18) | 57 (23) | 0.12 |
| >50 | 80 (32) | 66 (27) | |
| Race | |||
| White | 114 (46) | 95 (38) | 0.004 |
| Nonwhite | 12 (5) | 28 (11) | |
| Smoking | |||
| Negative | 61 (32) | 66 (35) | 0.39 |
| Positive | 35 (18) | 29 (15) | |
| Tumor stage | |||
| T1 | 107 (43) | 95 (38) | 0.29 |
| T2 | 19 (8) | 26 (10) | |
| Surgical margin status | |||
| Negative | 106 (46) | 107 (47) | 0.34 |
| Close/Positive | 6 (3) | 10 (4) | |
| Estrogen receptor status | |||
| Negative | 32 (13) | 53 (22) | 0.002 |
| Positive | 89 (37) | 64 (27) | |
| Progesterone receptor status | |||
| Negative | 36 (17) | 54 (25) | 0.02 |
| Positive | 69 (32) | 54 (25) | |
| HER2 status | |||
| Negative | 106 (46) | 95 (41) | 0.006 |
| Positive | 7 (3) | 21 (9) | |
| Triple-negative status | |||
| No | 98 (43) | 83 (37) | 0.01 |
| Yes | 15 (7) | 30 (13) | |
| Received adjuvant hormone therapy | |||
| No | 60 (24) | 69 (28) | 0.16 |
| Yes | 65 (26) | 52 (21) | |
| Received adjuvant chemotherapy | |||
| No | 89 (36) | 71 (29) | 0.049 |
| Yes | 37 (15) | 50 (20) | |
Univariate analysis showed that IFIT1 expression approached significance for improved LRFS (hazard ratio 0.369, p = 0.055) (Table 3). Age greater than 50 predicted for improved LRFS (hazard ratio 0.333, p = 0.016) and improved DMFS (hazard ratio = 0.438, p = 0.05), but also predicted a trend toward poorer overall survival (hazard ratio = 2.31, p = 0.055). In addition, T2 tumors (hazard ratio = 2.237, p = 0.01) and lack of hormonal therapy (hazard ratio = 0.334, p = 0.03) predicted poorer DMFS.
Table 3.
Univariate Analysis of IFIT1 Expression
| Characteristic | LRFS
|
DMFS
|
CSS
|
OS
|
||||
|---|---|---|---|---|---|---|---|---|
| p | HR | p | HR | p | HR | p | HR | |
| Age (years) | ||||||||
| ≤ 50 (Ref.) | 0.016 | 0.333 | 0.05 | 0.438 | 0.39 | 1.484 | 0.06 | 2.31 |
| >50 | ||||||||
| Race | ||||||||
| White (Ref.) | 0.99 | 0.989 | 0.84 | 0.884 | 0.27 | 0.32 | 0.16 | 0.236 |
| Black | ||||||||
| Smoking history | ||||||||
| Negative (Ref.) | 0.37 | 1.602 | 0.36 | 1.67 | 0.03 | 3.041 | 0.18 | 1.795 |
| Positive | ||||||||
| Tumor stage | ||||||||
| T1 (Ref.) | 0.34 | 1.519 | 0.01 | 2.237 | 0.15 | 1.745 | 0.09 | 1.773 |
| T2 | ||||||||
| Surgical margin status | ||||||||
| Negative (Ref.) | 0.38 | 1.935 | 0.68 | 0.655 | 0.82 | 0.796 | 0.53 | 0.524 |
| Close/Positive | ||||||||
| Adjuvant hormone therapy | ||||||||
| No (Ref.) | 0.23 | 0.58 | 0.03 | 0.334 | 0.08 | 0.411 | 0.42 | 0.726 |
| Yes | ||||||||
| Adjuvant chemotherapy | ||||||||
| No (Ref.) | 0.99 | 1.005 | 0.47 | 1.361 | 0.27 | 0.544 | 0.21 | 0.534 |
| Yes | ||||||||
| ER status | ||||||||
| Negative (Ref.) | 0.30 | 1.637 | 0.24 | 0.617 | 0.59 | 1.284 | 0.47 | 1.344 |
| Positive | ||||||||
| PR status | ||||||||
| Negative (Ref.) | 0.69 | 1.182 | 0.38 | 0.691 | 0.95 | 1.028 | 0.88 | 1.061 |
| Positive | ||||||||
| HER2 status | ||||||||
| Negative (Ref.) | 0.70 | .79 | 0.19 | 2.086 | 0.99 | 0.994 | 0.62 | 0.693 |
| Positive | ||||||||
| Triple-negative status | ||||||||
| No (Ref.) | 0.65 | 1.243 | 0.93 | 1.047 | 0.48 | 0.642 | 0.71 | 0.829 |
| Yes | ||||||||
| IFIT1 expression | ||||||||
| Negative (Ref.) | 0.055 | 0.369 | 0.83 | 0.905 | 0.49 | 0.702 | 0.82 | 0.909 |
| Positive | ||||||||
LRFS, local relapse-free survival; DMFS, distant metastasis-free survival; CSS, cause-specific survival; OS, overall survival; Ref., reference group; HR, hazard ratio; p, p-value; ER, estrogen receptor; PR, progesterone receptor; HER2, Human Epidermal Growth Factor Receptor 2.
Kaplan-Meier survival analysis shows that IFIT1 expression is associated with improved LRFS (94% versus 85%, p < 0.05) (Fig. 2). In addition subset analysis of patients who are triple-negative shows improved LRFS and DFS time (100% versus 53%, p = 0.004 and 87% versus 49%, p < 0.05, respectively) (Fig. 3 and Table 4). Within the IFIT1-positive cohort, there were 19 local relapses and 32 distant metastases. Within the IFIT1-negative cohort, there were 24 local relapses and 30 distant metastases.
Figure 2.
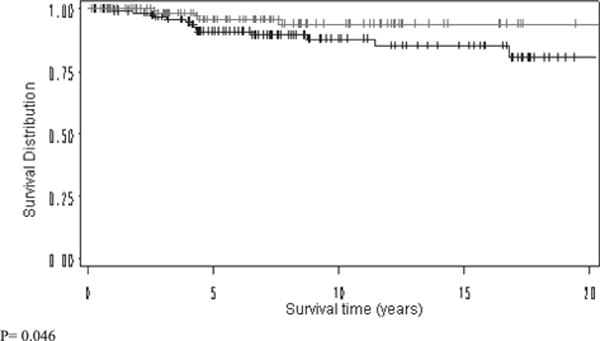
Kaplan-Meier Breast relapse-free survival analysis of IFIT1 positive (red) and negative (black) cohorts.
Figure 3.
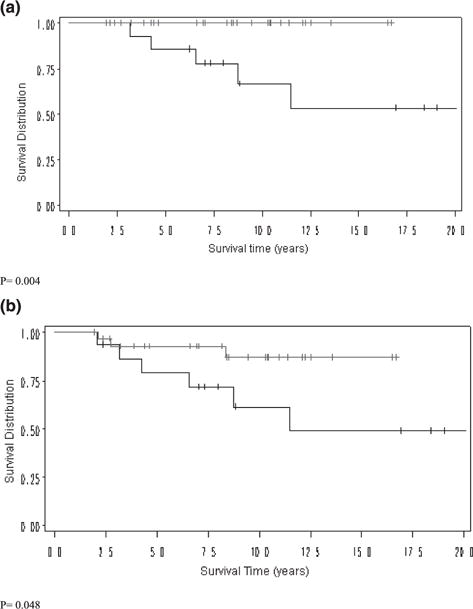
Kaplan-Meier survival analysis of IFIT1 positive (red) and negative (black) cohorts in the triple-negative subset (a) Breast relapse-free survival, triple-negative subset (b) Disease-Free Survival, triple-negative subset.
Table 4.
Patient Outcomes (% surviving) at 10-year Follow-Up, Log-rank Analysis
| IFIT1 positive | IFIT1 negative | p-value | |
|---|---|---|---|
| Overall survival | 87.4 | 86.8 | 0.82 |
| Local relapse-free survival | 93.8 | 85.4 | 0.046 |
| Local relapse-free survival, triple negative | 100 | 53.4 | 0.004 |
| Distant metastasis-free survival | 90.1 | 88.1 | 0.83 |
| Disease-free survival | 84.1 | 78.9 | 0.21 |
| Disease-free survival, triple negative | 87.0 | 49.2 | 0.048 |
| Cause-specific survival | 91.6 | 88.4 | 0.49 |
Multivariate analysis using the Cox proportional hazards model included variables from univariate analysis with a p-value ≤ 0.20. IFIT1 expression was found to be significant as an independent prognostic marker for LRFS (p = 0.04) but not DMFS, CSS or OS (Table 5).
Table 5.
Multivariate Analysis of IFIT1 Expression
| LRFS p |
DMFS p |
CSS p |
OS p |
|
|---|---|---|---|---|
| T stage | – | 0.03 | 0.38 | 0.055 |
| Age | 0.005 | 0.68 | – | 0.045 |
| HER2 | – | 0.19 | – | – |
| IFIT1 | 0.04 | 0.48 | 0.87 | 0.53 |
| Adjuvant hormonal therapy | – | 0.02 | 0.15 | – |
| Smoking history | – | – | 0.04 | 0.10 |
| Race | – | – | – | 0.99 |
LRFS, local relapse-free survival; DMFS, distant metastasis-free survival; DFS, disease-free survival; CSS, cause-specific survival; OS, overall survival; p, p-value; T stage, tumor stage; HER2, Human Epidermal Growth Factor Receptor 2.
DISCUSSION
The treatment of early-stage breast cancer with radiation therapy has been shown to improve local control (8,9). In addition, resistance to radiation therapy is implicated in local failure after breast-conserving surgery (10,11). Local regional recurrence (LRR) is a significant predictor of distant metastasis and overall survival (14,15). Molecular markers used in genomic profiles allow for individualized treatments by identifying patients with disease that is sensitive or resistant to treatment. Tailoring treatment improves outcomes as patients who are likely to have occult metastasis and/or resistant disease are given intensified treatment while over treatment is avoided in patients who do not need treatment or show response to less toxic regimens. Several recent studies are examining the use of molecular markers associated with distant metastasis to predict radiation resistance (16–18). Identifying patients at high risk for LRR in advance is desirable as larger excision volume, additional radiation boost, brachytherapy, and/or additional adjuvant modalities may optimize patient care and quality of life, including avoidance of salvage mastectomy (19,20). Refined risk stratification using these markers would also equally benefit low-risk patients who could avoid unnecessary treatments.
Weichselbaum et al. created a classifier based on DNA microarray analysis of the expression of seven IFN-related DNA damage resistance (IRDS) genes, including IFIT1. To the best of our knowledge, this is the only study that examines IFIT1 in cancer. The classifier was examined in the NKI-295 cohort (a series of 295 patients with stage I/II breast cancer treated at the Netherlands Cancer Institute between 1984 and 1995 (8)) and shown to be a therapy predicative marker for adjuvant chemotherapy (8). It was further cross-validated in several independent breast cancer data sets. This confirmed that the IRDS gene classifier is a therapy predicative marker that performs across a heterogeneous patient population. Within the NKI295 cohort, the subset of patients treated with adjuvant radiation therapy was analyzed and the IRDS classifier significantly contributed to prediction accuracy of LRR. IRDS (+) patients who received adjuvant radiation therapy exhibited an increased rate of LRR. In a multivariable Cox model, the IRDS classifier score is independently associated with LRR. Analysis of importance scores using random survival forest (RSF) or a Cox model reveals that the IRDS significantly contributes to prediction accuracy for LRR compared to age, mastectomy, grade 2/3, chemotherapy, ER-negative status, tumor size, and nodal status.
The IFIT1 gene is located on human chromosome 10 and contains two ISREs in its promoter. IFIT1 is 56 kDa cytoplasmic protein that contains 10 tetratricopeptide repeat (TPR) motifs. TPR is a degenerate 34 amino acid motif that folds into a helix-turn-helix and the resulting cassette mediates protein-protein interactions. Specifically, IFIT1 inhibits translation by binding to eukaryotic initiation factor-3 (eIF3) preventing formation of the eIF3-GTP-Met-tRNA ternary complex (21). IFIT1 has been implicated in the antiviral actions of IFNs against viruses such as hepatitis C, west nile, and lymphocytic choriomeningitis (22,23). A recent study shows that it inhibits human papillomavirus DNA replication by binding to the viral protein E1 (24).
The various cellular functions and protein interactions of IFIT1 are not fully understood (21). The multiple TPR sequences have the potential to interact or be modified by other proteins through phosphorylation or ISGylation. Future studies may show that IFIT1 interacts with other IFN-induced or cancer-specific proteins. Most importantly, the mechanism by which IFIT1 independently induces resistance to radiation is not understood. Weichselbaum et al. used shRNA to knockdown IFIT1 and this resensitized the Nu61 cancer cells to doxorubicin in vitro but response to radiation was not reported. A future study should examine the response of Nu61 cancer cells to radiation after IFIT1 knockdown. Weichselbaum et al. determined that IFIT1 is upregulated in the surviving fraction of 34 different cell lines in the NCI60 panel after 2 Gy radiation treatment in vitro. IFIT1 may be clearly associated with radiation resistance, but the Weichselbaum et al. analysis does not prove it is necessary or sufficient for radiation resistance.
The results in our study demonstrate that IFIT1 positivity is associated with significantly improved LRFS. This is contradictory to the previous Weichselbaum et al. study and may be due to the differences in the study populations and design. In contrast with the cohort of this study, the 243 patients in the NKI295 cohort that received adjuvant radiation therapy included a smaller proportion of patients receiving adjuvant hormonal therapy (46% versus 14%), a lower mean age (44 versus 55), and included node-positive patients (57.6% versus 0% in this study). In addition, Weichselbaum et al. examined mRNA expression of IFIT1 and our study examined protein expression. Protein expression may be a better measure of IFIT1 as IFIT1 inhibits translation and cell growth in a dose-dependant manner and may regulate its own translation (25). The degree of inhibition is likely dictated by the relative abundance of eIF3 and IFIT1 in the cell and induction kinetics (25,26). IFIT1 has been shown to have rapid and transient induction which may be partly due to an autoregulatory loop wherein IFIT1 accumulation inhibits its own translation and partly by its rapid turnover (25). Finally, the Weichselbaum et al. study does not independently identify IFIT1 as a prognostic marker for breast cancer, but uses it as a component of a seven gene classifier. This is a strength of their study, as one molecular marker may only be marginally useful in predicting prognosis, but co-expression of several markers may be significant. However, if the individual marker has a relative risk conflicting with the overall model, it may be masked by the other markers and overlooked.
The mechanism by which IFIT1 may cause radiation sensitivity is likely related to its ability to inhibit translation and therefore cellular replication and growth. It is possible that IFIT1 overexpression may keep cancer cells in radiation-sensitive phases of the cell cycle. IFIT1 may inhibit the translation of DNA repair proteins and thus prevent sublethal DNA damage repair. A recent study shows that IFIT1 interacts with IFIT2 which is a pro-apoptotic factor (27). In summary, IFIT1 may function to increase cellular sensitivity to radiation and this improves the local regional control of early-stage, node-negative breast cancer patients.
This study is the first tissue microarray analysis of IFIT1 expression in breast cancer or any cancer, to the best of our knowledge. The strengths of this analysis include the relatively homogenous study population: All 282 patients had stage I/II, node-negative disease treated with breast-conserving surgery and radiation therapy at a single institution. The cohort was followed up for a median of over 7 years. In addition, the study determined the protein expression of IFIT1 rather than mRNA expression as was done previously. Finally, in univariate and multivariate analysis revealed that IFIT1 expression was independently associated with local recurrence-free survival. We recognize that our study is retrospective, and should be considered an exploratory analysis of IFIT1 in breast cancer. Further study of prospective design in a larger cohort of heterogeneous patients and/or patients with characteristics similar to our cohort is necessary to validate IFIT1 as a marker for LRR. Finally, studies to determine the cellular actions of IFIT1 that result in radiation resistance or sensitivity are also needed. If IFIT1 is proven in multiple data sets to have independent prognostic significance, it will help to risk stratify patients for treatment and possibly open the door for research into novel therapies that modulate IFIT1. This study demonstrates the association of IFIT1 positivity with improved LRFS in a sizeable population of early-stage, node-negative patients with breast cancer.
Acknowledgments
The Breast Cancer Research Foundation provided support to BGH for this research.
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
The authors report no disclosures and no conflict of interest.
References
- 1.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 3.Bektas N, Noetzel E, Veeck J, et al. The ubiquitin-like molecule interferon-stimulated gene 15 (ISG15) is a potential prognostic marker in human breast cancer. Breast Cancer Res. 2008;10:R58. doi: 10.1186/bcr2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borden EC, Williams BR. Interferon-stimulated genes and their protein products: what and how? J Interferon Cytokine Res. 2011;31:1–4. doi: 10.1089/jir.2010.0129. [DOI] [PubMed] [Google Scholar]
- 5.Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 6.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–63. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 7.Weichselbaum RR, Ishwaran H, Yoon T, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci. 2008;105:18490–5. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 9.Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst. 2004;96:115–21. doi: 10.1093/jnci/djh013. [DOI] [PubMed] [Google Scholar]
- 10.Recht A, Houlihan MJ. Conservative surgery without radiotherapy in the treatment of patients with early-stage invasive breast cancer. A review Ann Surg. 1995;222:9–18. doi: 10.1097/00000658-199507000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredriksson I, Liljegren G, Palm-Sjovall M, et al. Risk factors for local recurrence after breast-conserving surgery. Br J Surg. 2003;90:1093–102. doi: 10.1002/bjs.4206. [DOI] [PubMed] [Google Scholar]
- 12.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 13.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100:229–35. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 14.Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE, Jr, Jeong JH, Tan-Chiu E, Fisher B, Wolmark N. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–37. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 15.Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247–54. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Haffty BG. Molecular and genetic markers in the local-regional management of breast cancer. Semin Radiat Oncol. 2002;12:329–40. doi: 10.1053/srao.2002.35252. [DOI] [PubMed] [Google Scholar]
- 17.Haffty BG, Glazer PM. Molecular markers in clinical radiation oncology. Oncogene. 2003;22:5915–25. doi: 10.1038/sj.onc.1206704. [DOI] [PubMed] [Google Scholar]
- 18.Nuyten DS, Kreike B, Hart AA, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;8:R62. doi: 10.1186/bcr1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haffty BG, Goyal S. Molecular subtyping of early-stage breast cancer: implications for radiation therapy. Int J Radiat Oncol Biol Phys. 2010;77:1293–5. doi: 10.1016/j.ijrobp.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Haffty BG, Buchholz TA. Molecular predictors of locoregional recurrence in breast cancer: ready for prime time? J Clin Oncol. 2010;28:1627–9. doi: 10.1200/JCO.2009.27.1080. [DOI] [PubMed] [Google Scholar]
- 21.Fensterl V, Sen GC. The ISG56/IFIT1 gene family. J Interferon Cytokine Res. 2011;31:71–8. doi: 10.1089/jir.2010.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wacher C, Muller M, Hofer MJ, et al. Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses. J Virol. 2007;81:860–71. doi: 10.1128/JVI.01167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Pflugheber J, Sumpter R, Jr, et al. Alpha interferon induces distinct translational control programs to suppress hepatitis C virus RNA replication. J Virol. 2003;77:3898–912. doi: 10.1128/JVI.77.7.3898-3912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terenzi F, Saikia P, Sen GC. Interferon-inducible protein, P56, inhibits HPV DNA replication by binding to the viral protein E1. EMBO J. 2008;27:3311–21. doi: 10.1038/emboj.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J, Hui DJ, Merrick WC, Sen GC. A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J. 2000;19:6891–9. doi: 10.1093/emboj/19.24.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terenzi F, Hui DJ, Merrick WC, Sen GC. Distinct induction patterns and functions of two closely related interferon-inducible human genes, ISG54 and ISG56. J Biol Chem. 2006;281:34064–71. doi: 10.1074/jbc.M605771200. [DOI] [PubMed] [Google Scholar]
- 27.Stawowczyk M, Van Scoy S, Kumar KP, Reich NC. The interferon stimulated gene 54 promotes apoptosis. J Biol Chem. 2011;286:7257–66. doi: 10.1074/jbc.M110.207068. [DOI] [PMC free article] [PubMed] [Google Scholar]


